Abstract
Volume recruitment from the splanchnic compartment is an important physiological response to stressors such as physical activity and blood loss. In the setting of heart failure (HF), excess fluid redistribution from this compartment leads to increased cardiac filling pressures with limitation in exercise capacity. Recent evidence suggests that blocking neural activity of the greater splanchnic nerve (GSN) could have significant benefits in some patients with heart failure (HF) by reducing cardiac filling pressures and improvement in exercise capacity. However, to date the long-term safety of splanchnic nerve modulation (SNM) in the setting of HF is unknown. SNM is currently used in clinical practice to alleviate some forms of chronic abdominal pain. A systematic review of series where permanent SNM was used as a treatment for chronic abdominal pain indicates that permanent SNM is well tolerated, with side-effects limited to transient diarrhea or abdominal colic and transient hypotension. The pathophysiological role of the GSN in volume redistribution, the encouraging findings of acute and chronic pilot SNM studies and the safety profile from permanent SNM for pain provides a strong basis for continued efforts to study this therapeutic target in HF.
Keywords: splanchnic vasoconstriction, sympathetic nervous system, adverse effects
Introduction
Elevated intracardiac pressures at rest and during activity are a fundamental feature of heart failure (HF) with both reduced (HFrEF) and preserved ejection fraction (HFpEF) (1–3). Elevated filling pressures at rest or during exercise are linked closely to HF progression and end-organ damage including heart, kidneys, liver and lungs. Exercise-related increases in left atrial pressure lead to elevated pulmonary venous pressure with transudation of fluid into the pulmonary capillary interstitium (i.e., pulmonary edema) (4). Elevated right sided filling pressures may contribute to acute kidney dysfunction (cardio-renal syndrome), abdominal bloating and peripheral edema. In addition, elevated right sided pressures may contribute to increased left heart filling pressures due to pericardial restraint and ventricular interaction and coronary turgor effect (5). A multitude of mechanisms contribute to elevated filling pressures at rest and with exercise, including reduced inotropic and chronotropic reserves and impaired relaxation. Through a blunted vasodilatory response, the vascular system additionally contributes via an increased pulmonary arterial resistance and decreased pulmonary arterial compliance. More recently the concept of volume redistribution has gained attention as a cause of elevated filling pressures and cardiac decompensation (3,6).
Sympathetically-mediated splanchnic vasoconstriction results in rapid shifts of blood from the splanchnic compartment to the heart and lungs, which is a component of the normal physiological responses during exercise. However, in patients with HF the rapid shift of blood volume from the splanchnic to central vasculature results in exaggerated rise in cardiac filling pressures, thereby exacerbating exercise intolerance and possibly also precipitating HF decompensation (7,8). Modulation of splanchnic nerve activity has therefore been developed as a potential therapeutic option in HF patients to reduce the redistribution of volume and to improve symptoms and outcomes. Here we provide a mechanistic overview of splanchnic nerve modulation (SNM) in HF, and summarize recent pilot studies of this therapy in HF. Given currently ongoing and future planned research studies investigating permanent SNM as a treatment for HF, we provide a systematic review of the safety of ablation of the splanchnic nerves in non-HF conditions to guide further development of this therapeutic approach in HF.
Role of the Splanchnic Nerves and Splanchnic Compartment in Heart Failure
Accumulating evidence suggests that the hemodynamic abnormalities and physical limitations in HF are driven in part by a neurohormonal mediated decrease in vascular capacitance (7,8). Given the well-recognized relationship between cardiac afterload and cardiac performance, (9) much attention has been given to the arterial system. However, the venous system, as the main storage reservoir for central blood volume, is a major contributor to the pathophysiology of HF. The large capacitance of the venous system allows veins to store around 70% of the total blood volume (10,11) with the remaining 30% located in the arteries. The highly-vascularized organs of the splanchnic compartment (i.e. liver, spleen and mesenteric vasculature) contain the majority of intravascular blood volume making it the main source of vascular capacitance both in animals (10,12–14) and in humans (10,13,15–19). In contrast, only around 10% of the total blood volume is located in the extremities at rest. The splanchnic vasculature is innervated with a vast network of adrenergic neurons (20), which is far greater on the venous compared to the arterial system (21). Sympathetic fibers are the main regulator of splanchnic vascular tone (22) and thus can provide both rapid and chronic compensatory mechanisms for physiological stressors (i.e., orthostasis due to gravity, exercise) through volume redistribution in and out of the splanchnic vascular compartment (23–27).
Experimentally, increased sympathetic tone (direct nerve stimulation, catecholamine infusion, or mediated via reflex mechanisms such as baroreflex activation) rapidly shifts blood volume from the splanchnic to the central vascular compartment (heart and lungs) (28–31). Direct splanchnic nerve stimulation in animals and humans provides evidence for significant hemodynamic changes induced by acute splanchnic vascular constriction. Across multiple animal species splanchnic nerve stimulation results in an acute increase in preload and afterload with resultant increase in cardiac output and blood pressure (27,32,33). These changes, driven by a translocation of blood volume out of the splanchnic vascular compartment, occur within seconds of stimulation onset and subsides within minutes after the stimulation has ended. In a human study of four adults with well-controlled hypertension, splanchnic nerve stimulation for ~80 seconds raised cardiac output from 3.6 to 6.4 l/min, mean arterial blood pressure from 58.8 to 121 mmHg, stroke volume from 57.4 to 81.8 ml, heart rate from 62 to 84.8 beats/min, and central venous pressure from 7.2 to 13.6 mmHg (27). Increases in hemodynamic indices occurred within 10 seconds of stimulation which subsequently gradually returned to baseline (within minutes) after stimulation was interrupted. In a patient with compensated HFpEF and underlying atrial fibrillation a minimally invasive catheter-based 40-second stimulation of the greater splanchnic nerve (GSN) resulted in an acute rise of arterial blood pressure (85/45 to 123/75 mmHg), mean central venous pressures (6 to 8 mmHg), mean pulmonary arterial pressure (14 to 21 mmHg), and left atrial pressure (7 to 14 mmHg). Peak left atrial pressures occurred at the end the stimulation, and following stimulation, intracardiac and central vascular pressures dropped transiently before rising again to sustained levels for >3 minutes. The findings support the notion that GSN-mediated sympathetic activation of the splanchnic vascular compartment may precipitate cardiac decompensation via volume redistribution into the central compartment and increased afterload (3,7).
