Abstract
Objective:
While patients with late-life depression (LLD) often exhibit microstructural white matter alterations that can be identified with diffusion tensor imaging (DTI), there is a dearth of information concerning the links between DTI findings and specific cognitive performance, as well as between DTI measures and antidepressant treatment outcomes.
Design:
Neuroimaging and cognitive tests were conducted at baseline in 71 older adults participating in a larger, 8-week duration antidepressant randomized controlled trial. Correlations between DTI measures of white matter integrity evaluated with tract-based spatial statistics, baseline neurocognitive performance, and prospective antidepressant treatment outcome were evaluated.
Results:
Fractional anisotropy (FA), an index of white matter integrity, was significantly positively associated with better cognitive function as measured by the Initiation/Perseveration subscale of the Dementia Rating Scale in the bilateral superior longitudinal fasciculus (SLF), bilateral SLF-temporal, and right corticospinal tract (CST). An exploratory analysis limited to these tracts revealed that increased FA in the right CST, right SLF, and right SLF-temporal tracts was correlated with a greater decrease in depressive symptoms. Increased FA in the right CST predicted a greater chance of remission, while increased FA in the right CST and the right SLF predicted a greater chance of treatment response.
Conclusions:
In LLD subjects, white matter integrity was positively associated with executive function in white matter tracts which act as key connecting structures underlying the cognitive control network. These tracts may play a role as a positive prognostic factor in antidepressant treatment outcome.
Keywords: Diffusion Tensor Imaging, White Matter Integrity, Executive Function, Dementia Rating Scale, Treatment Outcome, Late-Life Depression
OBJECTIVE
Two contributing factors to antidepressant treatment non-response are the presence of white matter hyperintensities (WMHs) seen on T2-weighted magnetic resonance imaging (MRI) scans (1) and executive dysfunction (2). WMHs are observed in the majority of older adults with depression, are associated with a more chronic clinical course (1), and predict lower response to antidepressant treatment (3). Executive dysfunction is common in late-life depression (LLD) and also predicts poorer response to antidepressant medication and higher relapse rates (2,4). The vascular depression hypothesis was proposed to explain the pathogenesis of the subtype of LLD characterized by WMHs, executive dysfunction, and resistance to antidepressant medication. The vascular depression hypothesis postulates that cerebrovascular disease is involved with the pathogenesis and persistence of geriatric depressive symptoms through structural damage to white matter (WM), leading to disruption in cortical and subcortical circuits, specifically in frontostriatal regions, that are implicated in mood regulation and cognition, particularly executive function (5).
While macrostructural changes in cerebral WM are characterized by WMH burden, diffusion tensor imaging (DTI) acts as a complementary approach by capturing microstructural changes in WM tracts, which is indexed by anisotropic measures such as fractional anisotropy (FA) (6). DTI provides a means to further elucidate the vascular depression hypothesis by yielding greater specificity concerning tract integrity. One DTI study found that LLD patients exhibited lower fractional anisotropy FA values in anterior cingulate cortex (ACC) white matter, as well as the superior and middle frontal gyri when compared to healthy controls, supporting the idea that these WM alterations reflect an impairment of the dorsolateral prefrontal and the anterior cingulate circuits (7), both of which are considered subsets of the corticostriatal network (8). DTI studies using tract-based spatial statistics (TBSS) (9) and tractography (10) methods found reduced WM integrity in additional WM tracts including the cingulum, uncinate fasciculus (UNC), corpus callosum, anterior thalamic radiation (ATR), corticospinal tract (CST), and superior longitudinal fasciculus (SLF) in LLD patients compared to healthy controls (11, 12).
While there appear to be identifiable WM alterations in LLD patients compared to healthy controls, the few studies available linking DTI findings to specific cognitive performance deficits and antidepressant treatment outcome yielded inconsistent results. Several DTI studies found that the integrity of certain WM tracts was positively associated with cognitive performance in older adults with depression, including on tests of executive function, episodic and working memory, processing speed, and language (11, 13). Alexopoulos et al. (2002) found that decreased FA in the WM lateral to the anterior cingulate, which contains fibers of the anterior cingulate and dorsolateral pathways, was associated with a low remission rate (14). A separate study by these investigators found that lower FA in multiple frontal limbic brain areas, including the dorsolateral prefrontal cortex, ACC, posterior cingulate cortex, insula, and the genu of corpus callosum, was associated with non-remission in LLD patients (15). In contrast, a 12-week trial of sertraline found that higher FA values in the superior frontal gyri bilaterally and the ACC were significantly associated with failure to remit (16) and a greater decline in FA within the ACC from baseline to follow up was significantly associated with improvement in depression severity (17). These inconsistencies in the literature highlight the need for additional DTI studies to assess the relationship between WM integrity and neurocognitive function, as well as treatment response.
In this study, we evaluated the correlations between WM integrity measured by DTI using TBSS, baseline cognitive performance, and prospective antidepressant response in LLD patients. Neurocognitive performance was comprehensively assessed, with a focus on executive function, processing speed, and episodic memory, as deficits in all of these areas have been observed in LLD patients (18). Associations between WM integrity and clinical outcomes after medication treatment were also analyzed. Additionally, we conducted an exploratory analysis of clinical response and remission in tracts where a relationship between WM integrity and neurocognitive function was observed. We hypothesized that WM integrity would be correlated with both baseline cognitive performance and antidepressant response, with increased FA positively associated with both cognitive performance and clinical outcomes.
