Abstract
Background:
Signifying the two-compartments/one-disease paradigm, allergic rhinoconjunctivitis (ARC) and asthma (AA) are prevalent, comorbid conditions triggered by environmental factors (e.g., house dust mites (HDMs)). However, despite the ubiquity of triggers, progression to severe ARC/AA is infrequent, suggesting either resistance or adaptation.
Objective:
Determine if ARC/AA severity relates to maladaptive responses to disease triggers.
Methods:
Adults with HDM-associated ARC were challenged repetitively with HDMs in an aeroallergen challenge chamber; associated mechanistic traits were identified.
Results:
HDM challenges evoked maladaptive (persistently higher ARC symptoms), adaptive (progressive symptom reduction), and resilient (resistance to symptom induction) phenotypes. Symptom severity in the natural environment was an imprecise correlate of the phenotypes. Nasal airway traits, defined by low inflammation-effectual epithelial integrity, moderate inflammation-effectual epithelial integrity, and higher inflammation-ineffectual epithelial integrity, were hallmarks of the resilient, adaptive, and maladaptive evoked phenotypes, respectively. Highlighting a crosstalk mechanism, peripheral blood inflammatory tone calibrated these traits: ineffectual epithelial integrity correlated with CD8+ T-cells, whereas airway inflammation correlated with CD8+ T-cells and eosinophils. Hallmark peripheral blood maladaptive traits were increased NK and CD8+ cells, lower CD4+ MAIT cells, and deficiencies along the TLR-IRF-IFN antiviral pathway. Maladaptive traits tracking HDM-associated ARC also contributed to AA risk and severity models.
Conclusions:
Repetitive challenges with HDMs revealed that maladaptation to disease triggers may underpin ARC/AA disease severity. A combinatorial therapeutic approach may involve reversal of loss-of-beneficial-function traits (ineffectual epithelial integrity, TLR-IRF-IFN deficiencies), mitigation of gain-of-adverse-function traits (inflammation), and blocking of a detrimental crosstalk between the peripheral blood and airway compartments.
Clinical Implications:
The wide variation in allergic rhinoconjunctivitis disease severity may be underpinned by an imbalance in epithelial integrity and inflammation manifesting as maladaptation to disease triggers such as house dust mites.
Keywords: Aeroallergen challenge chamber, house dust mites, allergic rhinoconjunctivitis, evoked phenotypes, maladaptation
Capsule Summary:
Repetitive aeroallergen challenges coupled to mechanistic studies identify evoked phenotypes of allergic rhinoconjunctivitis that are mediated by differing levels of epithelial integrity and inflammatory traits.
INTRODUCTION
Allergic rhinoconjunctivitis (ARC) and allergic asthma (AA) are environmentally triggered comorbid conditions that share mechanistic correlates, accounting for the two-airway (upper and lower) compartments/one-disease paradigm (1, 2). Despite the ubiquity of aeroallergens such as Dermatophagoides pteronyssinus (house dust mites [HDMs]), the basis for the heterogeneity in severity of HDM-associated perennial ARC (HDM-PARC) with or without AA is unknown. Since ARC/AA are environmentally triggered conditions, this heterogeneity may relate to differences in how a person responds to environmental triggers. To capture host x environment interactions in real-time, as opposed to cross-sectional analysis, which omits induced or evoked responses, we used an aeroallergen challenge chamber (ACC) to repeatedly deliver a fixed concentration of HDMs to adults with HDM-PARC without physician-diagnosed AA (3, 4). We tested the hypothesis that mild, moderate, and severe HDM-PARC is attributable to resilience, adaptation, and maladaptation to repeated exposures to HDMs, respectively.
Trained immunity attributable to transcriptional and/or epigenetic reprogramming of innate cells, such as natural killer (NK) cells, epithelial cells (ECs), and innate lymphoid cells (ILCs), may allow tissues to adapt to inflammation by reacting faster to a secondary assault with an identical or heterologous antigenic challenge (5–9). Erosion of trained immunity may confer susceptibility to some infectious (e.g., tuberculosis) and other diseases (5–10). By analogy, we theorized that differences in the host’s proclivity to preserve vs. erode trained immunity in response to repetitive exposures to aeroallergens may contribute to the resilient or adaptive vs. maladaptive phenotypes, respectively (Figure 1A). This framework is supported by GWAS in persons with atopic diseases (e.g., ARC, AA, and atopic dermatitis) that identified two major classes of genes that influence (i) epithelial integrity (Epiintegrity; e.g., Filaggrin, IL33), and (ii) inflammatory responses, such as T helper (Th) 2 skewing (e.g., IL13) (11–17) (Figure 1B). Hence, we posited that maladaptation may be mediated by two factors: ineffective trained immunity manifesting as ineffectual airway Epiintegrity and a proinflammatory profile. We considered effectual Epiintegrity to signify an epithelium that manifests transcriptomic features that are proxies for preservation of epithelial barrier and immunity functions.
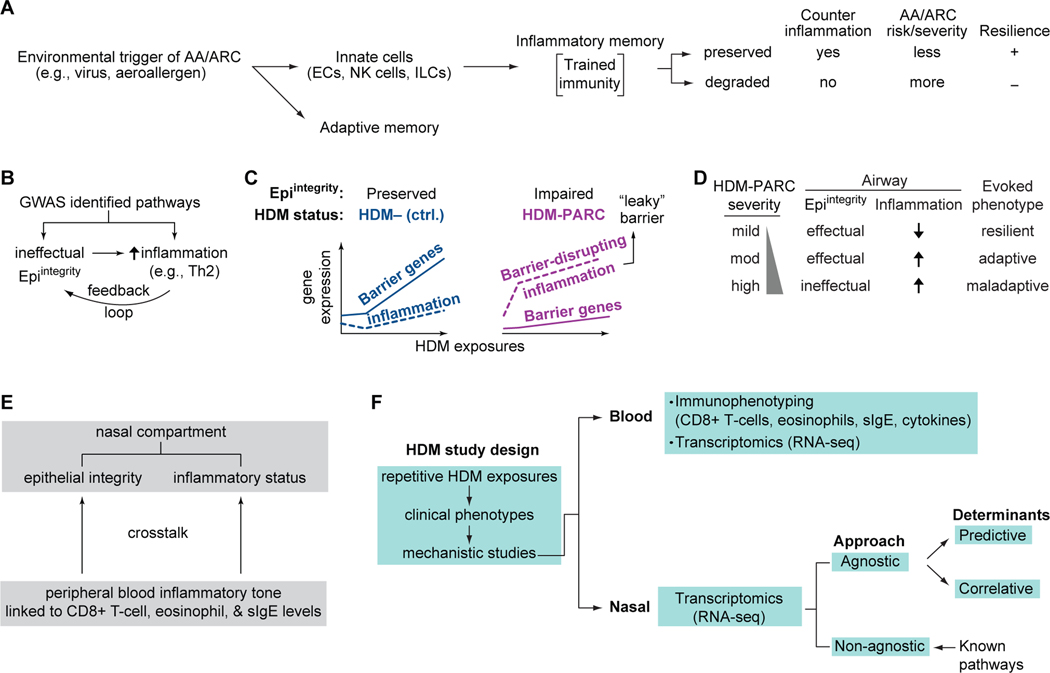
Figure 1. Concepts and models tested.
(A) Conceptual framework of allergic asthma/allergic rhinoconjunctivitis (AA/ARC) pathogenesis; resistance or adaptation vs. maladaptation to inflammation induced by environmental triggers signifies preservation vs. degradation of inflammatory memory. (B) Feedback loop of epithelial integrity (Epiintegrity) and inflammation. (C) Model based on our findings (18) showing that the imbalance between Epiintegrity and airway allergic inflammation distinguished non-atopic, non-allergic controls (ctrl.) and persons with HDM-associated perennial ARC (HDM-PARC). (D) Association of airway Epiintegrity and inflammation status with mild, moderate (mod), and high HDM-PARC severity and evoked phenotypes. (E) Intercompartment crosstalk. (F) HDM study design. EC, epithelial cells; ILCs, innate lymphoid cells; NK, natural killer; slgE, HDM-specific IgE; Th2, T-helper 2.
We previously showed that an imbalance between airway Epiintegrity and inflammation distinguished non-atopic, non-HDM allergic controls and HDM-PARC persons repetitively challenged with HDMs in an ACC (18). Transcriptomic analyses of nasal samples revealed that controls upregulated pathways reflective of effectual Epiintegrity and mounted a muted inflammatory response; the converse pattern was observed in HDM-PARC persons (Figure 1C). In these HDM-PARC persons, we addressed five questions.
First, do HDM-PARC persons manifest resilience, adaptation, and maladaptation to repetitive HDM exposures (evoked phenotypes)? Second, do conjoint Epiintegrity-inflammation traits underpin the evoked phenotypes (Figure 1D)? Third, since the inflammatory tone in peripheral blood linked to levels of eosinophils and other processes (IgE- and CD8+ T-cell-associated inflammation) influences allergic inflammation (19, 20), does a heightened inflammatory tone accentuate ineffectual Epiintegrity and airway inflammation (crosstalk; Figure 1E)? Fourth, does cross-sectional phenotyping outside the ACC convey an imprecise assessment of disease severity vs. the phenotypes evoked in an ACC? Finally, in accord with the 2-airway compartments/1-disease paradigm, are the mechanistic correlates of AA risk and severity overrepresented in HDM-PARC persons with the maladaptive phenotype?
METHODS
Study design and definitions
The study had two stages: stage I focused on a four-phase ACC study in HDM-PARC persons (Figure 1F) and stage II focused on translating findings to asthma. Stage I studies evaluated controls (n=15) and HDM-PARC (n=23) persons whose characteristics are described (18, 21). The IntegReview Institutional Review Board (Austin, TX; Biogenics Research Chamber HDM study 005) and University of Texas Health Science Center at San Antonio Institutional Review Board approved the study. Participants underwent two sets of ACC exposures (ACC-I, ACC-II) separated by a 38-day observation period. ACC-I and ACC-II comprised 3-hour exposures to finely ground body powder of Dermatophagoides pteronyssinus on 4 consecutive days (Figure 2A,B). ACC-I was preceded by a 4-day run-in period (Figure 2A).
