Abstract
The Glasgow Prognostic Score (GPS) has been established as a useful resource to evaluate inflammation and malnutrition and predict prognosis in several cancers. However, its prognostic significance in patients with heart failure (HF) is not well established. To investigate the association between the GPS and mortality in patients with HF, we assessed 870 patients who were 20 years old and more and had been admitted for acute decompensated HF. The GPS ranged from 0 to 2 points as previously reported. Over the 18-month follow-up (follow-up rate, 83.9%), 143 patients died. Increasing GPS was associated with higher HF severity assessed by New York Heart Association functional class and B-type natriuretic peptide (BNP) levels. Kaplan–Meier analysis showed significant associations for mortality and increased GPS. In multivariate analysis, compared to the GPS 0 group, the GPS 2 group was associated with high mortality (hazard ratio 2.92, 95% confidence interval 1.77–4.81, p < 0.001) after adjustment for age, sex, blood pressure, HF history, HF severity, hemoglobin, renal function, sodium, BNP, left ventricular ejection fraction, and anti-HF medications. In conclusion, high GPS was significantly associated with worse prognosis in patients with HF. Inflammation-based assessment by the GPS may enable simple evaluation of HF severity and prognosis.
Subject terms: Cardiology, Prognostic markers
Introduction
Heart failure (HF) is predominantly a disease of the elderly1. The prevalence of multi-morbidity also increases with age2, with elderly patients having five or six comorbidities in addition to HF3–5. Frequently coexisting malnutrition also affects the prognosis of patients with HF6. Thus, broader interventions beyond HF management are necessary for aged patients, including the treatment of concurrent decompensated chronic conditions, reduction of polypharmacy, minimization of disability, and prescription of physical exercise and nutritional supplementation7,8.
Recently, inflammation in addition to neurohormonal activation has been recognized to play an important role in the pathogenesis of both HF with reduced ejection fraction (rEF) and preserved ejection fraction (pEF)9–14. Emerging pathophysiological models for HF in the elderly suggest that coexisting proinflammatory cardiovascular and noncardiovascular conditions lead to systemic microvascular endothelial inflammation, global cardiac and skeletal muscle inflammation, and subsequent fibrosis15,16. Indeed, increases in inflammatory markers such as C-reactive protein (CRP) have been associated with the severity and prognosis of HF10,11,17–22.
The Glasgow Prognostic Score (GPS) is a simple and well-established nutritional and inflammatory assessment tool for patients with malignancy that incorporates serum albumin (Alb) and CRP23. Previous studies have validated the GPS as a reliable prognostic indicator in patients with several kinds of cancers23–27. We therefore hypothesized that such inflammation-based assessment could also be a prognostic indicator for patients with HF. The aim of this study was to investigate the prognostic ability of the GPS for mortality in patients with HF.
Results
Study population
The 870 enrolled patients were categorized into the GPS 0 group (n = 394), GPS 1 group (n = 348), or GPS 2 group (n = 128) based on biochemical measurements (Fig. 1). The baseline patient characteristics stratified by GPS are shown in Table 1. The median [25th, 75th percentile] age of the cohort was 81 [72, 87] years, and 45.4% of patients were female. Patients in the GPS 2 group had significantly lower body mass index, higher heart rate, lower proportion of dyslipidemia and more moderate to severe HF (New York Heart Association [NYHA] functional class III or IV) versus the GPS 1 and 0 groups. The etiology of heart failure and traditional coronary risk factors except for dyslipidemia, including hypertension, and diabetes mellitus, were comparable among the groups. Regarding laboratory data, creatinine, estimated glomerular filtration rate (eGFR), sodium, and hemoglobin A1c values were all similar among the groups. Alb, hemoglobin, and low-density lipoprotein cholesterol tended to decrease, while CRP and B-type natriuretic peptide (BNP) tended to increase, as GPS increased. In echocardiographic examinations, patients in the GPS 0 group had the lowest left ventricular ejection fraction (LVEF). HFpEF (LVEF ≥ 50%) and HFrEF (LVEF < 40%) patients were most prevalent in the GPS 1 group and GPS 0 group, respectively. The use of angiotensin-converting enzyme inhibitor (ACEI) and/or angiotensin-receptor blocker (ARB) and beta-blockers was the most frequent in the GPS 0 group, with no significant differences noted for aldosterone antagonists or loop diuretics.
Figure 1.
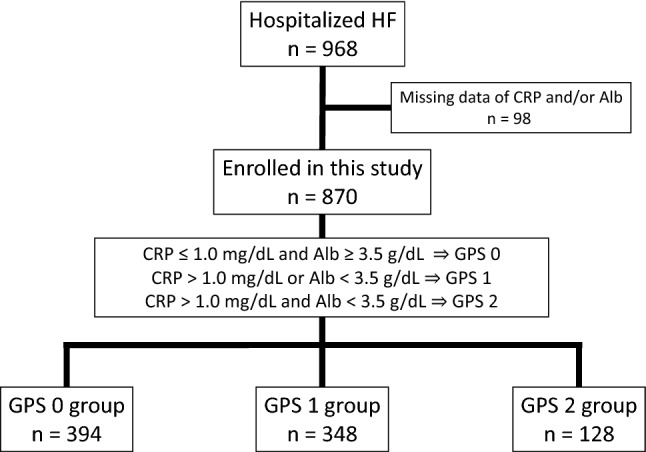
Flow diagram of study recruitment. Alb albumin, CRP C-reactive protein, GPS Glasgow Prognostic Score, HF heart failure.
Table 1.