METHODS
Subjects
Data for this study were collected as part of a larger, 8-week randomized controlled trial examining mechanisms of antidepressant non-response in LLD (19, in press). The study was conducted in the Clinic for Aging, Anxiety, and Mood Disorders at the New York State Psychiatric Institute (NYSPI), approved by the NYSPI Institutional Review Board, and registered on Clinicaltrials.gov (NCT01931202). Eligible subjects were men and women aged 60–90 years old who met Diagnostic and Statistical Manual IV (DSM-IV) criteria for non-psychotic major depressive disorder (MDD), had a 24-item Hamilton Rating Scale for Depression (HRSD-24) score ≥ 16, and were willing to and capable of providing informed consent and complying with study procedures. Subjects were excluded from participation for a current comorbid Axis I DSM IV disorder (other than nicotine dependence, adjustment disorder, or anxiety disorders), substance abuse or dependence within the past 12 months, lifetime psychosis or mania, or probable Alzheimer’s disease, vascular dementia, or Parkinson’s disease. Other exclusion criteria included a Mini Mental State Examination score < 24, significant suicidality, adverse reaction or non-response to escitalopram and duloxetine, current treatment with psychotherapy, antidepressants, anti-psychotics, or mood stabilizers, Clinical Global Impression (CGI) score of 7 at baseline, or acute, severe, or unstable medical illness.
Study Design
The parent randomized controlled trial utilized differing probabilities of receiving active medication as a means of studying the placebo effect-component of treatment response in LLD (19, in press). As results from the study showed that depressive symptom change did not differ significantly between randomized groups, the sample is combined here for the purposes of evaluating correlations between white matter integrity measures, baseline cognitive performance, and prospective antidepressant response. Briefly, subjects were screened for eligibility at baseline and scheduled for neuroimaging procedures to take place prior to a Week 0 appointment at which study medication was initiated. Thereafter, subjects returned weekly for clinical assessments and medication management. Escitalopram 10mg was the default treatment option, though individuals who had previously failed or had difficulty tolerating escitalopram were started on duloxetine 30mg per day. If subjects did not meet remission criteria by Week 4 (HRSD-24 ≤ 7), escitalopram was increased to 20mg (or duloxetine to 90mg). Subjects unable to tolerate the increased dose of medication had their dosage reduced to the maximum previously tolerated dose. Subjects returned for 8 weekly visits.
Clinical and Neurocognitive Assessments
A Structured Clinical Interview Diagnostic for DSM-IV TR (SCID) was performed at baseline to confirm subject eligibility. The HRSD-24 was performed at every study visit, and change on the HRSD-24 was defined a priori as the primary clinical outcome measure. Response (≥ 50% decrease in baseline HRSD-24 score or CGI-Improvement score of 1 or 2 at Week 8) and remission (HRSD-24 ≤ 7 at Week 8) were defined for secondary analyses. Other secondary outcomes included weekly 16-item Quick Inventory for Depressive Symptomatology Self Report (QIDS-SR), CGI-Severity, and CGI-Improvement scales, a rating scale for treatment-emergent side effects, and weekly pill counts (to ensure compliance).
All neurocognitive tasks were measured at baseline. The Stroop Color-Word Test was used to quantify both response inhibition and a component of executive function. Responses from the incongruent condition (termed ‘Stroop Interference’ here) require reading color-names printed in inconsistent colors (e.g. the word “red” is printed in green ink and the subject must answer “green”) and were used to measure response inhibition, a constituent executive function. Executive function measurement was supplemented by the Initiation/Perseveration (I/P) subscale of the Dementia Rating Scale (DRS) (20), which consists of items that assess verbal generative fluency, auditory articulation of vowel and consonant patterns, double alternating motor movements, and simple graphomotor skills. Processing speed was measured with the Digit Symbol subtest from the Wechsler Adult Intelligence Scale-III (WAIS-III) and episodic memory was evaluated through the Logical Memory component of the Wechsler Memory Scale-Revised (WMS-R).
DTI Data Acquisition
DTI enables the mapping of WM tracts through the measurement of the diffusion of water molecules, which can either be isotropic and diffuse equally in all directions in the absence of barriers, or be anisotropic and diffuse along the axis of existing barriers (21). WM tracts in the brain form organized boundaries along which water will diffuse thus enabling anisotropic diffusion, which is often indexed via FA. An intact boundary will direct diffusion along one axis, resulting in a higher FA value, while an abnormal tract will result in less anisotropic diffusion, resulting in a lower FA value (22). In addition to FA, mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) can all be computed using DTI. Generally, FA is considered a primary and overall measure of WM integrity, while RD and AD are sensitive to demyelination and axonal degeneration, respectively (21). MD is thought to be sensitive to cellularity, edema and necrosis (23). Therefore, we used DTI to conduct analysis on white matter integrity and its role in cognitive function as well as treatment outcome.
