Abstract
Introduction:
Central nervous system (CNS) diseases affect a large portion of the population, however, few therapeutic options are available. Furthermore, to date, clinical trials have been largely unsuccessful due to difficulty in targeting the undruggable, toxic proteins that underly many CNS disorders. PROteolysis Targeting Chimeras (PROTACs) are a rapidly emerging technology that has been proposed as a potential treatment option for various CNS diseases by hijacking the endogenous protein degradation process.
Areas Covered:
Herein, the authors discuss how the application of PROTACs may be beneficial in the treatment of major CNS diseases. They further discuss the main advantages and disadvantages of using PROTACs in the CNS, focusing on potential limitations such as their transient nature, localization, blood-brain barrier permeability and proteasome dysfunction.
Expert opinion:
It is evident that PROTACs have significant potential as a therapeutic tool for the treatment of CNS diseases and there is preliminary evidence suggesting that PROTACs could be successful in a clinical setting. Nevertheless, numerous limitations exist that must be overcome before this technology can be applied as a successful therapeutic for CNS disorders. Importantly, more in vivo studies are needed to determine the feasibility and effectiveness of using PROTACs in the brain.
Keywords: Alzheimer’s disease, Brain, Central Nervous System, Degradation, PROTAC
1. Introduction
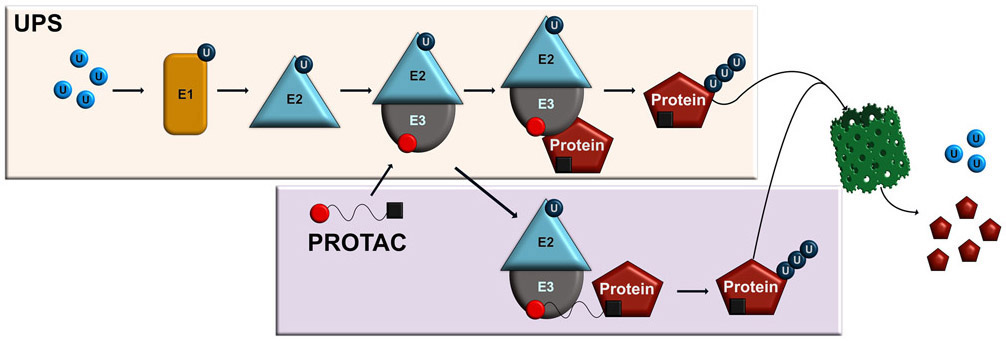
PROteolysis Targeting Chimeras (PROTACs) are a rapidly expanding field with significant potential for the treatment of undruggable protein targets1 that has been developed over the last two decades through the significant contributions of several labs1-4. PROTACs target proteins of interest (POI) for degradation by utilizing processes controlled by the highly conserved ubiquitin-proteasome system (UPS), which in cells is employed to degrade misfolded, nonfunctional and other undesired proteins and is critically involved in a variety of cellular processes, such as gene transcription5, protein transport6, synaptic plasticity and memory7, and the pathophysiology of various neurodegenerative diseases8-10 and cancers11. The UPS works through sequential conjugation of ubiquitin to a target substrate via a series of three ubiquitin-associated enzymes, E1, E2 and E3, the latter of which has substrate specificity and can lead to the target being ubiquitinated, recognized and degraded by the proteasome12-14. PROTACs essentially hijack this process, working in conjunction with the UPS system to degrade target substrates (Figure 1). This process works by PROTACs binding the POI ligand with an E3 ligase through a linker, leading to POI ubiquitination and subsequent degradation by the proteasome15-17. The cereblon (CRBN) and von Hippel-Lindau (VHL) ligands are the two most commonly used E3 ligases when designing PROTACs, although efforts are being made to identify other E3 ligases that are compatible for PROTAC-mediated targeting18. PROTACs have significant potential as a means of degrading unwanted, toxic and foreign proteins in the cell that are difficult to target via traditional pharmacological approaches and have proved successful for targeting drug-resistant proteins, indicating their potential for treating various forms of cancer and neurodegenerative diseases17.
Figure 1. Mechanistic approach for PROTACs to hijack the ubiquitin proteasome system for targeted protein degradation.
First, a free ubiquitin pool exists in the cell. Once a free ubiquitin molecule binds the ubiquitin activating enzyme (E1), the ubiquitin becomes activated. The activated ubiquitin is then transferred to the ubiquitin conjugating enzymes (E2), which subsequently conjugates to the ubiquitin ligase (E3). The E3 ligase can then recognize the target protein and transfer the ubiquitin. This process continues to polyubiquitinate the protein, which leads to degradation by the proteasome. The PROTAC contains a recognition domain for the E3 ligase and the protein of interest which allows it to act as a linker. In this way, the PROTAC can recruit the E3 ligase to the protein of interest for directed degradation. After polyubiquitination occurs, the target protein is degraded by the proteasome and the ubiquitin returns to the free ubiquitin pool.