As a consequence of the above findings, the splanchnic vascular compartment and its control via the GSN have been identified as a potential therapeutic target in HF. Evidence from animal models suggests that central venous capacitance is reduced in HF. In a canine model of acute systolic HF induced by cardiac ischemia, the splanchnic vascular volume was decreased (34) (Figure 1). Baroreflex-mediated venoconstriction accounted for the majority (~ 80%) of the observed increase left ventricular end-diastolic pressure, while left ventricular dysfunction accounted for a minority (~ 20%) of the left ventricular end diastolic pressure increase (34,35). The shift in blood volume is seen not only in acute HF but also in animal models of chronic HF. Reduction of total vascular capacitance has been observed across a number of animal models of chronic HF (~50%) (36–38). Notably, vascular capacitance tends to return to normal once ventricular function and neurohormonal imbalance are allowed to recover in the above animal models. Whether cardiac insults always predate a change in sympathetic and vascular tone or whether changes in vascular tone and volume shifts are an early derangement in the HF cascade is however unknown.
Figure 1. Impact of heart failure on splanchnic vascular pressure-volume relation (capacitance).
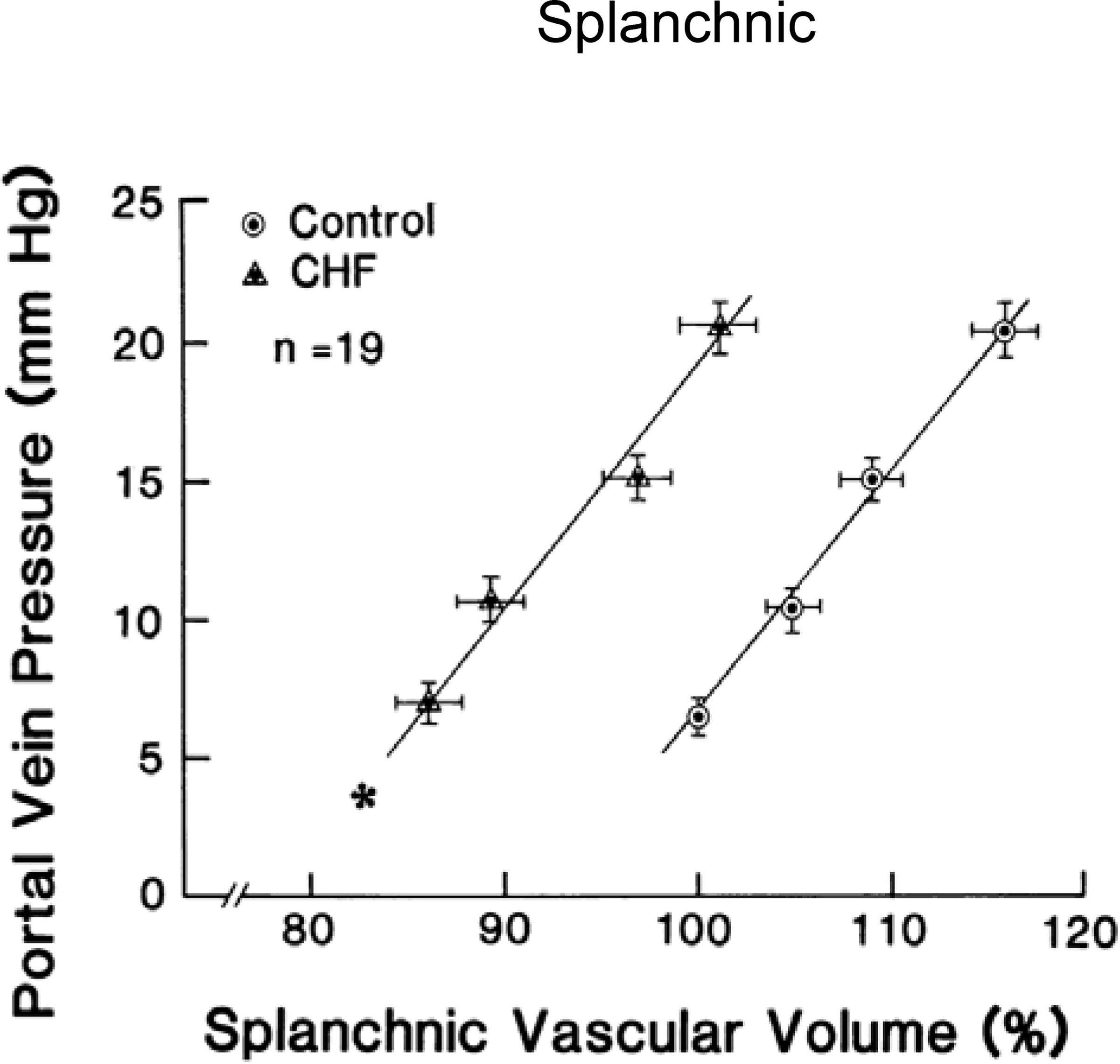
Left: Graph showing effect of acute congestive heart failure (CHF) on the splanchnic vascular pressure–volume relation. Acute CHF produced a significant parallel leftward shift of the splanchnic vascular pressure–volume relation which signifies a reduced vascular capacitance. These are the pooled results in 19 animals. From (34).
In experimental HF, stressed blood volume can be reduced via pharmacological vasodilation through a redistribution of fluid back into the splanchnic compartment (39). Nitroglycerin (a predominantly venodilator) was most effective to reduce stressed blood volume (~ 30%) (40). Agents targeting primarily arterial conductance (e.g., hydralazine) have the least effect on splanchnic capacitance (~ 0%) and are less effective at reducing cardiac filling pressures (34,38,41). Combined arterial and venous dilators such as angiotensin converting enzyme inhibitors have an intermediary effect on venous capacitance and reduction in cardiac filling pressures (34,40,42).
Direct evidence to substantiate the contribution of venous capacitance in humans with HF is lacking due to the complexity of required experimental techniques. It is widely recognized that pure arterial dilators, such as hydralazine, are ineffective in the treatment of HF (43). In contrast, mixed arterial and venous dilators such as angiotensin converting enzyme inhibitors, angiotensin receptor blockers or the combination of hydralazine and nitrates are some of the most effective therapies in current use (44). Although a large fraction of the benefits of angiotensin converting enzyme inhibitors, and angiotensin receptor blockers lays in the anti-hormonal properties of these agents the disproportionate effect on preload reduction via venodilation could be a critical component of their effectiveness. Computer-based simulation analyses have extended the findings from animals to humans by showing that a decrease in venous capacitance with subsequent increased in stressed blood volume would be the most likely contributor explaining an increase in left atrial pressures (7,8). It is important to note that in the absence of venous capacitance changes, changes in afterload, diastolic dysfunction, and heart rate alone or in combination are insufficient to explain cardiac decompensation. Along the same lines, an increase in cardiac output in response to primary afterload reduction has a very small effect on pulmonary venous pressures, and does not relieve pulmonary congestion (45,46). Interestingly, to date studies that focused on acute pre and afterload reduction in acute HF failed to demonstrate superiority over standard of care which heavily relies on diuretic management (47).