DTI data were acquired on a GE Discovery MR750 3.0 Tesla whole body scanner (GE Medical Systems, Waukesha, Wisconsin) using a spin-echo echo planar imaging (SE-EPI) sequence with the following parameters: 30 non-collinear spatial directions at b value = 1000 s/mm2, three baseline images at b = 0 s/mm2, TR/TE = 9500 ms /86.6 ms, flip angle = 90 degrees, field of view = 24 cm, matrix size = 132 × 128 (machine-interpolated to 256 × 256 for post-processing, in-plane resolution of 0.94 mm), 60 axial slices, slice thickness = 2 mm without gap, number of excitations (NEX) = 2, scan time for each excitation was 5 minutes 13.5 seconds and we averaged 2 excitations for each subject.
DTI Data Preprocessing and Analysis
DTI data were processed using FMRIB Software Library (FSL) version 6.0.1 (Oxford, UK) (24). Specifically, DTI data were corrected for subject movement, eddy current-induced distortion, outlier replacement, and within-volume (or “slice-to-volume”) movement using FSL Eddy (25). Brain Extraction Tool (26) was used to extract a brain mask from the eddy corrected image in order to exclude skull and non-brain tissue. Diffusion tensor was fitted using FSL DTIFIT for each voxel and the fitted diffusion tensors were used to generate the FA and color encoded FA images. Data quality was assessed by visually inspecting the eddy corrected diffusion images and the color encoded FA images (27) to exclude subjects who had motion-corrupted or signal loss DTI data. We also visually checked the extracted brain masks to make sure that skull and non-brain tissue were completely removed.
We then ran TBSS (9) on the FA images for those subjects who passed the quality control. All FA images were aligned to a 1×1×1mm standard space using nonlinear registration on the adult-derived target image FMRIB58_FA provided by FSL. A mean FA image was created by averaging the aligned FA images across all subjects and was skeletonized to generate a mean FA skeleton. The FA threshold for the skeletonization was 0.20 to exclude gray matter regions from the analyses. We then project all subjects’ FA images onto the mean FA skeleton which was then used for statistical analyses. Nonlinear warps and skeleton projection were then also applied to MD, AD, and RD images for statistical analyses.
Statistical Analysis
First, we investigated associations between WM integrity and baseline neurocognitive assessments, including response inhibition (Stroop Interference), executive functions (I/P subscale of the DRS), processing speed (Digit Symbol), and episodic memory (Logical Memory). Permutation-based nonparametric inference (n =10,000) was used to perform statistical analyses on FA as well as on MD, AD, and RD using FSL Randomize version 2.9 (28), which were corrected for multiple comparisons with threshold-free cluster enhancement (TFCE) (family-wise error [FWE]-corrected p<0.05) (29). The Johns Hopkins University White-Matter Tractography Atlas (30) was used to label WM tracts.
Permutation-based nonparametric inference (n = 10,000) also was used to investigate associations between WM integrity (FA, MD, AD, and RD) and clinical outcome (post-pre HRSD-24 change, post-pre QIDS-SR change, and post-pre CGI-Severity change), which were corrected for multiple comparisons with TFCE (FWE-corrected p<0.05). Besides using linear regression models (p<0.05, uncorrected) of continuous measures, we also explored categorical outcomes of clinical response (≥ 50% decrease in baseline HRSD-24 score or Week 8 CGI-Improvement score = 1, 2) and remission (Week 8 HRSD-24 ≤ 7) using FA in tracts where significant associations of FA with baseline neurocognitive assessments were detected (TFCE, FWE-corrected p<0.05).
RESULTS
Subject Disposition and Characteristics
Seventy-one subjects with DTI scans were included in the analyses (one subject was removed from the dataset due to server signal loss during DTI data acquisition and three others were removed due to drop out). Demographic data, cognitive data, depressive symptom scores, and medication information are reported in Table 1.
Table 1.
Baseline and post treatment (Week 8) subject characteristics across demographic, clinical, and neurocognitive measures
| Demographics | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristic | n | Mean ± SD or % | |||
| Age | 71 | 69.4 ± 6.95 | |||
| Sex | |||||
| Male | 24 | 33.8 | |||
| Female | 47 | 66.2 | |||
| Race | |||||
| Asian | 1 | 1.4 | |||
| Black | 9 | 12.7 | |||
| White | 51 | 71.8 | |||
| >1 | 4 | 5.6 | |||
| Unknown | 6 | 8.5 | |||
| Ethnicity | |||||
| Not Hispanic/ Latino | 60 | 84.5 | |||
| Hispanic/Latino | 11 | 15.5 | |||
| Years of education | 62 | 16.7 ± 2.85 | |||
| Cognitive Measures | |||||
|
| |||||
| Assessment | n | Mean ± SD or % | |||
|
| |||||
| Stroop Interference | 69 | 44.6 ± 9.18 | |||
| Initiation/Perseveration subscale of Dementia Rating Scale | 71 | 35.4 ± 3.15 | |||
| Digit Symbol (WAIS-III) | 71 | 41.6 ± 9.53 | |||
| Logical Memory (WMS-R) delayed raw score | 71 | 13.1 ± 4.35 | |||
| Depression Measures | |||||
|
| |||||
| Characteristic | Baseline | Post treatment | Post-pre treatment difference | ||
|
| |||||
| n | Mean ± SD or % | n | Mean ± SD or % | p-valuea(t-statistic) | |
|
| |||||
| 24-item Hamilton Rating Scale for Depression | 71 | 20.3 ± 6.53 | 68 | 13.5 ± 7.93 | <.001 (−6.65) |
| Quick Inventory of Depressive Symptomatology Self Report | 71 | 10.8 ± 4.26 | 67 | 6.75 ± 4.95 | <.001 (−6.33) |
| Clinical Global Impressions—Severity | 71 | 3.9 ± 0.74 | 67 | 2.9 ± 1.42 | <.001 (−6.47) |
| Response and Remission Rates | |||||
|
| |||||
| Characteristic | n | % | |||
|
| |||||
| Response rates (≥ 50% decrease in 24-item Hamilton Rating Scale for Depression at post-treatment) | 24/68 | 35.3 | |||
| Remission rates (≤ 7 24-Hamilton Rating Scale for Depression at post-treatment) | 20/68 | 29.4 | |||
| Clinical Global Impressions—Improvement (score of 1 or 2 at post-treatment) | 31/67 | 46.3 | |||
| Medication | |||||
|
| |||||
| Medication | n | % | |||
|
| |||||
| Escitalopram | 28/71 | 39.4 | |||
| Duloxetine | 43/71 | 60.6 | |||
Paired t-test; df = 67, 66, 66 for 24-item Hamilton Rating Scale for Depression, Quick Inventory of Depressive Symptomatology Self Report, and Clinical Global Impressions-Severity, respectively. The df differ by measure due to the differing number of missing values for each depression measure that was evaluated.
