Case
A 49-year-old woman presented with a 1-day history of sharp left-sided pleuritic chest pain radiating to the back and associated with shortness of breath. Her past medical history was significant for fistulizing Crohn’s disease (CD) diagnosed in 2011, status post hemicolectomy. She was on prednisone intermittently over the first few years after diagnosis and reportedly had never been on aminosalicylates (mesalamine or sulfasalazine), thiopurines such as azathioprine or 6-mercaptopurine, or methotrexate. At the time of presentation, she was not taking prednisone or any other corticosteroids. She was started on infliximab therapy a year prior to admission and had initially received infusions every 8 weeks prior to switching to every 6 weeks. Additionally, she had missed her most recent dose that was due 3 weeks prior due to insurance issues, so it had been 9 weeks since her last dose. She denied any preceding viral prodromal symptoms including fever, chills, myalgia, headache, cough, rhinitis, nausea, or vomiting and also denied any previous sick contacts or recent travel.
On physical exam, vital signs were unremarkable except for tachycardia with heart rate of 104 beats per minute. Lungs were clear to auscultation bilaterally. Cardiac examination revealed normal S1 and S2 with no murmurs, rubs, or gallops. Examination of the extremities revealed mild swelling and tenderness to palpation over the wrists, metacarpophalangeal and proximal interphalangeal joints bilaterally without warmth or erythema and decreased range of motion in bilateral wrists in both passive and active flexion and extension. There were no signs of oral ulcers, photosensitivity, neurological disorder, or rash (malar or discoid).
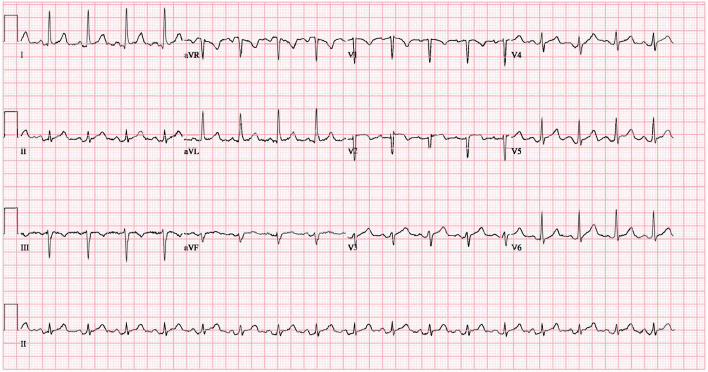
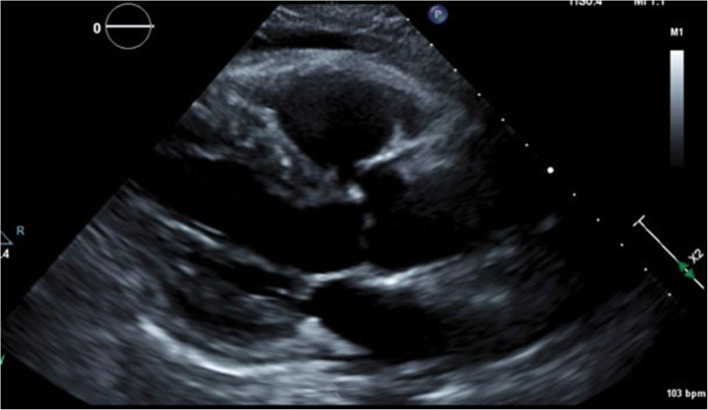
Electrocardiogram showed sinus tachycardia, left ventricular hypertrophy (LVH), diffuse ST segment elevation, PR elevation in AVR, and PR depression elsewhere consistent with pericarditis (Fig. 1). Echocardiogram revealed mild concentric LVH, grade II diastolic dysfunction, trace mitral and tricuspid regurgitation, and a moderate pericardial effusion with no sign of cardiac tamponade (Fig. 2). She was started on colchicine and indomethacin for pericarditis. Upon further questioning to identify and rule out other causes of pericarditis, she reported pain, swelling, and morning stiffness in both wrists and multiple metacarpophalangeal and proximal interphalangeal joints for the preceding 4 months.
Figure 1.
EKG findings consistent with pericarditis.
Figure 2.
Echocardiogram.