Patient characteristics.
| Overall | GPS 0 | GPS 1 | GPS 2 | p-value | |
|---|---|---|---|---|---|
| (n = 870) | (n = 394) | (n = 348) | (n = 128) | ||
| Age (years) | 81 [72, 87] | 77 [67, 84] | 84 [77, 88] | 84 [78, 88] | < 0.001 |
| Female, n (%) | 395 (45.4) | 165 (41.9) | 182 (52.3) | 48 (37.5) | 0.002 |
| Body mass index (kg/m2) | 21.0 [18.9, 23.9] | 21.7 [19.5, 24.5] | 20.5 [18.5, 23.1] | 20.2 [18.6, 23.5] | < 0.001 |
| Heart rate (beats/min) | 69 [60, 80] | 69 [60, 79] | 69 [60, 80] | 70 [65, 81] | 0.061 |
| Systolic blood pressure (mmHg) | 112 [101, 126] | 111 [101, 124] | 113 [102, 128] | 114 [101, 126] | 0.30 |
| Hypertension, n (%) | 556 (63.9) | 240 (60.9) | 223 (67.0) | 83 (64.8) | 0.173 |
| Dyslipidemia, n (%) | 229 (26.3) | 118 (29.9) | 87 (25.0) | 24 (18.8) | 0.034 |
| Diabetes mellitus, n (%) | 256 (29.4) | 118 (29.9) | 104 (29.9) | 34 (26.6) | 0.76 |
| NYHA class III or IV, n (%) | 170 (19.5) | 61 (15.5) | 77 (22.1) | 32 (25.0) | 0.017 |
| Prior HF hospitalization, n (%) | 273 (31.4) | 133 (33.8) | 95 (27.3) | 45 (35.2) | 0.099 |
| Ischemic etiology, n (%) | 229 (26.3) | 98 (24.9) | 95 (27.3) | 36 (28.1) | 0.66 |
| Valvular disease, n (%) | 267 (30.7) | 131 (33.2) | 104 (29.9) | 32 (25.0) | 0.199 |
| Atrial fibrillation, n (%) | 448 (51.5) | 213 (54.2) | 171 (49.1) | 64 (50.0) | 0.23 |
| Laboratory data | |||||
| CRP (mg/dL) | 0.23 [0.10, 0.71] | 0.12 [0.05, 0.26] | 0.30 [0.12, 0.60] | 2.27 [1.49, 3.84] | < 0.001 |
| Alb (g/dL) | 3.4 [3.1, 3.8] | 3.8 [3.6, 4.0] | 3.2 [3.0, 3.4] | 3.0 [2.7, 3.2] | < 0.001 |
| Hemoglobin (g/dL) | 12.0 [10.5, 13.7] | 13.0 [11.3, 14.6] | 11.3 [10.1, 12.8] | 11.0 [10.0, 12.4] | < 0.001 |
| Creatinine (mg/dL) | 1.08 [0.86, 1.41] | 1.09 [0.88, 1.39] | 1.07 [0.85, 1.46] | 1.10 [0.82, 1.56] | 0.87 |
| eGFR (mL/min/1.73 m2) | 45.0 [33.0, 58.3] | 46.1 [34.9, 59.0] | 44.8 [32.1, 57.2] | 45.1 [31.2, 60.5] | 0.27 |
| Sodium (mEq/L) | 139 [137, 141] | 139 [137, 141] | 140 [137, 141] | 139 [136, 141] | 0.176 |
| LDL cholesterol (mg/dL) | 94 [76, 116] | 97 [78, 121] | 93 [76, 114] | 88 [72, 109] | 0.027 |
| Hemoglobin A1c (%) | 6.0 [5.6, 6.5] | 6.0 [5.7, 6.5] | 5.9 [5.6, 6.4] | 6.0 [5.6, 6.3] | 0.079 |
| BNP (pg/mL) | 288 [136, 527] | 221 [111, 452] | 327 [158, 558] | 353 [160, 754] | < 0.001 |
| Echocardiographic data | |||||
| LAD (cm) | 4.5 [4.0, 5.0] | 4.5 [4.0, 5.0] | 4.5 [4.0, 5.0] | 4.5 [3.9, 4.9] | 0.41 |
| LVDd (cm) | 5.0 [4.4, 5.8] | 5.3 [4.5, 6.0] | 4.9 [4.3, 5.5] | 4.8 [4.4, 5.6] | < 0.001 |
| LVDs (cm) | 3.6 [2.9, 4.6] | 4.0 [3.0, 4.9] | 3.4 [2.7, 4.3] | 3.3 [2.8, 4.2] | < 0.001 |
| LVEF (%) | 49.0 [35.0, 62.0] | 44.0 [33.0, 58.0] | 54.0 [40.0, 63.0] | 53.0 [35.8, 67.2] | < 0.001 |
| LVEF ≥ 50%, n (%) | 415 (47.7) | 147 (38.0) | 202 (59.1) | 66 (52.8) | < 0.001 |
| LVEF < 40%, n (%) | 286 (32.9) | 160 (41.3) | 84 (24.6) | 42 (33.6) | < 0.001 |
| Medications | |||||
| ACEI and/or ARB, n (%) | 603 (69.3) | 292 (74.1) | 227 (65.2) | 84 (65.6) | 0.012 |
| Beta-blockers, n (%) | 630 (72.4) | 304 (77.2) | 251 (72.1) | 75 (58.6) | < 0.001 |
| Aldosterone antagonists, n (%) | 469 (53.9) | 230 (58.4) | 180 (51.7) | 59 (46.1) | 0.070 |
| Loop diuretics, n (%) | 733 (84.3) | 334 (84.8) | 298 (85.6) | 101 (78.9) | 0.30 |
| Mortality | |||||
| All-cause death, n (%) | 143 (16.4) | 35 (8.9) | 66 (19.0) | 42 (32.8) | < 0.001 |
| Cardiac death, n (%) | 86 (9.9) | 26 (6.6) | 38 (10.9) | 22 (17.2) | 0.002 |
| Noncardiac death, n (%) | 54 (6.2) | 8 (2.0) | 27 (7.8) | 19 (14.8) | < 0.001 |
| Unknown death, n (%) | 3 (0.3) | 1 (0.2) | 1 (0.3) | 1 (0.8) | |
Continuous variables are summarized as the median and interquartile range. Comparisons between patient groups were performed using the Kruskal–Wallis test for continuous variables and by means of contingency table analysis and Fisher’s exact test for categorical variables. ACEI angiotensin-converting enzyme inhibitor, Alb albumin, ARB angiotensin-receptor blocker, BNP B-type natriuretic peptide, CRP C-reactive protein, Dd end-diastolic diameter, Ds end-systolic diameter, EF ejection fraction, eGFR estimated glomerular filtration rate, GPS Glasgow Prognostic Score, HF heart failure, LAD left atrial dimension, LDL low-density lipoprotein, LV left ventricular, NYHA New York Heart Association.