Splanchnic Nerve Modulation for the Management of Heart Failure: Pilot Study Results
The safety and efficacy of temporary SNM was investigated in two small proof of concept studies in patients with decompensated HF (Splanchnic HF-1 – ClinicalTrials.gov #: NCT02669407; N=11) (48,49), and chronic HF (Splanchnic-HF 2 – NCT03453151; N=15) (50). In patients with advanced HFrEF hospitalized for an acute decompensated HF, bilateral temporary SNM with a needle based injection of lidocaine at the GSN (duration of action <90 min) lowered resting right- and left-sided filling pressures and improved cardiac output without procedure- or nerve block-related complications (48,49). In patients with ambulatory, chronic HF (mostly HFrEF), SNM with ropivacaine (duration of action <24 h) reduced resting pulmonary capillary wedge pressure (PCWP) from 28.3±7.6 to 20.3±9.5 mmHg (p<0.001) and peak exercise PCWP from 34.8±10.0 to 25.1±10.7 mmHg (p<0.001). The reduction in filling pressures was associated with improvement in the cardiac index at peak exercise from 3.4±1.2 to 3.8±1.1 L/min/m2 (p=0.011) and peak oxygen consumption (VO2) from 9.1±2.5 to 9.8±2.7 ml/kg/min (p=0.053). The majority of the intracardiac pressure drop was explained by a reduction in estimated stressed blood volume (8). Bilateral GSN block did result in symptomatic orthostatic hypotension in 4 out of 5 patients which required fluid administration to resolve, supporting the concept of substantial central vascular underfilling. The subsequent 10 patients underwent unilateral SNM without symptomatic orthostatic hypotension, suggesting an incremental effect of bilateral over unilateral SNM. One patient reported loose stools for the first 24 hours following temporary bilateral SNM. There were no other cases of gastrointestinal side effects in any of the 15 patients.
A European two-center study investigated the feasibility of permanent surgical right-sided SNM for the treatment of HFpEF (Surgical Resection of the Greater Splanchnic Nerve in Subjects Having Heart Failure With Preserved Ejection Fraction – ClinicalTrials.gov #: NCT03715543, N=11). The study included patients with NYHA III with elevated PCWP at rest or during exercise. The primary endpoint of reduction in PCWP at 3 months was met. Patient experienced a mean 6.2 mmHg (95% CI −12.2 to −0.2; p<0.05) reduction in PCWP at 20W exercise which carried over to peak exercise (−5.1 mmHg; 95% CI −10.1 to −0.1; p<0.05). There was also improvement in NYHA class and quality of life at 12 months. Neither orthostasis nor gastrointestinal dysmotility were observed in any patients up to 12 months. Taken together, these observational, open-label studies suggest that splanchnic sympathetic nerve modulation may serve a potential therapeutic use in both acute and chronic HF irrespective of left ventricular ejection fraction (Figure 2).
Figure 2. Effect of temporary splanchnic nerve modulation on intra-cardiac and arterial pressure.
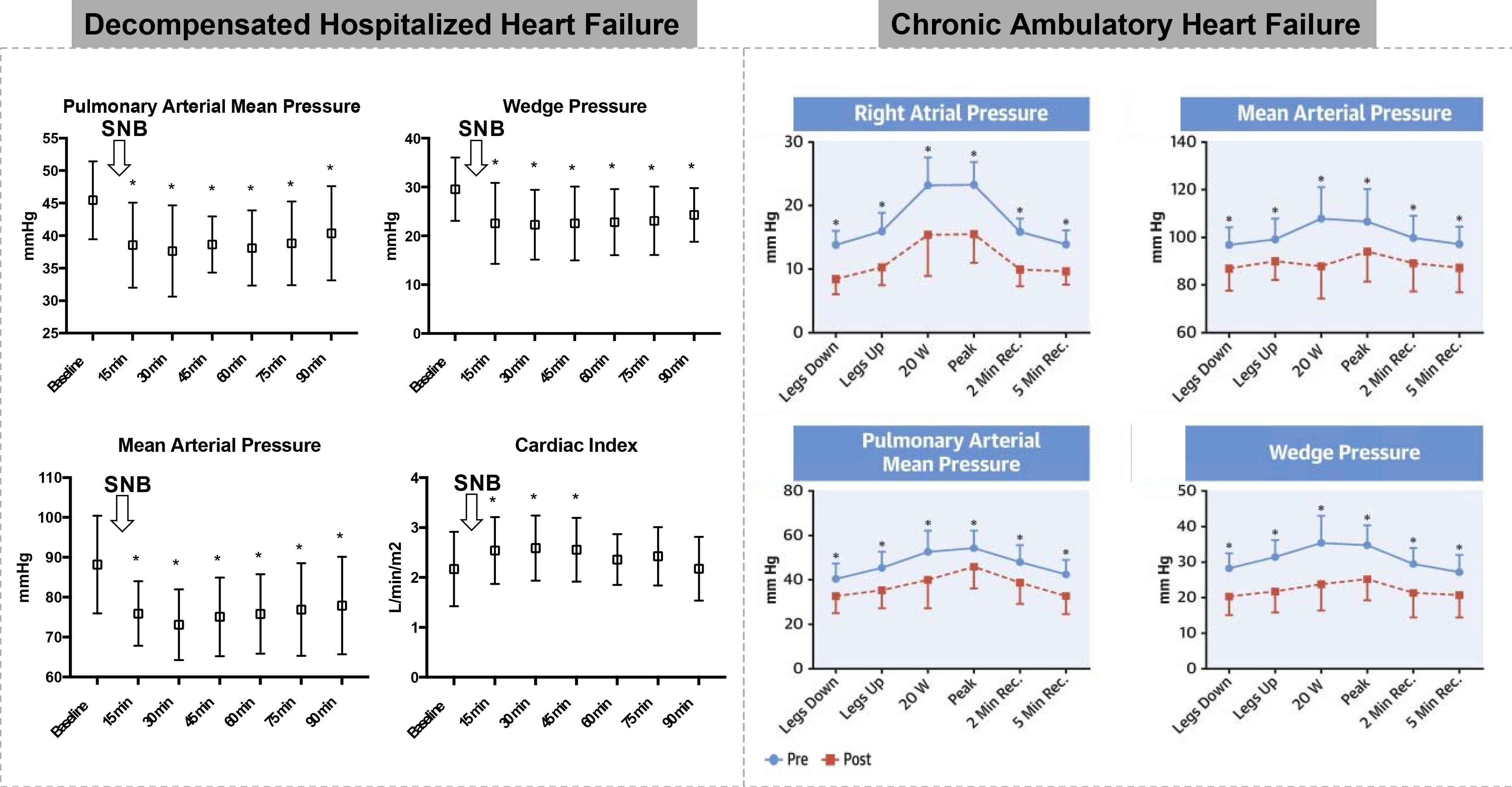
Findings from the Splanchnic HF-1 (left) and Splanchnic HF-2 studies (right)
Safety of Splanchnic Nerve Modulation: A Systematic Review
Splanchnic nerve modulation is used currently for several indications, including intractable abdominal pain due to severe pancreatitis or carcinoma (51–54). Surgical denervation of splanchnic nerves via surgical splanchnicectomy also has been used as a treatment for uncontrolled hypertension in the 1930–1950s. While this procedure was shown to be efficacious in reducing blood pressure, the discovery of effective antihypertensive medications made it obsolete (55). Long-term pharmacological blockade of the celiac plexus/splanchnic nerves with botulinum toxin also has been reported to be safe and effective in a case of refractory hypertension (56).