Workup was initiated to evaluate for other possible causes of pericarditis. Hepatitis viral serology and interferon gamma release assay were negative. Initial laboratory studies revealed normal serum creatinine, white blood, cells and platelet counts and slightly low hemoglobin at 10.4 g/dL (normal 11.5–14.7 g/dL). Later, urinalysis revealed small amount of protein. Pertinent inflammatory markers, rheumatologic, and other laboratory values are included in Table 1. Total blood complement level was normal in addition to complement C3 and C4. Further workup was done which showed positive double-stranded DNA (dsDNA) antibody by Crithidia and anti-histone antibodies. A diagnosis of drug-induced lupus erythematosus (DILE) causing pericarditis with pericardial effusion due to infliximab was made in view of the temporal association of symptoms and positive clinical and serological evidence. Follow-up studies showed positive anti-TNF-α antibodies. Infliximab was discontinued and the patient was started on corticosteroids with improvement in her symptoms with plans to eventually taper and switch to vedolizumab. Approximately 1 year later, she is currently taking prednisone 5 mg daily. Her CD remains well controlled with no flare-ups per her gastroenterologist. Additionally, her joint pain and swelling have significantly improved since discontinuing infliximab and she has not had any recurrent episodes of pericarditis or worsening of joint pain. She currently follows with rheumatology every 3 months and plans to switch to vedolizumab if needed for continued flare-ups.
Table 1.
Pertinent Inflammatory, Rheumatologic, and Other Laboratory Values
| Laboratory test | Result | Reference value |
|---|---|---|
| Erythrocyte sedimentation rate | >80 mm/h | 0–20 |
| C-reactive protein | 6.2 mg/dL | <0.5 |
| Antinuclear antibody | 1:1280 speckled | <1:40 |
| Rheumatoid factor | 19 IU/mL | <14 |
| Histone antibodies, S | 5.8 U | <1.0 |
| Anti-smith antibodies | <0.2 AI | <1.0 |
| Anti-RNP antibodies | <0.2 AI | <1.0 |
| SS-A | <0.2 AI | <1.0 |
| SS-B | <0.2 AI | <1.0 |
| Jo-1 | <0.2 AI | <1.0 |
| SCL-70 | <0.2 AI | <1.0 |
| Complement C3 | 155 mg/dL | 90–180 |
| Complement C4 | 24 mg/dL | 10–40 |
| Complement, total | 73 U/mL | 30–75 |
| Endomysial antibodies | Negative | Negative |
| Anti-CCP antibodies | 0.5 U/mL | <3.0 |
| Anti-dsDNA by Crithidia | Positive | Negative |
| ANCA | Negative | Negative |
RNP, ribonucleoprotein; AI, antibody index; CCP, cyclic citrullinated peptide; dsDNA, double-stranded DNA; ANCA, antineutrophil cytoplasmic antibodies
Discussion
Crohn’s disease is a systemic inflammatory disorder affecting the gastrointestinal tract with extra intestinal manifestations and associated immune disorders. Due to its debilitating symptoms that affect the quality of life, aggressive treatment to induce and maintain remission is warranted. The treatment of CD is rapidly advancing with the introduction of anti-tumor necrosis factor-alpha (TNF-α) agents, such as infliximab, that can induce and maintain remission effectively.1
Infliximab is a monoclonal anti-TNF-α antibody which is widely used in the treatment of inflammatory bowel disease and other rheumatologic diseases.2 It was the first biological response modifier to be used in the treatment of inflammatory bowel disease. It is a genetically engineered chimeric (75% human and 25% murine), monoclonal, immunoglobulin (IgG1) antibody that inhibits human TNF-α.2 Infliximab has the ability to induce complement-dependent cytotoxicity in cells expressing TNF-α and downregulate the inflammatory mechanisms in the entire mucosal layer.3 It is administered intravenously, typically as an initial three-dose induction over 6 weeks, followed by a maintenance schedule of every 8 weeks.3 Based on the results of landmark clinical trials in the treatment of CD, it remains a cornerstone of therapy in the management of moderate, severe, fulminant, and fistulizing forms of disease.1
Common adverse reactions of infliximab include infusion reactions, reactivation of tuberculosis, and serum sickness, with less common adverse reactions including hematologic or bronchogenic malignancies.2 Uncommonly, it has the potential to cause an autoimmune response producing symptoms similar to those of systemic lupus erythematosus (SLE).4 This is extremely rare with an estimated prevalence of 0.19–0.22% for infliximab in post marketing studies.4 Pericarditis is another rare complication of infliximab.5 After exclusion of other causes, it was determined that our patient had pericarditis with pericardial effusion secondary to drug-induced lupus erythematosus (DILE).
