Abstract
Cannabidiol, a non-intoxicating phytocannabinoid, has potential therapeutic effects over a broad range of disorders. Recently, there has been increased interest in CBD, as several studies showed promising anticonvulsant efficacy with few side effects. In 2018, a CBD-based oral solution, Epidiolex®, was approved by the FDA to treat two severe forms of pediatric epilepsy, Dravet syndrome, and Lennox-Gastaut syndrome. Although only these two syndromes are recognized indications for CBD, it has been consumed in an unregulated fashion for a variety of indications including chronic pain, muscle stiffness, inflammation, anxiety, smoking cessation, and even cancer. While CBD legislation in the USA is confusing due to the differences in state and federal laws, CBD has proliferated in the US market in several forms such as CBD oil or capsules, hemp oil/extract, and also as an ingredient in several dietary supplements, syrups, teas, and creams. With the ever-increasing use of CBD and its widespread availability to the general public, it is important to examine and report on possible drug–drug interactions between CBD and other therapeutic agents as well as addictive substances such as alcohol and tobacco. A detailed literature search for CBD’s possible interactions was conducted using online databases. As expected, CBD has been reported to interact with anti-epileptic drugs, antidepressants, opioid analgesics, and THC, but surprisingly, it interacts with several other common medications, e.g. acetaminophen, and substances including alcohol. This review provides a comprehensive list of interacting drugs. The possible mechanisms for these drug–drug interactions are presented in table format. Given the growing popularity of CBD as a medication and the dearth of available information on CBD drug–drug interactions, it is critical to be aware of current drug–drug interactions and it will be important to investigate the impact of CBD upon concomitant medication use in future randomized, controlled trials.
KEY WORDS: cannabidiol, drug–drug interactions, cannabinoids, mechanism, cytochrome P450
INTRODUCTION
The cannabis plant has been used to treat a variety of ailments for many centuries and includes multiple species, of which Cannabis indica and Cannabis sativa are best known1. Δ9-Tetrahydrocannabinol (THC) is the major psychoactive ingredient, and cannabidiol is a non-intoxicating ingredient. Cannabis sativa usually has a higher THC:CBD ratio than Cannabis indica. Thus, sativa strains often have more psychotropic effects whereas indica strains are more sedating2. As of July 2020, 33 states and the District of Columbia have medical cannabis laws and 11 states and the District of Columbia have recreational cannabis laws. Due to the recent change in cannabis laws, CBD consumer sales have skyrocketed; they are expected to increase from half a billion in 2018 to $1.8 billion in 20223. As CBD has gained more popularity and expanded unregulated use, its drug–drug interactions remain largely unknown. CBD is known to interact with cytochrome P450 drug metabolizing enzymes, and this affects co-administration of CBD with other pharmaceutical drugs that are also inhibited or metabolized by these enzymes4. The consequence of the lack of information on drug–drug interactions is an inadequate knowledge of their potential adverse reactions when consumed together. Interactions, either additive or synergistic, or contraindications are largely undescribed and are a major health concern. As evidenced from drug interaction databases such as the Medscape Drug Interaction Checker, which healthcare professionals and researchers primarily use to check for drug interactions, searches for CBD interactions typically yield few results. Therefore, a comprehensive detailed review is warranted to provide insight into this topic.
METHODS
We conducted a detailed online literature search of the databases Pubmed and Google Scholar (1975 to March 2020), along with the drug interaction databases Medscape Drug Interaction Checker and Drug Bank using the terms, cannabidiol (or CBD) with interactions (n = 19,943), narcotics (n = 4070); anti-depressants (n = 440); AED (1246); alcohol (n = 1810); drug. In addition, CBD with specific drug names (acetaminophen (n = 1776) and morphine (6034), for example) were also searched. The results regarding drug interactions from the search were extracted and summarized by 1 author (PB). This review’s focus is not just limited to adverse effects but also any possible effects that could be attributable to CBD–drug interactions by simultaneous use either prescribed or consumed nonmedically. When examining CBD’s interactions with nicotine, there were several references available on cannabis or marijuana as a whole plant with nicotine/smoke, but none for CBD and nicotine/smoke. Cannabis/marijuana plant–drug interactions are beyond the scope of this review.
CANNABIDIOL’S MECHANISM OF ACTION
CBD is a non-psychotomimetic phytocannabinoid that has broad range of possible therapeutic effects including anxiolytic, antidepressant, anticonvulsant, neuroprotective, anti-inflammatory and immunomodulatory properties without any stimulant or convulsant properties5. CBD attenuates brain damage associated with neurogenerative or ischemic conditions. It affects synaptic plasticity and facilitates neurogenesis. The mechanism of these effects involves multiple pharmacological targets6. In animal models, CBD (a) blocks or reduces the spread of generalized seizures induced by maximal electroshock or γ-aminobutyric acid (GABA)–inhibiting drugs, (b) blocks simple partial seizures induced by the topical application of convulsant metals on the cortex, and (c) increases the seizure threshold for electrical kindling. CBD increased the potency of AEDs in animal models of partial and generalized motor seizures, but inhibited the action of AEDs in animal models of absence seizures7. CBD attenuated GABA release from ventral pallidum neurons, restoring the normal function of this system in psychotic patients8. CBD can also increase adult neurogenesis in mice, and this effect has been shown to be dependent on CB1 receptors9. CBD can act as a serotonin 1A receptor (5HT1A) agonist. Aripiprazole, an atypical antipsychotic, acts as a partial agonist at this receptor, an effect that could, together with its actions on D2 and 5-HT2A receptors, contribute to the therapeutic effects of this drug.
