Abstract
We describe a case of stress-induced cardiomyopathy following epoprostenol withdrawal. A patient with pulmonary arterial hypertension presented with a malfunctioning Hickman catheter. Inappropriate withdrawal of epoprostenol resulted in shock. Evaluation confirmed stress-induced cardiomyopathy. Restarting epoprostenol resolved the electrocardiographic and echocardiographic abnormalities. This case meets Taskforce on Takotsubo Syndrome Stress-Induced Cardiomyopathy criteria. (Level of Difficulty: Beginner.)
Key Words: acute heart failure, cardiogenic shock, epoprostenol, pulmonary arterial hypertension, stress cardiomyopathy
Abbreviations and Acronyms: ECG, electrocardiogram; LV, left ventricle; RV, right ventricle
Graphical abstract
This report describes a case of stress-induced cardiomyopathy following epoprostenol withdrawal. A patient with pulmonary arterial hypertension presented…
History of Presentation
The patient is a 67-year-old woman with a history of scleroderma-associated pulmonary arterial hypertension, maintained on long-term epoprostenol infusion, and secondary adrenal insufficiency. She presented to an outside hospital for evaluation of an epoprostenol infusion pump alarm. In the emergency department, the epoprostenol infusion was discontinued out of concern for a malfunctioning Hickman catheter. No provisions were made to infuse the drug by other means. Within 12 h, the patient developed hypotension and hypoxia; she was transferred to Tufts Medical Center in Boston, Massachusetts, for management of cardiogenic shock. Home medications included the following: furosemide, 20 mg daily; gabapentin, 100 mg 3 times a day; hydrocortisone, 10 mg every 12 h; omeprazole, 40 mg daily; and epoprostenol (Veletri), 44 ng/kg/min by continuous intravenous infusion.
Learning Objectives
-
•
The clinician will appreciate clinical nuances of patients with drug-induced stress cardiomyopathy.
-
•
The clinician will understand the evaluation and management of patients with stress cardiomyopathy and cardiogenic shock.
Before transfer, her systolic blood pressure was 50 mm Hg, requiring infusions of dobutamine (5 μg/kg/min) and norepinephrine (Levophed) (3 μg/min). She also received a hydrocortisone sodium succinate intravenous injection once. At Tufts, alternative access was established, and she was immediately restarted on epoprostenol at one-half her home dose, with subsequent up-titration to her full dose. Her blood pressure returned to baseline, and the dobutamine and Levophed infusions were discontinued. Because of hypoxia, she required 4 l of supplemental oxygen by nasal cannula.
Physical examination was notable for an erythematous rash consistent with long-term epoprostenol therapy, telangiectasias, and sclerosis consistent with scleroderma. The lungs were clear to auscultation, and the cardiac examination revealed a loud P2 but was otherwise unremarkable. There was no peripheral edema or jugular venous distention. There was an intact left anterior chest wall Hickman catheter without signs of infection.
Past Medical History
Her medical history included scleroderma, pulmonary arterial hypertension, secondary adrenal insufficiency, gastroesophageal reflux disease, and neuropathy.
Differential Diagnosis
The differential diagnosis included myocardial infarction, septic shock, stress cardiomyopathy, and acute right ventricular failure.
Investigations
Laboratory values were significant for an elevated troponin I value of 5.96 ng/ml and a B-type natriuretic peptide value of 2,075 pg/ml. An electrocardiogram (ECG) (Figure 1) showed normal sinus rhythm, low-voltage, right ventricular hypertrophy with a repolarization abnormality, a prolonged QT interval, and diffuse T-wave inversions that were more pronounced than in an earlier ECG (Figure 2).
Figure 1.
Admission Electrocardiogram
Figure 2.