Slightly more than 300 cases of DILE associated with biologic agents have been reported in the literature, with most cases occurring in patients with rheumatoid arthritis compared to inflammatory bowel disease and other underlying diseases.6, 7 The incidence of DILE with anti-TNF-α is exceedingly rare but is most common with infliximab, with an estimated incidence of 0.19–0.22% for infliximab, 0.18% for etanercept, and 0.10% for adalimumab.4, 5 Rarely, DILE has been associated with other biologic agents. According to recent data published from the BIOGEAS registry, a prospective registry of biologic agents in autoimmune disease, out of 369 cases of DILE, only a small number (n = 10 or 3%) have been attributed to agents other than anti-TNF-α therapy. This includes ustekinumab (n = 1) which is also used in the treatment of CD, as well as other biologics not used in the treatment of CD such as bevacizumab (n = 3), efalizumab (n = 3), rituximab (n = 2), and ipilimumab (n = 1).7 Although a rare occurrence, this data highlights the stronger association of DILE with anti-TNF-α agents compared to other biologics.
Drug-induced lupus erythematosus typically affects middle-aged women with a median time to onset of 11 months, which mimics the time of onset in our patient. The reported range of time to onset is less than 1 month to more than 4 years.4 Typical clinical features of anti-TNF-α-associated DILE include cutaneous or systemic manifestations.4–7 Cutaneous features may present in the form of malar rash, discoid rash, mucosal ulcers, and alopecia. Systemic features include fever, myalgias, polyarthritis, or arthralgias. Renal involvement in the form of immune complex-mediated glomerulonephritis can occur, and thus, it is important to screen with urinalysis and urine protein quantification. Other rare clinical characteristics of anti-TNF-α-associated DILE may develop such as serositis with pleurisy, pleural effusions, deep vein thrombosis, life-threatening pneumonitis, and neuritis.4 Common presentations of DILE caused specifically by infliximab include symmetric large joint arthralgia and high titers of ANA and anti-dsDNA antibody as seen in our patient. The ANA is positive in 79% and anti-dsDNA antibodies are positive in 72% of the cases of anti-TNF-α DILE.8, 9 These antibodies are less common in DILE due to hydralazine or procainamide. Interestingly, our patient also had positive anti-histone antibodies. Anti-histone antibodies have been shown to be positive in approximately 95% of patients with classic DILE due to hydralazine or procainamide but only 17–57% positive in patients with anti-TNF-α DILE.4, 10 Although presence of these antibodies may aid in the diagnosis, they are not pathognomonic for DILE as they have been reported in up to 75% of patients with idiopathic SLE.4 All of these are important considerations when trying to establish diagnosis.
The mechanism by which DILE manifests secondary to anti-TNF-α therapy is largely unknown, but some hypotheses have been proposed that involve autoantibody formation.11 First, autoantibody formation may be due to immunogenic plasma nucleosome accumulation from dysregulation of apoptosis. Second, increased infections with anti-TNF-α therapy may lead to lymphocyte activation and autoantibody production. Finally, suppression of T helper type 1 (TH1) response by anti-TNF-α agents causing a shift to TH2 predominant response may contribute to autoantibody development. Regardless of mechanism, autoantibody development appears to be a class effect of anti-TNF-α agents.11
Development of pericarditis with or without pericardial effusion due to infliximab is an even more rare manifestation. We have identified 9 cases of infliximab-induced pericarditis based on our literature search (Table 2).6, 12–19 However, only three of these cases were associated with DILE due to infliximab based on autoimmune workup.6, 13, 16 Two cases were in males and one had developed DILE after being re-challenged with infliximab following an episode of pericarditis while the other had negative anti-histone antibodies.6, 16 In the other case, the patient had developed pericarditis but did not complain of DILE-associated symptoms.13 Our case presentation was unique given the onset of pericarditis coinciding with the onset of DILE and associated symptoms along with positive anti-histone antibodies. In other cases of infliximab-induce pericarditis, there is not definitive evidence of DILE. The proposed mechanism behind infliximab-induced pericarditis in these patients includes direct cardiac toxicity, immunoglobulin E-mediated allergic reaction, humoral antibody response, and cell-mediated hypersensitivity delayed reaction, causing a serum sickness-like reaction.14 Some of the other cases reported were associated with infection, pericardial tamponade, or hemorrhagic effusions, none of which was seen in our patient.
Table 2.