Abbreviations: WAIS-III: Wechsler Adult Intelligence Scale-III, WMS-R: Wechsler Memory Scale-Revised, SD: standard deviation, df: degrees of freedom.
Associations between WM Integrity and Baseline Neurocognitive Function
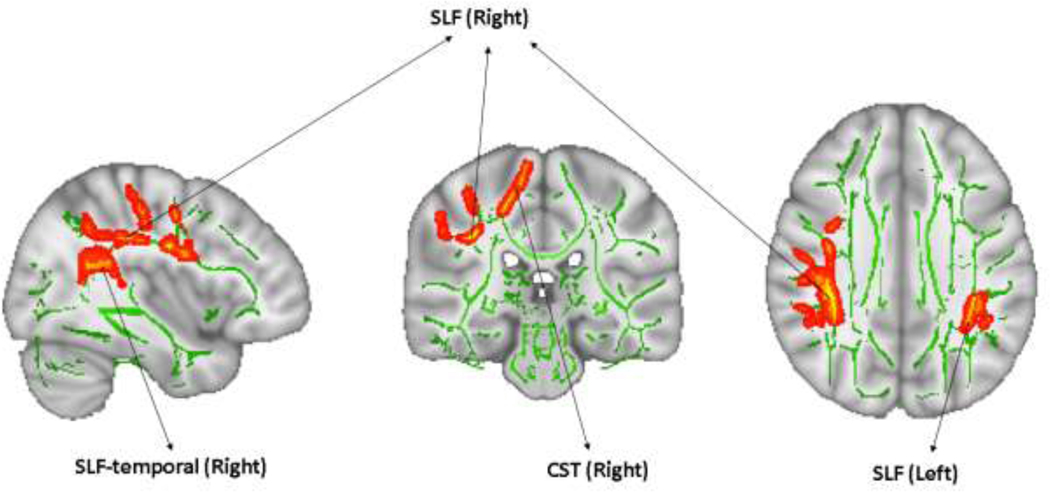
FA was positively associated with the I/P subscale of the DRS in two clusters encompassing the bilateral SLF, bilateral SLF-temporal, and right CST (Figure 1 and Table 2), i.e., increased FA in these tracts was positively associated with higher scores on the I/P subscale of the DRS. FA was not associated with Stroop Interference (TFCE, FWE-corrected, minimum p=0.165, t-statistic=3.18, df=67), WAIS-III Digit Symbol (TFCE, FWE-corrected, minimum p=0.097, t-statistic=3.48, df=69), or WMS-R Logical Memory (TFCE, FWE-corrected, minimum p=0.284, t-statistic=4.82, df=69).
Figure 1. Association between fractional anisotropy (FA) and baseline neurocognitive function.
Clusters in which FA was significantly positively associated with executive function as measured by I/P subscale of the DRS in LLD patients (TFCE, FWE-corrected p<0.05). Regions showing clusters are overlaid on the mean FA skeleton (green) and MNI152 T1 images. The results are “thickened” using tbss_fill script in TBSS toolbox and displayed in radiological orientation. Abbreviations: I/P subscale of DRS: Initiation/Perseveration subscale of the Dementia Rating Scale, LLD: late-life depression, TFCE: threshold-free cluster enhancement, FWE: family-wise error, TBSS: tract-based spatial statistics, CST: corticospinal tract, SLF: superior longitudinal fasciculus, SLF-temporal: superior longitudinal fasciculus temporal, Left: left hemisphere, Right: right hemisphere.
Table 2. Associations between fractional anisotropy (FA) and baseline neurocognitive function.