The central nervous system (CNS), representing the brain, spinal cord and all associated nerves, exerts executive control over every bodily function. There are more than 600 known diseases that affect the CNS, including a wide variety of neurological, neurodevelopmental and neurodegenerative disorders19, and it is estimated that nearly 1/6 of the world’s population suffers from a CNS disorder20. Unlike the peripheral nervous system (PNS), the CNS poses unique challenges that often hinder drug discovery efforts21, resulting in clinical failure rates that can reach near 100% for some disorders. Some of this failure can be attributed to a poor understanding of the disease itself, making it difficult to correctly target the major cellular mechanisms that underly it. However, in other cases drug development has been largely unsuccessful even when the underlying pathophysiology is well understood, such as in Huntington’s disease. Furthermore, diseases that result from mutations or malfunctioning of proteins expressed in both the PNS and CNS pose unique challenges in regards to localizing the treatment specifically to the CNS. Consequently, even some of the most promising treatments for various CNS disorders continue to fail and, to date, an overwhelming number of major CNS disorders, such as Alzheimer’s disease, lack an effective treatment option.
PROTACs have the potential to treat a wide variety of diseases22, 23, and have been proposed as the next “blockbuster” therapy that could treat Alzheimer’s disease and other disorders of the CNS that have undruggable protein targets17, 24. Yet whether PROTACs are the answer for CNS drug development remains unclear due to a lack of empirical evidence that they can treat certain disorders of the nervous system in vivo. In this perspective we briefly review evidence that PROTACs could be a potential treatment for CNS disorders, focusing on the most prevalent neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis) and glioblastomas, all of which still lack effective therapeutic strategies. Next, we discuss the potential limitations that might limit PROTAC effectiveness and drug development for CNS disorders and conclude by providing our expert opinion on whether PROTACs are a “game-changer” for CNS drug development. For a more detailed discussion on the design of PROTACs, we refer the reader to recent reviews on this topic25, 26.
2. Disorders of the CNS: Could PROTACS be the answer for neurodegenerative disorders?
Neurodegenerative disorders are one of the largest class of CNS diseases, affecting more than 10 million people worldwide each year. These disorders have debilitating outcomes and are considered among the most difficult diseases to treat. While the pathophysiology of these disorders can be complex, often times they are associated with accumulation of toxic proteins that lead to severe, progressive neuronal death, such is the case with Alzheimer’s and Huntington’s diseases. For this reason, PROTACs have become an attractive approach to potentially treat these disorders. In this section we will briefly discuss recent evidence suggesting that PROTACs have significant potential to treat the 4 major neurodegenerative disorders: Alzheimer’s, Parkinson’s and Huntington’s diseases and amyotrophic lateral sclerosis (ALS). For a more detailed discussion on this topic we refer the reader to several recent reviews17, 25-30.
2.1. Alzheimer’s disease
Alzheimer’s disease affects more than 10% of the population over the age of 70 and is the leading cause of dementia world-wide. However, despite a number of promising candidates, drug development has been largely unsuccessful for the treatment of this disorder. A hallmark characteristic of Alzheimer’s disease is abnormal accumulation of tau protein throughout the brain31, which results in significant and progressive cell death. Recently, PROTACs have been employed to target and degrade intracellular tau. For example, a recent study designed a PROTAC peptide that used Keap1-Cul3 ubiquitin E3 ligase to bind tau, leading to ubiquitination and subsequent degradation32. Another study combined PROTAC technology and positron emission tomography (PET)33 and developed 25 hetero-bifunctional molecules by linking PET tracer T807, which binds to pathological tau, and E3-ligase recruiting ligand pomalidomide to target tau for degradation. One molecule, called QC-01-175, would preferentially target tau for proteasome-dependent degradation in frontotemporal dementia (FTD) patient-derived neurons as opposed to the wild-type (WT) neurons. While these in vitro studies do not fully embody in vivo outcomes, these data demonstrate the potential of PROTACs to target the tau protein and potentially be used as a drug treatment in patients with Alzheimer’s disease. Notably, one study examined the ability of PROTACs to target tau in the brain of a mouse model of Alzheimer’s disease. Several E3 ligases and chimeric molecules that could penetrate cells and bind to tau were generated, of which molecule TH006 was demonstrated to permeate the cells and induce tau degradation via an increase in polyubiquitination34. Importantly, TH006 was administered to mouse model of Alzheimer’s disease using a combined intranasal approach with intravenous injection for 10 days, which reduced tau in the hippocampus and cerebral cortex. Additionally, Arvinas, a pioneering company in PROTACs, has recently reported preclinical evidence of a PROTAC that targets pathological tau. This PROTAC was able to cross the blood-brain barrier and degrade more than 95% of pathological tau, without altering wildtype tau, in the mouse brain 24-hours after parenteral administration. Together, these in vivo studies demonstrate the ability of PROTACs to effectively work in the brain, where abnormal tau accumulation is present, indicating the potential PROTACs could have in treating even difficult to target CNS diseases, such as Alzheimer’s disease.