Sympathetic and parasympathetic control of the upper abdominal viscera (e.g., pancreas, liver, duodenum) is mediated by the GSN, lesser splanchnic nerve, and least splanchnic nerve, which are bilateral, pre-ganglionic nerves originating from the 5th to 8th, 9th to 10th, and 11th thoracic ganglia, respectively, and synapse in the celiac plexus (57) (Figure 3). These bundles also contain visceral afferent pain fibers, which is why splanchnic (unilateral or bilateral) as well as celiac plexus block was proposed as a therapy for intractable abdominal pain caused by several conditions, including cancer of the abdominal viscera, chronic benign abdominal pain (chronic pancreatitis), and portal vein thrombosis refractory to pharmacological treatment. The effectiveness of this therapeutic intervention to control abdominal pain has been demonstrated in numerous studies.
Figure 3. Splanchnic nerve anatomy.
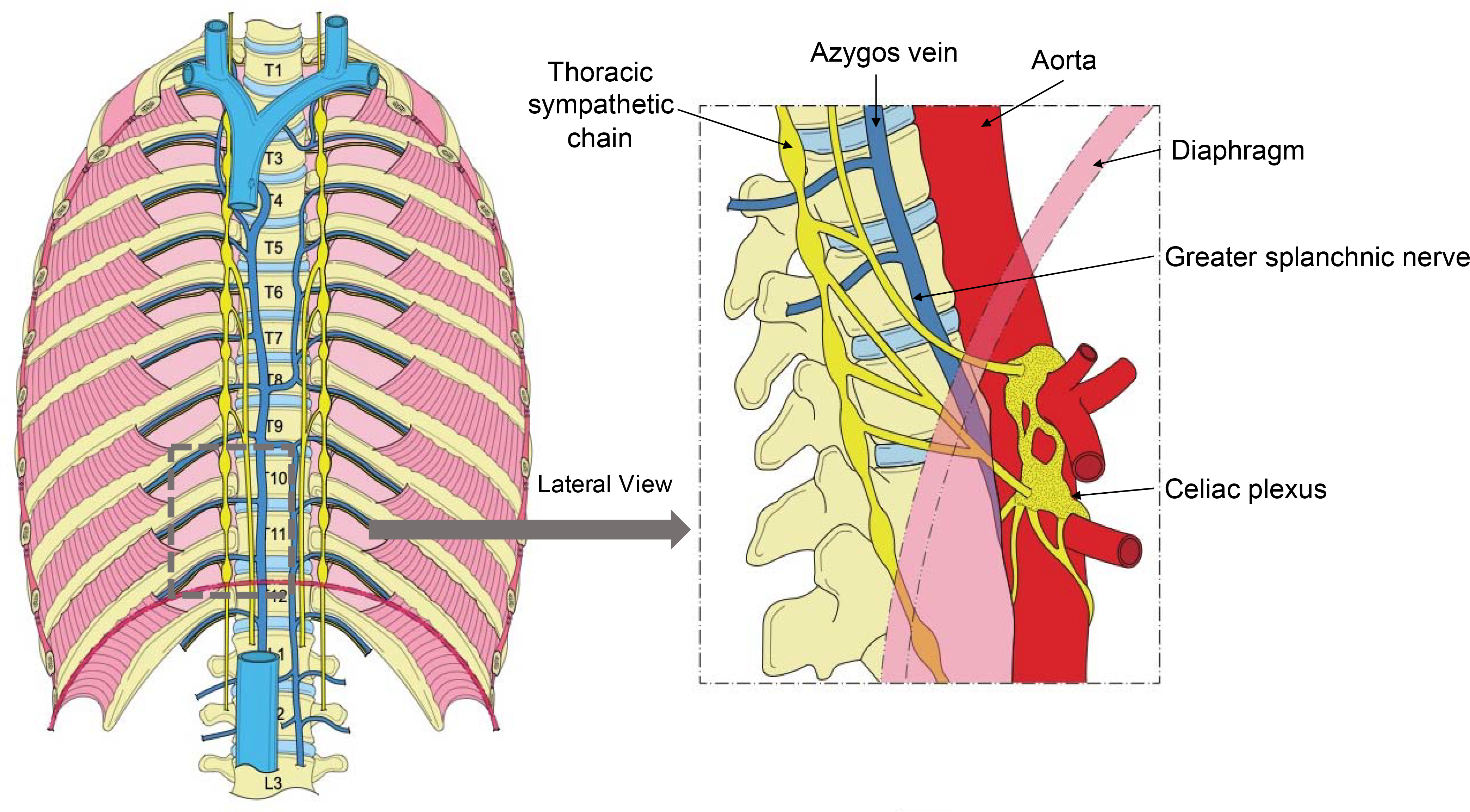
Anterior and lateral view of the supradiaphragmatic and infradiaphragmatic course of the splanchnic nerve
The initial approach taken to interrupt signaling to and from the visceral organs was through open surgical splanchnicectomy. The approach has since evolved to minimally invasive thoracoscopic splanchnicectomies and, more recently, percutaneous interventions applying chemical or radiofrequency (RF) ablation techniques. There are 2 different categories of potential side effects that can occur in response to splanchnic ablation: (1) physiological side effects following interruption of efferent and afferent autonomic tone to the visceral vasculature; and (2) procedural complications related to the intervention itself.
A published systematic review on the side effects of SNM via nerve ablation involved 170 patients undergoing thoracoscopic GSN resection (57). The most common complications related to the procedure included intercostal neuralgia (n = 23, 7%), pulmonary atelectasis (n = 6, 1.9%), and chylothorax (n = 4, 1.3%). The only sequelae to SNM reported in this systematic review was orthostatic hypotension (n = 4, 1.3%). There were no reports of diarrhea.