Clusters in which FA was significantly positively associated with executive function as measured by the I/P subscale of the DRS.
| Cluster size (Voxels) | MIN p valuea | Max T | Mean T (SD) | MAX X (mm) | MAX Y (mm) | MAX Z (mm) | Major tractsb |
|---|---|---|---|---|---|---|---|
| 3336 | 0.019 | 5.276 | 2.281 (0.704) | 35 | −41 | 31 | SLF (Right), SLF-temporal (Right), CST (Right) |
| 376 | 0.034 | 5.028 | 3.099 (0.673) | −37 | −38 | 30 | SLF (Left), SLF-temporal (Left) |
Threshold-free cluster enhancement, family-wise error-corrected p<0.05, df= 69
Johns Hopkins University White-Matter Tractography Atlas (30)
Abbreviations: I/P subscale of DRS: Initiation/Perseveration subscale of the Dementia Rating Scale, CST: corticospinal tract, SLF: superior longitudinal fasciculus, SLF-temporal: superior longitudinal fasciculus temporal, Left: left hemisphere, Right: right hemisphere.
Notes: Voxels: the number of voxels in each cluster. MIN p value: the value of the minimum p value within the cluster. Max T: the value of the maximum t value within the cluster. Mean T (SD): the value of the mean (standard deviation) t value within the cluster. MAX X/Y/Z (mm): the location of the voxel with minimum p value in the standard space coordinates (mm).
In an exploratory analysis, RD, but not AD (TFCE, FWE-corrected, minimum p=0.441, t-statistic=5.08, df=69) or MD (TFCE, FWE-corrected, minimum p=0.353, t-statistic=4.18, df=69), was significantly negatively associated with scores on the I/P subscale of the DRS in clusters (TFCE, FWE-corrected, minimum p=0.019, t-statistic=5.276, df=69). within the bilateral ATR and CST, right UNC, SLF, SLF-temporal, inferior fronto-occipital fasciculus (IFO), and inferior longitudinal fasciculus (ILF), and forceps major. Lower RD in these tracts was associated with higher scores on the I/P subscale of the DRS.
Associations between WM Integrity and Antidepressant Response
Scores on the primary depression outcome (HRSD-24) decreased from pre- to post-treatment. Response rates, defined as at least a 50% decrease in HRSD-24 score at post-treatment or a CGI-Improvement score of 1 or 2 at post-treatment, were 35.3% (24/68) and 45.6% (31/68), respectively, while the remission rate, defined as achieving a post-treatment HRSD-24 score of at least 7, was 29.4% (20/68). Statistically significant improvements were found for other secondary clinical measures, including CGI-Severity and QIDS-SR (Table 1). Dividing the sample into subjects receiving escitalopram (n = 28) and those receiving duloxetine (n = 43) did not change the overall pattern of results observed.
Using permutation-based nonparametric inference, there were no associations found between FA, MD, AD, or RD and clinical outcome (post-pre HRSD-24, post-pre QIDS-SR, and post-pre CGI-Severity).
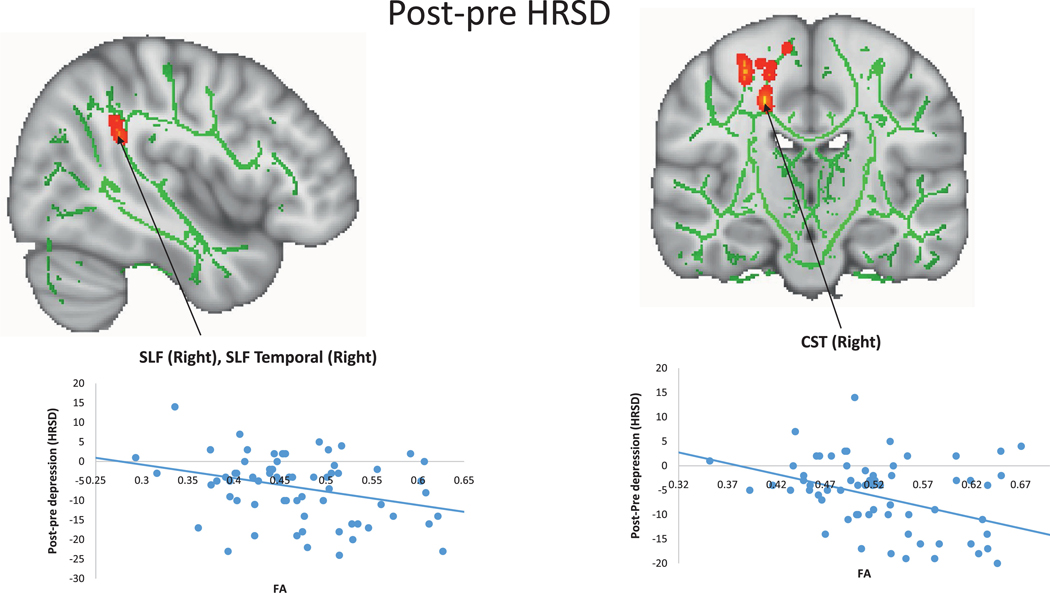
In an exploratory analysis, using linear regression (LR) models (p<0.05, uncorrected) and limiting the association analyses in tracts where significant associations between FA and the I/P subscale score of the DRS were detected, negative associations of FA with post-pre HRSD-24 were detected in the SLF, SLF-temporal, and CST, all in the right hemisphere (LR model, minimum p=0.0014, uncorrected; t-statistic=−3.35; df=65). Scatterplots of these associations revealed that higher FA values in those tracts predicted greater improvement with treatment (Figure 2). Negative associations of FA with post-pre QIDS-SR (LR model, minimum p=0.0006, uncorrected; t-statistic=−3.60; df=64) and post-pre CGI-Severity (LR model, minimum p=0.0074, uncorrected; t-statistic=−2.77; df=64) were also detected in the right CST. Scatterplots of these associations revealed that higher FA values in those tracts predicted greater improvement with treatment. Positive associations of increased FA with remission were detected in the right CST, with increased FA predicting greater chance of remission (LR model, minimum p=0.0028, uncorrected; t-statistic=2.99, df=66), and significant positive associations of FA with response were detected in the right SLF and right CST, with elevated FA in those tracts predicting greater chance of response (LR model, minimum p=0.0009, uncorrected; t-statistic=3.33, df=66).