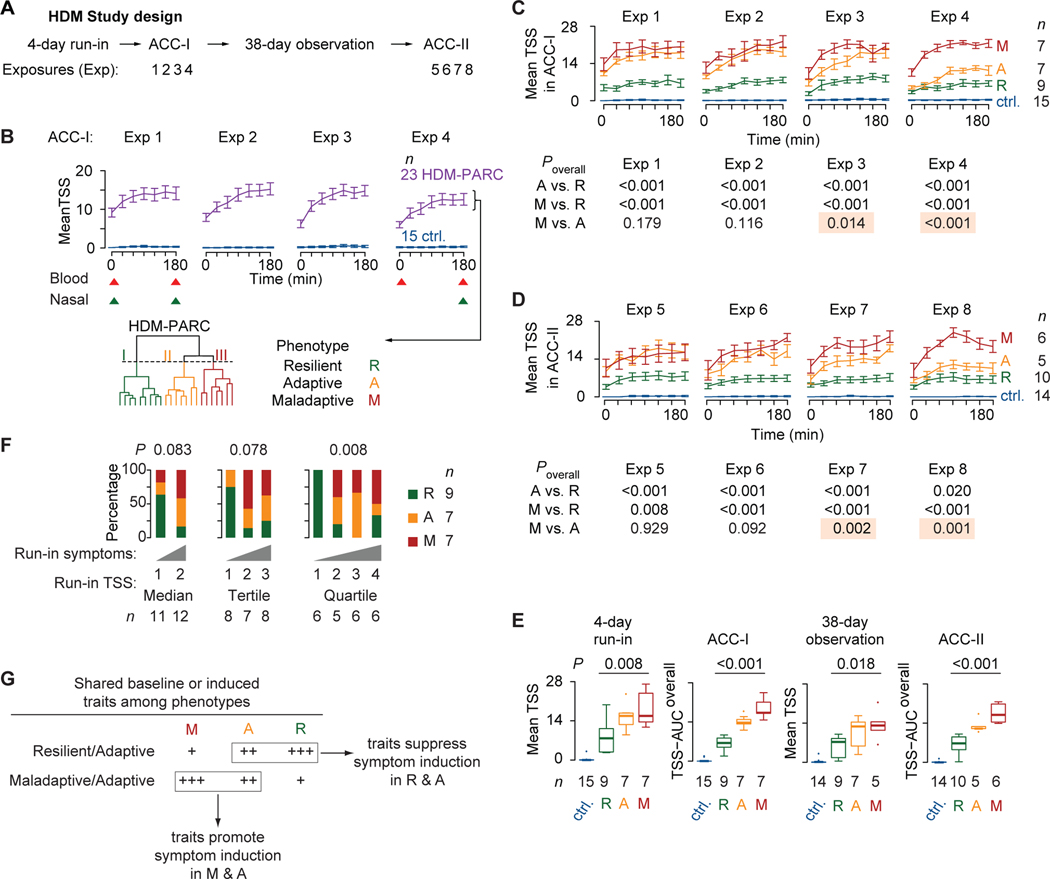
Figure 2. Study design and HDM-PARC phenotypes.
(A) Study design. (B) Top: Mean (SEM) total symptom score (TSS) in controls (ctrl.) and HDM-PARC participants during exposures (Exp) 1–4 in ACC-I; triangles indicate sampling (red, blood; green, nasal brushing). Bottom: Hierarchical clustering of all 28 ACC-I TSS recordings in HDM-PARC participants identified three phenotypes. (C) ACC-I mean (SEM) TSS in controls and HDM-PARC participants stratified by phenotypes (resilient [R], adaptive [A], maladaptive [M]). (D) ACC-II (Exp 5–8; panel A) mean (SEM) TSS in controls and HDM-PARC participants stratified by R, A, or M phenotypes. (E) Mean TSS in the 4-day run-in and 38-day observation phases, and TSS-area under the curve overall (TSS-AUCoverall) in ACC-I and ACC-II in controls and HDM-PARC participants stratified by R, A, or M phenotypes. (E) P, determined by likelihood ratio test. (F) Proportion of participants with each of the HDM-PARC phenotypes according to whether their 4-day run-in average TSS recordings were above (1st) or below (2nd) the median, classified to the 1st, 2nd, or 3rd tertiles, or the 1st, 2nd, 3rd, or 4th quartiles. P, determined by Fisher’s exact test. (G) Model of shared traits among the HDM-PARC phenotypes. Methods for deriving significance values in Supplement.
Symptom severity was measured by the total symptom score (TSS), a participant-recorded metric that captures severity (range: 0 [least] to 4 [worst]) of four nasal and three ocular symptoms (Table E1) (18, 21). To assess overall disease severity, the TSS-AUCoverall metric was generated to capture the overall TSS area under the curve (AUC) of the mean daily AUCs of TSS recorded during the 4 ACC-I exposure days. To conduct mechanistic studies, whole peripheral blood and nasal cells were sampled in ACC-I (Figure 1F). Results of the initial whole peripheral blood immunophenotyping guided two additional rounds of immunophenotyping performed on biobanked PBMCs. Peripheral blood samples for immunophenotyping were obtained before and after exposures 1 and 4. For gene expression (RNA-seq) analyses, samples were obtained before and after exposure 1 and after exposure 4 (Figure 2B; red triangles). RNA-seq analyses were performed on nasal cells obtained by nasal brushings before and after exposure 1 and after exposure 4 (18) (Figure 2B; green triangles). Using conditional reprogramming technology, nasal cells obtained after exposure 8 in ACC-II were propagated ex vivo for the cultivation of nasal ECs (22, 23). In stage II studies, to address questions related to the 2-compartments/1-disease paradigm, we examined publicly available gene expression datasets.
Immunophenotyping
Methods for flow cytometry in whole peripheral blood (Table E2) and PBMCs (Table E3–E4) were as described previously (18) and in supplementary methods; antibody panels are in Table E5. Plasma cytokine levels were evaluated [Luminex LX200 platform; Milliplex high-sensitivity T-cell panel (HSTCMAG-28SK- Millipore-Sigma)]. Plasma HDM-specific IgE (sIgE) levels were evaluated (assayed by ThermoFisher by ImmunoCAP assay).
Hierarchical Clustering
Hierarchical clustering was performed to derive (i) phenotypes using all 28 TSS measures recorded at 30-minute intervals in HDM-PARC persons in ACC-I (method: Ward’s D linkage and Manhattan distances), and (ii) CD8-CD4 balance clusters using baseline CD8+ T-cell and CD4:CD8 T-cell ratio values (method: Euclidean distances and Ward’s D linkage).
RNA-sequencing
RNA-seq was performed on nasal cells, peripheral blood, and ECs propagated ex vivo (Table E6) using methods described previously (18) and in the supplementary methods.
Bioinformatics
To generate z-scores of a gene signature, the log2-normalized expression of each gene within the signature was z-transformed (mean centered then divided by standard deviation) across all samples and then averaged for all genes in the signature (supplementary methods; Table E7–E8). Correlation network analysis was performed as described in the supplementary methods. Analyses of publicly available transcriptomic datasets (Table E9) were performed on log2-transformed and/or quantile normalized data or DESeq normalized data. Detailed bioinformatic and statistical methods are outlined in the supplementary methods, and Table E10 describes the statistical methods for each figure panel.
RESULTS
Resilient, adaptive, and maladaptive HDM-PARC phenotypes
In the ACC, controls resisted symptom induction, whereas HDM-PARC persons classified into three clusters (Figure 2B, bottom). TSS trajectories of these clusters identified three evoked phenotypes corresponding to resilient, adaptive, and maladaptive disease severity patterns in HDM-PARC (Figure 2C). Hierarchy of baseline TSS was: controls < [resilient < adaptive ~ maladaptive phenotypes]. The resilient phenotype and controls resisted increases in TSS in the ACC. Although both adaptive and maladaptive phenotypes associated with higher and similar baseline TSS, repetitive HDM exposures unmasked contrasting trajectory patterns (Figure 2C). The adaptive phenotype was distinguished from the maladaptive phenotype by declining TSS that became apparent during exposure 3. The TSS trajectory patterns of the adaptive and resilient phenotypes were similar in exposure 4, whereas trajectory patterns of the maladaptive and adaptive phenotypes were similar in exposures 1 and 2 (Figure 2C).
The evoked phenotypes were reproducible in ACC-I and ACC-II (Figure 2C,D). The evoked resilient, adaptive, and maladaptive phenotypes associated with lower, intermediate, and higher symptom severity, respectively, during the 4-day run-in and 38-day observation window (Figure 2E). In contrast, use of TSS recorded in the natural environment (i.e., 4-day run-in phase; Figure 2A) to predict phenotypes would have resulted in confounding. Persons with higher symptom severity in the run-in phase (third or fourth quartile of run-in TSS) represented a conflation of individuals with the resilient, adaptive, and maladaptive phenotypes (Figure 2F).
Mechanistic model
How might one explain these trajectory patterns? We posited that mechanistic correlates (traits) in the airways and peripheral blood overrepresented in the resilient and adaptive phenotypes (shared resilient-adaptive traits) may promote suppression of symptom induction, whereas traits overrepresented in the adaptive and maladaptive phenotypes (shared adaptive-maladaptive traits) may promote symptom induction (Figure 2G). We thus focused on identifying the shared resilient-adaptive vs. maladaptive-adaptive traits.
Peripheral blood traits in HDM-PARC phenotypes
We examined eosinophil levels by phenotype, as they are a biomarker of ARC/AA and Th2-high AA (24–26). Additionally, because HDM exposures associated with changes in CD4+ and CD8+ T-cell levels and CD4:CD8 T-cell ratios (18), we inquired whether these variables differed by phenotype. At baseline, eosinophil levels were lower in the resilient vs. adaptive and maladaptive phenotypes (P=0.023 and 0.015, respectively), and eosinophil levels did not increase with HDM exposures in the resilient phenotype (Figure 3A). Eosinophil expansion occurred mainly in the maladaptive phenotype (P=0.008 for pre-exposure 1 vs. post-exposure 4; Figure 3A).
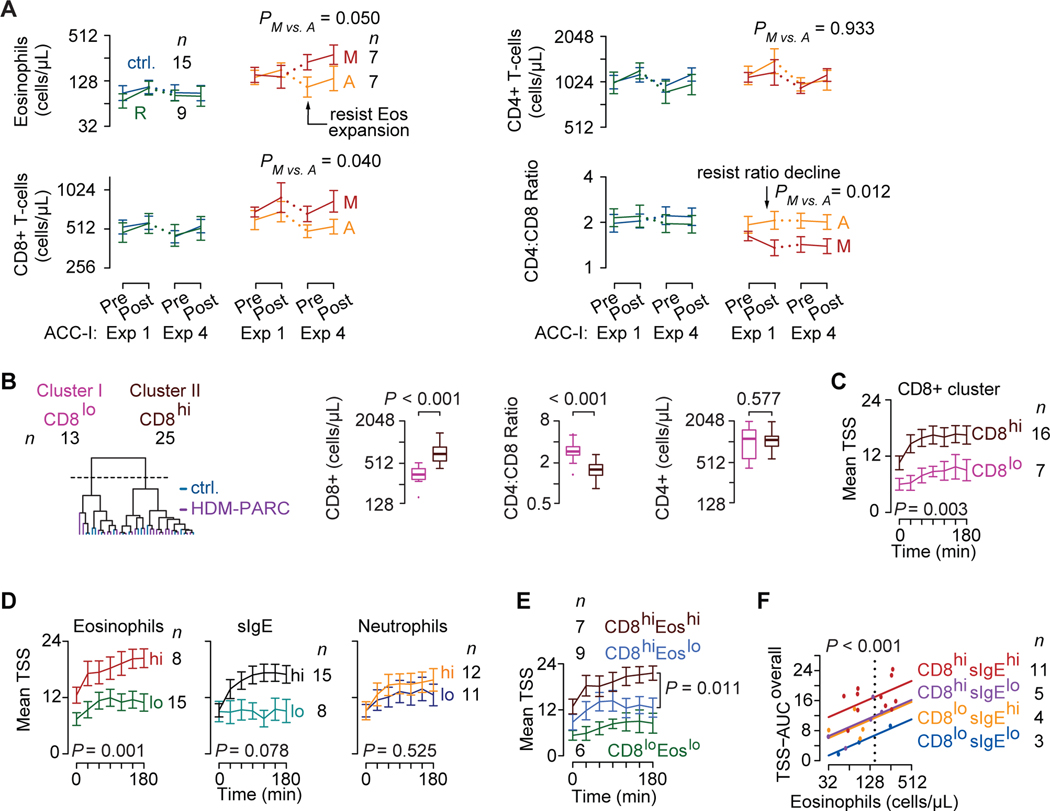
Figure 3. Peripheral blood traits and HDM-PARC symptom severity in the ACC.