2.2. Parkinson’s disease
Parkinson’s disease is a neurodegenerative disorder that affects 1-3% of the population over 80 years of age. The main characteristic of Parkinson’s disease is accumulation of alpha-synuclein protein (α-syn), which leads to the formation of Lewy-bodies35. A recent investigation demonstrated the ability of PROTACs technology to target α-syn36. This study combined an α-syn protein binding domain, a cell-penetrating domain, and a proteasome-targeting motif to generate a cell-permeable PROTAC, which was able to target α-syn for degradation by the proteasome in a time- and dose-dependent manner in primary neurons and neuroblastoma cells. The reduction in α-syn lead to decreased mitochondrial dysfunction and cell toxicity, indicating the potential of the PROTAC as a potential strategy to treat Parkinson’s disease. Importantly, though these results are exciting, this in vitro study will need further validation to determine if this method has potential clinical applications.
2.3. Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disorder that is caused by an excessive expansion of a CAG trinucleotide repeat, which leads to the mutant huntingtin (mHtt) protein that often aggregates and leads to cell death37,38. Recently, two PROTACs, labeled 1 and 2, were shown to target mHtt for degradation in fibroblast cells derived from two patients with Huntington’s disease37. Both PROTACs 1 and 2 successfully reduced mHtt levels in the fibroblasts with the former having dose-dependent effects, though wild-type Htt also had reduced levels without changes in gene transcription. Furthermore, this PROTAC-mediated reduction of mHtt levels occurred via proteasome-dependent degradation. Interestingly, these PROTACs were able to lead to degradation of the mHtt protein without knowing the specific ligand that was targeted. This demonstrates that PROTAC technology can be used to target mutant, aggregate-prone proteins that lead to neurodegenerative disorders, even if the specific ligand is unknown. Similar to those PROTACs described in previous sections, further validation with in vivo studies will be necessary to know the full potential of this technology for treating Huntington’s disease. Interestingly, degradation of mHtt has been reported by using an autophagosome-tethering compound (ATTEC), which utilizes the macroautophagy degradation pathway. This study found that ATTECs were able to pass through the blood-brain barrier and distinguish between mHtt and WT Htt during targeted degradation, which ultimately led to the rescue of multiple Huntington’s disease-related phenotypes in both drosophila and mice39. This approach is a comparable alternative to PROTACs, as it utilizes a pathway for protein degradation to target a POI with a small molecule, and further demonstrates the potential for targeting mHtt for degradation with PROTACs or PROTAC-like technology.
2.4. Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic Lateral Sclerosis (ALS) is a multisystem, progressive neurodegenerative disorder with a median survival of less than 5 years after onset40, 41. One common characteristic of ALS is the aggregation of TDP-43 protein in the cytoplasm, although more than 20 genes have been associated with the disease40. Previously, TDP-43 has been targeted by hydrophobic tagging and various other methods, indicating its targeting potential42, 43. In 2018, Zfn179 E3 ligase was discovered to specifically ubiquitinate TDP-43 and was able to regulate clearance of TDP-43 aggregates44. While still in its early stages, several efforts are currently underway testing the potential of PROTAC-mediated degradation of TDP-43 to control ALS disease progression.
Aggregation of mutant SOD1 is associated with familial ALS. One study generated a Dorfin-CHIP PROTAC containing the hydrophobic portion of Dorfin, an E3 protein that binds to the mutant SOD1, and the U-box domain of the C-terminal of Hsc70-interacting protein (CHIP), which has strong E3 activity45. The Dorfin-CHIPL chimeric protein was able to specifically target mutant SOD1, but not WT SOD1, for ubiquitination and decreased aggregation formation. Together, these studies indicate the potential for PROTACs as a therapeutic for ALS, though more research is needed.
3. Disorders of the CNS: Could PROTACS be the answer for glioblastomas?
Glioblastoma is an aggressive, malignant brain tumor that typically leads to death within 15-18 months46. Although glioblastomas are heavily studied, treatment options remain limited and usually consist of surgery and chemotherapy, which still results in less than ideal survival rates. Recently, histone deacetylase 6 (HDAC6) has been implicated as a target for glioblastoma treatment47 as overexpression of this protein promotes cell proliferation and contributes to drug resistance in cancer. However, while readily available, HDAC inhibitors are often non-selective and result in unfavorable effects. To combat this, the small molecule chemical compound, J22352, was synthesized to selectively target HDAC6 and was able to significantly decrease HDAC6 expression and inhibit cell proliferation in glioblastoma. Importantly, this small molecule acted in a PROTAC-like manner, promoting the ubiquitination and subsequent degradation of HDAC6 by the proteasome. This study provides compelling evidence that HDAC6 can be targeted for degradation by synthetic small molecules, indicating the potential of PROTACs for the treatment of glioblastoma.
4. Advantages of using PROTACs to treat CNS disorders
As described above, PROTACS have significant potential to treat a variety of CNS disorders. Importantly, they have several advantages over traditional approaches that make them uniquely suited for the treatment of CNS diseases28. The first major advantage, as described in detail above, is the ability of PROTACs to control targeted degradation of undruggable proteins in the CNS. Additionally, PROTACs bypass the potential toxic effects that can be created by pharmacological approaches which simply inactivate a target protein without also controlling turnover. Furthermore, the specificity of PROTACs is especially high in comparison to other approaches, with numerous examples coming from molecules designed to degrade mutant, but not wild-type, proteins. Finally, PROTACs have substoichiometric catalytic activity48, allowing administration of very low concentrations to be sufficient enough to degrade a target protein. Collectively, these data suggest that PROTACs could be a very effective method to target and destroy undruggable proteins that characterize the pathophysiology of many CNS disorders.