Figure 2. Association between fractional anisotropy (FA) and post-pre treatment HRSD-24.
Significant negative associations of FA with post-pre HRSD-24 were detected in the SLF, SLF-temporal, and CST, all within the right hemisphere. FA values are plotted on the x axis, and post-pre HRSD-24 are plotted on the y axis. The scatter shows increased FA was correlated with greater decrease in HRSD-24 score. More negative numbers of post-pre HRSD-24 are considered better. These results are “thickened” using tbss_fill script in TBSS toolbox and displayed in radiological orientation. Abbreviations: HRSD-24: 24-item Hamilton Rating Scale for Depression, SLF: superior longitudinal fasciculus, SLF-temporal: superior longitudinal fasciculus temporal, CST: corticospinal tract, Right: right hemisphere, TBSS: tract-based spatial statistics.
CONCLUSIONS
We examined correlations between DTI measures of WM integrity, baseline neurocognitive performance, and antidepressant treatment outcome. We observed a statistically significant positive relationship between FA and executive function as measured by the I/P subscale of the DRS in the bilateral SLF and SLF-temporal fiber tracts, as well as in the right CST. In exploratory analyses, positive associations between FA and clinical outcomes (response, remission), and negative associations between FA and post-pre HRSD-24 score were also detected in the tracts that showed a significant relationship with the I/P subscale of DRS. Increased baseline FA in the right SLF, SLF-temporal portion, and CST was associated with a greater decrease in HRSD-24 score, increased FA in the right CST predicted a greater chance of remission, and increased FA in the right CST and the right SLF predicted a greater chance of treatment response.
A positive relationship between FA and executive function (I/P subscale of the DRS) was observed in the bilateral SLF and SLF-temporal fiber tracts as well as in the right CST. The SLF is a bidirectional pathway that is primarily involved in the transmission of speech and language, but appears to also play a role in attention, memory, emotion, visuospatial processing and numerical cognition (31). The SLF-temporal fiber bundle, which connects the temporal lobe with the dorsolateral prefrontal cortex, has classically been linked to language functioning in the left hemisphere and visuospatial and semantic processing in the right hemisphere (32). The CST, which originates from neurons predominantly within the primary motor, premotor, and somatosensory cortices and terminates in the lower motor neurons of the spinal cord, is largely responsible for controlling voluntary distal movements (33), specifically the movements required for fine motor skills such as hand function. Given the I/P subscale of the DRS measures multiple coordinated cognitive skills that require executive function, including semantic fluency, articulation, and motor skills, it is intuitive that better performance on the I/P subscale of the DRS would reflect integrity within these tracts.
While the SLF and SLF-temporal WM tracts are implicated in broader neural networks involved in depression, there is a paucity of data related to the potential role of the CST in neural circuitry associated with mood disorders. Indeed, existing literature on the white matter integrity of the CST itself in LLD is scarce, and its implication in geriatric depression has largely been overlooked. A recent MRI study found that WM volume of the right CST in LLD patients was significantly higher than that of the healthy controls and was positively correlated with depression severity (34). This WM integrity finding warrants further study using DTI methodology to elucidate the characteristics of CST microstructural WM integrity in LLD, as well as its role in pathology and resulting clinical manifestations.
Although executive function as measured by the I/P subscale of the DRS was associated with FA, our primary measure of executive function, the Stroop Color-Word Task, did not show a significant association. This observation may seem surprising as both are considered measures of executive function, it is important to note that the two tests are measuring different cognitive functions. In particular, the I/P subscale of the DRS is a measure of fluency, articulation and graphomotor skills (35) whereas the Stroop task is a measure of response inhibition. Indeed, the construct of executive function is incredibly broad, as it encompasses a set of higher-order cognitive functions that include a number of disparate cognitive functions including planning, organization, problem solving, cognitive flexibility (e.g., set shifting and switching), updating and monitoring of working memory representations, error monitoring, and response inhibition (36). Some have even gone so far as to question the utility of the construct, given that the components of this vast concept are so disparate (37).