Characteristics of Infliximab-Associated Pericarditis Cases
| Ref. | Age/sex | Medical condition | Onset (in relation to starting infliximab) | Presenting symptoms | Associated with DILE | ANA | Anti-DsDNA | Anti-histone antibodies | Other |
|---|---|---|---|---|---|---|---|---|---|
| 12 | 57/M | CD | Two months | Chest pain, fever, malaise | No | N/A | N/A | N/A |
•Salmonella pericarditis •Pericardial tamponade •Large effusion |
| 13 | 30/F | CD | 12 months | Sharp chest pain, dyspnea | Yes | 1:2560 | Pos | Pos |
•Pericardial tamponade •Large effusion |
| 14 | 14/M | UC | During induction | Sharp chest pain | No | 1:160 (pos) | Neg | Neg | •Mild right ventricular wall detachment |
| 15 | 60/M | UC | One week | Shortness of breath, joint pain | No | Neg | Neg | N/A |
•Pericardial tamponade •Moderate to large hemorrhagic effusion |
| 16 | 41/M | UC | 19 months | Chest pain, dyspnea | Yes | 1:2560 | Pos | Neg |
•Pericardial tamponade •Large effusion |
| 17 | 68/F | RA | 40 days | Leg edema | No | N/A | N/A | N/A |
•Pericardial tamponade •Large effusion |
| 18 | 59/M | UC | Prior to 3rd dose | Chest pain | No | Neg | Neg | Neg | •No effusion |
| 6 | 39/M | CD | 8 months | Chest pain, nausea, weakness | Yes, after re-trial | Pos | Pos | Pos |
•Large effusion •DILE symptoms developed after pericarditis |
| 19 | 57/M | RA | 3 weeks | Anorexia, nausea | No | N/A | N/A | N/A |
•Peptostreptococcal pericarditis •Large effusion |
CD, Crohn’s disease; DILE, drug-induced lupus erythematosus; ANA, antinuclear antibody; UC, ulcerative colitis; RA, rheumatoid arthritis
Diagnostic criteria for anti-TNF-α DILE do not exist. However, one should consider the diagnosis in a patient with signs and symptoms of SLE which have a temporal association with the use of infliximab. Additionally, application of the American College of Rheumatology criteria for idiopathic SLE may exclude diagnosis of DILE secondary to anti-TNF-α therapy. In most cases, DILE is diagnosed based on temporal association of the offending agent, characteristic signs and symptoms, as well as resolution of symptoms upon discontinuation of the drug.11 Our patient was diagnosed with infliximab-induced DILE based on pericarditis and inflammatory arthritis, both known manifestations of SLE, along with positive ANA, anti-dsDNA antibodies, and anti-histone antibodies. Furthermore, she developed symptoms 1 year after starting infliximab, which fits the timeline most often reported. She also had a gradual resolution of symptoms after stopping infliximab and switching to prednisone. At the time of presentation, no other medication in her history suggested a cause for DILE.
Management of infliximab-associated DILE involves immediate discontinuation of infliximab and treatment of serositis or arthritis with corticosteroids. Vedolizumab is approved for use in moderate to severe IBD who have failed conventional and anti-TNF-α therapy. Vedolizumab does not act on the TNF-α pathway, has not been associated with DILE, and possesses a favorable safety profile overall.7, 20, 21 Furthermore, vedolizumab has been used successfully in a patient who experienced pericarditis due to infliximab-induced DILE without recurrence.13 For this reason, vedolizumab has been considered for our patient although she has not started as she is currently maintained on low-dose corticosteroids which she is tolerating well.