Relationship between GPS and clinical characteristics
Age, BNP, and LVEF correlated positively with GPS (r = 0.300, p < 0.001; r = 0.182, p < 0.001; and r = 0.170, p < 0.001, respectively), whereas hemoglobin correlated negatively with GPS (r = − 0.322, p < 0.001). There were no significant correlations between GPS and systolic blood pressure, eGFR, or sodium (Table 2).
Table 2.
Univariate correlations between the GPS and baseline indices.
| Variable | Spearman’s r | p-value |
|---|---|---|
| Age (years) | 0.300 | < 0.001 |
| Systolic blood pressure (mmHg) | 0.050 | 0.139 |
| Hemoglobin (g/dL) | − 0.332 | < 0.001 |
| eGFR (mL/min/1.73 m2) | − 0.049 | 0.152 |
| Sodium (mEq/L) | − 0.005 | 0.90 |
| BNP (pg/mL) | 0.182 | < 0.001 |
| LVEF (%) | 0.170 | < 0.001 |
Correlations between GPS and clinical and laboratory indices were evaluated by Spearman’s rank test. BNP B-type natriuretic peptide, eGFR estimated glomerular filtration rate, GPS Glasgow Prognostic Score, LVEF left ventricular ejection fraction.
Association of GPS with mortality
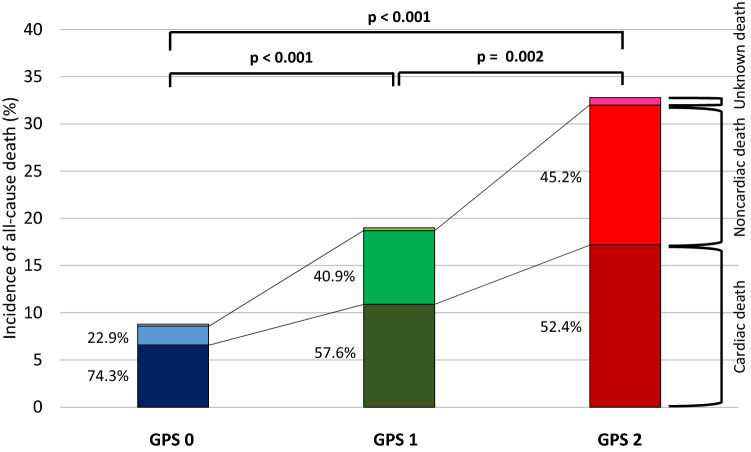
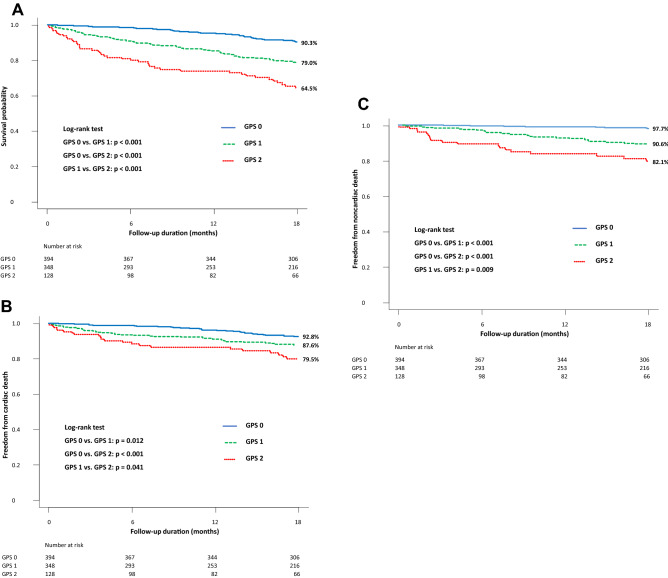
Of the 870 patients, 143 (16.4%) died during the 18-month (548-day) follow-up period. Follow-up rates at 6 months, 12 months and 18 months were 94.0%, 89.2% and 83.9%, respectively. Incidence of all-cause mortality increased significantly with increasing GPS (GPS 0 vs. 1 vs. 2: 8.9% vs. 19.0% vs. 32.8%; GPS 0 vs. 1 or 2: both p < 0.001 and GPS 1 vs. 2: p = 0.002) (Table 1, Fig. 2). Moreover, there were significant differences in cardiac death (p = 0.002) and noncardiac death (p < 0.001) among the 3 groups (Table 1). Cardiac death accounted for 74.3%, 57.6% and 52.4% of all-cause death in the GPS 0, 1 and 2 groups, respectively. On the other hand, noncardiac death accounted for 22.9%, 40.9% and 45.2% of all-cause death in the GPS 0, 1, and 2 groups, respectively (Fig. 2). In Kaplan–Meier survival analysis, patients in the GPS 2 group had a significantly lower survival probability than did those in the GPS 1 group (64.5% vs. 79.0%, log-rank p < 0.001) and the GPS 0 group (90.3%, p < 0.001). GPS 1 patients also had a significantly lower survival probability than did GPS 0 patients (p < 0.001) (Fig. 3A). As with all-cause mortality, cardiac mortality and noncardiac mortality increased significantly with increasing GPS (Fig. 3B,C). Multivariate Cox proportional hazards analysis adjusted for age, sex, systolic blood pressure, NYHA functional class, prior HF hospitalization, hemoglobin, eGFR, sodium, BNP, LVEF, and the use of ACEI and/or ARB, beta-blockers, or aldosterone antagonists revealed that the hazard ratio for mortality in GPS 1 and 2 patients relative to GPS 0 patients were 1.53 (95% confidence interval [CI] 0.96–2.43, p = 0.071) and 2.92 (95% CI 1.77–4.81, p < 0.001), respectively (Table 3). C-statistics for all-cause-mortality were greater in baseline model plus GPS (0.754, p = 0.046) than baseline model alone (0.733). The net reclassification improvement (NRI) and integrated discrimination improvement (IDI) for all-cause mortality also significantly increased after addicting GPS to baseline model (0.249, p = 0.006; 0.029, p < 0.001, respectively) (Table 4).