MECHANISMS BEHIND CANNABIDIOL’S INTERACTIONS WITH OTHER MEDICATIONS
CBD is extensively metabolized by CYP450 enzymes in the liver, in particular by the isoforms CYP3A4 and CYP2C1910. Furthermore, CBD is able to inhibit CYP2C19, CYP2D6, and CYP2C9, and may inhibit members of the CYP3 family11,12, leading to potential pharmacologic interactions with other drugs13,14. In animal models, repetitive administration of CBD may induce members of the CYP2B family4. Studies in mice have shown that CBD inactivates cytochrome P450 isozymes in the short-term, but can induce them after repeated administration. This is similar to their induction by phenobarbital, thereby strongly suggesting a role for the 2b subfamily of isozymes of cytochrome P450. Another study showed this effect to be mediated by upregulation of mRNA for CYP3A, 2C, and 2B10 after repeated CBD administration15.
CBD is metabolized via the CYP3A4 enzyme, and approximately 60% of clinically prescribed medications are also metabolized through CYP3A4. In particular, drugs such as ketoconazole, itraconazole, ritonavir, and clarithromycin inhibit CYP3A416 and this could lead to the increased levels of CBD when consumed together. CBD may increase serum concentrations of cyclosporine, sildenafil, antihistamines, haloperidol, antiretrovirals, and some statins (atorvastatin and simvastatin but not pravastatin or rosuvastatin)17. Interaction of these drugs with CYP3A4 leads to slower CBD degradation and can consequently lead to higher CBD levels that are pharmaceutically active for long periods of time. In contrast, phenobarbital, rifampicin, carbamazepine, and phenytoin induce CYP3A4, causing reduced CBD bioavailability.
GPR55 (G protein-coupled receptor 55) is highly expressed in large dorsal root ganglion neurons (added now) and, upon activation by agonists (e.g., THC), increases intracellular calcium in these neurons that may lead to neuronal excitability18. CBD is reported to function as GPR55 antagonist and suppresses GPR55’s activities. The GPR55-dependent mechanism plays a major role in CBD’s anti-psychotic and anti-epileptic activities19. The therapeutic effects of CBD on inhibiting the neurotransmission in Dravet syndrome mouse model were mediated by its antagonism of GPR5520.
CBD inhibition of the BCRP (Breast Cancer Resistance Protein) efflux function in the placental cotyledon warrants further research of co-administration of CBD with known BCRP substrates such as nitrofurantoin, cimetidine, and sulfasalazine21.
The Medscape Drug Interaction Checker database22 was searched for CBD’s interactions with other drugs and the results are tabulated in Table 1.
Table 1.
Cannabidiol–Psychoactive Drug Interactions22
| Name of drug | Class of drug | Mechanism of interaction | Clinical suggestions when co-administered with CBD |
|---|---|---|---|
| Carbamazepine | Anti-epileptic drug | Will decrease the level or effect of cannabidiol by affecting hepatic/intestinal enzyme CYP3A4 metabolism. | Consider an increase in cannabidiol dosage (based on clinical response and tolerability) when co-administered. |
| Lamotrigine | Anti-epileptic drug | CBD will increase the level or effect of lamotrigine by inhibiting UGT2B7 activity. | Consider reducing the dose when concomitantly using UGT2B7 substrates such as morphine, losartan, diclofenac, tamoxifen, and ibuprofen. |
| Oxcarbazepine | Anti-epileptic drug | Will decrease the level or effect of CBD by affecting hepatic/intestinal enzyme CYP3A4 metabolism. | Consider an increase in CBD dosage (based on clinical response and tolerability) when co-administered with a strong CYP3A4 inducer. |
