Abstract
Background
Tofacitinib is an oral, small-molecule JAK inhibitor for the treatment of ulcerative colitis (UC). Creatine kinase (CK) levels and CK-related adverse events (AEs) in tofacitinib-treated patients with UC were evaluated.
Methods
Data were analyzed for three UC cohorts: Induction (phase 2 and 3 induction studies); Maintenance (phase 3 maintenance study); Overall [patients who received tofacitinib 5 or 10 mg twice daily (b.d.) in phase 2, phase 3, or open-label, long-term extension studies; data at November 2017]. Clinical trial data for tofacitinib-treated patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis are presented for contextualization.
Results
Week 8 mean change from baseline CK with tofacitinib 10 mg b.d. induction therapy was 91.1 U/L (95% CI, 48.1–134.1) versus 19.2 U/L (8.5–29.9) with placebo. Among patients completing induction with 10 mg b.d. and re-randomized to 52 weeks of maintenance therapy, mean increases from induction baseline to the end of maintenance were 35.9 (8.1–63.7), 90.3 (51.9–128.7), and 115.6 U/L (91.6–139.7), with placebo, 5 and 10 mg b.d., respectively. The incidence rate (unique patients with events per 100 patient-years) for AEs of CK elevation in the tofacitinib-treated UC Overall cohort was 6.6 versus 2.2, 6.5, and 3.7 for tofacitinib-treated patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis, respectively. No serious AEs of CK elevation or AEs of myopathy occurred in UC studies.
Conclusions
In patients with UC, CK elevations with tofacitinib appeared reversible and not associated with clinically significant AEs. UC findings were consistent with tofacitinib use in other inflammatory diseases.
Trial Registration
NCT00787202; NCT01465763; NCT01458951; NCT01458574; NCT01470612; NCT01262118; NCT01484561; NCT00147498; NCT00413660; NCT00550446; NCT00603512; NCT00687193; NCT01059864; NCT01164579; NCT00976599; NCT01359150; NCT02147587; NCT00960440; NCT00847613; NCT00814307; NCT00856544; NCT00853385; NCT01039688; NCT02187055; NCT00413699; NCT00661661; NCT01710046; NCT00678210; NCT01276639; NCT01309737; NCT01241591; NCT01186744; NCT01163253; NCT01877668; NCT01882439; NCT01976364.
Electronic supplementary material
The online version of this article (10.1007/s10620-020-06560-4) contains supplementary material, which is available to authorized users.
Keywords: Ulcerative colitis, Inflammatory bowel disease, Tofacitinib, Creatine kinase, Safety
Introduction
Creatine and its associated enzyme creatine kinase (CK) play a central role in energy metabolism through the cellular mediation of adenosine triphosphate demand [1]. The CK-M cytosolic isoform of CK is expressed primarily in skeletal and cardiac muscle, while the CK-B cytosolic isoform is present in non-muscle tissues [1].
Elevations in CK may be indicative of neuromuscular conditions, including myositis, myopathy, and rhabdomyolysis [2, 3]. CK elevations may also occur due to exercise, endocrine disorders, and the use of prescription drugs and supplements [4]. CK levels may be impacted by gender, ethnicity, and age [2, 5–7], and accordingly, defining normal reference ranges for CK is challenging. A number of studies have demonstrated that manufacture-determined assay reference ranges categorize a substantial proportion of otherwise asymptomatic subjects as having elevated CK levels [8–10].
Tofacitinib is an oral, small-molecule Janus kinase (JAK) inhibitor for the treatment of ulcerative colitis (UC). Elevations in CK have been observed with JAK inhibitors, including tofacitinib [11–13], baricitinib [14], and upadacitinib [15–17], during the treatment of a range of inflammatory diseases. CK elevations with tofacitinib have been shown to be reversible with tofacitinib withdrawal [18] and not associated with myopathies [19]. Asymptomatic, drug-related CK elevations have also been observed with infliximab, a tumor necrosis factor inhibitor (TNFi) used to treat inflammatory bowel disease [20]. However, the origin and implications of CK elevations in patients with UC have not been fully explored.
In this analysis of data from tofacitinib UC clinical studies, we aimed to characterize changes in CK levels, understand the potential clinical significance of these changes, and evaluate possible risk factors. We also present data from tofacitinib-treated patients with rheumatoid arthritis (RA), psoriasis (Pso), and psoriatic arthritis (PsA) to contextualize the data from the UC program.
Methods
Tofacitinib UC Studies
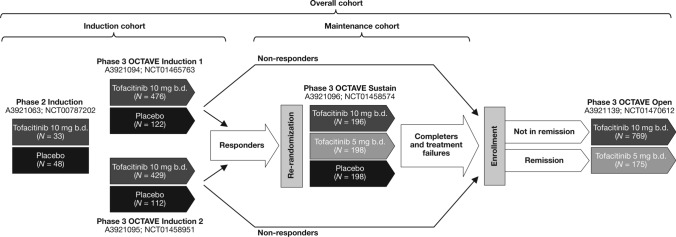
Data for patients with UC were obtained from an 8-week, double-blind, placebo-controlled phase 2 induction study (A3921063, NCT00787202) [21]; two identical, 8-week, double-blind, placebo-controlled phase 3 induction studies (OCTAVE Induction 1 and 2, NCT01465763 & NCT01458951) [12]; a 52-week, double-blind, placebo-controlled phase 3 maintenance study (OCTAVE Sustain, NCT01458574) [12]; and an ongoing, open-label, long-term extension (OLE) study (OCTAVE Open, NCT01470612; data as of November 2017 data cutoff) [22] (Fig. 1). Study design and results from these studies have been previously reported [12, 21, 22].
Fig. 1.