5. Limitations of using PROTACs to treat CNS disorders
The studies described in Sections 2 and 3 are just a few of the available evidence suggesting that PROTACs have a high potential for treating CNS disorders, as they can target aberrant proteins for degradation. However, considering the complications of treating CNS disorders, there are a number of limitations that could prevent PROTAC drug development and clinical application. For example, one major issue is blood-brain barrier permeability, which can be a limiting factor for many pharmacological approaches. As discussed above, one study evaluated PROTAC in vivo efficacy and saw effects in the brain after intravenous and intranasal administration34, suggesting that PROTACs may be able to effectively penetrate the blood-brain barrier. Nevertheless, each PROTAC is designed for a specific target, which means using different E3 ligases and linkers that could further link to other molecules and have varied molecular weights, all of which can impact blood-brain barrier permeability and efficiency28, 49. Another potential limitation is localization of the PROTAC to specific brain regions. To successfully use a PROTAC, the E3 ligase must be expressed in the target region(s), however, some E3 ligases, including CRBN50, are differentially expressed across brain regions. This differential expression pattern can lead to complications when treating diseases that span brain regions. For instance, in Alzheimer’s disease, tau accumulation occurs in a progressive manner across brain regions, starting in the locus coeruleus and entorhinal cortex and finishing in the primary visual cortex51. This means that depending on disease progression, PROTACs would need to target tau specifically in some brain regions. Besides the potential absence of E3 ligase expression in the affected brain regions, such localized drug manipulations are not yet possible, though promising new technology suggests that this could be possible in the future52.
PROTACs are transient by nature53, but evidence suggests they can be effective in low, infrequent doses, specifically in cases of slow protein synthesis, and last longer than traditional inhibitor drugs54. Still, continuous administration throughout a patient’s life would be required when the disease has a genetic basis (Figure 2). For example, while PROTAC-mediated degradation of mHtt could clear the protein and reduce cellular toxicity, the effect would only be temporary as the disease-causing trinucleotide repeat expansion in Htt would continue to produce new mHtt protein. Furthermore, many CNS disorders are associated with broad reductions in proteasome catalytic activity55, meaning that even if a PROTAC can ubiquitinate the POI it still may not be possible to degrade it. Importantly, PROTACs cannot solve this problem of proteasome downregulation and instead are greatly limited by it since they rely on the endogenous proteasome function to degrade the target protein. Consequently, for PROTACs to be effective at treating some CNS disorders, they may have to be combined with approaches that can stimulate proteasome function. There is evidence that 1-[1-(4-fluorophenyl)-2,5-dimethylpyrrol-3-yl]-2pyrrolidin-1-ylethanone (IU1), a small-molecule inhibitor of the ubiquitin-specific protease 14 (USP14), can successfully enhance protein degradation in vitro56 and in mouse brain tissue following intraperitoneal injections57. However, IU1 does not directly stimulate the proteasome, instead leading to increased protein degradation of select substrates regulated by USP14. Consistent with this, one study found that when IU1 was directly infused into the amygdala, proteasome activity was not altered58. This evidence suggests that IU1 is unlikely to broadly enhance proteasome function in the brain, which would be needed in severe states of reduced protein degradation that are present in many diseases, such as Alzheimer’s disease. Furthermore, while promising new data does suggest that CRISPR-dCas9 technology could be used to broadly stimulate proteasome function in the brain59, this is still in its early stages. Thus, reduced proteasome function remains a barrier for PROTAC-mediated degradation of mutant proteins, especially when treating CNS disorders that span multiple brain regions.
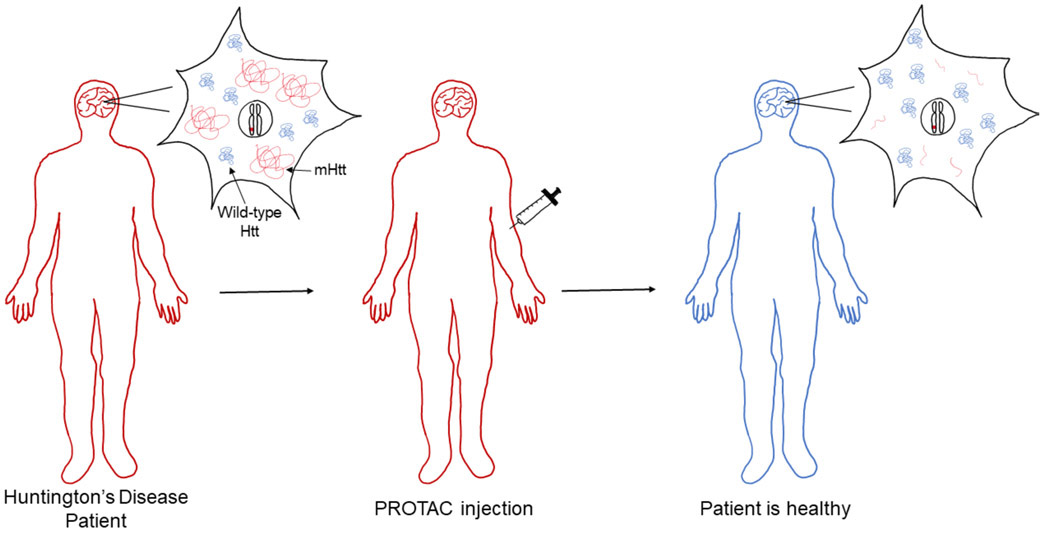
Figure 2. Theoretical schematic demonstrating PROTACs as a therapeutic for Huntington’s disease.