(A) Trajectories of log2 cell counts of eosinophils (Eos), CD8+ T-cells, CD4+ T-cells, and the CD4:CD8 ratio at pre- and post-exposure (Exp) 1 and 4 in controls (ctrl.) and HDM-PARC participants stratified by phenotypes (resilient [R], adaptive [A], maladaptive [M]). (B) Left: CD8-CD4 balance clusters identified by hierarchical clustering of baseline CD8+ T-cell counts and CD4:CD8 ratio in controls (blue) and HDM-PARC (purple) participants. Right: Baseline CD8+ and CD4+ T-cell counts and CD4:CD8 T-cell ratio stratified by the CD8-CD4 balance clusters. (A-B) Y-axes depicted as exponentiation of log2 scale. (C-E) ACC-I mean (SEM) total symptom score (TSS) during Exp 1 in HDM-PARC participants stratified by (C) CD8-CD4 balance clusters (CD8hi vs. CD8lo), (D) eosinophil (Eoshi vs. Eoslo), HDM-specific IgE (sIgEhi vs. sIgElo), and neutrophil (neutrophilhi vs. neutrophillo) strata, and (E) CD8hiEoshi, CD8hiEoslo, and CD8loEoslo groups; CD8loEoshi not shown (n = 1). (F) TSS-AUCoverall modeled as a function of log2 eosinophil cell counts by CD8+ (CD8-CD4 balance clusters) and sIgE strata in HDM-PARC participants. Hi, higher; Lo, lower. Cutoffs for peripheral blood traits and methods for deriving significance values in Supplement.
During exposure 1, the maladaptive and adaptive phenotypes associated with higher CD8+ levels vs. the resilient phenotype (P=0.013 and 0.159, respectively; Figure 3A). Compared with the maladaptive phenotype, resilient and adaptive phenotypes showed resistance to CD8+ T-cell expansion and declines in ratio values. Mirroring the lack of symptom induction in the resilient phenotype and controls, eosinophil, CD4+, CD8+, and ratio values were similar in these study groups. These findings suggested that higher eosinophils and CD8+ T-cells represent shared maladaptive-adaptive traits and HDM exposures induce expansion of these cell types in the maladaptive vs. resilient and adaptive phenotypes.
CD8higher: independent determinant of disease severity
We next determined whether an imbalance in CD8+ and CD4+ T-cell levels not captured by absolute levels of CD8+ T-cells or the CD4:CD8 ratio alone may constitute a shared maladaptive-adaptive trait. Unsupervised hierarchal clustering of baseline (pre-exposure 1) CD8+ T-cell levels and CD4:CD8 ratios identified two clusters: cluster I was characterized by lower CD8+ T-cell levels and higher CD4:CD8 ratios, and cluster II showed the opposite pattern (Figure 3B). CD4+ T-cell levels were comparable. Clusters I and II were designated as CD8lower and CD8higher strata (Figure 3B, left-most).
CD8higher, eosinophilhigher, and sIgEhigher but not neutrophilhigher strata associated with higher TSS at baseline and/or after HDM exposures (Figure 3C,D; Figure E1). CD8+, eosinophils, and sIgE strata had additive and independent effects (Figure 3E,F; Figure E2). Baseline CD8+ strata and levels of eosinophils and sIgE explained (r2) approximately 31%, 33%, and 29%, respectively, of the variability in TSS-AUCoverall; together, these three traits explained approximately 60% of the variability in TSS-AUCoverall (Table E11). Thus, the disease-amplifying effects associated with eosinophilhigher occurred mainly in the context of CD8higher, as CD8higher-eosinophilhigher associated with maximal disease severity. Correspondingly, higher allergy-relevant (e.g., IL-5) cytokine levels in eosinophilhigher occurred in the context of CD8higher (Figure E3; Table E12). Thus, lower cytokine levels were observed in persons classifying to the CD8lower-eosinophillower group, whereas the CD8higher-eosinophilhigher and CD8higher-eosinophillower groups had similar cytokine levels (Figure E3). Differences in cytokine levels became apparent only after ACC exposures. Collectively, these findings suggest that CD8higher contributed independently to both HDM-PARC severity and peripheral blood cytokine inflammatory status.
Peripheral blood inflammatory milieu in phenotypes
CD8higher, eosinophilhigher, and sIgEhigher strata were disproportionately overrepresented in the adaptive and maladaptive vs. resilient phenotypes (Figure 4A), suggesting that these strata represent shared maladaptive-adaptive traits. sIgE strata did not associate with the phenotypes. In initial immunophenotyping, we evaluated 14 additional traits, including monocytes, CD19+ cells, ILCs, and Tregs (Table E2). Baseline levels of ILC2 cells expressing the IL-33 receptor ST2 (ILC2-ST2+) was the only other trait associated with symptom severity across all 4 exposures in ACC-I (Table E2). Baseline levels of ILC2-ST2+ cells, which are implicated in AA (27, 28), explained approximately 15% of the variability in TSS-AUCoverall (r2=0.15; P=0.055) (Table E11) and the proportion of these cells was higher in the maladaptive phenotype (Figure 4A; Figure E4).
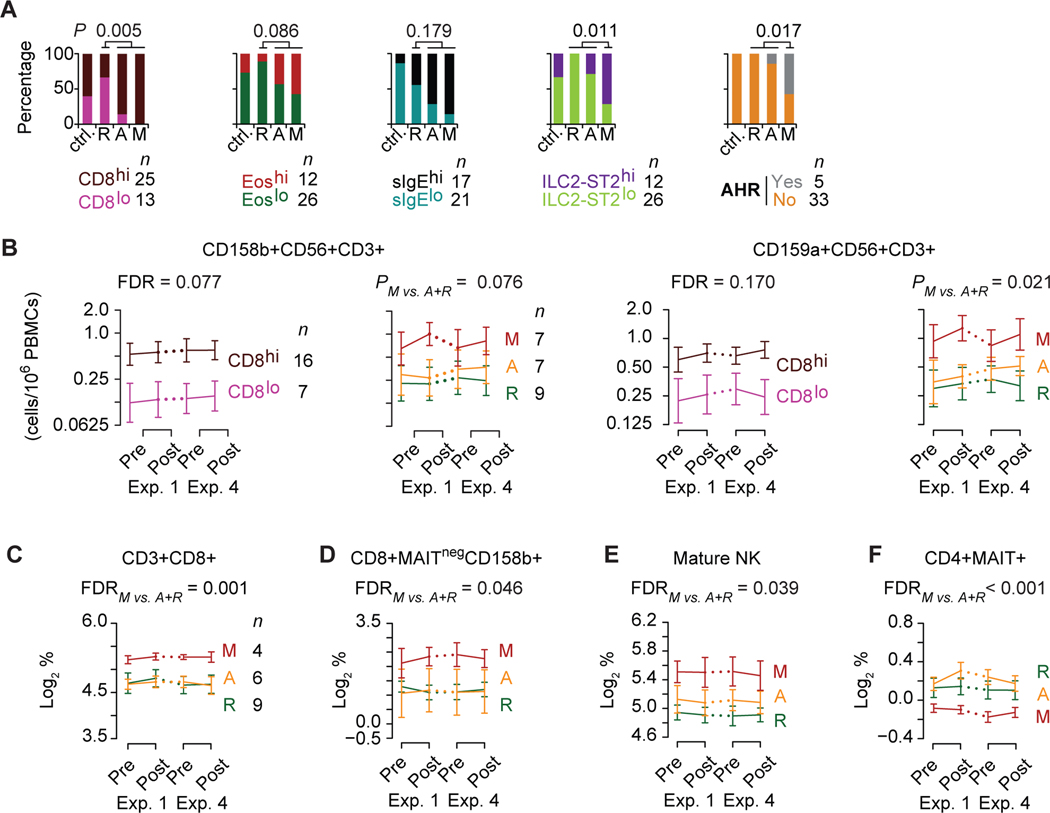
Figure 4. Peripheral blood traits and HDM-PARC phenotypes.
(A) Proportion of controls (ctrl.) and HDM-PARC participants stratified by phenotypes (resilient [R], adaptive [A], maladaptive [M]) with the indicated peripheral blood trait strata at baseline [pre-exposure (Exp) 1] and who developed symptoms of airway hyperresponsiveness (AHR) in ACC-I. P, determined by Fisher’s exact test comparing R vs. A+M, or M vs. R+A. Cutoff for ILC2-ST2+ cells evaluated as a categorical variable: ILC2-ST2hi: ≥ 11.47 log2-cells/μL (B) Trajectories (cell counts/1×106 PBMCs) of (left) CD158b+CD56+CD3+ and (right) CD159a+CD56+CD3+ cells at pre- and post-Exp 1 and 4 in HDM-PARC participants stratified by CD8hi and CD8lo, and HDM-PARC phenotypes (R, A, M). P, for difference across all 4-time points comparing CD8hi vs. CD8lo or M vs. A+R. (C-F) Trajectories of percent (log2-transformed) abundance within PBMCs of (C) CD8+ among CD3+ T-cells, (D) CD158b+ among CD8+MAITNeg T-cells, (E) mature NK cells, and (F) MAIT+ among CD4+ T cells at pre- and post-Exp 1 and 4 in HDM-PARC phenotypes (R, A, M). (C-F) P, for the difference across all 4 time points comparing M vs. A+R. FDR, false discovery rate; Hi, higher; Lo, lower. Methods for deriving significance values in Supplement.
Maladaptive phenotype and asthma-like features
In ACC-I, five HDM-PARC participants developed symptoms consistent with airway hyperresponsiveness commonly observed in asthma (e.g., shortness of breath, wheezing, decline in FEV1 (21)); of these, four individuals had the maladaptive phenotype (Figure 4A). Thus, the maladaptive phenotype associated with asthma-like clinical features and mechanistic hallmarks of AA (higher levels of eosinophils, sIgE, and ILC2-ST2+ cells).