Exploratory analyses, limited to only the tracts that showed a significant relationship with the I/P subscale of the DRS, demonstrated positive associations between FA (right SLF, SLF-temporal, and CST) and clinical outcomes (response, remission), and negative associations between FA (right SLF, SLF-temporal, and CST) and post-pre HRSD-24 score. These tracts have not been identified as playing a role in treatment response to date. Previous DTI studies have documented the role of the ACC in antidepressant nonresponse (14–17). The association between decreased FA in the right SLF, SLF-temporal, and CST, however, is consistent with previous studies comparing LLD subjects to healthy controls (12). Although exploratory, our findings raise the possibility that these WM tracts play a role in antidepressant treatment response, but further study is required.
This study has several limitations worth mentioning, including small sample size, limited executive function battery, and lack of post-treatment cognitive assessment. Future studies should investigate cognitive performance measures both at baseline and after treatment has concluded. Although T2-weighted FLAIR MRI data were collected for each participant, WMHs were estimated regionally instead using a voxelwise approach, which rendered us unable to adjust for the potential effect of WMHs on FA in the significant voxels. It is also important to note that the results obtained in TBSS analysis did not remain significant after correcting for all baseline neurocognitive measures, WM integrity measures, and outcome measures, which may result in an inflated type 1 error rate. Limitations regarding DTI methodology must also be addressed. Although DTI based methods for identifying WM microstructural abnormalities are highly sensitive, the regions within the brain where WM fibers cross can result in observed anisotropic changes without any underlying WM abnormalities (21). More recent recommendations for DTI data acquisition that were not employed in the current study include topup scans to correct inhomogeneity distortions for DTI data (38) and use of multi-shell (multiple b values) and isotropic voxels to reduce the crossing fiber issue (39). Additionally, in TBSS specifically, it is sometimes difficult to differentiate between structures in close proximity, which may limit anatomical specificity (40). Future studies of LLD patients should employ DTI tractography to further probe the WM microstructural characteristics that were uncovered within this analysis, further enhancing our understanding of anatomical connectivity in LLD.
Highlights.
1) What is the primary question addressed by this study?
This study investigated the relationship between white matter integrity, cognitive functioning, and antidepressant treatment outcome in older adults.
2) What is the main finding of this study?
Greater integrity (fractional anisotropy [FA]) in frontostriatal white matter tracts was positively associated with better performance on the Initiation/Perseveration subscale of the Mattis Dementia Rating Scale. Higher FA in these tracts also was correlated with more depressive symptom improvement during treatment as well as higher response and remission rates.
3) What is the meaning of the finding?
White matter integrity was positively associated with executive function as measured by the Dementia Rating Scale Initiation/Perseveration subscale in white matter tracts underlying the cognitive control network, which may play a role in antidepressant treatment outcome.
Acknowledgements:
This study was funded by the National Institute of Mental Health, R01 MH102293 (Rutherford B.).
Source of Funding: Rutherford B. is receiving a grant (R01 MH102293) from the National Institute of Mental Health. For the remaining authors none were declared.
Footnotes
Conflicts of Interest: The authors report no conflicts with any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lavretsky H, Lesser IM, Wohl M, et al. : Clinical and neuroradiologic features associated with chronicity in late-life depression. Am J Geriatr Psychiatry 1999; 7:309–316. doi: 10.1097/00019442-199911000-00006 [DOI] [PubMed] [Google Scholar]
- 2.Kalayam B, Alexopoulos GS: Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry 1999; 56:713–718. doi: 10.1001/archpsyc.56.8.713 [DOI] [PubMed] [Google Scholar]
- 3.Rushia SN, Shehab AAS, Motter JN, et al. : Vascular depression for radiology: A review of the construct, methodology, and diagnosis. World J Radiol 2020; 12:48–67. doi: 10.4329/wjr.v12.i5.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexopoulos GS, Kiosses DN, Heo M, et al. : Executive dysfunction and the course of geriatric depression. Biol Psychiatry 2005; 58:204–210. doi: 10.1016/j.biopsych.2005.04.024 [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos GS, Meyers BS, Young RC, et al. : “Vascular depression” hypothesis. Arch Gen Psychiatry 1997; 54:915–922. doi: 10.1001/archpsyc.1997.01830220033006 [DOI] [PubMed] [Google Scholar]
- 6.Tadayonnejad R, Yang S, Kumar A, et al. : Multimodal brain connectivity analysis in unmedicated late-life depression. PLoS One 2014; 9(4):e96033. doi: 10.1371/journal.pone.0096033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae JN, MacFall JR, Krishnan KRR, et al. : Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry 2006; 60:1356–1363. doi: 10.1016/j.biopsych.2006.03.052 [DOI] [PubMed] [Google Scholar]
- 8.Tadayonnejad R, Ajilore O: Brain network dysfunction in late-life depression: a literature review. J Geriatr Psychiatry Neurol 2014; 27:5–12. doi: 10.1177/0891988713516539 [DOI] [PubMed] [Google Scholar]
- 9.Smith SM, Jenkinson M, Johansen-Berg H, et al. : Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- 10.Yendiki A, Panneck P, Srinivasan P, et al. : Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform 2011; 5:23. doi: 10.3389/fninf.2011.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mettenburg JM, Benzinger TL, Shimony JS, et al. : Diminished performance on neuropsychological testing in late life depression is correlated with microstructural white matter abnormalities. Neuroimage 2012; 60:2182–2190. doi: 10.1016/j.neuroimage.2012.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sexton CE, Allan CL, Le Masurier M, et al. : Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Arch Gen Psychiatry 2012; 69:680–689. doi: 10.1001/archgenpsychiatry.2011.1862 [DOI] [PubMed] [Google Scholar]