Figure 2.
Incidence of all-cause death stratified by GPS and cause of deaths. Higher incidence of all-cause, cardiac and noncardiac mortality was observed with increasing GPS. GPS Glasgow Prognostic Score.
Figure 3.
Kaplan–Meier survival curve of all-cause, cardiac and noncardiac mortality stratified by GPS. (A) Higher GPS was associated with worse prognosis in the overall cohort. With increasing the GPS, (B) cardiac mortality and (C) noncardiac mortality increased. GPS Glasgow Prognostic Score.
Table 3.
Univariate and multivariate cox proportional hazards analysis to identify prognostic ability of the GPS.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| GPS 0 | Reference | Reference | ||
| GPS 1 | 2.41 (1.60–3.63) | < 0.001 | 1.53 (0.96–2.43) | 0.071 |
| GPS 2 | 4.62 (2.95–7.24) | < 0.001 | 2.92 (1.77–4.81) | < 0.001 |
| Age (years) | 1.07 (1.05–1.09) | < 0.001 | 1.06 (1.04–1.09) | < 0.001 |
| Female, n (%) | 0.86 (0.62–1.21) | 0.39 | 0.67 (0.46–0.99) | 0.042 |
| Systolic blood pressure (mmHg) | 0.99 (0.981–0.999) | 0.032 | 0.99 (0.981–1.001) | 0.064 |
| NYHA class III or IV, n (%) | 2.13 (1.50–3.03) | < 0.001 | 1.33 (0.89–1.98) | 0.166 |
| Prior HF hospitalization, n (%) | 1.83 (1.32–2.55) | < 0.001 | 1.29 (0.90–1.87) | 0.168 |
| Hemoglobin (g/dL) | 0.79 (0.72–0.85) | < 0.001 | 0.89 (0.81–0.99) | 0.030 |
| eGFR (mL/min/1.73 m2) | 0.97 (0.96–0.98) | < 0.001 | 0.98 (0.97–0.99) | 0.002 |
| Sodium (mEq/L) | 0.96 (0.916–1.001) | 0.056 | 0.98 (0.94–1.03) | 0.41 |
| BNP (pg/mL) | 1.00 (1.000–1.001) | < 0.001 | 1.00 (1.000–1.001) | 0.052 |
| LVEF (%) | 0.99 (0.983–1.004) | 0.191 | 0.99 (0.977–1.002) | 0.103 |
| ACEI and/or ARB, n (%) | 0.83 (0.59–1.18) | 0.30 | 1.04 (0.72–1.51) | 0.82 |
| Beta-blockers, n (%) | 0.68 (0.48–0.96) | 0.029 | 0.90 (0.61–1.34) | 0.62 |
| Aldosterone antagonists, n (%) | 0.89 (0.63–1.22) | 0.44 | 1.04 (0.72–1.50) | 0.83 |
Data presented are hazard ratios and 95% confidence intervals. ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin-receptor blocker, BNP B-type natriuretic peptide, CI confidence interval, eGFR estimated glomerular filtration rate, GPS Glasgow Prognostic Score, HF heart failure, HR hazard ratio, LVEF left ventricular ejection fraction, NYHA New York Heart Association.
Table 4.
Discrimination of each predictive model for all-cause mortality using C-statistics, NRI and IDI.
| Predictive models | C-statistics (95% CI) | p value | NRI | p value | IDI | p value |
|---|---|---|---|---|---|---|
| Baseline model | 0.733 (0.687–0.778) | Reference | Reference | Reference | ||
| + GPS | 0.754 (0.710–0.798) | 0.046 | 0.249 | 0.006 | 0.029 | < 0.001 |
Baseline model included age, sex, hypertension, diabetes mellitus, atrial fibrillation, New York Heart Association functional class. CI confidence interval, GPS Glasgow Prognostic Score, IDI integrated discrimination improvement, NRI net reclassification improvement.