The prior systematic review included a relatively small number of patients and was published more than 10 years ago. Therefore, we performed an updated and expanded systematic review. The literature search was performed without language restriction using electronic databases of PubMed and the Cochrane library from January 1st, 1990, until September 30th, 2020 (30 years). The following search terms were used: “Splanchnic nerve block” [Mesh] OR “ Splanchnic nerve block” [tiab] OR “Splanchnic nerve resection” [tiab] OR “Splanchnic nerve ablation” [tiab] OR “splanchnicectomy” [tiab]. We did not restrict the study population. We considered studies that used both unilateral and bilateral nerve resections/ablation. We only considered reports/studies that presented permanent resection/ablation of the GSN and not merely temporary blockade. Articles were excluded if they reported pre-clinical data, meeting abstracts, or were duplicates. We abstracted narrative and quantitative safety results as stated in each article. Extracted data included procedural approach, number of patients, length of follow up, side effects and procedural complications.
The original search yielded 169 results (Supplementary Figure 1 for PRIMSA chart). Three authors reviewed the publications and applied the above criteria (MF, JM, AB). Disagreements were decided by a majority vote. The review identified a final sample of 54 publications that reported side-effects / complications subsequent to SNM with a total of 1,511 patients (see Supplementary Table 1 for the complete list of studies). Bilateral permanent SNM was performed in 1,144 patients and unilateral blockade in 385 patients. A summary of the side-effect / complication profile is presented in Table 1. Our systematic review of these patients (none of whom had a known history of HF) suggests that the side effects of permanent SNM (surgical or interventional) are restricted to a 48 hour period post-procedure and were reported almost exclusively in patients undergoing bilateral blockade. Notably, side effects were in many studies not systematically evaluated, thus short-term and long-term side effects might have been underreported. From the published literature and our own systematic review (Supplementary Table 1) the most common short-term side effects include gastrointestinal symptoms (7.7%) followed by orthostatic hypotension (6.4%). Of note, transient orthostatic hypotension can be prevented by aggressive pre-procedural hydration (54). In general, modern approaches to block sympathetic innervation to the visceral organs is associated with minimal patient morbidity and no reported cases of procedural-related mortality. All reported side-effects or complications of SNM and celiac plexus block are summarized in Table 2. While the complications for celiac ganglion block are fundamentally similar, for the purposes of this review we will focus only on complications arising from SNM (see Supplementary Table 2 for systematic review of celiac block). Detailed information on side effects and complications identified in our systematic review are outlined below.
Table 1.
Summary of GSN ablation/resection related physiological side effects.
| Side-effect | Prevalence |
|---|---|
| Diarrhea/Abdominal Colic | 7.7 % (n=116) |
| Acute (<3 days) | 6.9% (n=105) |
| Transient (<7 days) | <1% (n = 11) |
| Orthostatic Hypotension | 6.4% (n=96) |
| Acute (<2 days) | 6% (n=91) |
| Transient (< 4 months) | <0.5% (n=4) |
| Chronic (>4 months) | <0.1% (=1) |
| Nausea/Vomiting (<2 days) | 3% (n=45) |
| TOTAL | n = 1,511 |
Table 2.
Side effect profile / complications due to Splanchnic Nerve or Celiac Plexus Blocks
| Splanchnic only | Celiac only | Both | |
|---|---|---|---|
| Physiological | |||
| Hypotension | X | ||
| Diarrhea | X | ||
| Urinary abnormalities | X | ||
| Failure of ejaculation | X | ||
| Procedural | |||
| Pain during or after procedure | X | ||
| Vascular trauma | X | ||
| Pneumothorax | X | ||
| Chylothorax | X | ||
| Peritonitis | X | ||
| Retroperitoneal hematoma | X | ||
| Paraplegia | X | ||
| Paresthesia of lumbar somatic nerve | X |
Physiological Side-Effects
Transient diarrhea and transient hypotension (with or without an orthostatic component) are the most common physiological side effects of SNM via ablation/resection. The physiological complications are listed below in the order of reported frequency of occurrence.
Gastrointestinal hypermotility (diarrhea) and abdominal colic is the most common reported side effect of splanchnic ablation/resection. Self-limited diarrhea (lasting 36 to 72 hours) without any electrolyte or hemodynamic disturbances has been reported. However, there have been rare reports of severe and persistent diarrhea (58). Similar to hypotension, diarrhea is regarded to be a strong indication of successful nerve block. To understand the expected hazard of gastrointestinal hypermotility in the intended HF population, it is again important to recognize that the reported data on the safety of SNM were almost exclusively in patients diagnosed with advanced abdominal cancer. These patients tend to be chronically constipated from high-dose opioid therapy, and rather than diarrhea, most patients simply experience improved bowel motility (52).
-
Hypotension is the second most commonly reported complication of the SNM and is usually transient and without clinical sequelae. It is typically associated with orthostatic hypotension (drop in systolic blood pressure of >20 mmHg with upright posture), leading to transient symptoms like dizziness and blurred vision. Hypotension in this context can usually be prevented by the intravenous administration of 500 to 1000 mL of crystalloid prior to or immediately following SNM (59). Without intravenous fluid prophylaxis, clinically significant (albeit transient) orthostatic hypotension can be expected in a majority of patients, a finding that points to an important interplay between volume status and adrenergic control of this vascular bed. Longer term orthostatic hypotension events reported in the literature were limited to <48 hours with 5 reports of hypotension persisting for weeks after SNM. Some authors have suggested that hypotension is a reliable indication of successful SNM (52) although it is generally masked due to the prudent practice of hydration prior to the procedure.
To understand the expected prevalence of orthostatic hypotension in HF patients, it is important to recognize that the data regarding safety of SNM reported in the literature were generated almost exclusively in patients diagnosed with advanced abdominal cancer. This patient population is typically characterized by severely reduced oral intake due to a variety of causes related to their cancer or treatment resulting in dehydration. The consequential intravascular volume depletion in these patients makes them more susceptible to orthostatic hypotension in the presence or absence of GSN ablation. Vasodilation of the splanchnic vasculature following SNM increases vascular capacitance and results in the redistribution of blood from the central veins to the splanchnic bed, which can result in further decreases in cardiac preload and reduced arterial pressures. Thus, patients with pre-existing intravascular volume depletion will more likely suffer orthostasis hypotension following SNM. In contrast, patients with HF typically exhibit intravascular and extravascular hypervolemia, and would therefore be expected to experience less clinically relevant reductions in blood pressure and resultant orthostatic hypotension with arterial and venodilation (60–62).
