Abstract
In patients with constrictive pericarditis, a characteristic reduction in the regional longitudinal strain seen in the areas of the left ventricular free wall and relative sparing of the septal longitudinal strain values create an easily recognizable bullseye plot pattern that can be described as “hot septum.” (Level of Difficulty: Beginner.)
Key Words: constriction, echocardiography, longitudinal strain
Graphical abstract
In patients with constrictive pericarditis, a characteristic reduction in the regional longitudinal strain seen in the areas of the left ventricular…
History of Presentation
A 78-year-old man presented to the outpatient clinic complaining of a 2-week history of progressive exertional shortness of breath, decreased appetite, abdominal distention, and lower extremity edema. His blood pressure was 135/80 mm Hg, heart rate was 91 beats/min, and oxygen saturation was 96% on room air. Physical examination revealed jugular venous distention, ascites, and marked (4+) peripheral edema.
Learning Objectives
-
•
Regional variations in longitudinal strain between the free and septal walls in the setting of thickened pericardium creates a bullseye appearance of a “hot septum,” which suggests pericardial constriction.
-
•
Improvement in the free-wall longitudinal strain values in patients with constrictive pericarditis may be used as a marker for recovery and treatment effectiveness.
Past Medical History
Four weeks before the current presentation, the patient was hospitalized with new-onset shortness of breath and was found to have moderate-to-large pericardial effusion. The pericardial effusion was drained percutaneously providing symptomatic relief. The workup for the cause of pericardial effusion was unrevealing except for markedly elevated inflammatory markers, so the cause of effusion was presumed to be acute idiopathic pericarditis. He was discharged on a nonsteroidal anti-inflammatory agent and colchicine.
Differential Diagnosis
The patient presented with typical symptoms of heart failure. Physical signs were suggestive of marked systemic congestion. Although the differential diagnosis of heart failure syndrome is broad, the recent history of inflammatory pericardial effusion requiring drainage put pericardial disease high on the list.
Investigations