Subgroup analysis
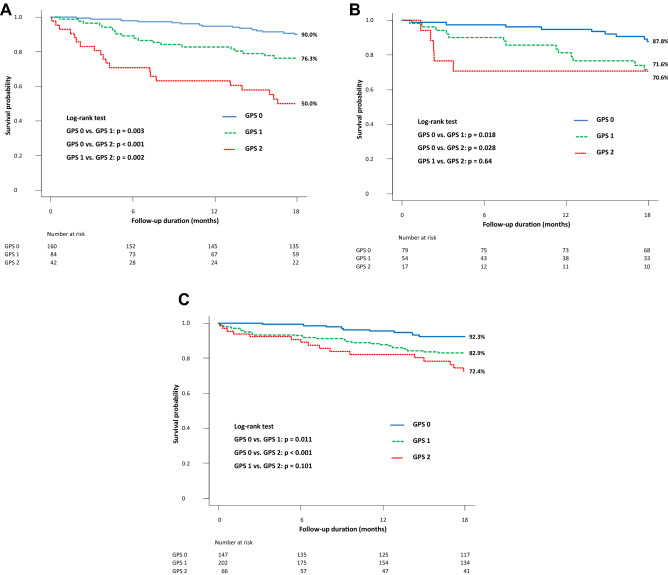
To evaluate the association of GPS with mortality according to HF subtype, the cohort was divided into the HFpEF (LVEF ≥ 50%), HF with mid-range EF (HFmrEF) (LVEF ≥ 40% and < 50%), and HFrEF (LVEF < 40%) groups. In HFrEF patients, incidence of all-cause death increased significantly with increasing GPS (GPS 0 vs. 1 vs. 2: 9.4% vs. 22.6% vs. 47.6%; GPS 0 vs. 1 or 2: p = 0.006 or p < 0.001 and GPS 1 vs. 2: p = 0.007) (Fig. S1A online). Although there was no significant difference between three groups, mortality tended to increase with GPS in the HFmrEF subgroup (GPS 0 vs. 1 or 2: 11.4% vs 24.1% or 29.4%, p = 0.061 or p = 0.121; GPS 1 vs. 2: p = 0.75) (Fig. S1B online). The GPS 0 group had lower mortality rate than the GPS 1 group (6.8% vs. 15.3%, p = 0.018) and the GPS 2 group (vs. 24.2%, p = 0.001). There was no significant difference between the GPS 1 and 2 group (p = 0.134) (Fig. S1C online). In Kaplan–Meier analysis, survival probability decreased significantly with increasing GPS in HFrEF patients (GPS 0 vs. 1 vs. 2: 90.0% vs. 76.3% vs. 50.0%; log-rank test: GPS 0 vs. 1 or 2: p = 0.003 or p < 0.001 and GPS 1 vs. 2: p = 0.002) (Fig. 4A). In HFmrEF patients, those with GPS 0 had a higher survival probability than did those with GPS 1 or 2 (87.8% vs. 71.6% or 70.6%; p = 0.018 or p = 0.028). There was no significant difference in mortality between patients with GPS 1 and 2 (p = 0.64) (Fig. 4B). Similarly, in HFpEF patients, those with GPS 0 had a higher survival probability than did those with GPS 1 or 2 (92.3% vs. 82.9% or 72.4%; p = 0.011 or p < 0.001). There was no significant difference in mortality between patients with GPS 1 and 2 (p = 0.101) (Fig. 4C).
Figure 4.
Kaplan–Meier survival curve of patients by HF subtype. (A) Survival probability decreased significantly with increasing GPS in HFrEF patients. (B) HFmrEF patients with GPS 0 had significantly higher survival probability than did those with GPS 1 or 2. (C) Although HFpEF patients with GPS 0 had significantly higher survival probability than did those with GPS 1, no other remarkable differences were seen. GPS Glasgow Prognostic Score, HFmrEF heart failure with mid-range ejection fraction, HFpEF heart failure with preserved ejection fraction, HFrEF heart failure with reduced ejection fraction.
Discussion
This study demonstrated the prognostic significance of the GPS for predicting mortality in patients with HF. Specifically, it showed that: (1) patients with higher GPS had significantly higher severity of HF assessed by NYHA functional class and BNP levels than did patients with lower GPS, (2) age, BNP, and LVEF all correlated positively with GPS, (3) high GPS was significantly associated with a worse prognosis, (4) the GPS improve the predictive accuracy of all-cause mortality compared to other predictive model that did not require any form of laboratory testing, (5) both cardiac mortality and noncardiac mortality increased with increasing GPS, (6) better prognosis were observed in the GPS 0 group in all HF subtype groups and (7) significant associations between GPS and mortality were more evident in patients with HFrEF.
The GPS has been identified as a reliable predictor of mortality in several cancers, including non-small-cell lung cancer, gastro-esophageal cancer, and hepatocellular carcinoma23–25. Chronic activation of the systemic inflammatory response induces cancer progression. Inflammation leads to the accumulation of regulatory T lymphocytes and chemokines as well as the stimulation of cytokines, which produce CRP, increase neutrophils, and induce an inappropriate immune response. As a result, tumor angiogenesis, invasion, and metastasis are promoted25. Moreover, systemic inflammation causes malnutrition leading to decreased body weight, fatigue, loss of lean mass, declines in activities of daily living, and poor survival24. It is also considered that the progression of sarcopenia and malnutrition due to systemic inflammation may contribute to poor outcomes in patients with HF. Indeed, our study showed that high GPS was significantly associated with a worse prognosis in patients with HF. These are consistent with a previous report by Namiuchi et al., which found that higher GPS was an independent risk factor for higher mortality among admitted patients with acute decompensated HF28. However, the present study analyzed the GPS for risk-stratification in patients with HF in an older and larger cohort than the above report28. A few studies have reported the prognostic value of the GPS in HF according to HF subtype. Cho et al. recently demonstrated the utility of the modified GPS for predicting survival in HFrEF patients29 and Bolat et al. reported the modified GPS as the novel predictor of clinical outcome including all-cause mortality in HFpEF patients30. Our findings supported their studies29,30. In the present study, as GPS increased, mortality had tendency to increase in patients with HFrEF and HFpEF. In particular, the tendency was evident and significant in patients with HFrEF. Additionally, in all subtype of HF including HFmrEF, low GPS was suggested to associate with better prognosis. Further assessment of nutrition and inflammation is needed for a broader HF population age spectrum and more analyses according to HF subtype.