Prior Electrocardiogram
A transthoracic echocardiogram revealed a normal-sized left ventricle (LV) with severely reduced systolic function and an ejection fraction of 25% to 30% (Video 1). The interventricular septum was asynchronous and flattened, consistent with right ventricular pressure and volume overload. The middle and apical segments of the LV were relatively more hypokinetic than the basal segments. The right ventricle (RV) was severely dilated, and the systolic function was severely reduced (Video 2). The right atrium was severely dilated. There was mild tricuspid regurgitation with an estimated right ventricular systolic pressure of 30 to 40 mm Hg. A previous echocardiogram did not show dysfunction of the LV or RV. Prior left ventricular ejection fraction was 54%. To exclude coronary artery disease as the cause of the decompensation, left-sided heart catheterization was performed. It revealed a right-dominant circulation with a 30% mid-right coronary artery stenosis and a 30% mid-left anterior descending artery stenosis, consistent with mild nonobstructive coronary artery disease. The remainder of the coronary circulation was patent; thus, no intervention was performed.
Online Video 1.
Hypokinetic Left Ventricle off Epoprostenol
Online Video 2.
Hypokinetic Right Ventricle off Epoprostenol
Management
The patency of the Hickman catheter was re-established with a guidewire, saline flushes, and tissue plasminogen activator infusion under sterile conditions, and the intravenous epoprostenol was transitioned to the catheter. Home medications were reinitiated. An angiotensin-converting enzyme inhibitor and beta-blocker were withheld because of the patient’s low baseline blood pressure. A treatment strategy of supportive care in addition to reinitiation of the previously withdrawn medication was thought to be the most appropriate in this case.
Discussion
Initial descriptions of Takotsubo, or stress, cardiomyopathy arose in the early 1990s by researchers in Japan who coined the term for the likeness of the LV in patients diagnosed with the syndrome to an octopus trap (1). The classic description on imaging of the LV includes apical hypokinesis or akinesis and ballooning with hyperdynamic basal systolic function (2). However, other variants have been documented in several diagnosed individuals, with estimations of right ventricular involvement reaching as high as 50% (3).
The Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology summarized the diagnostic criteria for this clinical syndrome as the following (4): 1) transient regional wall motion abnormalities of myocardium of the LV or RV; 2) wall motion abnormalities usually extending beyond a single epicardial vascular distribution; 3) absence of culprit atherosclerotic coronary artery disease or other pathological conditions to explain the dysfunction of the LV; 4) new and reversible ECG abnormalities; 5) significantly elevated serum natriuretic peptide; 6) positive but relatively small elevation in cardiac troponin; and 7) recovery of ventricular systolic function on cardiac imaging at follow-up. On this basis, as well as a recent international Takotsubo expert consensus definition, the current clinical case meets the diagnostic criteria for stress cardiomyopathy (5).
The pathophysiology of ventricular dysfunction is thought to involve a cardiotoxic neurohumoral catecholamine surge in response to a stressor (6). Therefore, the syndrome is known to be preceded by intense emotional or physical stress (7). Common psychological triggers include domestic abuse, divorce, catastrophic medical diagnoses, financial losses, and the death of relatives. Our patient had no such identifiable psychological or emotional stresses. Physical triggers include illnesses such as infections, diarrhea, vomiting, pheochromocytoma, anaphylaxis, and cancer (8).
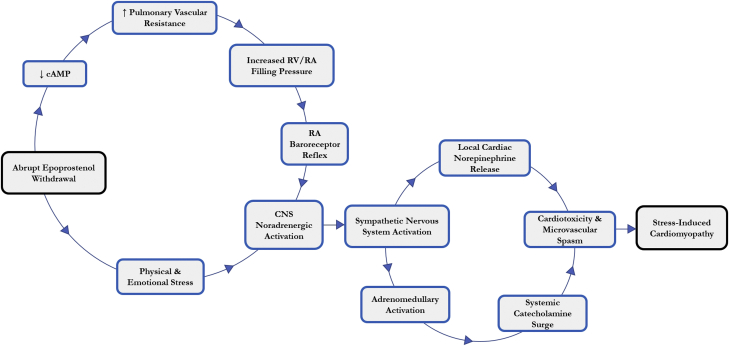
Additionally, several medications, including exogenous catecholamines, sympathomimetic agents, vasoconstrictors, chemotherapeutic agents, and antidepressants, have been reported as precipitating factors of stress cardiomyopathy (9). Similar to the current case, an estimated 8% of cases of drug-induced stress cardiomyopathy have been documented in circumstances of withdrawal. The agents most commonly implicated after drug cessation are opioids, alcohol, and beta-blockers (9). In this case, we hypothesize that the patient developed acute pulmonary vasoconstriction after discontinuation of intravenous epoprostenol, thus resulting in a sudden increase in right ventricular afterload (Figure 3). Right atrial baroreceptor reflex activation of the sympathetic nervous system likely resulted in local myocardial norepinephrine release from cardiac nerve endings, as well as increased circulating catecholamines from sympathetic adrenomedullary circuits. This catecholamine surge, in response to acute pulmonary vasoconstriction, is likely responsible for the observed reversible myocardial dysfunction (10).