PBMC immunophenotyping: CD8+ and NK cells in maladaptive phenotype
Because CD8+ and NK cells share common lineages (29) and NK cells may contribute to development of AA (30–32), we performed two additional immunophenotyping screens in PBMCs with the objective of determining which CD4+, CD8+, NK, NKT, and mucosal-associated invariant T cells (MAIT) lymphocyte subsets differed by CD8higher strata and/or phenotypes (Table E3–E4). In the first PBMC screen, we evaluated overall markers for NK/NKT cell subsets; of the 38 subsets evaluated, only 2 subsets (at FDR<0.20) associated with CD8higher in both HDM-PARC persons and controls: NKT-cells (CD3+CD56+) expressing the CD158b or CD159a (NKG2A) markers (Figure 4B; Table E3). Proportions of these lymphocyte subsets were also higher in the maladaptive vs. adaptive and resilient phenotypes (Figure 4B) and did not differ significantly by eosinophil or sIgE strata (Figure E5). In the second PBMC immunophenotyping screen (Table E4), we focused on specific traits within the CD3+ T-cell compartment, including NKT and MAIT cells. The proportions of CD3+CD8+ T-cells in PBMCs were greater in the CD8higher strata but not in the eosinophilhigher or sIgEhigher strata and in maladaptive vs. resilient and adaptive phenotypes (Figure 4C; Figure E5).
Only two of the specific non-MAIT T-cell or NK cell traits [CD8+MAITneg T-cells expressing CD158b+ and mature NK cells (CD56+CD16+) expressing CD158b+] had higher proportions in the maladaptive phenotype (Figure 4D,E; Figure E5).
We examined MAIT cells (Vα7.2-Jα33) because lower levels of this subset of innate-like T lymphocytes associate with asthma (33, 34). CD4+ MAIT cells were underrepresented in the maladaptive vs. resilient and adaptive phenotypes (Figure 4F). CD8+CD159a+ MAIT cells were also found to associate with the maladaptive phenotype (Table E4). Thus, lymphocyte subsets (CD8+, NK/NKT) expressing natural cytotoxicity receptors are overrepresented, whereas CD4+ MAIT cells are underrepresented in the maladaptive phenotype. Hence, lymphocyte subsets whose levels were similar in the resilient and adaptive vs. maladaptive phenotype represent shared resilient-adaptive traits.
Maladaptive phenotype and AA risk: shared cellular determinants
The maladaptive phenotype associated with clinical (airway hyperresponsiveness) and cellular (CD8+ and NK cell lymphocyte subsets) features linked to asthma. To corroborate this link, we used a gene expression approach to determine whether transcriptomic proxies for peripheral blood cellular correlates of asthma risk were manifest in the maladaptive phenotype. Altman et al identified a 108-gene signature correlating with NK cells that, in PBMCs of asthma-prone children monitored in the Urban Environment and Childhood Asthma (URECA) cohort, associated with subsequent development of asthma (30). We noted two sets of genes in this signature correlating with NK-like cells (Table E7–E8). One set, designated as the NK/asthmaAltman signature, correlated with gene signatures associated with resting (NKresting) and activated (NKactivated) NK cells (Table E7–E8; Figure E6). The other set, designated as the NKT/asthmaAltman signature, correlated with a gene signature for NKT cells (Table E7–E8; Figure E6). Additionally, because of the shared lineage of NK and CD8+ T-cells (29), our findings (Figure 4B-F), and the contributions of effector memory CD8+ T-cell subsets in asthma pathogenesis (35), we evaluated a gene signature for effector memory CD8+ T-cells (CD8EM).
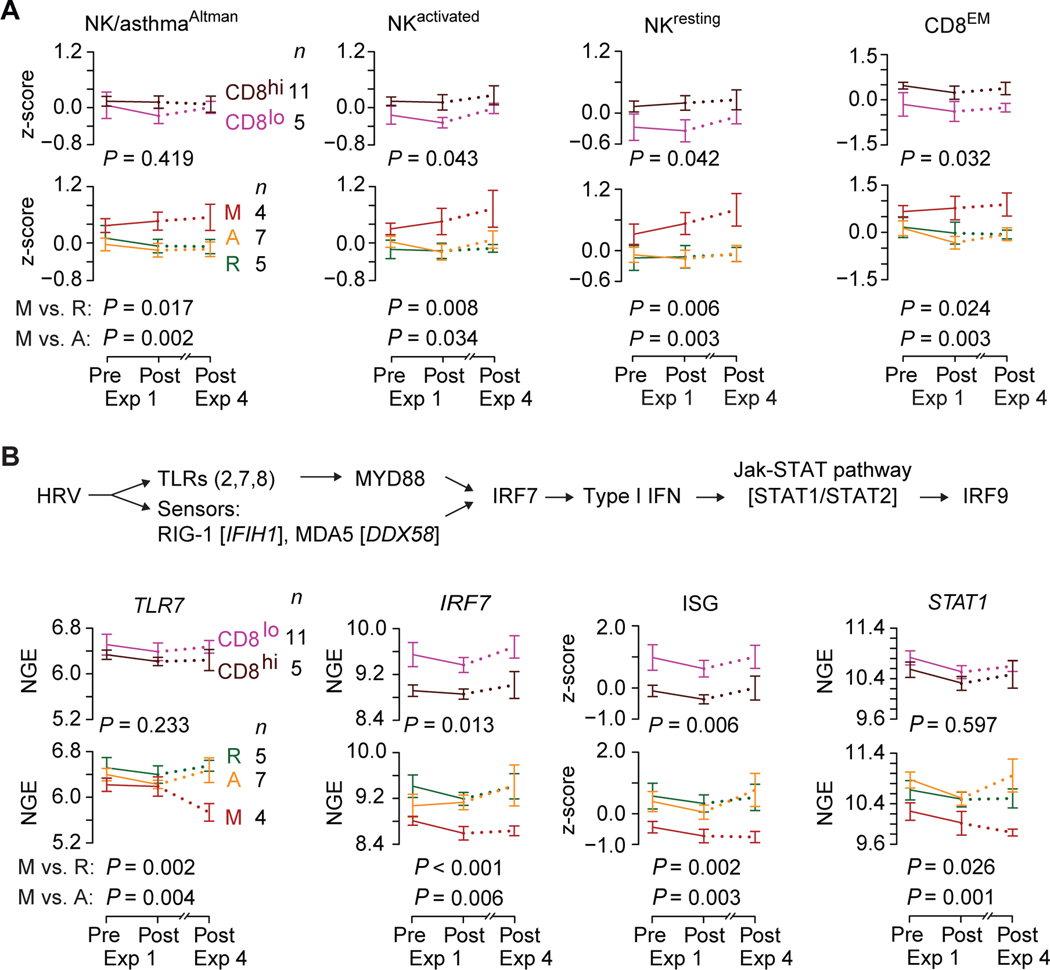
The expression levels (z-scores) of transcriptomic proxies for NKT cells (NKT/asthmaAltman and NKT signatures) did not differ significantly by phenotypes (Figure E7). In contrast, expression of transcriptomic proxies for NK cells (NK/asthmaAltman, NKactivated and NKresting signatures) and CD8+ effector memory T-cells (CD8EM signature) were higher in the maladaptive phenotype (Figure 5A, bottom row; Figure E8). Expression of the NK cell- and CD8+-related gene signatures did not differ significantly between the resilient and adaptive phenotypes. Additionally, with the exception of the NK/asthmaAltman signature, expression levels of NKactivated, NKresting, and CD8EM gene signatures were higher in peripheral blood transcriptomes indexed to CD8higher but not the eosinophil or sIgE strata (Figure 5A; top row; Figure E8). Transcriptomes from children in the URECA cohort who subsequently developed asthma had higher expression of the NKactivated, NKresting, and CD8EM signatures (Figure E9). These findings suggested (i) a commonality in peripheral blood cellular determinants (NK cells and CD8+ T-cells) that contribute to the maladaptive phenotype and AA risk and (ii) that lower expression of the transcriptomic proxies for NK and CD8+ T-cells is a shared resilient-adaptive trait.
Figure 5. Expression of AA risk correlates in whole blood transcriptomes of persons with HDM-PARC.
Trajectories of (A) NK/asthmaAltman, NKactivated, NKresting, and CD8EM gene signature scores (z-scores), and (B) TLR7, IRF7, and STAT1 log2-transformed normalized gene expression (NGE), and interferon-stimulated genes (ISG) signature score (z-scores) in whole blood transcriptomes of HDM-PARC participants obtained at pre- and post-exposure (Exp) 1 and post-Exp 4 of ACC-I stratified by CD8hi and CD8lo, and HDM-PARC phenotypes (resilient [R], adaptive [A], maladaptive [M]). (top) schema representing genes involved in the human rhinovirus (HRV) pathway. P, for an overall difference between groups (CD8lo vs. CD8hi, M vs. R, or M vs. A). Hi, higher; Lo, lower. Methods for deriving significance values in Supplement.
Maladaptive phenotype and AA risk: shared molecular determinants in peripheral blood
To examine whether molecular determinants associated with increased AA risk contributed to the maladaptive phenotype, we focused on genes associated with antiviral immunity, especially against human rhinovirus (HRV). This focus was based on the observation that wheezing in association with HRV infection is a risk factor for AA (36, 37). The TLR-IRF-IFN pathway (Figure 5B, top) plays a role in HRV immunity (38, 39), and deficiencies along this pathway (including TLR7) have been linked to AA risk and/or severity (40–43). We found that several HRV-associated TLRs (2, 7, 8), IRFs (7, 9), signaling mediators (MYD88), sensors (IFIH1, DDX58), and downstream effectors (STAT1/2), as well as a gene signature of IFN-stimulated genes (ISGs) (used as a proxy for type I IFN levels (44)) had lower expression in the maladaptive vs. adaptive and resilient phenotypes (Figure 5B; Figure E10; Table E7–E8). Expression of several genes along this pathway was lower in peripheral blood transcriptomes indexed to the CD8higher stratum and, in some instances, in the eosinophilhigher stratum (Figure 5B; Figure E10). Expression of genes along the TLR-IRF-IFN pathway did not differ significantly by sIgE stratum (Figure E10).
We examined an 18-gene signature tracking an IRF7/TNF-driven gene network overrepresented in asthma-resistant (Amish; n=27) vs. -susceptible (Hutterites; n=29) children (42). Expression of this asthma resistance-tracking signature was lower in the maladaptive vs. adaptive and resilient phenotype as well as in transcriptomes indexed to the CD8higher but not eosinophilhigher or sIgEhigher stratum (Figure E11). Collectively, these findings suggest that the maladaptive phenotype associated with transcriptional deficiencies along the antiviral TLR-IRF-IFN pathway, which is also observed in AA (4043); these deficiencies may relate to CD8higher, which is overrepresented in persons with the maladaptive phenotype (Figure 4A). Hence, greater preservation of the TLR-IRF-IFN pathway represented a shared resilient-adaptive trait.
TLR7 deficiency in nasal cells of maladaptive phenotype
The decrease in TLR7 expression in peripheral blood in persons with the maladaptive phenotype was an “induced” trait apparent only after repeated HDM exposures (Figure 5B). A similar induced deficiency was observed in the nasal cell transcriptome obtained from persons with the maladaptive phenotype (Figure E11). In contrast, TLR7 deficiency was a “fixed” trait in the airway epithelium derived from patients with severe asthma vs. controls or those with non-severe asthma (Figure E11). Hence, ACC challenges unveiled inducible maladaptive mechanistic traits in HDM-PARC persons and suggest that disease progression may relate to a switch from an inducible to a fixed trait.