In patients with HF, hypoalbuminemia is caused by multiple factors, including anorexia, malabsorption, high energy demand, and cytokine-induced increases in metabolic rate31. Hypoalbuminemia may exacerbate HF severity by promoting peripheral and pulmonary congestion and cause increased oxidative stress and inflammation to create a vicious cycle of worsening inflammation, HF, and hypoalbuminemia31. HF also provokes inflammation by wall stress and the release of cardiac cytokines and other inflammatory mediators from the heart and other organs. Cytokines evoke myocardial remodeling and pump failure, resulting in the progression of HF14.
This study demonstrated that the GPS was useful for estimating the prognosis of patients with HF. Additionally, the GPS was associated with the severity of HF because the number of patients with NYHA functional class III or IV and levels of BNP increased with increasing GPS and BNP correlated positively with GPS, which indices are recognized to reflect severity of HF5,32. The GPS can predict mortality using only two routinely measured parameters, CRP and Alb, both of which can be easily obtained in clinical practice. Several nutritional indicators, such as the GNRI, PNI, and CONUT scores, as well as inflammatory markers including CRP, have been reported to individually associate with HF prognosis and severity6,10,11,17–22. Prognosticators that consist of both nutritional and inflammatory markers would therefore appear most useful for patients with HF. Since the GPS reflects systemic inflammation (elevated CRP) and malnutrition (hypoalbuminemia), it may be considered appropriate to reflect the severity of HF. Moreover, nutritional assessment alone isn’t enough to evaluate severity of inflammation and treat it, which is one of the causes of malnutrition. Similarly, inflammation assessment alone isn’t enough to evaluate how much inflammation affects nutritional status. Assessment of combining nutritional status and inflammation allow not only nutritional intervention but also approach chronic inflammatory diseases in the background of malnutrition. Finally, the improvement of patient GPS by the early management of nutrition and inflammation may therefore improve also prognosis.
In this study, all-cause mortality was chosen as primary endpoint. Along with the aging society, the number of elderly HF patients is increasing33,34. Although the most common cause of death in patients with HF is cardiac death regardless of HF subtype35,36, it has been reported that the proportion of noncardiac death in elderly HF patients is higher than in younger HF patients37. Thus, it may be important to predict all-cause mortality as well as cardiac mortality, especially in elderly HF patients. Furthermore, because of increasing frailty and progression of HF in elderly patients, palliative and end-of-life care has been needed for them. However, it is often difficult to determine when to introduce palliative care for elderly HF patients, because of their clinical course before death which differ from cancer patients. Patients with HF have repeated acute exacerbations and diminished their physical function. Each exacerbation can lead to death, but usually symptoms at that time improve with treatment. Whereas, their conditions rapidly deteriorate before they die38,39. Hence, it is also difficult to recognize that they are at the end-of-life40. To predict not cardiac mortality but all-cause mortality by using the GPS may help introducing palliative care for elderly HF patients at the appropriate time.
This study had several limitations. First, the sample size was relatively limited; fewer significant differences in the subgroup analyses may have been caused by underpowered statistics. Second, since we evaluated a cohort of admitted patients with HF, our results might not be generalizable to patients with stable HF. Third, our analysis was based on a single data point taken at discharge, with no further testing during follow-up. Therefore, the impact of GPS improvement on mortality is unknown and requires further study. Fourth, the GPS is associated with acute infection or chronic inflammation such as cancer and collagen disease. There were probably no patients with acute infection in this study, because their Alb and CRP were measured at discharge, and at that time they had no clinical symptoms and physical findings of infection. However, we could not exclude patients with cancer or collagen disease. Finally, because mortality data in this study was obtained from participating institutes instead of a governmental database, our data did not accurately assess mortality in our geographic region. Despite these limitations, however, inflammation-based assessment using the GPS appears useful for the risk stratification of mortality in patients with HF.
In conclusion, high GPS was associated with worse prognosis in patients with HF. Combined assessment of inflammation and nutrition may improve the evaluation of HF severity and prognosis and assist in patient treatment.
Methods
Study design and patient population
The present multi-center, prospective, observational study was conducted in Nagano Prefecture, Japan. The inclusion criteria were 20 years of age or more and admission for acute decompensated HF. Patients with acute coronary syndrome (ACS) were excluded. After providing informed consent, subjects were enrolled between July 2014 and December 2018 during a compensated HF state before discharge. We recorded medical history, HF etiology, comorbidities, socio-economic background, medications, and examination findings at discharge that included electrocardiogram (ECG), echocardiography, and blood test results when HF compensated and causes of HF such as arrythmia, anemia and infection were cured. HF and ACS were diagnosed by attending physicians based on symptoms, ECGs, echocardiography, laboratory data, chest X-rays, and coronary angiograms according to the Framingham criteria41 and ACC/AHA guidelines42, respectively. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or ongoing therapy of for hypertension. Dyslipidemia was defined as a low-density lipoprotein cholesterol concentration ≥ 140 mg/dL, a high-density lipoprotein cholesterol concentration < 40 mg/dL, a triglyceride concentration ≥ 150 mg/dL, a previous diagnosis of dyslipidemia or current treatment for lipid lowering agents. Diabetes mellitus was defined as a hemoglobin A1c level ≥ 6.5%, fasting plasma glucose ≥ 126 mg/dL, 2-h plasma glucose level after a 75 g oral glucose tolerance test ≥ 200 mg/dL, casual plasma glucose ≥ 200 mg/dL, a previous diagnosis of diabetes mellitus or treatment with oral hypoglycemic agents or insulin injection. LVEF was measured by the biplane modified Simpson’s method, which recommended by the American Society of Echocardiography43. Details of the GPS have been described previously. Patients with both elevated CRP (> 1.0 mg/dL) and hypoalbuminemia (< 3.5 g/dL) were allocated a score of 2. Patients in whom only one of these biochemical abnormalities was present were allocated a score of 1. Patients in whom neither of these abnormalities existed were allocated a score of 0 (Fig. 1)23,44. Enrolled patients were categorized into the GPS 0, 1, or 2 group based on their GPS at discharge. The study protocol was approved by the Shinshu University Institutional Review Board (approval number: 4237). Informed consent was obtained from all patients. This study was performed in accordance with the Declaration of Helsinki, and is registered with the University Hospital Medical Information Network (UMIN 000024470).