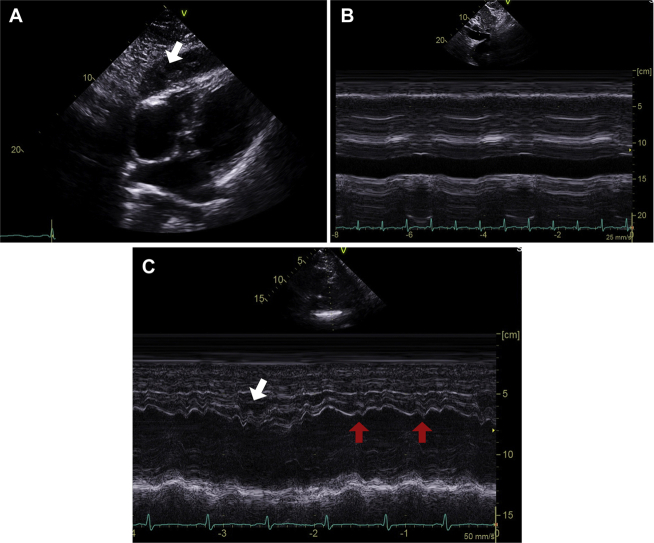
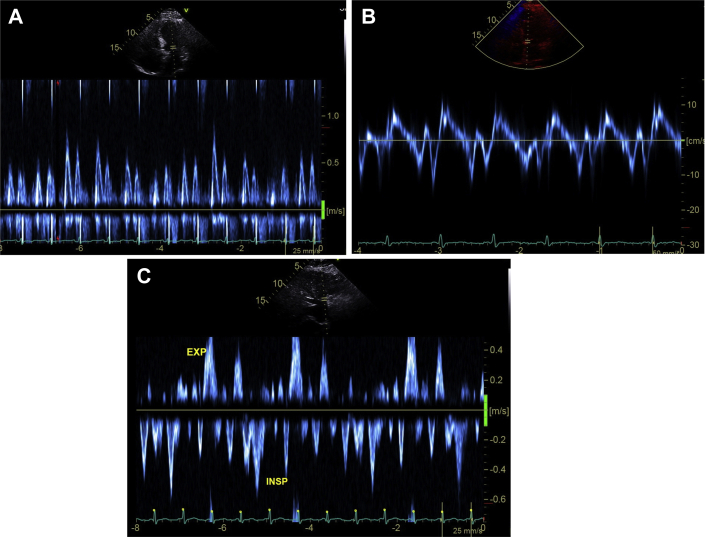
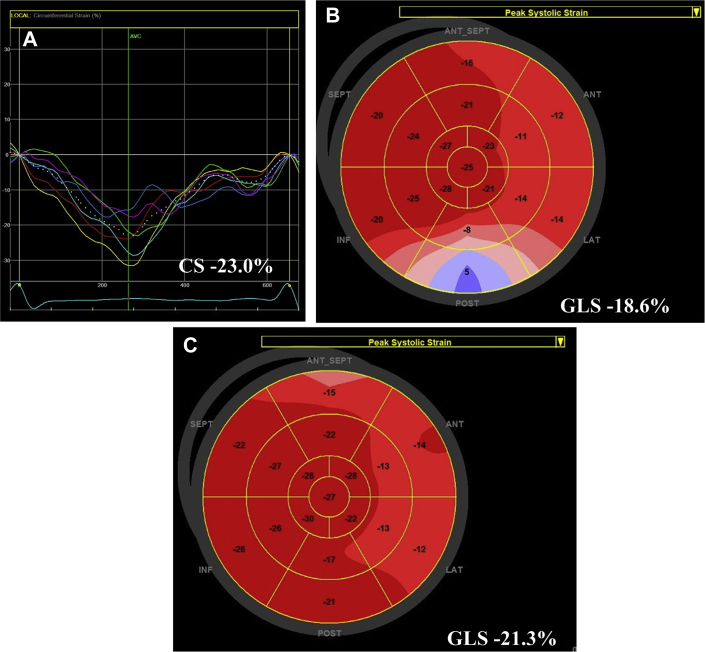
The patient was referred for echocardiographic evaluation. Two-dimensional echocardiogram demonstrated marked pericardial thickening with predominantly soft echo densities in the pericardial space that was most prominent over the inferior wall of the right ventricle (exceeding 2 cm) (Figure 1A, Video). The inferior vena cava was distended and noncollapsible (Figure 1B), suggestive of elevated right atrial pressure (Figure 2A). Examination of the septal motion showed both beat-to-beat shudder (Figure 1C) and leftward respiratory septal shift during inspiration (Figure 1C). Left ventricular ejection fraction by Simpson’s biplane planimetry was 59%. Spectral Doppler evaluation showed marked variation (>30%) of the transmitral flow velocities (Figure 2A). Tissue Doppler evaluation showed preserved septal e′ velocity (Figure 2B). Expiratory diastolic flow reversal in the hepatic veins was also observed (Figure 2C). Speckle tracking echocardiography (GE EchoPac software version 201, GE Healthcare, Chicago, Illinois) demonstrated relatively preserved mid-ventricular circumferential strain (−23%) (Figure 3A) and global longitudinal strain (−18.6%), but a significant regional variation in the longitudinal strain values (Figure 3B). The observed pattern of longitudinal strain was consistent with relatively preserved septal longitudinal strain (“hot septum”) and reduced strain values in the anterolateral and inferolateral segments. Constrictive physiology was diagnosed on the basis of comprehensive echocardiographic evaluation.
Figure 1.
Echocardiographic Findings Suggestive of Constrictive Physiology
Marked thickening of pericardium (A, arrow), M-mode findings of distended and noncollapsible inferior vena cava (B), and diastolic septal shudder (C, red arrows), as well as respiratory septal shift (C, white arrow).
Online Video 1.
Marked thickening of pericardium over the inferior wall of the right ventricle is seen in the modified subcostal view. Diastolic septal shudder can also be appreciated.
Figure 2.
Doppler Findings Suggestive of Constrictive Physiology
Transmitral flow variation (A), preserved septal e′ velocity (B), and expiratory (EXP) flow reversal in hepatic veins (C). ISP = inspiratory.