Nasal compartment traits: approach
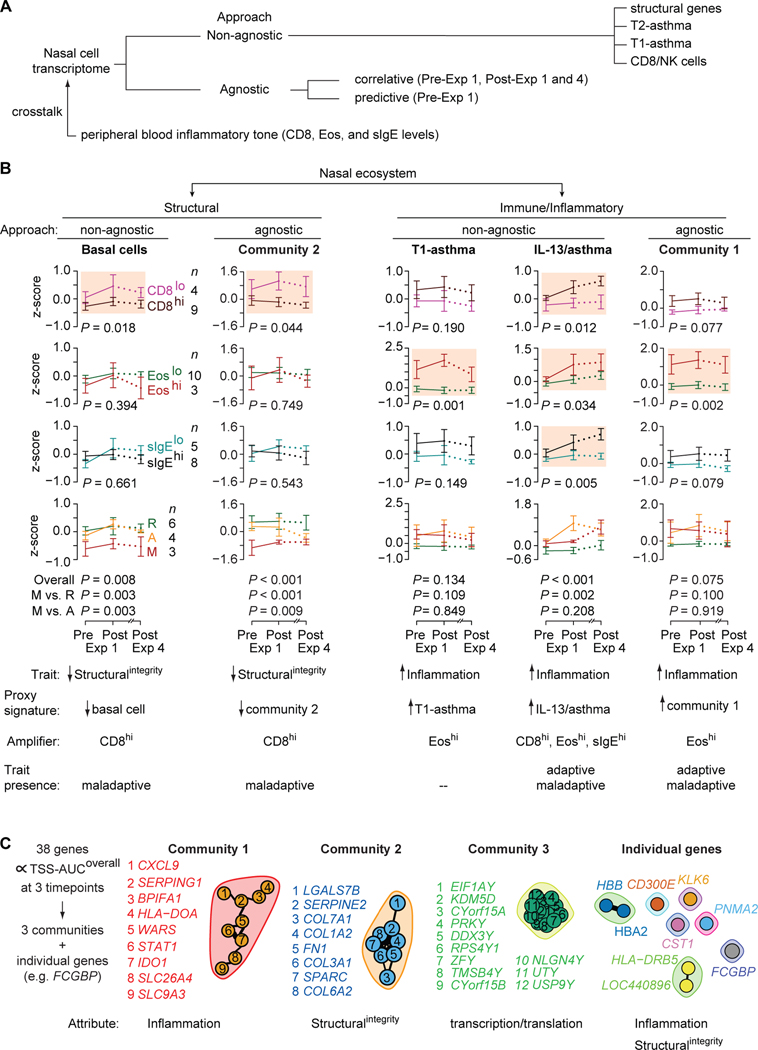
To test the mechanistic framework that conjoint airway Epiintegrity-inflammation traits coupled with a crosstalk (Figure 1D,E) undergirds the phenotypes, we used (i) a non-agnostic approach wherein we examined expression of transcriptomic proxies for the structural integrity and inflammatory status of the nasal compartment, and (ii) an agnostic (hypothesis-free) approach to identify predictive and correlative gene signatures (Figure 6A). To assess crosstalk, we examined whether expression levels of gene signatures in nasal cell transcriptomes differed according to baseline peripheral blood CD8+ T-cell, eosinophil, and sIgE strata.
Figure 6. Non-agnostic and agnostic approaches to evaluate nasal cell transcriptomes of persons with HDM-PARC.
(A) Non-agnostic vs. agnostic approaches used to study the nasal cell transcriptomes obtained from HDM-PARC persons. (B) Trajectories of (left to right) structural [basal cells, Community 2] and immune/inflammatory [T1-asthma, IL-13/asthma, Community 1] gene signature scores (z-scores) in nasal cell transcriptomes of HDM-PARC participants at pre- and post-exposure (Exp) 1 and post-Exp 4 stratified by CD8hi vs. CD8lo, Eoshi vs. Eoslo, sIgEhi vs. sIgElo, and HDM-PARC phenotypes (resilient [R], adaptive [A], maladaptive [M]). P, for the difference overall or between the indicated groups. Shaded plots highlight significant differences in signature scores by the indicated peripheral blood traits. Hi, higher; Lo, lower. (C) (Left) 38 correlative genes that, in the nasal cell transcriptomes associated with TSS-AUCoverall at pre- and post-Exp 1 and post-Exp 4. (Right) Depiction of gene communities and single genes. The nodes represent the genes and the edges represent significant correlations (false discovery rate <0.05). Methods for deriving significance values in Supplement.
Nasal non-agnostic approach: transcriptomic proxies for structural integrity
Ordovas-Montanes et al. (45) identified transcriptomic proxies for six structural attributes of the nasal compartment: basal, apical, glandular, and ciliated ECs; fibroblasts; and endothelial cells, (Table E7–E8). We determined whether expression levels of these proxies differed in nasal cell transcriptomes categorized according to CD8+, eosinophil, and sIgE strata and by phenotype (Figure 6B; Figure E12). We found that expression levels of the proxies for basal cell and fibroblasts were lower in nasal cell transcriptomes of participants with the maladaptive phenotype and CD8higher strata.
Non-agnostic approach: transcriptomic proxies for inflammatory pathways
Next, we examined the expression levels of transcriptomic proxies for three inflammatory pathways: T2-high asthma, T1-high asthma, and CD8+/NK cell-associated inflammation (Figure 6B). To monitor Th2 skewing, we derived three Th2high/asthma gene signatures (IL13/asthma, benralizumab, and T2-asthmaup) comprising genes that (i) correlated with asthma and IL13 expression in ECs (46); (ii) had decreased expression levels in peripheral blood after treatment with benralizumab, a Th2-targeting agent (47); and (iii) were Th2-related and upregulated in bronchial epithelium of asthmatics in a meta-analysis (48). The T1-asthma pathway was monitored via the T1-asthma signature, comprising 13 core hub genes associated with T1-high-associated asthma (e.g., CXCL9, IDO1, STAT1) (49). Based on findings in Figure 5A and Figure E8, CD8+- and NK cell-associated inflammation was monitored via the CD8EM, NK/asthmaAltman, and NKactivated signatures.
Expression patterns of the T1-asthma, CD8EM, NK/asthmaAltman, and NKactivated signatures did not differ significantly by phenotype; however, these signatures were constitutively higher at baseline and induced during HDM exposures in nasal cell transcriptomes linked to eosinophilhigher but not CD8higher or sIgEhigher strata (Figure 6B, T1-asthma signature; Figure E13 for other signatures). Expression levels of the three Th2high/asthma signatures in nasal cell transcriptomes showed that (i) signature levels at baseline were similar and did not differ by CD8higher, eosinophilhigher, and sIgEhigher strata or phenotype; (ii) HDM exposure induced higher signature levels in nasal cell transcriptomes linked to the CD8higher, eosinophilhigher, and sIgEhigher strata; and (iii) induced expression was highest in the adaptive phenotype (Figure 6B, IL-13/asthma signature; Figure E13 for other signatures).
Crosstalk between peripheral blood and nasal compartments
In support of a cross-talk, higher peripheral blood levels of: (i) CD8+ T-cells associated with lower levels of transcriptomic proxies for structural integrity; (ii) eosinophils associated with higher levels of proxies for T1-high asthma; and (iii) CD8+, eosinophil, and sIgE levels associated with higher induced levels of proxies for T2-high asthma (Figure 6B, bottom). These findings suggest that higher levels of transcriptomic proxies for structural integrity represent a shared resilient-adaptive trait, whereas higher levels of transcriptomic proxies for Th2 inflammation are a shared maladaptive-adaptive trait.
Agnostic approach: correlative genes
A three-step process was applied to identify correlative genes. First, we identified genes whose expression levels correlated with overall symptom severity (TSS-AUCoverall) at pre- and post-exposure 1 and post-exposure 4 (Figure 6A,C). Second, we determined whether expression of the correlative genes associated with the phenotypes. Third, we examined whether the expression of the correlative genes were subject to a crosstalk. Of the 38 correlative genes identified, 29 clustered into 1 of 3 gene network communities (Figure 6C; Table E13). Communities 1 and 2 were overrepresented with genes involved in inflammation and structural integrity, respectively (Figure 6C). A literature review failed to identify studies showing an association between community 3 genes and AA/ARC. Of the remaining 9 genes, several had documented associations with airway inflammation relevant to AA pathogenesis (e.g., FCGBP (50, 51), CST1 (52), and KLK6 (53, 54); Figure 6C).
The community 2 gene signature was enriched for genes involved in airway structural integrity (e.g., COL3A1 (55), COL1A2 (56); Figure 6C). Congruently, the expression and crosstalk patterns of the community 2 gene signature resembled those of the transcriptomic proxies for basal cells and fibroblasts (Figure 6B, community 2; Figure E12). The community 1 signature was enriched for genes in the T1-asthma signature (e.g., CXCL9, IDO1, STAT1; Table E8), and the expression and crosstalk patterns of the community 1 signature resembled those of the T1-asthma, CD8EM, NK/asthmaAltman, and NKactivated signatures (Figure 6B; Figure E13). Consistent with the observation that FCGBP is expressed by mucin-secreting cells and is rapidly induced by IL-13 in lung ECs (50), the expression and crosstalk patterns of FCGBP resembled those of the three Th2high/asthma signatures (Figure E14).
Agnostic approach: predictive genes
We next identified predictive genes, i.e., whose expression before exposure to HDMs (baseline) predicted TSS-AUCoverall (Figure 6A,7A; Table E14). Of the 122 predictive genes identified, baseline expression levels of 69% (n = 84) and 31% (n = 38) of these genes associated with lower and higher TSS-AUCoverall, respectively (Figure 7A). The top biological process gene ontology (GO) terms associated with genes predicting lower TSS-AUCoverall had attributes that influence Epiintegrity (e.g., epidermis development) (Figure 7A; Table E15). Baseline expression of the genes in the top GO terms was lower in the maladaptive phenotype vs. the resilient and adaptive phenotypes as well as controls (Figure 7A; Figure E15). Thus, at baseline, the maladaptive phenotype was characterized by lower expression of genes that broadly contribute to Epiintegrity compared with 3 study groups that either resisted symptom induction (controls and resilient HDM-PARC phenotype) or showed adaptation to repetitive HDM exposures.
Figure 7. Agnostic approach to identify genes that predict HDM-PARC-associated disease severity and phenotypes.
(A) (Left) Identification of 122 genes whose baseline nasal cell expression levels in HDM-PARC persons associated with lower vs. higher TSS-AUCoverall. (Right) pre-exposure (Exp) 1 gene signature scores (z-scores) of the top 2 GO terms of the 84 genes comprising the 69% in controls (ctrl.) and HDM-PARC persons stratified by phenotypes (resilient [R], adaptive [A], maladaptive [M]). (B) Correlation heatmap of the 122 genes resulting in six clusters. Associations with lower or higher TSS-AUCoverall depicted (color-coded vertical bars; left). (C) Venn diagram of clusters IV-VI genes and 140 genes previously identified (18) to be upregulated in controls vs. HDM-PARC persons. (D) Venn diagram of the 122 genes (panel A) whose expression levels showed the same direction (up/down) uniquely in CD8hi vs. CD8lo, Eoshi vs. Eoslo, or shared. (Left) Top GO terms of the CD8–58 and CD8/Eos-56 genes. (E) Distribution of the six clusters (panel B) in the CD8/Eos-56 and CD8–58 gene sets associated with higher or lower TSS-AUCoverall. (F) Median (IQR) of indicated gene signature scores (z-scores) at pre-Exp 1 in nasal cell transcriptomes of controls and HDM-PARC participants stratified by phenotypes. Statistical methods in Supplement.