- 13.Alves GS, Karakaya T, Fußer F, et al. : Association of microstructural white matter abnormalities with cognitive dysfunction in geriatric patients with major depression. Psychiatry Res 2012; 203:194–200. doi: 10.1016/j.pscychresns.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 14.Alexopoulos GS, Kiosses DN, Choi SJ, et al. : Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry 2002; 159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929 [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. : Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry 2008; 165:238–244. doi: 10.1176/appi.ajp.2007.07050744 [DOI] [PubMed] [Google Scholar]
- 16.Taylor WD, Kuchibhatla M, Payne ME, et al. : Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One 2008; 3:e3267. doi: 10.1371/journal.pone.0003267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor WD, Macfall JR, Boyd B, et al. : One-year change in anterior cingulate cortex white matter microstructure: relationship with late-life depression outcomes. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry 2011; 19:43–52. doi: 10.1097/JGP.0b013e3181e70cec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor WD, Aizenstein HJ, Alexopoulos GS: The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry 2013; 18:963–974. doi: 10.1038/mp.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutherford BR, Choi CJ, Choi J et al. : Slowed processing speed disrupts expectancy-based placebo effects in late life depression. Am J Geriatr Psychiatry, 2021; Article in Press. doi: 10.1016/j.jagp.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 20.Mattis S Dementia Rating Scale Professional Manual. Odessa, FL: Psychological Assessment Resources, 1988. [Google Scholar]
- 21.Alexander AL, Lee JE, Lazar M, et al. : Diffusion tensor imaging of the brain. Neurother J Am Soc Exp Neurother 2007; 4:316–329. doi: 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenton ME, Hamoda HM, Schneiderman JS, et al. : A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012; 6:137–192. doi: 10.1007/s11682-012-9156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander AL, Hurley SA, Samsonov AA, et al. : Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect 2011; 1:423–446. doi: 10.1089/brain.2011.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Woolrich MW, et al. : Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004; 23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 25.Andersson JLR, Graham MS, Drobnjak I, et al. : Towards a comprehensive framework for movement and distortion correction of diffusion MR images: within volume movement. Neuroimage 2017; 152:450–466. doi: 10.1016/j.neuroimage.2017.02.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith SM: Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155. doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He X, Liu W, Li X, et al. : Automated assessment of the quality of diffusion tensor imaging data using color cast of color-encoded fractional anisotropy images. Magn Reson Imaging 2014; 32:446–456. doi: 10.1016/j.mri.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols TE, Holmes AP: Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002; 15:1–25. doi: 10.1002/hbm.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SM, Nichols TE: Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44:83–98. doi: 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- 30.Oishi K, Faria AV, van Zijl PC, et al. : MRI Atlas of Human White Matter. Academic Press Inc., 2011. [Google Scholar]
- 31.Conner AK, Briggs RG, Rahimi M, et al. : A connectomic atlas of the human cerebrum—chapter 10: tractographic description of the superior longitudinal fasciculus. Oper Neurosurg 2018; 15:S407–S422. doi: 10.1093/ons/opy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catani M, Thiebaut de Schotten M: A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008; 44:1105–1132. doi: 10.1016/j.cortex.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 33.Welniarz Q, Dusart I, Roze E: The corticospinal tract: evolution, development, and human disorders. Dev Neurobiol 2017; 77:810–829. doi: 10.1002/dneu.22455 [DOI] [PubMed] [Google Scholar]
- 34.Lam CLM, Liu H-L, Huang C-M, et al. : The neural correlates of perceived energy levels in older adults with late-life depression. Brain Imaging Behav 2019; 13:1397–1405. doi: 10.1007/s11682-018-9940-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marson DC, Dymek MP, Duke LW, et al. : Subscale validity of the mattis dementia rating scale. Arch Clin Neuropsychol 1997; 12:269–275. doi: 10.1016/S0887-6177(96)00003-0 [DOI] [PubMed] [Google Scholar]
- 36.Pimontel MA, Culang-Reinlieb ME, Morimoto SS, et al. : Executive dysfunction and treatment response in late-life depression. Int J Geriatr Psychiatry 2012; 27:893–899. doi: 10.1002/gps.2808 [DOI] [PubMed] [Google Scholar]
- 37.Salthouse TA: Relations between cognitive abilities and measures of executive functioning. Neuropsychology 2005; 19:532–545. doi: 10.1037/0894-4105.19.4.532 [DOI] [PubMed] [Google Scholar]
- 38.Andersson JLR, Skare S, Ashburner J: How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003; 20:870–888. doi: 10.1016/S1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- 39.Jbabdi S, Behrens TEJ, Smith SM: Crossing fibres in tract-based spatial statistics. Neuroimage 2010; 49:249–256. doi: 10.1016/j.neuroimage.2009.08.039 [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Luo Y, Mok VCT, et al. : Tractography atlas-based spatial statistics: statistical analysis of diffusion tensor image along fiber pathways. Neuroimage 2016; 125:301–310. doi: 10.1016/j.neuroimage.2015.10.032 [DOI] [PubMed] [Google Scholar]