Overview of the tofacitinib UC clinical studies and cohorts included in this analysis. Clinical response in OCTAVE Induction 1 and 2 was defined as a decrease from induction study baseline total Mayo score of ≥ 3 points and ≥ 30%, plus a decrease in rectal bleeding subscore of ≥ 1 point, or an absolute rectal bleeding subscore of 0 or 1. Study A3921139 (OCTAVE Open) is ongoing. Remission was defined as a total Mayo score ≤ 2 with no individual subscore > 1, and a rectal bleeding subscore of 0 b.d. twice daily, N number of patients in each treatment group included in the cohort analysis, UC ulcerative colitis
UC Analysis Cohorts
The UC analyses included patients who received treatment with placebo, tofacitinib 5 mg twice daily (b.d.), and tofacitinib 10 mg b.d.
Data were analyzed for three UC cohorts. The Induction cohort included patients who received placebo or tofacitinib 10 mg b.d. in phase 2 or phase 3 induction studies. The Maintenance cohort included patients who received placebo, tofacitinib 5 mg b.d., or tofacitinib 10 mg b.d. in OCTAVE Sustain. The Overall cohort included patients who received at least one dose of tofacitinib 5 or 10 mg b.d. in any phase 2, phase 3, or OLE study (OLE ongoing at the time of analysis; data as of November 2017 data cutoff).
For tofacitinib-treated patients with UC, mean change from induction study baseline CK level over time was evaluated for two phases: the induction phase (patients from the Induction cohort) and the maintenance phase (patients in OCTAVE Sustain who previously received tofacitinib 10 mg b.d. induction therapy).
Rheumatoid Arthritis, Psoriasis, and Psoriatic Arthritis Cohorts
Data for tofacitinib-treated patients with RA, Pso, and PsA were evaluated for contextualization with the UC cohorts. The RA Overall cohort included tofacitinib-treated patients from phase 1, phase 2, phase 3, phase 3b/4, and OLE studies (OLE ORAL Sequel main study was completed; database locked at time of analysis: March 2017) [23–44]. The Pso Overall cohort included tofacitinib-treated patients from phase 2, phase 3, and OLE studies (all of which were completed as of August 2016) [11, 18, 45–48]. The PsA Overall cohort included tofacitinib-treated patients from phase 3 and OLE studies (OLE ongoing at the time of analysis; data as of January 2017) [13, 49, 50]. The RA, Pso, and PsA studies included in these analyses are summarized in Supplementary Table S1.
Creatine Kinase Evaluations and Analyses
In the UC OCTAVE phase 3 and OLE studies, CK levels were monitored periodically during treatment. CK levels were evaluated in OCTAVE Induction 1 and 2 at baseline and at weeks 2, 4, and 8; in OCTAVE Sustain at baseline and at weeks 4, 8, 16, 24, 32, 40, and 52; and in the OLE at baseline and at months 1, 2, 4, 6, 9, and 12. CK values were not routinely monitored in the phase 2 induction study A3921063. Mean changes (and 95% confidence intervals [CIs]) from induction study baseline CK levels were calculated. In addition, Pearson correlation coefficients for change from baseline CK levels and C-reactive protein (CRP) were calculated for tofacitinib- and placebo-treated groups at weeks 4 and 8 in the UC Induction cohort, and at weeks 8, 24, and 52 in the UC Maintenance cohort.
In all studies, adverse events (AEs) related to CK elevation were defined as AEs that were reported by the investigator(s) and coded to the Medical Dictionary for Regulatory Activities (MedDRA) preferred term (PT) of “blood creatine phosphokinase increased.” The criteria for determining whether an abnormal laboratory test finding should be reported as an AE were as follows: The test result was associated with accompanying symptoms; or required additional diagnostic testing or medical/surgical intervention; or resulted in a change in study dosing or discontinuation from the study, significant additional concomitant drug treatment, or other therapy; or if the test result was considered to be an AE by the investigator or sponsor.
Incidence rates (IRs; unique patients with events per 100 patient-years) and 95% CIs for AEs of CK elevation were calculated using an exact Poisson method. Analysis of IRs excludes data from the phase 2 induction study A3921063 as CK levels were not routinely monitored in that study.
In the OCTAVE studies, any single CK elevation > 3 × the upper limit of normal (ULN) required re-testing within 3–5 days; patients with two sequential CK elevations > 10 × ULN discontinued the study unless the causality was known not to be medically serious (e.g., exercise-induced). ULN for CK was approximately 170 U/L.
Risk factors for CK elevations ≥ 3 × ULN and ≥ 5 × ULN in the UC Overall cohort were evaluated using a Cox regression model. Backward stepwise regression (factors were retained in the final models if significant at the 0.05 level) was used for the evaluation. The following potential factors were included in the initial models: age, predominant dose of tofacitinib received (5 mg b.d. vs. 10 mg b.d., defined by average total daily dose < 15 mg and ≥ 15 mg, respectively), gender, body mass index (BMI), race, baseline CRP, extent of UC, duration of UC, prior TNFi failure, baseline corticosteroid use, prior immunosuppressant use, and geographic region.
All analyses in the UC Induction and Maintenance cohorts were based on the study treatment received. In the UC Overall cohort, analyses were performed based on all patients who received at least one dose of tofacitinib 5 mg b.d. or 10 mg b.d. In the RA, Pso, and PsA Overall cohorts, analyses were performed based on all patients who received at least one dose of tofacitinib.
Results
Patients
Tofacitinib exposure, patient demographics, and CK levels at baseline are presented for the UC, RA, Pso, and PsA cohorts in Table 1.
Table 1.