Endpoint and follow-up
The study endpoint was all-cause mortality. Relationships between mortality rates and GPS were statistically assessed. We followed patients prospectively and collected relevant clinical data at the time of scheduled follow-ups by telephone. Survival status was confirmed by chart review.
Statistical analysis
Continuous variables are summarized as the mean ± standard deviation if normally distributed and as the median and interquartile range otherwise. Normality was assessed by the Shapiro–Wilk W test. Categorical variables are presented as numbers and percentages. Comparisons between patient groups were performed using the Kruskal–Wallis test for continuous variables and by means of contingency table analysis and Fisher’s exact test for categorical variables. Correlations between GPS and clinical and laboratory indices were evaluated by Spearman’s rank test. Kaplan–Meier survival curves were calculated from baseline to the time of death for comparisons using the log-rank test. Cox proportional hazards regression analysis was conducted to identify the prognostic ability of the GPS. The multivariate analysis model was adjusted for age, sex, systolic blood pressure, NYHA functional class, prior HF hospitalization, hemoglobin, eGFR, sodium, BNP, LVEF, and the use of ACEi and/or ARB, beta-blockers, or aldosterone antagonists. These covariates were selected in advance which are recognized as prognostic factors in HF45–47. To evaluate whether the accuracy of predicting all-cause mortality would improve after the addition of the GPS into a baseline model with established risk factors, including age, sex, hypertension, diabetes mellitus, atrial fibrillation, NYHA functional class, C-statistics, NRI and IDI were calculated. The C-statistics is defined as the area under receiver-operating characteristic curves. A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed with R (The R Foundation for Statistical Computing, Vienna, Austria) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R48.
IRB information
This study was approved by the Ethics Committee of Shinshu University Hospital (approval number: 4237) and is registered with the University Hospital Medical Information Network (UMIN 000024470).
Supplementary Information
Acknowledgements
We thank the following 14 hospitals that participated in this study: Minaminagano Medical Center, Shinshu Ueda Medical Center, Nagano Red Cross Hospital, Matsumoto Medical Center, Nagano Municipal Hospital, Iida Municipal Hospital, Aizawa Hospital, Okaya City Hospital, Saku Central Hospital, Hokushin General Hospital, Suwa Red Cross Hospital, Matsushiro General Hospital, Shinshu Medical Center, and Asama Nanroku Komoro Medical Center. The authors also acknowledge the secretarial assistance of Minako Aono and thank Trevor Ralph for his editorial assistance.
Author contributions
T.I. and H.M. wrote the main manuscript text. K.O., K.M., T.T., M.K., K.K., S.H., and H.K. contributed to patients' enrollment. H.M., M.M., and K.K. conducted the research. All authors reviewed the manuscript.
Data availability
The deidentified participant data will be shared on a request basis. Please contact the corresponding author to request data sharing. All data, including patients’ clinical characteristics, outcome data, related documents on study protocol, and statistical analysis plan, will be available by digital media to medical doctors for all types of analyses for a period of 1 year following publication of the study.
Competing interests
Dr. Kuwahara has received lecture fee from Boehringer Ingelheim, Otsuka pharmaceutical, AstraZeneca, Mitsubishi Tanabe Pharma corporation, Bristol-Myers Squibb company, and Daiichi Sankyo company from 2017 to 2019.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94525-6.