Nausea and vomiting are also amongst the commonly reported transient side effects of SNM, limited at most to the first 72 hours after the procedure. These side effects can be explained by the sympathetic denervation of the splanchnic organs (similarly to the above point) and possibly also periprocedural anesthesia.
Endocrine function of the visceral organs such as the pancreas is not affected by denervation of the GSN as demonstrated by metabolic studies and hormone levels (63,64). In a single publication reporting the effects of bilateral splanchnicectomy on sixteen patients, a statistically significant but clinically benign reduction in adrenomedullary function was detected after the procedure (65). However, the approach used by surgeons in this study was to “transect all of the potentially nerve-bearing tissue on each side as well as the sympathetic chain itself at level T5,” so these observations are likely the effect of accessory nerve transection and not GSN blockade alone.
Procedural Complications of Splanchnic Nerve Modulation
Procedural complications of SNM that are reported in the literature are related to the approach (transhiatal, transthoracic, transdiscal) and the method of nerve block (surgical, chemical, RF ablation). Pain is by far the most commonly reported complication. It is generally self-limiting presumable from soft-tissue trauma, paresthesias, or chemical irritation and usually manifests as a dull back ache or pleuritic pain, after celiac or splanchnic nerve modulation, respectively. Other common complications include bleeding, nerve injury (intercostal neuralgia, paresthesia), and injury to organs in proximity of the GSN or celiac plexus including the lung (pneumothorax), bowel (bowel perforation, peritonitis), and thoracic duct (chylothorax). Commonly used neurolytic agents (alcohol and phenol) can cause inflammation, necrosis and untoward complications if they come into inadvertent contact with collateral vasculature, which may include the aorta and inferior vena cava. Some of the rare complications associated with splanchnic nerve ablation include damage to the spinal cord or its vascular supply.
Conclusions and Future Directions
In the syndrome of HF, deranged mechanisms underlying volume redistribution constitute an integral element of impaired fluid control and can be involved in the symptoms of exercise intolerance and underlie cardiac decompensation (66). Pilot investigations support the feasibility and safety of SNM in HF. Unilateral SNM prevents inappropriate central blood volume redistribution in HF (primary mode of action) and as a result lowers intracardiac pressures at rest and with exercise, effects that would be expected to improve symptoms and aerobic capacity. Temporary and permanent blockade of the splanchnic nerves is performed currently in clinical practice to alleviate abdominal pain, which allowed us to examine potential side effects and periprocedural complications in a large number of patients. Our systematic review of the anesthesia/surgical literature demonstrates that in patients with intractable pain SNM is well tolerated with side effects limited to transient gastrointestinal disturbance and orthostatic hypotension. The evidence to support short-term and long-term safety and tolerability of SNM in HF is very limited. Yet, the pathophysiological role of the GSN in volume redistribution, the encouraging findings of acute and chronic pilot SNM studies and the safety profile from permanent SNM for pain provides a strong basis for continued efforts to study this therapeutic target in HF. Future studies will need to address the long-term tolerability of SNM in larger randomized studies. Further, investigations into a potential differential benefit or tolerability of SNM in HFpEF vs HFrEF are needed. Whether unilateral SNM is preferred over bilateral SNM remains to be determined. Efforts to develop and test a minimally invasive approach to a permanent SNM are underway. An endovascular, transcatheter approach (Axon Therapies, New York, New York) is now available to perform permanent SNM in the setting of HF. The novel therapy targets unilateral GSN, which should minimize many of the periprocedural complications associated with surgical approaches to ablation.
Supplementary Material
Acknowledgments
Conflict of Interest Disclosures:
Fudim: Supported by an American Heart Association Grant, 17MCPRP33460225, NIH T32 grant 5T32HL007101, NIH K23 grant NHLBI K23HL151744, Bayer, Mario Family Award, Translating Duke Health Award; consulting fees from Axon Therapies, Daxor, NXT Biomedical. Sievert: 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Append Medical, Axon, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, Cardiac Success, Cardimed, Celonova, Contego, Croivalve, CVRx, Dinova, Edwards, Endobar, Endologix, Endomatic, Hangzhou Nuomao Medtech, Holistick Medical, Intershunt, K2, Lifetech, Magenta, Maquet Getinge Group, Medtronic, Metavention, Mitralix, Mokita, NXT Biomedical, Occlutech, Recor, Renal Guard, Terumo, Trisol, Vascular Dynamics, Vectorious Medtech, Venus, Venock, Vivasure Medical, Vvital Biomed. Engelman is an employee of Axon Therapies. Reddy is a consultant and recived equitey from Axon Therapies; he also has conflicts with other companies not related to this manuscript: Abbott (Consultant), Ablacon (Consultant, Equity), Acutus Medical (Consultant, Equity), Affera (Consultant, Equity), Apama Medical (Consultant, Equity), Aquaheart (Consultant, Equity), Atacor (Consultant, Equity), Autonomix (Consultant, Equity), Backbeat (Consultant, Equity), BioSig (Consultant, Equity), Biosense-Webster (Consultant), Biotronik (Consultant), Boston Scientific (Consultant), Cardiofocus (Consultant), Cardionomic (Consultant), CardioNXT / AFTx (Consultant), Circa Scientific (Consultant, Equity), Corvia Medical (Consultant, Equity), Dinova-Hangzhou Nuomao Medtech Co, Ltd (Consultant, Equity), East End Medical (Consultant, Equity), EBR (Consultant), EPD (Consultant, Equity), Epix Therapeutics (Consultant, Equity), EpiEP (Consultant, Equity), Eximo (Consultant, Equity), Farapulse (Consultant, Equity), Fire1 (Consultant, Equity), Impulse Dynamics (Consultant), Intershunt (Consultant, Equity), Javelin (Consultant, Equity), Kardium (Consultant, Equity), Keystone Heart (Consultant, Equity), LuxMed (Consultant, Equity), Manual Surgical Sciences (Equity), Medlumics (Consultant, Equity), Medtronic (Consultant), Middlepeak (Consultant, Equity), Newpace (Equity), Nuvera (Consultant, Equity), Philips (Consultant), Pulse Biosciences (Consultant), Sirona Medical (Consultant, Equity), Stimda (Consultant), Surecor (Equity), Thermedical (Consultant), Valcare (Consultant, Equity) and Vizaramed (Equity). Ponikowski: PP reports research funding from Vifor Pharma and serving as consultant for Vifor Pharma, Amgen, Abbott, Servier, Novartis, Berlin Chemie, Bayer, Pfizer, Cibiem, Coridea, Impulse Dynamics, Renal Guard Solutions. Shah: consults for AxonTherapies