Demographics and baseline disease characteristics for patients in the tofacitinib UC, RA, Pso, and PsA cohorts
| UC Induction cohort | UC Maintenance cohort | UC Overall cohort | RA Overall cohort | Pso Overall cohort | PsA Overall cohort | ||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo N = 282 | Tofacitinib 10 mg b.d. N = 938 | Placebo N = 198 | Tofacitinib 5 mg b.d. N = 198 | Tofacitinib 10 mg b.d. N = 196 | Tofacitinib all N = 1157 | Tofacitinib all N = 7061 | Tofacitinib all N = 3663 | Tofacitinib all N = 783 | |
| Exposure, patient-years | 44.8 | 156.2 | 100.4 | 146.2 | 154.3 | 2050.5 | 22 874.5 | 8954.6 | 1237.9 |
| Age (years), mean (SD) | 41.4 (14.4) | 41.3 (13.8) | 43.4 (14.0) | 41.9 (13.7) | 43.0 (14.4) | 41.3 (13.9) | 52.1 (12.0) | 44.8 (12.9) | 48.7 (12.0) |
| Female, n (%) | 127 (45.0) | 381 (40.6) | 82 (41.4) | 95 (48.0) | 86 (43.9) | 478 (41.3) | 5829 (82.6) | 1117 (30.5) | 428 (54.7) |
| Disease duration (years), mean (SD) | 8.2 (6.8) | 8.2 (7.0) | 8.8 (7.5) | 8.3 (7.2) | 8.7 (7.0) | 8.2 (7.0) | 8.0 (8.1) | 18.3a (11.9) | 7.7 (7.2) |
| Median (range) CK at baseline,e U/L | 61.0 (9.0–591.0) | 62.0 (7.0–3173.0) | 119.0 (7.0–1272.0) | 124.0 (20.0–826.0) | 117.0 (21.0–1948.0) | 63.0 (7.0–3173.0) | 57.0b (18.0–1985.0) | 94.0c (12.0–21 382.0) | 87.0d (18.0–1283.0) |
b.d. twice daily, CK creatine kinase, N number of evaluable patients in each treatment group or cohort, n number of patients in the specified category, PsA psoriatic arthritis, Pso psoriasis, RA rheumatoid arthritis, SD standard deviation, UC ulcerative colitis
aN = 3660
bN = 4656
cN = 3470
dN = 759
eFor the UC Induction and Overall cohorts, data from the phase 2 induction study (A3921063) are not included as CK was not routinely monitored in this study. UC Induction cohort, N = 234 for placebo and N = 905 for tofacitinib 10 mg b.d.; UC Overall cohort, N = 1124
Tofacitinib exposure was 2051, 22 875, 8955, and 1238 patient-years in the UC, RA, Pso, and PsA Overall cohorts, respectively. Median CK at baseline in the UC (63.0 U/L), RA (57.0 U/L), Pso (94.0 U/L), and PsA (87.0 U/L) Overall cohorts was generally similar. Mean age at baseline in the UC cohorts was generally lower than in the RA, Pso, and PsA Overall cohorts. Mean disease duration in the Pso Overall cohort was greater than in the UC cohorts, and in the RA and PsA Overall cohorts. Across the Overall cohorts, the RA cohort had a generally higher proportion of female patients (82.6%) than the UC (41.3%), Pso (30.5%), and PsA (54.7%) cohorts.
Changes in CK
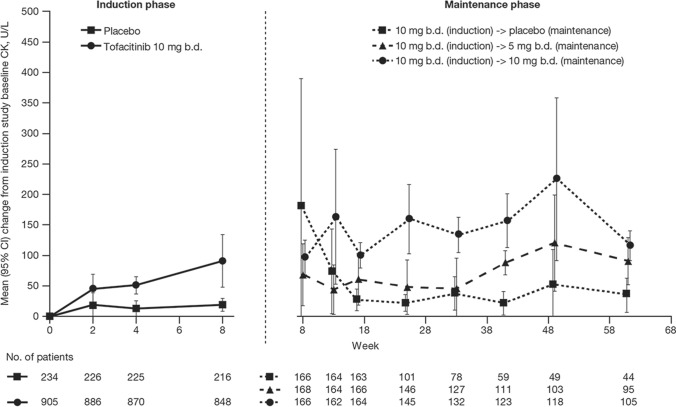
For tofacitinib-treated patients with UC, mean CK increased continually during the 8-week induction phase (Fig. 2). At week 8, mean change from induction study baseline CK for patients who received tofacitinib 10 mg b.d. was 91.1 U/L (95% CI, 48.1–134.1) versus 19.2 U/L (95% CI, 8.5–29.9) for patients who received placebo (Fig. 2). Among patients who completed induction treatment with tofacitinib 10 mg b.d., during 52 weeks of further treatment in the OCTAVE Sustain maintenance phase, there were a decrease in mean CK in patients who were switched to placebo, minimal changes in patients who received tofacitinib 5 mg b.d., and further small increases in patients who continued on tofacitinib 10 mg b.d. (Fig 2). Among patients completing induction with tofacitinib 10 mg b.d. and re-randomized to 52 weeks of maintenance therapy during OCTAVE Sustain, mean change from induction study baseline CK to the end of the maintenance phase was 35.9 U/L (95% CI, 8.1–63.7) with placebo, 90.3 U/L (95% CI, 51.9–128.7) with tofacitinib 5 mg b.d., and 115.6 U/L (95% CI, 91.6–139.7) with tofacitinib 10 mg b.d. (Fig 2).
Fig. 2.
Mean (95% CI) change from induction study baseline CK during the tofacitinib phase 3 UC induction and maintenance phases. For the induction phase, data from the phase 2 induction study (A3921063) are not included b.d. twice daily, CI confidence interval, CK creatine kinase, UC ulcerative colitis
In the UC Induction and Maintenance cohorts, correlations between change from induction study baseline CK versus change from induction study baseline CRP were weak at all time points evaluated. (Correlation coefficients ranged between 0.0082 and 0.1520.)