The patient is heterozygous for Huntington’s disease, meaning they carry one mutated allele (allele with red mark). This genotype causes the patient to develop Huntington's disease (red body) and leads to the synthesis of both wild-type Huntingtin protein (wild-type Htt; blue) and mutant Huntingtin protein (mHtt; red). To treat the patient, PROTACs are administered by injection. Once injected into the patient, the PROTACs pass through the blood-brain barrier and target mHtt for degradation. The PROTAC does not alter wild-type Htt or the heterozygous genotype. The patient is now healthy (blue body), but consistent treatment is required due to the genetic nature of the disease, which is not corrected by the PROTAC.
6. Conclusion
In summary, PROTACs have significant implications for drug discovery efforts focused on developing effective treatments for a wide range of CNS disorders. Importantly, due to their ability to target proteins that are largely “undruggable”, PROTACs may have a significant advantage over previous approaches that have ultimately failed in preclinical stages. However, as noted above, there are several boundaries to CNS PROTAC drug development that must be overcome before this potential can be accessed. Despite this, PROTACs have been and will continue to be a critical tool used in drug discovery efforts meant to develop treatments for complex CNS disorders.
7. Expert opinion
While only a small sample of the literature, the studies discussed here suggest that PROTAC technology has the potential to be a critical tool in treating CNS diseases. However, this work has been done almost exclusively outside of the brain, meaning that further research must be conducted to validate the ability of PROTACs to function in vivo. Importantly, while it has clearly been demonstrated that PROTACs have the ability to target aberrant proteins that lead to CNS diseases, in vitro studies often times do not translate to in vivo outcomes. Above we discussed evidence demonstrating that PROTACs have been developed that can successfully target tau, α-syn, mHtt, and SOD1. Additionally, a PROTAC-like molecule was developed which was able to target HDAC6, indicating that a wide-range of different molecules, some of which are considered to be undruggable, could be successfully degraded using PROTAC technology. Despite these exciting findings, it is important to note that, to date, only tau has been targeted in vivo, and this has not been independently validated by other labs. Considering that each PROTAC is designed differently, it is possible that some may not be successful at targeting proteins in the brain, leaving a significant question about whether such technology has the generalizability needed to treat a wide range of CNS disorders for which no effective therapies currently exist.
As discussed above, there are numerous limitations that must be overcome before PROTACs can truly be deemed a game changer for CNS drug discovery. To target the brain, PROTACs must be able to permeate through the blood-brain barrier, which could be potentially challenging because some PROTACs may be able to permeate, while others may not. Once in the brain, it may lack the ability to penetrate brain cells, which are notoriously difficult to transfect. Another significant barrier to drug discovery, in general, is localization of the drug to specific brain regions, which may be necessary in some cases. To date, there is no evidence indicating that PROTACs would be brain region specific. Further studies will need to examine PROTAC localization, as well as different administration strategies and the frequency required to control symptoms to determine if PROTACs would be suitable for treatment of CNS diseases, especially diseases with a genetic basis where mutant protein would consistently be synthesized. Another major concern, as stated above, is proteasome downregulation that is often seen in many CNS diseases, particularly those that are age-associated, which presents a significant hurdle since PROTACs work through the endogenous UPS system to degrade target proteins. Studies should evaluate the efficiency of PROTACs in a cellular environment that has abnormally low proteasome activity to determine if efficient targeted-degradation of a substrate can still occur. Perhaps in these cases of proteasome downregulation PROTACs may be used in conjunction with different technology that may enhance proteasome activity, although none currently exist. These limitations may be addressed as more in vivo studies are conducted, but to date, there is not enough evidence suggesting that PROTACs will work correctly if administered to the intact CNS.
Considering the ubiquitin proteasome system is only one pathway involved in regulating protein degradation, there are alternative options to PROTACs that utilize different pathways for small molecule-dependent POI degradation. ATTECs are one example mentioned above, which have been used to target mHtt as a potential therapeutic for Huntington’s disease. Another potential alternative, known as mallostery, utilizes allosteric regulation to cause misfolding of a protein. This misfolding activates the endoplasmic reticulum-associated degradation (ERAD) pathway to degrade the misfolded protein. It has been discovered that mallostery is utilized in yeast to control the HMG-CoA reductase, Hmg2, through the geranylgeranyl pyrophosphate molecule, which increased Hgm2 degradation by the ERAD pathway60. The Hampton lab, who coined the term mallostery, propose this pathway as another potential method for targeting undruggable proteins. These alternative methods for targeted protein degradation are beneficial for expanding the availability of therapeutics for CNS disorders and offer a complementary approach to PROTAC-mediated targeting.