Funding Sources: None
Abbreviations:
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- GSN
greater splanchnic nerve
- SNM
splanchnic nerve modulation
- PCWP
pulmonary capillary wedge pressure
- RF
radiofrequency
Footnotes
Trial Registration: N/A
REFERENCES
- 1.Pieske B, Tschope C, de Boer RA et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 2.Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation 2020;142:998–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fudim M, Hernandez AF, Felker GM. Role of Volume Redistribution in the Congestion of Heart Failure. Journal of the American Heart Association 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy YNV, Obokata M, Wiley B et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019;40:3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borlaug BA, Reddy YNV. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail 2019;7:574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail 2011;4:669–75. [DOI] [PubMed] [Google Scholar]
- 7.Burkhoff D, Tyberg JV. Why does pulmonary venous pressure rise after onset of LV dysfunction: a theoretical analysis. Am J Physiol 1993;265:H1819–28. [DOI] [PubMed] [Google Scholar]
- 8.Fudim M, Patel MR, Boortz-Marx R et al. Splanchnic Nerve Block Mediated Changes in Stressed Blood Volume in Heart Failure. JACC Heart Fail 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy YNV, Andersen MJ, Obokata M et al. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothe CF. Reflex control of veins and vascular capacitance. Physiol Rev 1983;63:1281–342. [DOI] [PubMed] [Google Scholar]
- 11.Thiriet M Physiology and Pathophysiology of Venous Flow. In: Lanzer P, editor PanVascular Medicine. Berlin, Heidelberg: Springer Berlin Heidelberg, 2015:569–589. [Google Scholar]
- 12.Greenway CV. Role of splanchnic venous system in overall cardiovascular homeostasis. Fed Proc 1983;42:1678–84. [PubMed] [Google Scholar]
- 13.Bell L, Hennecken J, Zaret BL, Rutlen DL. Alpha-adrenergic regulation of splanchnic volume and cardiac output in the dog. Acta Physiol Scand 1990;138:321–9. [DOI] [PubMed] [Google Scholar]
- 14.Greenway CV, Lister GE. Capacitance effects and blood reservoir function in the splanchnic vascular bed during non-hypotensive haemorrhage and blood volume expansion in anaesthetized cats. J Physiol 1974;237:279–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowell LB, Detry JM, Blackmon JR, Wyss C. Importance of the splanchnic vascular bed in human blood pressure regulation. J Appl Physiol 1972;32:213–20. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd JT, Vanhoutte PM. Role of the venous system in circulatory control. Mayo Clin Proc 1978;53:247–55. [PubMed] [Google Scholar]
- 17.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 1974;54:75–159. [DOI] [PubMed] [Google Scholar]
- 18.Risoe C, Simonsen S, Rootwelt K, Sire S, Smiseth OA. Nitroprusside and regional vascular capacitance in patients with severe congestive heart failure. Circulation 1992;85:997–1002. [DOI] [PubMed] [Google Scholar]
- 19.Manyari DE, Wang Z, Cohen J, Tyberg JV. Assessment of the human splanchnic venous volume-pressure relation using radionuclide plethysmography. Effect of nitroglycerin. Circulation 1993;87:1142–51. [DOI] [PubMed] [Google Scholar]
- 20.Rowell LB: Human Cardiovascular Control New York, Oxford University Press, 1993, pp 37–96. [Google Scholar]
- 21.Birch DJ, Turmaine M, Boulos PB, Burnstock G. Sympathetic innervation of human mesenteric artery and vein. J Vasc Res 2008;45:323–32. [DOI] [PubMed] [Google Scholar]
- 22.Barnes RJ, Bower EA, Rink TJ. Haemodynamic responses to stimulation of the splanchnic and cardiac sympathetic nerves in the anaesthetized cat. J Physiol 1986;378:417–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelman S Venous function and central venous pressure: a physiologic story. Anesthesiology 2008;108:735–48. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation 2007;116:1549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zile MR, Bennett TD, St John Sutton M et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008;118:1433–41. [DOI] [PubMed] [Google Scholar]
- 26.Bapna A, Adin C, Engelman ZJ, Fudim M. Increasing Blood Pressure by Greater Splanchnic Nerve Stimulation: a Feasibility Study. J Cardiovasc Transl Res 2019. [DOI] [PubMed] [Google Scholar]
- 27.Fudim M, Yalamuri S, Herbert JT, Liu PR, Patel MR, Sandler A. Raising the pressure: Hemodynamic effects of splanchnic nerve stimulation. Journal of applied physiology (Bethesda, Md : 1985) 2017;123:126–127. [DOI] [PubMed] [Google Scholar]
- 28.Shoukas AA, Sagawa K. Control of total systemic vascular capacity by the carotid sinus baroreceptor reflex. Circ Res 1973;33:22–33. [DOI] [PubMed] [Google Scholar]
- 29.Shoukas AA, Brunner MC. Epinephrine and the carotid sinus baroreceptor reflex. Influence on capacitive and resistive properties of the total systemic vascular bed of the dog. Circ Res 1980;47:249–57. [DOI] [PubMed] [Google Scholar]
- 30.Rothe CF. Physiology of venous return. An unappreciated boost to the heart. Arch Intern Med 1986;146:977–82. [PubMed] [Google Scholar]
- 31.Drees JA, Rothe CF. Reflex venoconstriction and capacity vessel pressure-volume relationships in dogs. Circ Res 1974;34:360–73. [DOI] [PubMed] [Google Scholar]
- 32.Greenway CV, Innes IR. Effects of splanchnic nerve stimulation on cardiac preload, afterload, and output in cats. Circ Res 1980;46:181–9. [DOI] [PubMed] [Google Scholar]
- 33.Brooksby GA, Donald DE. Dynamic changes in splanchnic blood flow and blood volume in dogs during activation of sympathetic nerves. Circ Res 1971;29:227–38. [DOI] [PubMed] [Google Scholar]
- 34.Wang SY, Manyari DE, Scott-Douglas N, Smiseth OA, Smith ER, Tyberg JV. Splanchnic venous pressure-volume relation during experimental acute ischemic heart failure. Differential effects of hydralazine, enalaprilat, and nitroglycerin. Circulation 1995;91:1205–12. [DOI] [PubMed] [Google Scholar]