We determined whether these 122 predictive genes clustered into groups associated with distinct functions and were subject to a crosstalk. To this end, we first generated a correlation matrix based on the gene expression levels. The 122 genes grouped into 6 clusters (I–VI) (Figure 7B; Table E14). Next, we determined whether genes in these clusters associated with overall symptom severity (TSS-AUCoverall). Except for one misclassified gene (LOC440496 in cluster II), genes with membership in clusters IV-VI vs. clusters I-III were correlated with lower vs. higher TSS-AUCoverall, respectively (Figure 7B, green vs. red vertical band; Table E14). Clusters IV-VI were overrepresented with genes that influenced attributes related to Epiintegrity. For example, clusters V and VI were enriched for genes implicated in airway remodeling (e.g., OSM (57), CHI3L1 (58–61)) and epithelial barrier (e.g., FLG (62, 63)), respectively. In contrast, clusters I-III were overrepresented with inflammation-related genes (Figure 7B). Genes implicated in T2-asthma were not represented. Hence, the predictive genes categorized into two broad functional classes: Epiintegrity vs. inflammation, correlating with disease mitigation vs. accentuation, respectively.
Cluster VI appeared to be enriched for “protective” genes involved in Epiintegrity. First, nearly 16% of the genes in cluster VI were associated with AA or AR risk identified by GWAS and included genes that cluster in the epidermal differentiation complex (e.g., FLG, RPTN, CRNN, and CRCT1) (64, 65) (Figure 7B; Table E14). Second, 70.5% of cluster VI genes overlapped with the 140 protective genes previously shown to be significantly upregulated in controls after HDM challenges (18); in contrast, five genes (15.2%) from cluster V, one gene (16.7%) from cluster IV, and none from clusters I-III overlapped (Figure 7C). These data reinforce the idea that genes whose expression promotes Epiintegrity and is higher in controls may underpin disease mitigation.
To assess crosstalk, we determined whether the expression of predictive genes differed according to peripheral blood CD8+ and eosinophil strata. Of the 122 predictive genes, 119 were differentially expressed (DE) between CD8+ and/or eosinophil strata with concordant direction of the associations (i.e., genes associated with higher vs. lower TSS-AUCoverall were also associated with CD8higher vs. CD8lower and/or eosinophilhigher vs. eosinophillower; Figure E16; Table E14). In this analysis, the 119 genes were distributed into 3 groups (Figure 7D): (i) 56 genes were DE between CD8higher and CD8lower as well as eosinophilhigher and eosinophillower strata (termed CD8/Eos-56 genes); (ii) 58 genes were uniquely DE between CD8higher vs. CD8lower strata (termed CD8–58 genes); and (iii) 5 genes were uniquely DE between eosinophilhigher vs. eosinophillower strata. The top GO terms for CD8–58 genes related mainly to Epiintegrity (e.g., epithelial cell differentiation); those for CD8/Eos-56 genes related mainly to inflammation (e.g., response to lipopolysaccharide and bacterium; Figure 7D). These findings obtained through an agnostic strategy reinforced a crosstalk as peripheral blood inflammatory tone linked to (i) CD8+ T-cell strata influenced expression of genes associated with Epiintegrity and inflammation status in the nasal compartment and (ii) eosinophil strata influenced expression of genes associated with inflammatory status but not Epiintegrity in the nasal compartment.
We next identified the subsets of the CD8–58 and CD8/Eos-56 genes that tracked symptom severity and determined their associations with phenotype. The subsets of CD8–58 and CD8/Eos-56 genes were categorized by expression levels associated with lower vs higher TSS-AUCoverall (CD8–58TSS-low and CD8/Eos-56TSS-low vs CD8–58TSS-high and CD8/Eos-56TSS-high signatures; Figure 7E; Table E8). The distribution of clusters I to VI in these signatures suggested that (i) the association of the CD8–58TSS-low and CD8/Eos-56TSS-low signatures with lower TSS-AUCoverall related mainly to overrepresentation of cluster VI and V genes, respectively (i.e., genes involved in epithelial barrier and airway remodeling, respectively), and (ii) the association of the CD8–58TSS-high and CD8/Eos-56TSS-high signatures with higher TSS-AUCoverall related mainly to genes associated with inflammation in clusters II and III, respectively (Figure 7B,E).
Reflecting their predictive capacity, baseline expression levels of genes in the (i) CD8/Eos-56TSS-low and CD8–58TSS-low signatures were lower in the maladaptive phenotype and (ii) CD8–58TSS-high and CD8/Eos-56TSS-high signatures were lower, intermediate, and higher in the resilient, adaptive, and maladaptive phenotypes, respectively (Figure 7F). Hence, an agnostic approach aimed at identifying predictive genes revealed that, at baseline, the maladaptive phenotype was defined by expression of genes associating with reduced Epiintegrity and heightened inflammation. In contrast, higher expression of genes associating with preservation of Epiintegrity represented a shared resilient-adaptive trait.
Inflammatory memory: transcriptional reprogramming
We posited that the observed crosstalk between peripheral blood inflammatory tone and Epiintegrity and inflammatory status in the nasal compartment relates to inflammatory memory (trained immunity) in airway ECs (Figure 1A). In this model, chronic exposure to a heightened CD8-, eosinophil-, and sIgE-associated peripheral blood inflammatory tone transcriptionally reprograms airway ECs, skewing them toward a lower Epiintegrity-higher inflammation status. Our results from ex vivo propagated ECs from controls and HDM-PARC persons support this concept (Supplementary note 1; Figure E17A–C).
Gene signatures and epithelial barrier function
One feature of Epiintegrity is preservation of barrier function, and transepithelial electrical resistance (TEER) measurements recorded under air-liquid interface are used to assess barrier function of ECs. Utilizing a dataset generated by Marshall et al (66), we found that the CD8–58TSS-low and CD8/Eos-56TSS-low gene signatures were higher in ECs with higher TEER values (Supplementary note 2; Figure E17D).
Combinatorial model of HDM-PARC phenotypes
We identified shared maladaptive-adaptive and resilient-adaptive traits in the peripheral blood and nasal compartment (Figure 8A). The shared maladaptive-adaptive traits related mainly to inflammation in blood and nasal cells, whereas the shared resilient-adaptive traits related mainly to innate cells in blood (lower levels of ILC2-ST2+ and specific CD8+ and NK subsets) and attributes in the nasal compartment that reflect superior Epiintegrity. Hence, consistent with our model (Figure 2G), overrepresentation of shared maladaptive-adaptive traits and underrepresentation of shared resilient-adaptive traits defined the maladaptive phenotype, whereas the converse representation of these traits defined the resilient phenotype (Figure 8A). Through additional studies, we found that genes in the nasal compartment associated with inflammatory status and Epiintegrity subclassified as follows (Supplementary note 3; Figure E18A). Inflammatory genes subclassified into groups linked to (i) activated CD8+ T-cells and NK cells, (ii) T1 asthma and IFN-stimulated genes, (iii) CD8+ T-cells and eosinophils, and (iv) T2 asthma (Figure E18A). Genes involved in Epiintegrity subclassified into three groups: genes involved in airway remodeling and epithelial barrier/repair were linked to NKT cells whereas those involved in structural integrity were linked to basal cells and fibroblasts (Supplementary note 3; Figure E18A).
Figure 8. Combinatorial model of HDM-PARC and translation of maladaptive traits to asthma severity.
(A) Combinatorial model summary of nasal cell and peripheral blood traits and clinical features of the HDM-PARC phenotypes. Hi, higher. (B) Median (IQR) gene signature scores (z-scores) in sputum samples obtained from controls and patients with asthma that clustered into transcriptional endotypes of asthma (TEA) clusters 1, 2, and 3 (source: GSE56396; (67)). P, determined by Welch’s t-test comparing TEA cluster 1 vs. 3. (Right) Study group characteristics. ICS, inhaled corticosteroids. (C) Schema of expression levels of the epithelial integrity- and inflammation-related pathways in HDM-PARC phenotypes (resilient [R], adaptive [A], maladaptive [M]). (D) Gain- and loss-of-function traits (GOF and LOF, respectively) that influence HDM-PARC and possibly asthma severity. (E) Summary schema representing number of GOF and LOF pathogenic hits impacting HDM-PARC phenotypes. Methods for deriving significance values in Supplement.
Translation of the maladaptive phenotype to AA
Stage II studies supported that gene signatures predictive of the maladaptive phenotype (Figure 7E,F) track asthma severity (Figure 8B) and a mouse model of HDM-associated lung injury (Figure E18B). Our analyses of a sputum gene expression dataset generated by Yan et al. (67) showed that worsening asthma severity associated with a progressive increase in levels of gene signatures that are predictive of the HDM-PARC maladaptive phenotype (Figure 8B). This was accompanied by increased expression of genes correlating with airway injury (e.g., FLG), Th2 skewing (IL4), activated NK cells, and NKT cells, but lower levels of TLR7 and interferon-stimulated genes (Figure E18C).
We evaluated a dataset by Tan et al. (68) of lung tissue profiles from mice after HDM-induced airway injury that resulted in eosinophilic, mixed (neutrophilic/eosinophilic), and neutrophilic inflammation (68). Expression of the predictive gene signatures was upregulated proportionate to the level of airway injury proxied by the inflammatory infiltrate (neutrophilic > mixed > eosinophilic; Figure E18B).
Discussion
Through a first-of-kind study involving repetitive challenges with HDMs, we introduce the concept of resilience, adaptation, and maladaptation to environmental triggers of AA/ARC as mediators of mild, moderate, and severe disease, respectively. Analysis of nasal cell transcriptomes (Figure 7F; Figure E18A) (i) identified a link between Epiintegrity and NKT cells and (ii) revealed that symptom severity and phenotype expression were related to contributions from three features of Epiintegrity (airway remodeling, epithelial barrier/repair, and structural integrity) as well as CD8+/NK-, T1/IFN-, CD8/eosinophil-, and T2-associated inflammation (Figure 8C, Figure E18A). Together, our OMICs data support the idea that conjoint Epiintegrity-inflammation traits coupled with a crosstalk underpin the phenotypes and the maladaptive phenotype shares clinical and mechanistic features of AA risk and severity. Additional findings (e.g., Figure 7F; Figure E18A) suggest that preservation of Epiintegrity without or with increased inflammation reflect resilient and adaptive traits, respectively, whereas ineffectual Epiintegrity coupled with heightened inflammation tracks the maladaptive phenotype (Figure 8C).