Clearly, PROTACs have a high potential for clearing toxic proteins that result in many of the phenotypes associated with various CNS disorders. However, a major limitation to PROTACs is that they are transient and would require continuous administration to a patient with a genetic mutation. This is where PROTACs would be better suited by being combined with other therapeutically-relevant technologies, such as CRISPR/Cas9, to treat complex CNS disorders. In this complementary approach, CRISPR/Cas9 could be used to edit the mutated gene, removing or correcting the portion that codes for the toxic protein, and PROTACs could be used to clear the toxic protein that had already been translated within the cell. For example, Huntington’s disease, which was discussed in detail above, is one disease that could be treatable by this combined method. In this case, the Htt gene is mutated by a trinucleotide repeat expansion, which leads to the production of an abnormally large protein that causes cellular toxicity. CRISPR/Cas9 could be employed to cause a targeted deletion of the expanded region of the gene, which would essentially correct the mutation. Alone, the CRISPR/Cas9 system would not be able to solve the problem of toxic protein accumulation though because said toxic protein would have already been translated. However, through the addition of PROTACs technology, the toxic proteins can be targeted for degradation, and no new toxic proteins will be translated due to the CRISPR/Cas9-mediated gene correction. Thus, the greatest therapeutic potential of PROTACs for CNS disorders could actually be achieved by combining this technology with other novel approaches, such as CRISPR/Cas9.
Given all of the evidence, it is clear that PROTACs can effectively target a protein for degradation via the UPS system. Whether this can occur in patients with CNS diseases remains unknown, but the prospect is exciting. Considering the low success with current treatment options and the grave prognosis for certain CNS disorders, PROTACs may very well be a game changer in the upcoming years. For now, though, Alzheimer’s disease, Parkinson’s disease, Amyotrophic lateral sclerosis, Huntington’s disease, and glioblastoma, among others, remain devastating CNS disorders with no successful treatment. As we look towards the future, PROTACs are a front-runner for promising research that may lead to an effective treatment for these, and various other, complex CNS disorders.
Article highlights.
PROTACs act as a linker for an E3 ligase, which allows for the precise targeting of proteins of interest for degradation via the proteasome.
PROTACs have significant potential as a therapeutic tool to treat Central Nervous System (CNS) disorders in which few, if any, treatment options are currently available.
The ability of PROTACs to destroy toxic, undruggable proteins associated with a variety of CNS disorders has been evaluated in vitro.
The ability of PROTACs to destroy toxic, undruggable proteins in the intact brain is fleeting.
While promising, PROTACs have a number of limitations that must be overcome before they can be used as the next major drug discovery tool to treat CNS disorders.
Acknowledgments
Funding:
This work was supported by National Institute of Health (NIH) grants MH120498, MH120569 MH122414 and MH123742.
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Contributor Information
Kayla Farrell, Department of Animal and Poultry Sciences, Virginia Polytechnic Institute and State University, VA, USA.
Timothy J. Jarome, Department of Animal and Poultry Science and the School of Neuroscience, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA.
References
- 1.Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proceedings of the National Academy of Sciences 2001;98(15):8554–59.*This study was the first to offer proof of concept for PROTAC technology.
- 2.Itoh Y, Ishikawa M, Naito M, Hashimoto Y. Protein knockdown using methyl bestatin-ligand hybrid molecules: design and synthesis of inducers of ubiquitination-mediated degradation of cellular retinoic acid-binding proteins. J Am Chem Soc 2010. April 28;132(16):5820–6. [DOI] [PubMed] [Google Scholar]
- 3.Buckley DL, Van Molle I, Gareiss PC, Tae HS, Michel J, Noblin DJ, et al. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1α interaction. J Am Chem Soc 2012. March 14;134(10):4465–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, et al. DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 2015;348(6241):1376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muratani M, Tansey WP. How the ubiquitin-proteasome system controls transcription. Nat Rev Mol Cell Biol 2003. March;4(3):192–201. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar RC, Wendland B. Ubiquitin: not just for proteasomes anymore. Curr Opin Cell Biol 2003. April;15(2):184–90. [DOI] [PubMed] [Google Scholar]
- 7.Jarome TJ, Helmstetter FJ. The ubiquitin-proteasome system as a critical regulator of synaptic plasticity and long-term memory formation. Neurobiol Learn Mem 2013. October;105:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 2001. May 25;292(5521):1552–5. [DOI] [PubMed] [Google Scholar]
- 9.Ciechanover A, Iwai K. The ubiquitin system: from basic mechanisms to the patient bed. IUBMB Life 2004. April;56(4):193–201. [DOI] [PubMed] [Google Scholar]
- 10.Dohm CP, Kermer P, Bähr M. Aggregopathy in neurodegenerative diseases: mechanisms and therapeutic implication. Neurodegener Dis 2008;5(6):321–38. [DOI] [PubMed] [Google Scholar]
- 11.Olanow CW, McNaught KS. Ubiquitin-proteasome system and Parkinson's disease. Mov Disord 2006. November;21(11):1806–23. [DOI] [PubMed] [Google Scholar]
- 12.Gong B, Radulovic M, Figueiredo-Pereira ME, Cardozo C. The Ubiquitin-Proteasome System: Potential Therapeutic Targets for Alzheimer’s Disease and Spinal Cord Injury. Frontiers in Molecular Neuroscience 2016. 2016-January-26;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998;67:425–79. [DOI] [PubMed] [Google Scholar]
- 14.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 2002. April;82(2):373–428. [DOI] [PubMed] [Google Scholar]
- 15.Burslem GM, Crews CM. Proteolysis-Targeting Chimeras as Therapeutics and Tools for Biological Discovery. Cell 2020. 2020/April/02/;181(1):102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu B, Zhou Y, Sun D, Yang Y, Liu Y, Li X, et al. PROTACs: New method to degrade transcription regulating proteins. European Journal of Medicinal Chemistry 2020. 2020/December/01/;207:112698. [DOI] [PubMed] [Google Scholar]
- 17.Sun X, Gao H, Yang Y, He M, Wu Y, Song Y, et al. PROTACs: great opportunities for academia and industry. Signal Transduction and Targeted Therapy 2019. 2019/December/24;4(1):64.*This is a thorough review of PROTAC technology and its potential applications.