Conclusion
Infliximab is widely used in the treatment of inflammatory bowel disease and can rarely be associated with DILE that presents as pericarditis. Clinicians should be aware of this fact and counsel patients about this rare but clinically significant complication. Further studies are needed to establish more definitive criteria for the diagnosis of anti-TNF-α induced lupus.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Previous Presentation
The abstract of this manuscript was presented as a poster presentation at the 2019 Alabama/Mississippi ACP Scientific Meeting, held at The University of Mississippi Medical Center in Jackson, MS.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lichtenstein GR, Loftus EV, Jr, Isaacs KI, Regeuiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 2.Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clin Pharmacokinet. 2007;46(8):645–660. doi: 10.2165/00003088-200746080-00002. [DOI] [PubMed] [Google Scholar]
- 3.Adegbola SO, Sahnan K, Warusavitarne J, Hart A, Tozer P. Anti-TNF therapy in Crohn’s disease. Int J Mol Sci. 2018;19(8):2244. doi: 10.3390/ijms19082244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almoallim H, Al-Ghamdi Y, Almaghrabi H, Alyasi O. Anti-Tumor Necrosis Factor- Induced Systemic Lupus Erythematosus. Open Rheumatol J. 2012;6:315–319. doi: 10.2174/1874312901206010315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos-Casals M, Roberto-Perez-Alvarez C, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9:188–193. doi: 10.1016/j.autrev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Burke JP, Kelleher B, Ramadan S, Quinlan M, Sugrue D, O’Donovan MA. Pericarditis as a complication of infliximab therapy in Crohn’s disease. Inflamm Bowel Dis. 2008;14(3):428–429. doi: 10.1002/ibd.20270. [DOI] [PubMed] [Google Scholar]
- 7.Perez-De-Lis M, Retamozo S, Florez-Chavez A, et al. Autoimmune diseases induced by biological agents. A review of 12,731 cases (BIOGEAS Registry) Expert Opin Drug Saf. 2017;16(11):1255–1271. doi: 10.1080/14740338.2017.1372421. [DOI] [PubMed] [Google Scholar]
- 8.Pereira VM, Andrade C, Figueira R, Faria G, Jasmins L. Infliximab-induced lupus: a case report. GE-Portuguese Journal of Gastroenterology. 2017;24(2):84–88. doi: 10.1159/000450877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizra M, Mizra M, Murugesan V, Olano A. Pericardial effusion due to infliximab therapy for ulcerative colitis. Case Rep Gastrointest Med 2018; Article 4324592. [DOI] [PMC free article] [PubMed]
- 10.Ramos-Casals M, Brito-Zeron P, Munoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine. 2007;86:242–251. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 11.Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis alpha agents. Semin Arthritis Rheum. 2008;37(6):381–387. doi: 10.1016/j.semarthrit.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Saddler K, Castro-Lainez MT, Deliz-Aguirre R, et al. Nontyphoidal Salmonella purulent pericarditis presenting with pericardial tamponade in a patient on infliximab therapy. IDCases. 2019;15:e00500. doi: 10.1016/j.idcr.2019.e00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naseer M, Kulairi Z, Kam M. Cardiac tamponade as a presenting manifestation of infliximab-induced lupus in patient treated for Crohn’s disease. ACG Case Rep. 2017;4:e1. doi: 10.14309/crj.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dipasquale V, Gramaglia SMC, Catena MA, Romano C. Pericarditis during infliximab therapy in paediatric ulcerative colitis. J Clin Pharm Ther. 2018;43(1):107–109. doi: 10.1111/jcpt.12586. [DOI] [PubMed] [Google Scholar]
- 15.Lather HD, Henry D, Kahlenberg JM. Hemorrhagic pericardial effusion with tamponade: a rare adverse effect of infliximab-case report and literature review. Case Reports in Rheumatology 2016; Article: 2576496. [DOI] [PMC free article] [PubMed]
- 16.Harnett DT, Chandra-Sekhar HB, Hamilton SF. Drug-induced lupus erythematosus presenting with cardiac tamponade: a case report and literature review. Can J Cardiol. 2014;30(2):247.e11–2. doi: 10.1016/j.cjca.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura Y, Izumi C, Nakagawa Y, Hatta K. A case of effusive–constrictive pericarditis accompanying rheumatoid arthritis: The possibility of adverse effect of TNF-inhibitor therapy. J Cardiol Cases. 2013;7(1):e8–e10. doi: 10.1016/j.jccase.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devasahayam J, Pillai U, Lacasse A. A rare case of pericarditis, complication of infliximab treatment for Crohn’s disease. Journal of Crohn’s and Colitis. 2012;6(6):730–731. doi: 10.1016/j.crohns.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Harney S, O’Shea FD, Fitzgerald O. Peptostreptococcal pericarditis complicating anti-tumour necrosis factor α treatment in rheumatoid arthritis. Ann Rheum Dis. 2002;61:653–654. doi: 10.1136/ard.61.7.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahidi N, Bressler B, Panaccione R. The role of vedolizumab in patients with moderate-to-severe Crohn’s disease and ulcerative colitis. Therapy Adv Gastroenterol. 2016;9(3):330–338. doi: 10.1177/1756283X16635081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66(5):839–851. doi: 10.1136/gutjnl-2015-311079. [DOI] [PMC free article] [PubMed] [Google Scholar]