- 35.Wang SY, Manyari DE, Tyberg JV. Cardiac vagal reflex modulates intestinal vascular capacitance and ventricular preload in anesthetized dogs with acute myocardial infarction. Circulation 1996;94:529–33. [DOI] [PubMed] [Google Scholar]
- 36.Ogilvie RI, Zborowska-Sluis D. Effect of chronic rapid ventricular pacing on total vascular capacitance. Circulation 1992;85:1524–30. [DOI] [PubMed] [Google Scholar]
- 37.Gay R, Wool S, Paquin M, Goldman S. Total vascular pressure-volume relationship in conscious rats with chronic heart failure. Am J Physiol 1986;251:H483–9. [DOI] [PubMed] [Google Scholar]
- 38.Raya TE, Gay RG, Aguirre M, Goldman S. Importance of venodilatation in prevention of left ventricular dilatation after chronic large myocardial infarction in rats: a comparison of captopril and hydralazine. Circ Res 1989;64:330–7. [DOI] [PubMed] [Google Scholar]
- 39.Smiseth OA, Manyari DE, Scott-Douglas NW et al. The effect of nitroglycerin on pulmonary vascular capacitance in dogs. American heart journal 1991;121:1454–9. [DOI] [PubMed] [Google Scholar]
- 40.Tyberg JV, Wang SY, Scott-Douglas NW et al. The Veins and Ventricular Preload. In: Maruyama Y, Hori M, Janicki JS, editors. Cardiac-Vascular Remodeling and Functional Interaction. Tokyo: Springer Japan, 1997:257–266. [Google Scholar]
- 41.Yamamoto J, Trippodo NC, Ishise S, Frohlich ED. Total vascular pressure-volume relationship in the conscious rat. Am J Physiol 1980;238:H823–8. [DOI] [PubMed] [Google Scholar]
- 42.Risoe C, Hall C, Smiseth OA. Effect of enalaprilat on splanchnic vascular capacitance during acute ischemic heart failure in dogs. Am J Physiol 1994;266:H2182–9. [DOI] [PubMed] [Google Scholar]
- 43.Franciosa JA, Weber KT, Levine TB et al. Hydralazine in the long-term treatment of chronic heart failure: lack of difference from placebo. American heart journal 1982;104:587–94. [DOI] [PubMed] [Google Scholar]
- 44.Taylor AL, Ziesche S, Yancy C et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049–57. [DOI] [PubMed] [Google Scholar]
- 45.Chatterjee K, Parmley WW, Massie B et al. Oral hydralazine therapy for chronic refractory heart failure. Circulation 1976;54:879–83. [DOI] [PubMed] [Google Scholar]
- 46.Kaye DM, Byrne M, Mariani J, Nanayakkara S, Burkhoff D. Identification of physiologic treatment targets with favourable haemodynamic consequences in heart failure with preserved ejection fraction. ESC Heart Fail 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozhuharov N, Goudev A, Flores D et al. Effect of a Strategy of Comprehensive Vasodilation vs Usual Care on Mortality and Heart Failure Rehospitalization Among Patients With Acute Heart Failure: The GALACTIC Randomized Clinical Trial. JAMA 2019;322:2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fudim M, Ganesh A, Green C et al. Splanchnic nerve block for decompensated chronic heart failure: splanchnic-HF. Eur Heart J 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fudim M, Jones WS, Boortz-Marx RL et al. Splanchnic Nerve Block for Acute Heart Failure. Circulation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fudim M, Boortz-Marx RL, Ganesh A et al. Splanchnic Nerve Block for Chronic Heart Failure. JACC Heart Fail 2020. [DOI] [PubMed] [Google Scholar]
- 51.Raj P Celiac plexus/splanchnic nerve blocks. Techniques in Regional Anesthesia and Pain Management 2001;5:102–115. . [Google Scholar]
- 52.Raj PP, Thomas J, Heavner J et al. The Development of a Technique for Radiofrequency Lesioning of Splanchnic Nerves. Current review of pain 1999;3:377–387. [DOI] [PubMed] [Google Scholar]
- 53.Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. The American journal of gastroenterology 2007;102:430–8. [DOI] [PubMed] [Google Scholar]
- 54.Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg 1995;80:290–5. [DOI] [PubMed] [Google Scholar]
- 55.Goldblatt H, Gross J, Hanzal RF. Studies on Experimental Hypertension : Ii. The Effect of Resection of Splanchnic Nerves on Experimental Renal Hypertension. The Journal of experimental medicine 1937;65:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SH, Lim DH, Lee JH, Chang K, Koo JM, Park HJ. Long-Term Blood Pressure Control Effect of Celiac Plexus Block with Botulinum Toxin. Toxins (Basel) 2016;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baghdadi S, Abbas MH, Albouz F, Ammori BJ. Systematic review of the role of thoracoscopic splanchnicectomy in palliating the pain of patients with chronic pancreatitis. Surgical endoscopy 2008;22:580–8. [DOI] [PubMed] [Google Scholar]
- 58.Gafanovich I, Shir Y, Tsvang E, Ben-Chetrit E. Chronic diarrhea--induced by celiac plexus block? Journal of clinical gastroenterology 1998;26:300–2. [DOI] [PubMed] [Google Scholar]
- 59.Fujita Y Splanchnic circulation following coeliac plexus block. Acta anaesthesiologica Scandinavica 1988;32:323–7. [DOI] [PubMed] [Google Scholar]
- 60.Soloveva A, Fedorova D, Villevalde S et al. Addressing Orthostatic Hypotension in Heart Failure: Pathophysiology, Clinical Implications and Perspectives. J Cardiovasc Transl Res 2020;13:549–569. [DOI] [PubMed] [Google Scholar]
- 61.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 2012;59:442–51. [DOI] [PubMed] [Google Scholar]
- 62.Gorelik O, Feldman L, Cohen N. Heart failure and orthostatic hypotension. Heart Fail Rev 2016;21:529–38. [DOI] [PubMed] [Google Scholar]
- 63.Myhre J, Hilsted J, Tronier B et al. Monitoring of celiac plexus block in chronic pancreatitis. Pain 1989;38:269–74. [DOI] [PubMed] [Google Scholar]
- 64.Ihse I, Zoucas E, Gyllstedt E, Lillo-Gil R, Andren-Sandberg A. Bilateral thoracoscopic splanchnicectomy: effects on pancreatic pain and function. Annals of surgery 1999;230:785–90; discussion 790–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buscher HC, Lenders JW, Wilder-Smith OH, Sweep CG, van Goor H. Bilateral thoracoscopic splanchnicectomy for pain in patients with chronic pancreatitis impairs adrenomedullary but not noradrenergic sympathetic function. Surgical endoscopy 2012;26:2183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fudim M, Sobotka PA, Dunlap ME. Extracardiac Abnormalities of Preload Reserve: Mechanisms Underlying Exercise Limitation in Heart Failure with Preserved Ejection Fraction, Autonomic Dysfunction, and Liver Disease. Circ Heart Fail 2021;14:e007308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