- 18.Ishida T, Ciulli A. E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones. SLAS DISCOVERY: Advancing the Science of Drug Discovery 2020:2472555220965528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siuly S, Zhang Y. Medical Big Data: Neurological Diseases Diagnosis Through Medical Data Analysis. Data Science and Engineering 2016. 2016/June/01;1(2):54–64. [Google Scholar]
- 20.Collaborators GBDN. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18(5):459–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gribkoff VK, Kaczmarek LK. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 2017. July 1;120:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakamoto KM. Protacs for treatment of cancer. Pediatr Res 2010. May;67(5):505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014. January 17;343(6168):301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scudellari M Protein-slaying drugs could be the next blockbuster therapies. Nature 2019. March;567(7748):298–300. [DOI] [PubMed] [Google Scholar]
- 25.Delport A, Hewer R. Inducing the Degradation of Disease-Related Proteins Using Heterobifunctional Molecules. Molecules 2019. September 8;24(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konstantinidou M, Li J, Zhang B, Wang Z, Shaabani S, Ter Brake F, et al. PROTACs- a game-changing technology. Expert Opin Drug Discov 2019. December;14(12):1255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.An S, Fu L. Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs. EBioMedicine 2018. October;36:553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Jiang X, Feng F, Liu W, Sun H. Degradation of proteins by PROTACs and other strategies. Acta Pharmaceutica Sinica B 2020. 2020/February/01/;10(2):207–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabrizi SJ, Ghosh R, Leavitt BR. Huntingtin Lowering Strategies for Disease Modification in Huntington’s Disease. Neuron 2019. 2019/March/06/;101(5):801–19. [DOI] [PubMed] [Google Scholar]
- 30.Au YZ, Wang T, Sigua LH, Qi J. Peptide-Based PROTAC: The Predator of Pathological Proteins. Cell Chem Biol 2020. June 18;27(6):637–39. [DOI] [PubMed] [Google Scholar]
- 31.Himmelstein DS, Ward SM, Lancia JK, Patterson KR, Binder LI. Tau as a therapeutic target in neurodegenerative disease. Pharmacology & therapeutics 2012;136(1):8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu M, Liu T, Jiao Q, Ji J, Tao M, Liu Y, et al. Discovery of a Keap1-dependent peptide PROTAC to knockdown Tau by ubiquitination-proteasome degradation pathway. European Journal of Medicinal Chemistry 2018. 2018/February/25/;146:251–59. [DOI] [PubMed] [Google Scholar]
- 33.Silva MC, Ferguson FM, Cai Q, Donovan KA, Nandi G, Patnaik D, et al. Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models. eLife 2019;8:e45457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu TT, Gao N, Li QQ, Chen PG, Yang XF, Chen YX, et al. Specific Knockdown of Endogenous Tau Protein by Peptide-Directed Ubiquitin-Proteasome Degradation. Cell Chem Biol 2016. April 21;23(4):453–61.**This study demonstrated the ability of PROTACs to cross the blood-brain barrier and target tau in two brain regions.
- 35.Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting Alpha-Synuclein as a Therapy for Parkinson's Disease. Front Mol Neurosci 2019;12:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu J, Ren X, Xue F, He Y, Zhang R, Zheng Y, et al. Specific Knockdown of α-Synuclein by Peptide-Directed Proteasome Degradation Rescued Its Associated Neurotoxicity. Cell Chem Biol 2020. June 18;27(6):751–62.e4. [DOI] [PubMed] [Google Scholar]
- 37.Tomoshige S, Nomura S, Ohgane K, Hashimoto Y, Ishikawa M. Discovery of Small Molecules that Induce the Degradation of Huntingtin. Angew Chem Int Ed Engl 2017. September 11;56(38):11530–33.**This study was able to target mHtt without knowing the specific target ligand, demonstrating the potential for PROTAC as a therpeutic for diseases with unknown ligands.