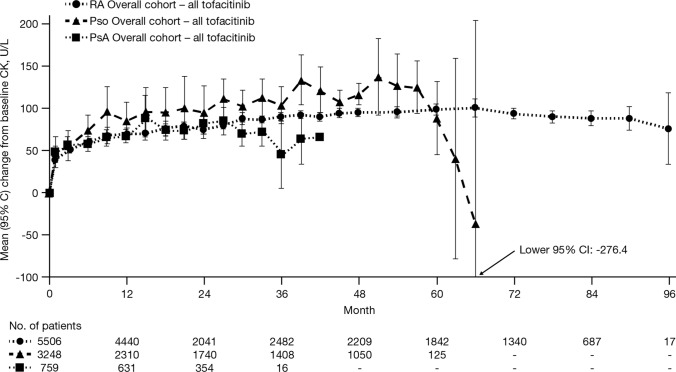
In the RA Overall cohort, mean change from baseline CK was generally stable over the duration of up to 96 months; mean change from baseline CK at month 90 (based on N = 248 patients with data available at this time point) was 88.3 U/L (95% CI, 74.6–101.9). In the Pso Overall cohort, mean change from baseline CK at month 57 (N = 319 at this time point) was 125.2 U/L (95% CI, 94.1–156.3). In the PsA Overall cohort, mean change from baseline CK at month 27 (N = 282 at this time point) was 84.7 U/L (95% CI, 68.5–100.9) (Fig. 3).
Fig. 3.
Mean (95% CI) change from baseline CK in the RA, Pso, and PsA Overall tofacitinib-treated cohorts. CI confidence interval, CK creatine kinase, PsA psoriatic arthritis, Pso psoriasis, RA rheumatoid arthritis
Adverse Events of CK Elevation
In the UC Induction cohort, AEs of CK elevation (i.e., investigator-determined AEs coded to the MedDRA PT of “blood creatine phosphokinase increased”) occurred in 2.8% of patients receiving tofacitinib 10 mg b.d. (n/N = 25/905) compared with 0.9% of placebo-treated patients (n/N = 2/234) (Table 2).
Table 2.
Summary of AEs of CK elevation and laboratory parameter abnormalities in the tofacitinib UC, RA, Pso, and PsA cohorts
| UC Induction cohort | UC Maintenance cohort | UC Overall cohort | RA Overall cohort | Pso Overall cohort | PsA Overall cohort | ||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo N = 234a | Tofacitinib 10 mg b.d. N = 905a | Placebo N = 198 | Tofacitinib 5 mg b.d. N = 198 | Tofacitinib 10 mg b.d. N = 196 | Tofacitinib all N = 1124a | Tofacitinib all N = 7061 | Tofacitinib all N = 3663 | Tofacitinib all N = 783 | |
| AE of CK elevation | |||||||||
| n (%) | 3 (1.3) | 25 (2.8) | 4 (2.0) | 6 (3.0) | 13 (6.6) | 126 (11.2) | 495 (7.0) | 529 (14.4) | 45 (5.7) |
| Number leading to discontinuation from the study, n (%) | 1 (33.3) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (7.7) | 2 (1.6) | 24 (4.8) | 29 (5.5) | 0 (0.0) |
| CK laboratory parameter abnormalities | |||||||||
| Any single CK elevation > 3 × ULN,b n (%) | 7 (3.0) | 39 (4.3) | 10 (5.1) | 15 (7.6) | 26 (13.3) | 210 (18.7) | 662 (9.4) | 864 (23.6) | 89 (11.4) |
| Two sequential CK elevations > 10 × ULN,b n (%) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 2 (1.0) | 9 (0.8) | 6 (0.1) | 11 (0.3) | 1 (0.1) |
AEs of CK elevation were investigator-determined AEs coded to the MedDRA PT of “blood creatine phosphokinase increased”
AE adverse event, b.d. twice daily, CK creatine kinase, N number of evaluable patients in each treatment group or cohort, n number of patients with response in the specified category, MedDRA Medical Dictionary for Regulatory Activities, PsA psoriatic arthritis, Pso psoriasis, PT preferred term, RA rheumatoid arthritis, UC ulcerative colitis, ULN upper limit of normal
aFor the UC Induction and Overall cohorts, data from the phase 2 induction study (A3921063) are not included as CK elevation was not routinely monitored in this study
bULN was approximately 170 U/L
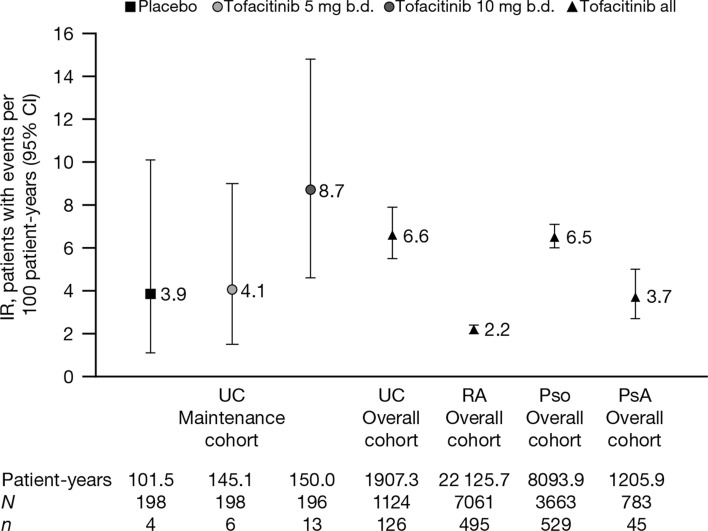
IRs for AEs of CK elevation in the UC Maintenance cohort are shown in Fig. 4. The IR for AEs of CK elevation with tofacitinib 10 mg b.d. (8.7 [95% CI, 4.6–14.8]) was numerically greater than with tofacitinib 5 mg b.d. (4.1 [95% CI, 1.5–9.0]) or placebo (3.9 [95% CI, 1.1–10.1]). The IR for AEs of CK elevation with all tofacitinib-treated patients in the UC Overall cohort (6.6 [95% CI, 5.5–7.9]) was within the range reported with tofacitinib treatment in the Maintenance cohort. IRs for discrete 6-month intervals in the UC Overall cohort are reported in Table 3 and did not increase over time. The IR in the UC Overall cohort was higher than the IR reported in the RA (2.2 [95% CI, 2.0–2.4]) and PsA (3.7 [95% CI, 2.7–5.0]) Overall cohorts and similar to that reported in the Pso Overall cohort (6.5 [95% CI, 6.0–7.1]) (Fig. 4).