We also introduce the concept of a crosstalk between mechanistic traits in the peripheral blood and nasal compartments that affects symptom severity, phenotype development, and expression of mechanistic traits. This crosstalk may reflect an aspect of inflammatory memory related to transcriptional reprogramming of ECs, the extent of which is dependent on CD8 T-cell-, eosinophil-, and IgE-associated peripheral blood inflammatory tone. These findings agree with Ordovas-Montanes et al (45) who demonstrated the ability of basal cells to “remember” the allergic inflammatory milieu. Thus, systemic inflammation may enforce maladaptation by impairing Epiintegrity and skewing ECs toward a pro-inflammatory state. Consistent with the 2-compartments/1-disease paradigm, we demonstrate that some of the immunologic and transcriptomic traits in the nasal and peripheral blood compartments that track the maladaptive HDM-PARC phenotype have been reported to predispose to development and severity of AA.
Detailed limitations of this study are discussed (Supplementary Note 4). While our study sample was small, several lines of evidence suggest that the findings are robust and non-confounded. First, congruent phenotypes were observed in ACC-I and ACC-II. Second, phenotypes were not attributable to differences in degree of sensitization to varied allergens (Table E16). Third, we found no association between home concentrations of HDM and induced symptom severity (21), suggesting that phenotypes were unlikely to be related to inter-individual differences in HDM exposures at home. Fourth, the mechanistic correlates identified via non-agnostic and agnostic approaches were similar, as were the features of predictive vs. correlative traits. Finally, we identified parallels between the traits associated with the maladaptive phenotype and AA risk/severity. Additionally, adaptation has been reported in persons with asthma experimentally challenged with rhinovirus (69), and maladaptation may reflect a heightened sensitivity to priming, a feature of atopy originally described by John Connell (70, 71).
In our mechanistic studies, we distinguished between induced/evoked phenotypes elicited by HDM challenges in an ACC vs. phenotypes defined by baseline symptom severity recorded outside the ACC in the natural setting (constitutive phenotypes; Figure 2F). These distinctions may be important for decoding AA/ARC pathogenesis in a non-confounded manner: the mechanistic correlates of constitutive phenotypes may be imprecise, as persons with low, moderate, or severe symptom severity in the constitutive setting are a conflation of distinct induced/evoked phenotypes (Figure 2F). For this reason, and since AA/ARC are environmentally triggered, predictive traits associated with induced/evoked phenotypes may have greater relevance for defining determinants of pathogenesis than traits associated with the constitutive phenotypes.
We suggest that mechanistic traits associated with maladaptation can be broadly categorized as those associated with gain of adverse function (GOF) marked by increased CD8-associated, CD8/eosinophil-associated, and Th2-skewed inflammation vs. loss of beneficial function (LOF) characterized mainly by ineffectual Epiintegrity as well as deficient TLR-IFN-IRF antiviral immunity pathway and MAIT cells in the peripheral blood (Figure 8D). Our findings suggest a disproportionate contribution of the inflammatory tone linked to peripheral blood CD8+ T-cells in phenotype generation. This is consistent with the findings in murine models showing that effector CD8+ T cells mediate inflammation and airway hyperresponsiveness (20, 72–76), and in humans, CD8+ T-cells contribute to asthma pathogenesis, lung function decline, and asthma-related death (20, 35, 77–82).
In accord with GWAS data (Figure 1B) and the concept of GOF or LOF traits (Figure 8D), we suggest evoked HDM-PARC phenotypes may relate to whether persons manifest 2 vs. 1 vs. 0 (minimal) pathogenic hits (Figure 8E). Two hits (GOF coupled with LOF) is a feature of the maladaptive phenotype correlating with severe disease; a single hit (mainly GOF with minimal LOF) is a feature of the adaptive phenotype correlating with moderate disease and adaptation to repetitive exposures to environmental disease triggers; and minimal hits is a feature of the resilient phenotype tracking mild disease (Figure 8E). This concept that 0, 1, and 2 hits contribute to disease severity has implications for personalized care, as those with 2 hits may require agents that suppress GOF traits and restore LOF traits (Figure 8E). Thus, our studies support a novel model of AA/ARC pathogenesis and point to novel therapeutic strategies.
Supplementary Material
Acknowledgments
We thank Puraskar Ingale, Ya-Guang Liu, Kim Summers, and Lauren Clark for technical help.
Declaration of funding sources: Supported by the Veterans Affairs (VA) Research Center for AIDS and HIV Infection and VA Center for Personalized Medicine grant IP1 CX000875, National Institutes of Health (NIH) MERIT grant R37AI046326, Doris Duke Distinguished Clinical Scientist Award, and Burroughs Welcome Clinical Scientist Award in Translational Research to SKA; the 59th Medical Wing Intramural Award (FA8650-17-2-6816) to JFO; the National Institutes of Health Clinical and Translational Science Award (UL1-TR002645) to RAC; LNS was supported by NIH grants K23 AI102970 and R01 HL125816 and AMS was supported by the NIH T32DE014318 COSTAR institutional research training grant. The views expressed are those of the authors and do not reflect the official views or policy of the Department of Defense or its components.
Abbreviations
- A
Adaptive
- ARC
Allergic Rhinoconjunctivitis
- AA
Allergic Asthma
- ACC
Aeroallergen Challenge Chamber
- AUC
Area Under the Curve
- EC
Epithelial Cell
- Epiintegrity
Epithelial Integrity
- FDR
False Discovery Rate
- FEV
Forced Expiratory Volume
- GOF
Gain-of-Function
- GWAS
Genome-Wide Association Studies
- HDM
House Dust Mite
- HDM–
Non-Atopic Non-HDM Allergic Controls
- HDM-PARC
HDM-Associated Perennial ARC
- HRV
Human Rhinovirus
- ILC
Innate Lymphoid Cell
- ISG
Interferon-Stimulating Genes
- M
Maladaptive
- MAIT
Mucosal-Associated Invariant T cells
- NK
Natural Killer
- NKT
Natural Killer T-cell
- LOF
Loss-of-Function
- PBMC
Peripheral Blood Mononuclear Cells
- R
Resilient
- RNA-seq
RNA-sequencing
- sIgE
HDM-Specific Immunoglobulin E
- Th2
T Helper 2
- TSS
Total Symptom Score
Footnotes
Disclosures and potential conflicts of interest: R.L.J. is the owner of the Biogenics Research Chamber. The rest of the authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63 Suppl 86:8–160. [DOI] [PubMed] [Google Scholar]
- 2.Togias A, Gergen PJ, Hu JW, Babineau DC, Wood RA, Cohen RT, et al. Rhinitis in children and adolescents with asthma: Ubiquitous, difficult to control, and associated with asthma outcomes. J Allergy Clin Immunol. 2019;143(3):1003–11e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis AK, Jacobs RL, Tenn MW, Steacy LM, Adams DE, Walker TJ, et al. Clinical standardization of two controlled allergen challenge facilities - The Environmental Exposure Unit and the Biogenics Research Chamber. Ann Allergy Asthma Immunol. 2019. [DOI] [PubMed] [Google Scholar]
- 4.Pfaar O, Calderon MA, Andrews CP, Angjeli E, Bergmann KC, Bonlokke JH, et al. Allergen Exposure Chambers (AEC): harmonizing current concepts and projecting the needs for the future - an EAACI Position Paper. Allergy. 2017. [DOI] [PubMed] [Google Scholar]
- 5.Hamada A, Torre C, Drancourt M, and Ghigo E. Trained Immunity Carried by Non-immune Cells. Front Microbiol. 2018;9:3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novakovic B, and Stunnenberg HG. I Remember You: Epigenetic Priming in Epithelial Stem Cells. Immunity. 2017;47(6):1019–21. [DOI] [PubMed] [Google Scholar]
- 7.Mulder WJM, Ochando J, Joosten LAB, Fayad ZA, and Netea MG. Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, et al. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature. 2017;550(7677):475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeken V, Verrall AJ, Netea MG, Hill PC, and van Crevel R. Trained innate immunity and resistance to Mycobacterium tuberculosis infection. Clin Microbiol Infect. 2019. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama T, Hirahara K, Onodera A, Endo Y, Hosokawa H, Shinoda K, et al. Th2 Cells in Health and Disease. Annu Rev Immunol. 2017;35:53–84. [DOI] [PubMed] [Google Scholar]
- 12.Irvine AD, McLean WH, and Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315–27. [DOI] [PubMed] [Google Scholar]
- 13.Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, and Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37(10):2779–86. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez F, Fritz JH, and Piccirillo CA. Pleiotropic Effects of IL-33 on CD4(+) T Cell Differentiation and Effector Functions. Front Immunol. 2019;10:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon ED, Simpson LJ, Rios CL, Ringel L, Lachowicz-Scroggins ME, Peters MC, et al. Alternative splicing of interleukin-33 and type 2 inflammation in asthma. Proc Natl Acad Sci U S A. 2016;113(31):8765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savenije OE, Mahachie John JM, Granell R, Kerkhof M, Dijk FN, de Jongste JC, et al. Association of IL33-IL-1 receptor-like 1 (IL1RL1) pathway polymorphisms with wheezing phenotypes and asthma in childhood. J Allergy Clin Immunol. 2014;134(1):170–7. [DOI] [PubMed] [Google Scholar]
- 17.Omraninava M, Eslami MM, Aslani S, Razi B, Imani D, and Feyzinia S. Interleukin 13 gene polymorphism and susceptibility to asthma: a meta-regression and meta-analysis. Eur Ann Allergy Clin Immunol. 2020. [DOI] [PubMed] [Google Scholar]
- 18.Ahuja SK, Manoharan MS, Harper NL, Jimenez F, Hobson BD, Martinez H, et al. Preservation of epithelial cell barrier function and muted inflammation in resistance to allergic rhinoconjunctivitis from house dust mite challenge. J Allergy Clin Immunol. 2017;139(3):844–54. [DOI] [PubMed] [Google Scholar]
- 19.Lambrecht BN, Hammad H, and Fahy JV. The Cytokines of Asthma. Immunity. 2019;50(4):975–91. [DOI] [PubMed] [Google Scholar]
- 20.Hinks TSC, Hoyle RD, and Gelfand EW. CD8(+) Tc2 cells: underappreciated contributors to severe asthma. Eur Respir Rev. 2019;28(154). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs RL, Andrews CP, Ramirez DA, Rather CG, Harper N, Jimenez F, et al. Symptom dynamics during repeated serial allergen challenge chamber exposures to house dust mite. J Allergy Clin Immunol. 2015;135(4):1071–5. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180(2):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palechor-Ceron N, Suprynowicz FA, Upadhyay G, Dakic A, Minas T, Simic V, et al. Radiation induces diffusible feeder cell factor(s) that cooperate with ROCK inhibitor to conditionally reprogram and immortalize epithelial cells. Am J Pathol. 2013;183(6):1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casciano J, Krishnan JA, Small MB, Buck PO, Gopalan G, Li C, et al. Value of peripheral blood eosinophil markers to predict severity of asthma. BMC Pulm Med. 2016;16(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz LE, Gleich GJ, Hartley BF, Yancey SW, and Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014;11(4):531–6. [DOI] [PubMed] [Google Scholar]
- 27.Bartemes KR, Kephart GM, Fox SJ, and Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134(3):671–8 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen R, Smith SG, Salter B, El-Gammal A, Oliveria JP, Obminski C, et al. Allergen-induced Increases in Sputum Levels of Group 2 Innate Lymphoid Cells in Subjects with Asthma. Am J Respir Crit Care Med. 2017;196(6):700–12. [DOI] [PubMed] [Google Scholar]