References
- 1.Curtis LH, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch. Intern. Med. 2008;168:418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 2.Marengoni A, et al. Aging with multimorbidity: A systematic review of the literature. Ageing Res. Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Caughey GE, Roughead EE, Shakib S, Vitry AI, Gilbert AL. Co-morbidity and potential treatment conflicts in elderly heart failure patients: A retrospective, cross-sectional study of administrative claims data. Drugs Aging. 2011;28:575–581. doi: 10.2165/11591090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Krum H, Gilbert RE. Demographics and concomitant disorders in heart failure. Lancet. 2003;362:147–158. doi: 10.1016/S0140-6736(03)13869-X. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Kinugawa S, Fukushima A. Malnutrition in heart failure: Important but undervalued issue. JACC Heart Fail. 2018;6:487–488. doi: 10.1016/j.jchf.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Fiatarone MA, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 8.Ng TP, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: A randomized controlled trial. Am. J. Med. 2015;128:1225–1236. doi: 10.1016/j.amjmed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 10.Vasan RS, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.CIR.0000057810.48709.F6. [DOI] [PubMed] [Google Scholar]
- 11.Anand IS, et al. C-reactive protein in heart failure: Prognostic value and the effect of Valsartan. Circulation. 2005;112:1428–1434. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- 12.Westermann D, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ. Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 13.Dick SA, Epelman S. Chronic heart failure and inflammation: What do we really know? Circ. Res. 2016;119:159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 14.Van Linthout S, Tschöpe C. Inflammation—Cause or consequence of heart failure or both? Curr. Heart Fail. Rep. 2017;14:251–265. doi: 10.1007/s11897-017-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redfield MM. Heart failure with preserved ejection fraction. N. Engl. J. Med. 2016;375:1868–1877. doi: 10.1056/NEJMcp1511175. [DOI] [PubMed] [Google Scholar]
- 16.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari R, et al. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92:1479–1486. doi: 10.1161/01.CIR.92.6.1479. [DOI] [PubMed] [Google Scholar]
- 18.Tsutamoto T, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J. Am. Coll. Cardiol. 1998;31:391–398. doi: 10.1016/S0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 19.Deswal A, et al. Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the Vesnarinone Trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.CIR.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 20.Kalogeropoulos A, et al. Inflammatory markers and incident heart failure risk in older adults. The Health ABC (Health, Aging, and Body Composition) Study. J. Am. Coll. Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zairis MN, et al. Multimarker strategy for the prediction of 31 days cardiac death in patients with acutely decompensated chronic heart failure. Int. J. Cardiol. 2010;141:284–290. doi: 10.1016/j.ijcard.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Milo-Cotter O, et al. Neurohormonal activation in acute heart failure: Results from VERITAS. Cardiology. 2011;119:96–105. doi: 10.1159/000330409. [DOI] [PubMed] [Google Scholar]
- 23.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer. 2003;89:1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crumley ABC, McMillan DC, McKernan M, McDonald AC, Stuart RC. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br. J. Cancer. 2006;94:637–641. doi: 10.1038/sj.bjc.6602998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita A, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br. J. Cancer. 2012;107:988–993. doi: 10.1038/bjc.2012.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, et al. Prognostic role of Glasgow prognostic score in patients with hepatocellular carcinoma. Medicine (Baltimore) 2015;94:2133. doi: 10.1097/MD.0000000000002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan SQ, et al. Glasgow prognostic score is superior to ECOG PS as a prognostic factor in patients with gastric cancer with peritoneal seeding. Oncol. Lett. 2018;15:4193–4200. doi: 10.3892/ol.2018.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namiuchi S, et al. The systemic inflammation-based Glasgow Prognostic Score as a prognostic factor in patients with acute heart failure. J. Cardiovasc. Med. 2015;16:409–415. doi: 10.2459/JCM.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 29.Cho A, et al. The inflammation-based modified Glasgow prognostic score is associated with survival in stable heart failure patients. ESC Heart Fail. 2020;7:654–662. doi: 10.1002/ehf2.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolat I, Biteker M. Modified Glasgow Prognostic Score is a novel predictor of clinical outcome in heart failure with preserved ejection fraction. Scand. Cardiovasc. J. 2020;54:174–178. doi: 10.1080/14017431.2019.1709656. [DOI] [PubMed] [Google Scholar]
- 31.Mene-Afejuku TO, et al. The relevance of serum albumin among elderly patients with acute decompensated heart failure. J. Geriatr. Cardiol. 2019;16:522–528. doi: 10.11909/j.issn.1671-5411.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponikowski P, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 33.Ambrosy AP, et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 34.Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur. J. Heart Fail. 2015;17:884–892. doi: 10.1002/ejhf.319. [DOI] [PubMed] [Google Scholar]
- 35.Lee DS, et al. A systematic assessment of causes of death after heart failure onset in the community. Circ. Heart Fail. 2011;4:36–43. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaduganathan M, et al. Mode of death in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2017;69:556–569. doi: 10.1016/j.jacc.2016.10.078. [DOI] [PubMed] [Google Scholar]
- 37.Sato M, et al. Clinical characteristics and prognostic factors in elderly patients with chronic heart failure—A report from the CHART-2 study. IJC Heart Vasc. 2020;27:100497. doi: 10.1016/j.ijcha.2020.100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lunney JR, Lynn J, Hogan C. Profiles of older medicare decedents. J. Am. Geriatr. Soc. 2002;50:1108–1112. doi: 10.1046/j.1532-5415.2002.50268.x. [DOI] [PubMed] [Google Scholar]
- 39.Goodlin SJ. Palliative care in congestive heart failure. J. Am. Coll. Cardiol. 2009;54:386–396. doi: 10.1016/j.jacc.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 40.Allen LA, et al. Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. J. Am. Med. Assoc. 2008;299:2533–2542. doi: 10.1001/jama.299.21.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham study. N. Engl. J. Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 42.Amsterdam EA, et al. 2014 AHA/ACC guideline for the management of patients with Non-ST-Elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;64:139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Nagueh SF, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br. J. Cancer. 2004;90:1704–1706. doi: 10.1038/sj.bjc.6601789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahimi K, et al. Risk prediction in patients with heart failure: A systematic review and analysis. JACC Heart Fail. 2014;2:440–446. doi: 10.1016/j.jchf.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Bello NA, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ. Heart Fail. 2014;7:590–595. doi: 10.1161/CIRCHEARTFAILURE.113.001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossignol P, Hernandez AF, Solomon SD, Zannad F. Heart failure drug treatment. Lancet. 2019;393:1034–1044. doi: 10.1016/S0140-6736(18)31808-7. [DOI] [PubMed] [Google Scholar]
- 48.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deidentified participant data will be shared on a request basis. Please contact the corresponding author to request data sharing. All data, including patients’ clinical characteristics, outcome data, related documents on study protocol, and statistical analysis plan, will be available by digital media to medical doctors for all types of analyses for a period of 1 year following publication of the study.