Fig. 4.
IRs (95% CI) for AEs of CK elevation in the UC Maintenance and Overall cohorts, and the RA, Pso, and PsA Overall cohorts. IRs for AEs of CK elevation in the UC Induction cohort are not shown due to the short duration (8 weeks) of the UC induction studies. For the UC Overall cohort, data from the phase 2 induction study (A3921063) are not included. AE adverse event, b.d. twice daily, CI confidence interval, CK creatine kinase, IR incidence rate, N number of evaluable patients in each treatment group or cohort, n number of unique patients with events, PsA psoriatic arthritis, Pso psoriasis, RA rheumatoid arthritis, UC ulcerative colitis
Table 3.
IRs for AEs of CK elevation in the UC Overall cohort by 6-month interval
| UC Overall cohort—Tofacitinib all | ||||||
|---|---|---|---|---|---|---|
| 0–6 months N = 1124 | >6–12 months N = 737 | >12–18 months N = 622 | >18–24 months N = 540 | >24–30 months N = 481 | >30 months N = 395 | |
| Exposure, patient-years | 475.4 | 335.0 | 289.3 | 254.2 | 223.3 | 330.1 |
| Number of patients with AEs of CK elevation, n (%) | 62 (5.5) | 17 (2.3) | 13 (2.1) | 9 (1.7) | 11 (2.3) | 14 (3.5) |
| IR (95% CI) | 13.0 (10.0–16.7) | 5.1 (3.0–8.1) | 4.5 (2.4–7.7) | 3.5 (1.6–6.7) | 4.9 (2.5–8.8) | 4.2 (2.3–7.1) |
Data from the phase 2 induction study (A3921063) are not included
AE adverse event, CI confidence interval, CK creatine kinase, IR incidence rate, N number of patients evaluable in the specified time period, n number of unique patients with events, UC ulcerative colitis
In the UC Overall cohort, 126 (11.2%) patients reported an AE of CK elevation; the majority of cases were mild to moderate in severity (as reported by the investigator), and there were no serious AEs of CK elevation (i.e., events that resulted in death, were life-threatening, resulted in a persistent or significant disability or incapacity, required patient hospitalization or prolongation of existing hospitalization, or resulted in a congenital anomaly or birth defect). No AEs of myopathy were reported in the UC program. One (0.1%) patient in the UC Overall cohort had an AE of myositis.
In the UC Overall cohort, for the 126 (11.2%) patients who reported an AE of CK elevation, the median time to event was 183 days (range 1–1579 days). In the RA Overall cohort, 495 (7.0%) patients had AEs of CK elevation, with a median time to event of 505 days (range 1–2873 days). In the Pso Overall cohort, 529 (14.4%) patients had AEs of CK elevation, with a median time to event of 266 days (range 1–1707 days). In the PsA Overall cohort, 45 (5.7%) patients with AEs of CK elevation, with a median time to event of 169 days (range 15–696 days).
In the tofacitinib UC clinical studies, one patient who received placebo during OCTAVE Sustain developed rhabdomyolysis on day 224 of the study. The patient had received 63 days of tofacitinib 10 mg b.d. in OCTAVE Induction 1 and was then re-randomized to receive placebo in OCTAVE Sustain. The AE of rhabdomyolysis was mild in severity, did not require drug discontinuation, and resolved on day 250 of OCTAVE Sustain. The patient had CK and serum creatinine within normal range at baseline of OCTAVE Induction 1. On day 56 of OCTAVE Induction 1 (during treatment with tofacitinib 10 mg b.d.), CK was 312 U/L and serum creatinine was within normal range. In OCTAVE Sustain (in which the patient was re-randomized to receive placebo), CK decreased to 103 U/L on day 56 of OCTAVE Sustain and was 248 U/L on day 224. Serum creatinine reached a peak value of 1.5 mg/dL on day 224. Given the marginal increase in CK and serum creatinine at the time of the event, the event did not meet the conventional definition of rhabdomyolysis [51]. The elapsed time between the last dose of tofacitinib and onset of the AE suggested this was unlikely to be related to tofacitinib treatment.
AEs of CK elevation that required discontinuation from the study occurred infrequently in the UC program; in the Overall cohort, two of the 126 patients (1.6%; both of whom were receiving tofacitinib 10 mg b.d. at the time of the event) were required to discontinue.
CK Laboratory Parameter Abnormalities
In the UC Induction and Maintenance cohorts, the proportion of patients meeting criteria for monitoring (any single CK elevation > 3 × ULN) was numerically higher with tofacitinib treatment versus placebo (Table 2). The proportion of patients meeting criteria for monitoring in the UC Overall cohort (18.7%) was greater than reported in the RA (9.4%) and PsA (11.4%) Overall cohorts and lower than reported in the Pso (23.6%) Overall cohort. The observed differences in age and gender across the disease cohorts in this analysis are potential confounders for these observations. The proportion of patients with two sequential CK elevations > 10 × ULN was ≤ 1.0% across each of the UC, RA, Pso, and PsA cohorts in this analysis.