- 29.Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, et al. Molecular definition of the identity and activation of natural killer cells. Nat Immunol. 2012;13(10):1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altman MC, Whalen E, Togias A, O’Connor GT, Bacharier LB, Bloomberg GR, et al. Allergen-induced activation of natural killer cells represents an early-life immune response in the development of allergic asthma. J Allergy Clin Immunol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karimi K, and Forsythe P. Natural killer cells in asthma. Front Immunol. 2013;4:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorska MM. Natural killer cells in asthma. Curr Opin Allergy Clin Immunol. 2017;17(1):50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinks TS. Mucosal-associated invariant T cells in autoimmunity, immune-mediated diseases and airways disease. Immunology. 2016;148(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandra S, Wingender G, Greenbaum JA, Khurana A, Gholami AM, Ganesan AP, et al. Development of Asthma in Inner-City Children: Possible Roles of MAIT Cells and Variation in the Home Environment. J Immunol. 2018;200(6):1995–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee N, You S, Shin MS, Lee WW, Kang KS, Kim SH, et al. IL-6 receptor alpha defines effector memory CD8+ T cells producing Th2 cytokines and expanding in asthma. Am J Respir Crit Care Med. 2014;190(12):1383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baraldo S, Contoli M, Bonato M, Snijders D, Biondini D, Bazzan E, et al. Deficient Immune Response to Viral Infections in Children Predicts Later Asthma Persistence. Am J Respir Crit Care Med. 2018;197(5):673–5. [DOI] [PubMed] [Google Scholar]
- 37.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119(5):1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stetson DB, and Medzhitov R. Type I interferons in host defense. Immunity. 2006;25(3):373–81. [DOI] [PubMed] [Google Scholar]
- 39.Ganjian H, Rajput C, Elzoheiry M, and Sajjan U. Rhinovirus and Innate Immune Function of Airway Epithelium. Front Cell Infect Microbiol. 2020;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rupani H, Martinez-Nunez RT, Dennison P, Lau LC, Jayasekera N, Havelock T, et al. Toll-like Receptor 7 Is Reduced in Severe Asthma and Linked to an Altered MicroRNA Profile. Am J Respir Crit Care Med. 2016;194(1):26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnston SL. IFN Deficiency in Asthma Attacks. Is Restoring Toll-like Receptor-7 Expression a New Treatment Approach in Severe Asthma? Am J Respir Crit Care Med. 2016;194(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375(5):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J, Message SD, Mallia P, Kebadze T, Contoli M, Ward CK, et al. Bronchial mucosal IFN-alpha/beta and pattern recognition receptor expression in patients with experimental rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2019;143(1):114–25e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider WM, Chevillotte MD, and Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32:513–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560(7720):649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poole A, Urbanek C, Eng C, Schageman J, Jacobson S, O’Connor BP, et al. Dissecting childhood asthma with nasal transcriptomics distinguishes subphenotypes of disease. J Allergy Clin Immunol. 2014;133(3):670–8e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sridhar S, Liu H, Pham TH, Damera G, and Newbold P. Modulation of blood inflammatory markers by benralizumab in patients with eosinophilic airway diseases. Respir Res. 2019;20(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai YH, Parker JS, Yang IV, and Kelada SNP. Meta-analysis of airway epithelium gene expression in asthma. Eur Respir J. 2018;51(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Modena BD, Bleecker ER, Busse WW, Erzurum SC, Gaston BM, Jarjour NN, et al. Gene Expression Correlated with Severe Asthma Characteristics Reveals Heterogeneous Mechanisms of Severe Disease. Am J Respir Crit Care Med. 2017;195(11):1449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007;36(2):244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis CC, Aronow B, Hutton J, Santeliz J, Dienger K, Herman N, et al. Unique and overlapping gene expression patterns driven by IL-4 and IL-13 in the mouse lung. J Allergy Clin Immunol. 2009;123(4):795–804e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singhania A, Wallington JC, Smith CG, Horowitz D, Staples KJ, Howarth PH, et al. Multitissue Transcriptomics Delineates the Diversity of Airway T Cell Functions in Asthma. Am J Respir Cell Mol Biol. 2018;58(2):261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zingkou E, Pampalakis G, Charla E, Nauroy P, Kiritsi D, and Sotiropoulou G. A proinflammatory role of KLK6 protease in Netherton syndrome. J Dermatol Sci. 2019;95(1):28–35. [DOI] [PubMed] [Google Scholar]
- 54.Komatsu N, Suga Y, Saijoh K, Liu AC, Khan S, Mizuno Y, et al. Elevated human tissue kallikrein levels in the stratum corneum and serum of peeling skin syndrome-type B patients suggests an over-desquamation of corneocytes. J Invest Dermatol. 2006;126(10):2338–42. [DOI] [PubMed] [Google Scholar]
- 55.Pascoe CD, Obeidat M, Arsenault BA, Nie Y, Warner S, Stefanowicz D, et al. Gene expression analysis in asthma using a targeted multiplex array. BMC Pulm Med. 2017;17(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Firszt R, Francisco D, Church TD, Thomas JM, Ingram JL, and Kraft M. Interleukin-13 induces collagen type-1 expression through matrix metalloproteinase-2 and transforming growth factor-beta1 in airway fibroblasts in asthma. Eur Respir J. 2014;43(2):464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller M, Beppu A, Rosenthal P, Pham A, Das S, Karta M, et al. Fstl1 Promotes Asthmatic Airway Remodeling by Inducing Oncostatin M. J Immunol. 2015;195(8):3546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bara I, Ozier A, Girodet PO, Carvalho G, Cattiaux J, Begueret H, et al. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. Am J Respir Crit Care Med. 2012;185(7):715–22. [DOI] [PubMed] [Google Scholar]
- 59.Konradsen JR, James A, Nordlund B, Reinius LE, Soderhall C, Melen E, et al. The chitinase-like protein YKL-40: a possible biomarker of inflammation and airway remodeling in severe pediatric asthma. J Allergy Clin Immunol. 2013;132(2):328–35e5. [DOI] [PubMed] [Google Scholar]
- 60.Gomez JL, Crisafi GM, Holm CT, Meyers DA, Hawkins GA, Bleecker ER, et al. Genetic variation in chitinase 3-like 1 (CHI3L1) contributes to asthma severity and airway expression of YKL-40. J Allergy Clin Immunol. 2015;136(1):51–8e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358(16):1682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holloway JW, Yang IA, and Holgate ST. Genetics of allergic disease. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S81–94. [DOI] [PubMed] [Google Scholar]
- 63.McAleer MA, and Irvine AD. The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol. 2013;131(2):280–91. [DOI] [PubMed] [Google Scholar]
- 64.Ferreira MA, Vonk JM, Baurecht H, Marenholz I, Tian C, Hoffman JD, et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat Genet. 2017;49(12):1752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waage J, Standl M, Curtin JA, Jessen LE, Thorsen J, Tian C, et al. Genome-wide association and HLA fine-mapping studies identify risk loci and genetic pathways underlying allergic rhinitis. Nat Genet. 2018;50(8):1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marshall AM, Pai VP, Sartor MA, and Horseman ND. In vitro multipotent differentiation and barrier function of a human mammary epithelium. Cell Tissue Res. 2009;335(2):383–95. [DOI] [PubMed] [Google Scholar]
- 67.Yan X, Chu JH, Gomez J, Koenigs M, Holm C, He X, et al. Noninvasive analysis of the sputum transcriptome discriminates clinical phenotypes of asthma. Am J Respir Crit Care Med. 2015;191(10):1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan HT, Hagner S, Ruchti F, Radzikowska U, Tan G, Altunbulakli C, et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy. 2018. [DOI] [PubMed] [Google Scholar]
- 69.Sinha A, Lutter R, Xu B, Dekker T, Dierdorp B, Sterk PJ, et al. Loss of adaptive capacity in asthmatic patients revealed by biomarker fluctuation dynamics after rhinovirus challenge. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Connell JT. Quantitative intranasal pollen challenge. II. Effect of daily pollen challenge, environmental pollen exposure, and placebo challenge on the nasal membrane. J Allergy. 1968;41(3):123–39. [DOI] [PubMed] [Google Scholar]
- 71.Sahin-Yilmaz AA, and Naclerio RM. John T. Connell and nasal priming. Journal of Allergy and Clinical Immunology. 2006;118(5):1190–2. [Google Scholar]
- 72.Raemdonck K, Baker K, Dale N, Dubuis E, Shala F, Belvisi MG, et al. CD4(+) and CD8(+) T cells play a central role in a HDM driven model of allergic asthma. Respir Res. 2016;17:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, et al. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol. 2004;172(4):2549–58. [DOI] [PubMed] [Google Scholar]
- 74.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, et al. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med. 2004;10(8):865–9. [DOI] [PubMed] [Google Scholar]
- 75.Miyahara N, Takeda K, Miyahara S, Taube C, Joetham A, Koya T, et al. Leukotriene B4 receptor-1 is essential for allergen-mediated recruitment of CD8+ T cells and airway hyperresponsiveness. J Immunol. 2005;174(8):4979–84. [DOI] [PubMed] [Google Scholar]
- 76.Hamelmann E, Oshiba A, Paluh J, Bradley K, Loader J, Potter TA, et al. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a marine model of airway sensitization. J Exp Med. 1996;183(4):1719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gelfand EW, and Dakhama A. CD8+ T lymphocytes and leukotriene B4: novel interactions in the persistence and progression of asthma. J Allergy Clin Immunol. 2006;117(3):577–82. [DOI] [PubMed] [Google Scholar]
- 78.den Otter I, Willems LN, van Schadewijk A, van Wijngaarden S, Janssen K, de Jeu RC, et al. Lung function decline in asthma patients with elevated bronchial CD8, CD4 and CD3 cells. Eur Respir J. 2016;48(2):393–402. [DOI] [PubMed] [Google Scholar]
- 79.Chung EH, Jia Y, Ohnishi H, Takeda K, Leung DY, Sutherland ER, et al. Leukotriene B4 receptor 1 is differentially expressed on peripheral T cells of steroid-sensitive and -resistant asthmatics. Ann Allergy Asthma Immunol. 2014;112(3):211–6e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Sullivan S, Cormican L, Faul JL, Ichinohe S, Johnston SL, Burke CM, et al. Activated, cytotoxic CD8(+) T lymphocytes contribute to the pathology of asthma death. Am J Respir Crit Care Med. 2001;164(4):560–4. [DOI] [PubMed] [Google Scholar]
- 81.Dakhama A, Collins ML, Ohnishi H, Goleva E, Leung DY, Alam R, et al. IL-13-producing BLT1-positive CD8 cells are increased in asthma and are associated with airway obstruction. Allergy. 2013;68(5):666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H, Wang H, Sokulsky L, Liu S, Yang R, Liu X, et al. Single Cell Transcriptomic Analysis Reveals Key Immune Cell Phenotypes in the lung of Asthma Exacerbation. J Allergy Clin Immunol. 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.