Figure 3.
Proposed Pathophysiology of Stress Cardiomyopathy as a Consequence of Epoprostenol Withdrawal
cAMP = cyclic adenosine monophosphate; CNS = central nervous system; RA = right atrial; RV = right ventricular.
There has been only 1 other reported case of epoprostenol withdrawal as the inciting factor for the development of stress cardiomyopathy (11). However, the patient in that report had concomitant coronary artery disease requiring percutaneous coronary intervention.
Follow-up
Within a month, the patient’s hypoxia had resolved such that supplemental oxygen could be discontinued. An echocardiogram at 1-month post-discharge revealed normal size and function of the LV with an ejection fraction of 60% (Video 3). The septum remained asynchronous, but the prior wall motion abnormalities had resolved. The RV was mildly dilated with normal systolic function (Video 4). Follow-up ECG had returned to baseline (Figure 4). This case represents stress-induced cardiomyopathy resulting from abrupt withdrawal of epoprostenol; resolution occurred over time after reinitiation of epoprostenol.
Online Video 3.
Left Ventricle on Epoprostenol With Normal Function
Online Video 4.
Right Ventricle on Epoprostenol With Normal Function
Figure 4.
Follow-Up Electrocardiogram
Conclusions
We conclude that withdrawal of a long-standing continuous infusion of epoprostenol can also be a trigger for stress-induced cardiomyopathy. As in other cases of this syndrome, supportive care, along with reintroduction of the withdrawn medication, is the treatment of choice.
Footnotes
Dr. Farber has been a consultant for and received honoraria from Accredo. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Sato H, Taiteishi H, Uchida T, Kodama K, Haze K, Hon M (editors). Takotsubo-type cardiomyopathy due to multivessel spasm. In: Clinical Aspects of Myocardial Injury: From Ischemia to Heart Failure. Tokyo, Japan: Kagakuhyouronsha; 1990:56–64.
- 2.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Scally C., Ahearn T., Rudd A. Right ventricular involvement and recovery after acute stress-induced (Tako-tsubo) cardiomyopathy. Am J Cardiol. 2016;117:775–780. doi: 10.1016/j.amjcard.2015.11.057. [DOI] [PubMed] [Google Scholar]
- 4.Lyon A.R., Bossone E., Schneider B. Current state of knowledge on Takotsubo syndrome: a position statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 5.Ghadri J.R., Wittstein I.S., Prasad A. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39:2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wittstein I.S., Thiemann D.R., Lima J.A. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 7.Sharkey S.W., Lesser J.R., Zenovich A.G. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 8.Dawson D.K. Acute stress-induced (takotsubo) cardiomyopathy. Heart. 2018;104:96–102. doi: 10.1136/heartjnl-2017-311579. [DOI] [PubMed] [Google Scholar]
- 9.Kido K., Guglin M. Drug-induced Takotsubo cardiomyopathy. J Cardiovasc Pharmacol Ther. 2017;22:552–563. doi: 10.1177/1074248417708618. [DOI] [PubMed] [Google Scholar]
- 10.Pelliccia F., Kaski J.C., Crea F., Camici P.G. Pathophysiology of Takotsubo syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 11.Cork D.P., Mehrotra A.K., Gomberg-Maitland M. Takotsubo cardiomyopathy after treatment of pulmonary arterial hypertension. Pulm Circ. 2012;2:390–394. doi: 10.4103/2045-8932.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]