Risk Factor Analysis for CK Laboratory Parameter Abnormalities in the UC Overall Cohort
Results of the multivariate Cox regression risk factor analyses for CK elevations ≥ 3 × ULN and ≥ 5 × ULN in the UC Overall cohort are summarized in Supplementary Table S2. Gender, age at onset, and geographic region were retained in the final model for CK elevations ≥ 3 × ULN and ≥ 5 × ULN. Additionally, race was also retained in the final model for CK elevations ≥ 3 × ULN (see Supplementary Table S2). Tofacitinib dose was evaluated but was not identified as a significant risk factor for CK elevations ≥ 3 × ULN and ≥ 5 × ULN in the final models.
Discussion
In this analysis of data from tofacitinib UC clinical studies, tofacitinib treatment in patients with moderately to severely active UC appeared to be associated with reversible CK elevations. Increases in CK began within the first two weeks of tofacitinib induction therapy and continued to week 8 of induction treatment. Continued therapy with tofacitinib beyond week 8 did not result in further clinically meaningful elevations in CK. Elevations in CK were not associated with clinically significant AEs such as rhabdomyolysis or myopathy. Few AEs of CK elevation required patients to discontinue participation in these studies. Multivariate Cox risk factor analysis identified male gender and younger age as significant risk factors for CK elevations ≥ 3 × ULN and ≥ 5 × ULN. Results in the tofacitinib UC program were generally consistent with observations in the tofacitinib RA, Pso, and PsA clinical development programs. Elucidation of the mechanism for the observed changes in CK requires further investigation.
Results of this integrated analysis are also consistent with previous analyses from the tofacitinib rheumatology and dermatology clinical development programs that specifically evaluated the effects of tofacitinib on CK levels [18, 19]. Analyses in patients with Pso demonstrated that increases in CK were readily reversible in patients who had their tofacitinib therapy withdrawn, and the degree of elevation itself was not clinically significant [18]. In patients with RA, the effect of tofacitinib on muscle biomarkers, including CK, was evaluated. In this analysis, mean CK levels plateaued following six months of tofacitinib treatment, remained within the normal reference range, and were not associated with clinical myopathy [19].
Our findings are also consistent with data for other therapies with an immunosuppressant mode of action used to treat inflammatory diseases, including the JAK inhibitors baricitinib [14], upadacitinib [15–17], and TNFi infliximab [20]. In RA clinical studies of baricitinib [14], dose-dependent increases in CK were observed within one week, which plateaued at 8–12 weeks. Median CK increase from baseline with the highest baricitinib dose (4 mg once daily) was 52 U/L at week 16. AEs related to muscle injury were infrequent, and no rhabdomyolysis associated with baricitinib was observed [14]. Elevated CK levels were among the most common AEs reported in clinical trials of upadacitinib in UC [15], ankylosing spondylitis [16], and RA [17]. In a registry study of infliximab-treated patients with IBD, 30.5% had elevated CK levels (absolute CK > 180 U/L), but none reported persistent symptoms potentially related to myopathy [20]. Other commonly used medications associated with increases in CK include antipsychotics and certain beta blockers [2], as well as statins, where CK elevations 2–10 × ULN occur in around 5% of users [4]. As prescription drugs are a common cause of CK elevation, it is recommended that affected patients are monitored, and their medications reviewed [4]. For patients with elevated CK levels with no apparent medical explanation, current guidelines recommend that a muscle biopsy be performed for further investigation if one or more of the following are present: CK elevation ≥ 3 × ULN, myopathic electromyogram, or if the patient is < 25 years of age [2].
In the findings of the Cox risk factor analysis performed in the present study, male gender and younger age were associated with increased risk for elevated CK levels, which is consistent with previously reported observations of higher CK activity among men compared with women [2, 5, 6, 8–10] and among younger subjects (particularly young adult males, where the difference may be driven by higher muscle mass in this group) versus older subjects [7]. Tofacitinib dose was not identified as a significant risk factor in this analysis.
Although the profile of CK elevations and AEs was generally similar across the different disease populations analyzed, comparisons among populations should be interpreted with caution due to differences in patient population characteristics. In addition, differences in the age and gender makeup of the patient populations—the mean age of the UC Overall cohort (41.3 years) was numerically lower than the RA (52.1), Pso (44.8), and PsA (48.7) Overall cohorts, and 83% of patients in the RA Overall cohort were female compared with 41%, 31%, and 55% in the UC, Pso, and PsA Overall cohorts—are of particular relevance, given the previously reported variation in the normal range of CK levels by age and gender [6, 8].
Advantages of this analysis include the large numbers of tofacitinib-treated patients enrolled across multiple disease populations, and the long duration of follow-up (> 22 000 patient-years in the RA Overall cohort). In addition, detailed data on CK and associated AEs were collected in each of the UC, RA, Pso, and PsA clinical trial programs. However, the approach of pooling potentially heterogeneous patient populations for analysis in the Overall cohorts is a potential limitation of this study, as patients’ prior treatment history and permitted concomitant medications were different in some of the trials pooled in the Overall cohorts. A further limitation is that some information relating to AEs of CK elevation was dependent upon interpretation and reporting by site investigators, and this may not have been reported in a uniform fashion.
In conclusion, these analyses of clinical trial data from patients with UC, RA, Pso, and PsA demonstrated that although CK elevations in tofacitinib-treated patients were a relatively common occurrence, they were minor, were reversible, and were not associated with clinically important signs or symptoms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank the patients, investigators, and trial teams who were involved in the tofacitinib clinical trial programs. Medical writing support, under the guidance of the authors, was provided by Daniel Binks, PhD, CMC Connect, McCann Health Medical Communications, and was funded by Pfizer Inc, New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464). John D. Isaacs is supported by the NIHR Newcastle Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Abbreviations
- AE
Adverse event
- b.d.