- 38.Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 2011. January;10(1):83–98. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Zhu C, Ding Y, Fei Y, Lu B. ATTEC: a potential new approach to target proteinopathies. Autophagy 2020. 2020/January/02;16(1):185–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masrori P, Van Damme P. Amyotrophic lateral sclerosis: a clinical review. European Journal of Neurology 2020;27(10):1918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gois AM, Mendonça DMF, Freire MAM, Santos JR. IN VITRO AND IN VIVO MODELS OF AMYOTROPHIC LATERAL SCLEROSIS: AN UPDATED OVERVIEW. Brain Res Bull 2020. June;159:32–43. [DOI] [PubMed] [Google Scholar]
- 42.Tomoshige S, Ishikawa M. PROTACs and Other Chemical Protein Degradation Technologies for the Treatment of Neurodegenerative Disorders. Angew Chem Int Ed Engl 2020. May 14. [DOI] [PubMed] [Google Scholar]
- 43.Malik R, Wiedau M. Therapeutic Approaches Targeting Protein Aggregation in Amyotrophic Lateral Sclerosis. Frontiers in Molecular Neuroscience 2020. 2020-June-09;13(98). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee Y-C, Huang W-C, Lin J-H, Kao T-J, Lin H-C, Lee K-H, et al. Znf179 E3 ligase-mediated TDP-43 polyubiquitination is involved in TDP-43- ubiquitinated inclusions (UBI) (+)-related neurodegenerative pathology. Journal of Biomedical Science 2018. 2018/November/08;25(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishigaki S, Niwa J-i, Yamada S-i, Takahashi M, Ito T, Sone J, et al. Dorfin-CHIP chimeric proteins potently ubiquitylate and degrade familial ALS-related mutant SOD1 proteins and reduce their cellular toxicity. Neurobiology of Disease 2007. 2007/February/01/;25(2):331–41. [DOI] [PubMed] [Google Scholar]
- 46.Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020;22(8):1073–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J-R, Yu C-W, Hung P-Y, Hsin L-W, Chern J-W. High-selective HDAC6 inhibitor promotes HDAC6 degradation following autophagy modulation and enhanced antitumor immunity in glioblastoma. Biochemical Pharmacology 2019. 2019/May/01/;163:458–71. [DOI] [PubMed] [Google Scholar]
- 48.Bondeson DP, Mares A, Smith IE, Ko E, Campos S, Miah AH, et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol 2015. August;11(8):611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu S, Cui D, Chen X, Xiong X, Zhao Y. PROTACs: An Emerging Targeting Technique for Protein Degradation in Drug Discovery. Bioessays 2018. April;40(4):e1700247. [DOI] [PubMed] [Google Scholar]
- 50.Higgins JJ, Tal AL, Sun X, Hauck SCR, Hao J, Kosofosky BE, et al. Temporal and Spatial Mouse Brain Expression of Cereblon, An Ionic Channel Regulator Involved in Human Intelligence. Journal of Neurogenetics 2010. 2010/January/01;24(1):18–26. [DOI] [PubMed] [Google Scholar]
- 51.Franzmeier N, Neitzel J, Rubinski A, Smith R, Strandberg O, Ossenkoppele R, et al. Functional brain architecture is associated with the rate of tau accumulation in Alzheimer’s disease. Nat Commun 2020. January 17;11(1):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rich MC, Sherwood J, Bartley AF, Whitsitt QA, Lee M, Willoughby WR, et al. Focused ultrasound blood brain barrier opening mediated delivery of MRI-visible albumin nanoclusters to the rat brain for localized drug delivery with temporal control. J Control Release 2020. August 10;324:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cromm PM, Crews CM. Targeted Protein Degradation: from Chemical Biology to Drug Discovery. Cell Chem Biol 2017. September 21;24(9):1181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mares A, Miah AH, Smith IED, Rackham M, Thawani AR, Cryan J, et al. Extended pharmacodynamic responses observed upon PROTAC-mediated degradation of RIPK2. Communications Biology 2020. 2020/March/20;3(1):140.*This study demonstrates the pharmocological potential of PROTACs.
- 55.Thibaudeau TA, Anderson RT, Smith DM. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat Commun 2018. March 15;9(1):1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee B-H, Lee MJ, Park S, Oh D-C, Elsasser S, Chen P-C, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 2010;467(7312):179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Min JW, Lü L, Freeling JL, Martin DS, Wang H. USP14 inhibitor attenuates cerebral ischemia/reperfusion-induced neuronal injury in mice. J Neurochem 2017. March;140(5):826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarome TJ, Kwapis JL, Hallengren JJ, Wilson SM, Helmstetter FJ. The ubiquitin-specific protease 14 (USP14) is a critical regulator of long-term memory formation. Learn Mem 2013. December 16;21(1):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devulapalli R, Jones N, Farrell K, Musaus M, Kugler H, McFadden T, et al. Males and females differ in the regulation and engagement of, but not requirement for, protein degradation in the amygdala during fear memory formation. Neurobiol Learn Mem 2021. February 17:107404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wangeline MA, Hampton RY. "Mallostery"-ligand-dependent protein misfolding enables physiological regulation by ERAD. J Biol Chem 2018. September 21;293(38):14937–50. [DOI] [PMC free article] [PubMed] [Google Scholar]