Figure 3.
2-Dimensional Speckle Tracking Echocardiographic Findings
Relatively preserved global circumferential strain (CS) (A) and global longitudinal strain (B), but marked regional variation in longitudinal strain values. Some recovery of global longitudinal strain (GLS) after treatment (C).
Management
Due to high suspicion for a prominent inflammatory component, the patient was initiated on a tapering corticosteroid therapy. This was followed by a marked symptomatic improvement including improved exercise tolerance and near-complete resolution of the peripheral edema.
Follow-Up
Repeat echocardiographic evaluation 3 months after the initial echocardiogram and 4 weeks after steroid discontinuation showed a significant reduction in pericardial thickness, normal-sized and collapsible inferior vena cava (suggestive of normal right atrial pressure), and estimated left ventricular ejection fraction of 61%. Importantly, there was overall improvement in global longitudinal strain (−21.3%) with significant recovery in regional strain values over the inferolateral segments (Figure 3C).
Discussion
Both a disproportionate reduction in circumferential strain compared with longitudinal strain and impaired left ventricular twist mechanics have been described in constrictive pericarditis, but these measures have not yet made it into routine clinical recommendations (1,2). On the other hand, global longitudinal strain and longitudinal strain pattern have been extensively studied and are considered reproducible (1,3). Regional variation in longitudinal strain values have been previously described in patients with constrictive pericarditis, that is, reduction in the left ventricular free wall strain as opposed to septal strain (4,5). This reduction in the free wall strain appears to correlate with pericardial thickness, and it is likely explained by pericardial adhesions as well as involvement of adjacent myocardium (4). In our patient, a characteristic reduction in the regional strain was seen in the areas of the left ventricular free wall, whereas septal and apical strain values were preserved; these changes resulted in a bullseye appearance of hot septum. It has also been shown that regional strain values in the left ventricular free wall may recover after successful pericardiectomy (4). In patients with a significant inflammatory component, medical therapy may result in significant improvement or resolution of constriction physiology. In our patient, the clinical improvement with medical therapy was associated with the recovery of the global longitudinal strain, mostly due to recovery of regional strain values in the inferolateral wall. Therefore, follow-up measurement of longitudinal strain should be considered in patients with constrictive pericarditis who are given a trial of anti-inflammatory therapy.
Conclusions
In patients with a significant inflammatory component, medical therapy may result in improvement of the constrictive physiology as reflected by the recovery of longitudinal strain.
Footnotes
Both authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
Appendix
For a supplemental video, please see the online version of this paper.
References
- 1.Claus P., Omar A.M., Pedrizzetti G., Sengupta P.P., Nagel E. Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications. J Am Coll Cardiol Img. 2015;8:1444–1460. doi: 10.1016/j.jcmg.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Sengupta P.P., Krishnamoorthy V.K., Abhayaratna W.P. Disparate patterns of left ventricular mechanics differentiate constrictive pericarditis from restrictive cardiomyopathy. J Am Coll Cardiol Img. 2008;1:29–38. doi: 10.1016/j.jcmg.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Mirea O., Pagourelias E.D., Duchenne J. Variability and reproducibility of segmental longitudinal strain measurement: a report from the EACVI-ASE Strain Standardization Task Force. J Am Coll Cardiol Img. 2018;11:15–24. doi: 10.1016/j.jcmg.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Kusunose K., Dahiya A., Popovic Z.B. Biventricular mechanics in constrictive pericarditis comparison with restrictive cardiomyopathy and impact of pericardiectomy. Circ Cardiovasc Imaging. 2013;6:399–406. doi: 10.1161/CIRCIMAGING.112.000078. [DOI] [PubMed] [Google Scholar]
- 5.Madeira M., Teixeira R., Costa M., Goncalves L., Klein A.L. Two-dimensional speckle tracking cardiac mechanics and constrictive pericarditis: systematic review. Echocardiography. 2016;33:1589–1599. doi: 10.1111/echo.13293. [DOI] [PubMed] [Google Scholar]