Twice daily
- BMI
Body mass index
- CI
Confidence interval
- CK
Creatine kinase
- CRP
C-reactive protein
- HR
Hazard ratio
- IR
Incidence rate
- JAK
Janus kinase
- MedDRA
Medical Dictionary for Regulatory Activities
- OLE
Open-label, long-term extension
- PsA
Psoriatic arthritis
- Pso
Psoriasis
- PT
Preferred term
- RA
Rheumatoid arthritis
- SD
Standard deviation
- TNFi
Tumor necrosis factor inhibitor
- UC
Ulcerative colitis
- ULN
Upper limit of normal
Author’s contribution
RP, JDI, LAC, WW, AM, KK, LW, GC, and CS were involved in the conception and design of the study/analyses, data interpretation, and manuscript drafting, reviewing, and development. RP (UC studies) and JDI (RA studies) were involved in the recruitment of study patients. All authors read and approved the manuscript.
Funding
These studies were sponsored by Pfizer Inc. Medical writing support was funded by Pfizer Inc.
Availability of data and materials
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Compliance with Ethical Standards
Conflict of interest
RP has received consulting fees from AbbVie, Amgen, Aptalis, AstraZeneca, Baxter, Biogen, Bristol-Myers Squibb, Celgene, Centocor, Cubist, Eisai, Elan, Ferring, Gilead, GlaxoSmithKline, Janssen, Merck, Pfizer Inc, Robarts Clinical Trials, Salix, Samsung Bioepis, Shire, Takeda, and UCB; has received research Grants from AbbVie, Ferring, Janssen, and Takeda; has received lectures and/or speaker bureau fees from AbbVie, Aptalis, AstraZeneca, Ferring, Janssen, Merck, Prometheus, Shire, and Takeda; and has received advisory board fees from Abbott, AbbVie, Amgen, Aptalis, AstraZeneca, Baxter, Bristol-Myers Squibb, Celgene, Centocor, Cubist, Eisai, Elan, Ferring, Genentech, GlaxoSmithKline, Janssen, Merck, Pfizer Inc, Salix, Schering-Plough, Shire, Takeda, and UCB. JDI has been a consultant for, and/or has received research Grants or speaker fees from, AbbVie, Amgen, Eli Lilly, Gilead, Merck, Pfizer Inc, Roche, and UCB. LAC has served as a consultant for Pfizer Inc; has received research Grants from BioRad, Pfizer Inc, and PredictImmune; and has received advisory board fees from Gilead and Janssen. WW, AM, KK, LW, GC, and CS are employees and stockholders of Pfizer Inc.
Conference presentation
These data were presented at the 2018 Annual Scientific Meeting of the American College of Gastroenterology, Philadelphia, PA, USA, October 5–10, 2018.
Ethical approval
All studies received appropriate Institutional Review Board and/or Independent Ethics Committee approval. All patients provided written informed consent.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
The original version of this article was revised due to retrospective Open Access order.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/10/2020
A Correction to this paper has been published: 10.1007/s10620-020-06638-z
Contributor Information
Remo Panaccione, Email: rpanacci@ucalgary.ca.
John D. Isaacs, Email: john.isaacs@ncl.ac.uk
Lea Ann Chen, Email: Lea.Chen@nyumc.org.
Wenjin Wang, Email: wenjin.wang@pfizer.com.
Amy Marren, Email: Amy.Marren@pfizer.com.
Kenneth Kwok, Email: kenneth.kwok@pfizer.com.
Lisy Wang, Email: Lisy.Wang@pfizer.com.
Gary Chan, Email: gary.chan@pfizer.com.
Chinyu Su, Email: chinyu.su@pfizer.com.
References
- 1.Kitzenberg D, Colgan SP, Glover LE. Creatine kinase in ischemic and inflammatory disorders. Clin Transl Med. 2016;5:31. doi: 10.1186/s40169-016-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyriakides T, Angelini C, Schaefer J, et al. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur J Neurol. 2010;17:767–773. doi: 10.1111/j.1468-1331.2010.03012.x. [DOI] [PubMed] [Google Scholar]
- 3.Bagley WH, Yang H, Shah KH. Rhabdomyolysis. Intern Emerg Med. 2007;2:210–218. doi: 10.1007/s11739-007-0060-8. [DOI] [PubMed] [Google Scholar]
- 4.Moghadam-Kia S, Oddis CV, Aggarwal R. Approach to asymptomatic creatine kinase elevation. Cleve Clin J Med. 2016;83:37–42. doi: 10.3949/ccjm.83a.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong ET, Cobb C, Umehara MK, et al. Heterogeneity of serum creatine kinase activity among racial and gender groups of the population. Am J Clin Pathol. 1983;79:582–586. doi: 10.1093/ajcp/79.5.582. [DOI] [PubMed] [Google Scholar]
- 6.Neal RC, Ferdinand KC, Ycas J, Miller E. Relationship of ethnic origin, gender, and age to blood creatine kinase levels. Am J Med. 2009;122:73–78. doi: 10.1016/j.amjmed.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Brancaccio P, Maffulli N, Limongelli FM. Creatine kinase monitoring in sport medicine. Br Med Bull. 2007;81–82:209–230. doi: 10.1093/bmb/ldm014. [DOI] [PubMed] [Google Scholar]
- 8.Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta. 1999;279:107–115. doi: 10.1016/s0009-8981(98)00180-6. [DOI] [PubMed] [Google Scholar]
- 9.Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø study. Neuromuscul Disord. 2011;21:494–500. doi: 10.1016/j.nmd.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Brewster LM, Mairuhu G, Sturk A, van Montfrans GA. Distribution of creatine kinase in the general population: implications for statin therapy. Am Heart J. 2007;154:655–661. doi: 10.1016/j.ahj.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Papp KA, Krueger JG, Feldman SR, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: long-term efficacy and safety results from 2 randomized phase-III studies and 1 open-label long-term extension study. J Am Acad Dermatol. 2016;74:841–850. doi: 10.1016/j.jaad.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. doi: 10.1056/NEJMoa1606910. [DOI] [PubMed] [Google Scholar]
- 13.Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. OLUMIANT (baricitinib): Highlights of Prescribing Information. 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924s000lbl.pdf. Accessed June 5, 2019.
- 15.Sandborn WJ, Ghosh S, Panes J, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. 2020;158:2139–2149. doi: 10.1053/j.gastro.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 16.van der Heijde D, Song IH, Pangan AL, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet. 2019;394:2108–2117. doi: 10.1016/S0140-6736(19)32534-6. [DOI] [PubMed] [Google Scholar]
- 17.Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71:1788–1800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 18.Bissonnette R, Iversen L, Sofen H, et al. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol. 2015;172:1395–1406. doi: 10.1111/bjd.13551. [DOI] [PubMed] [Google Scholar]
- 19.Isaacs JD, Zuckerman A, Krishnaswami S, et al. Changes in serum creatinine in patients with active rheumatoid arthritis treated with tofacitinib: results from clinical trials. Arthritis Res Ther. 2014;16:R158. doi: 10.1186/ar4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theodoraki E, Orfanoudaki E, Foteinogiannopoulou K, Koutroubakis IE. Asymptomatic hyperCKemia during infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:1266–1271. doi: 10.1093/ibd/izy088. [DOI] [PubMed] [Google Scholar]
- 21.Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:1079–1087. doi: 10.1016/j.cgh.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein GR, Loftus EV, Jr, Bloom S, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of ulcerative colitis: an interim analysis of an open-label, long-term extension study with up to 49 years of treatment [abstract] Am J Gastroenterol. 2018;113:5329. [Google Scholar]
- 23.Charles-Schoeman C, Fleischmann R, Davignon J, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67:616–625. doi: 10.1002/art.38974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kremer JM, Kivitz AJ, Simon-Campos JA, et al. Evaluation of the effect of tofacitinib on measured glomerular filtration rate in patients with active rheumatoid arthritis: results from a randomised controlled trial. Arthritis Res Ther. 2015;17:95. doi: 10.1186/s13075-015-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–1905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 26.Kremer JM, Cohen S, Wilkinson BE, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–981. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- 27.Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64:617–629. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Tofacitinib Study Investigators Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res Hoboken. 2011;63:1150–1158. doi: 10.1002/acr.20494. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study. Mod Rheumatol. 2015;25:514–521. doi: 10.3109/14397595.2014.995875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McInnes IB, Kim HY, Lee SH, et al. Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis. 2014;73:124–131. doi: 10.1136/annrheumdis-2012-202442. [DOI] [PubMed] [Google Scholar]
- 31.Conaghan PG, Østergaard M, Bowes MA, et al. Comparing the effects of tofacitinib, methotrexate and the combination, on bone marrow oedema, synovitis and bone erosion in methotrexate-naive, early active rheumatoid arthritis: results of an exploratory randomised MRI study incorporating semiquantitative and quantitative techniques. Ann Rheum Dis. 2016;75:1024–1033. doi: 10.1136/annrheumdis-2015-208267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle DL, Soma K, Hodge J, et al. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann Rheum Dis. 2015;74:1311–1316. doi: 10.1136/annrheumdis-2014-206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75:687–695. doi: 10.1136/annrheumdis-2014-207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winthrop KL, Wouters AG, Choy EH, et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol. 2017;69:1969–1977. doi: 10.1002/art.40187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–460. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 36.van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–570. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 37.Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 38.Kremer J, Li Z-G, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253–261. doi: 10.7326/0003-4819-159-4-201308200-00006. [DOI] [PubMed] [Google Scholar]
- 39.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 40.Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 41.Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390:457–468. doi: 10.1016/S0140-6736(17)31618-5. [DOI] [PubMed] [Google Scholar]
- 42.Wollenhaupt J, Silverfield J, Lee EB, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol. 2014;41:837–852. doi: 10.3899/jrheum.130683. [DOI] [PubMed] [Google Scholar]
- 43.Yamanaka H, Tanaka Y, Takeuchi T, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther. 2016;18:34. doi: 10.1186/s13075-016-0932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wollenhaupt J, Silverfield J, Lee EB, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in open-label, long-term extension studies over 9 years [abstract] Arthritis Rheumatol. 2017;69:683–684. [Google Scholar]
- 45.Krueger J, Clark JD, Suárez-Fariñas M, et al. Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: a randomized phase 2 study. J Allergy Clin Immunol. 2016;137:1079–1090. doi: 10.1016/j.jaci.2015.12.1318. [DOI] [PubMed] [Google Scholar]
- 46.Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668–677. doi: 10.1111/j.1365-2133.2012.11168.x. [DOI] [PubMed] [Google Scholar]
- 47.Papp KA, Menter MA, Abe M, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two, randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173:949–961. doi: 10.1111/bjd.14018. [DOI] [PubMed] [Google Scholar]
- 48.Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386:552–561. doi: 10.1016/S0140-6736(14)62113-9. [DOI] [PubMed] [Google Scholar]
- 49.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 50.Nash P, Coates LC, Kivitz AJ, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, up to 24 months in patients with active psoriatic arthritis: interim data from OPAL balance, an open-label, long-term extension study [abstract] Ann Rheum Dis. 2017;76:682. [Google Scholar]
- 51.Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care. 2016;20:135. doi: 10.1186/s13054-016-1314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.