| Phenobarbital | Anti-epileptic drug |
Will decrease the level or effect of CBD by affecting hepatic/intestinal enzyme CYP3A4 metabolism or CYP2C19 metabolism. CBD discreetly potentiates the anticonvulsant effect of phenobarbital. |
Consider an increase in CBD dosage (based on clinical response and tolerability) when co-administered with a strong CYP3A4 inducer. Consider reducing the dose of sensitive CYP2C19 substrates, as clinically appropriate, when co-administered. |
| Phenytoin | Anti-epileptic drug |
Will decrease the level or effect of CBD by affecting hepatic/intestinal enzyme CYP3A4/CYP2C19 metabolism. CBD may potentiate the anticonvulsant effects of phenytoin. |
Consider an increase in CBD dosage (based on clinical response and tolerability) when co-administered with a strong CYP3A4 inducer. Consider reducing the dose of sensitive CYP2C19 substrates, as clinically appropriate, when co-administered. |
|
Chlordiazepoxide Clonazepam Ethosuximide |
Benzodiazepine | CBD reduces the anticonvulsant effects of these drugs | Consider an increase in the dose of these drugs when co-administered. |
| Clobazam or Diazepam | Benzodiazepine | CBD will increase the level or effect of clobazam or diazepam by affecting hepatic enzyme CYP2C19 metabolism. CBD increases clobazam plasma concentrations. | Consider reducing the dose of sensitive CYP2C19 substrates, as clinically appropriate, when co-administered. |
| Lorazepam | Benzodiazepine | CBD will increase the level or effect of lorazepam by decreasing its metabolism and potentially inhibit UGT2B7 activity. | Consider reducing the dose when concomitantly using UGT2B7 substrates. |
| Morphine | Opioid | CBD will increase the level or effect of morphine. | CBD may potentially inhibit UGT2B7 activity. Consider reducing the dose when concomitantly using UGT2B7 substrates. |
| Desipramine | Tricyclic anti-depressant | Desipramine will increase the level or effect of CBD by affecting hepatic/intestinal enzyme CYP3A4 metabolism. | Consider reducing the CBD dose when co-administered with a moderate CYP3A4 inhibitor. |
| Imipramine | Tricyclic anti-depressant | CBD will increase the level or effect of imipramine by affecting hepatic enzyme CYP2C19 metabolism. | Consider reducing the dose of sensitive CYP2C19 substrates, as clinically appropriate, when co-administered. |
| Trimipramine | Tricyclic anti-depressant | CBD will increase the level or effect of trimipramine by affecting hepatic enzyme CYP2C19 metabolism. | Consider reducing the dose of sensitive CYP2C19 substrates, as clinically appropriate, when co-administered. |
CB1 receptors are located in the central nervous system and CB2 receptors are mostly found in the peripheral system23. Due to the lipophilic nature of CBD and THC, these compounds bind to these receptors and exert several pharmacological activities. CBD is a CB1 antagonist, a negative allosteric modulator at CB2, and an agonist at the transient receptor potential cation channel subfamily V member 1 (TRPV1) and serotonin 1A (5-HT1A) receptors, resulting in anxiolytic, antipsychotic, anticonvulsant, antioxidant, analgesic, and immunomodulatory functions, some of which buffer the harmful effects of THC like psychosis24. In particular, CB1, TRPV1, and 5HT1A are thought to be related to psychosis, anxiety, and pain, respectively. As reported by several researchers, CBD appears to have minimal analgesic activity25. In addition, evidence supporting CBD’s efficacy in treating psychiatric disorders remain scarce26.
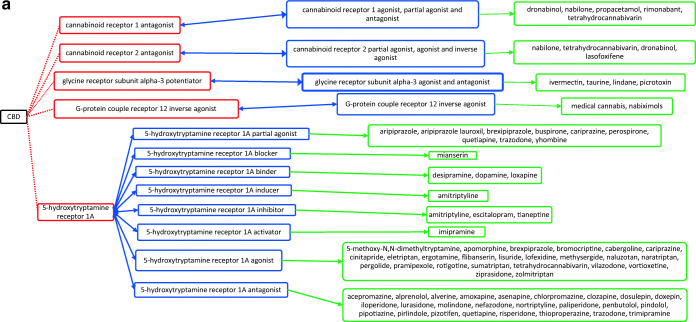
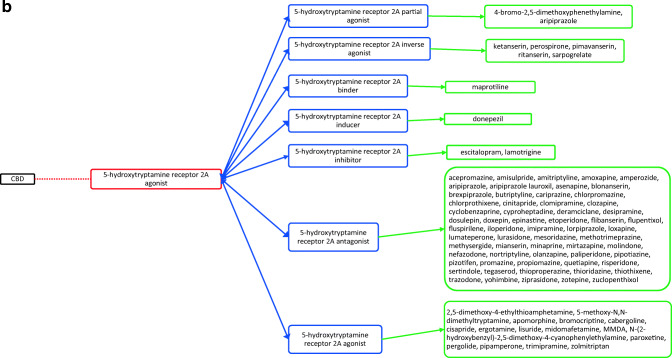
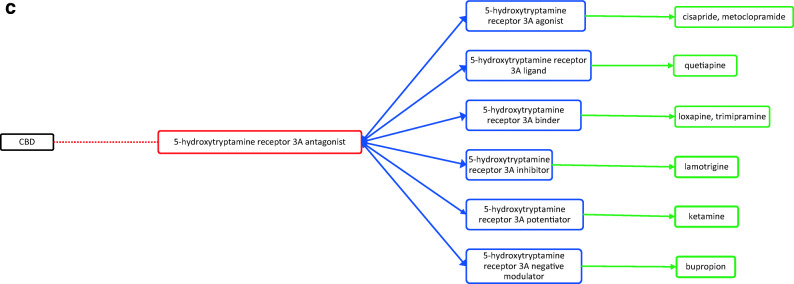
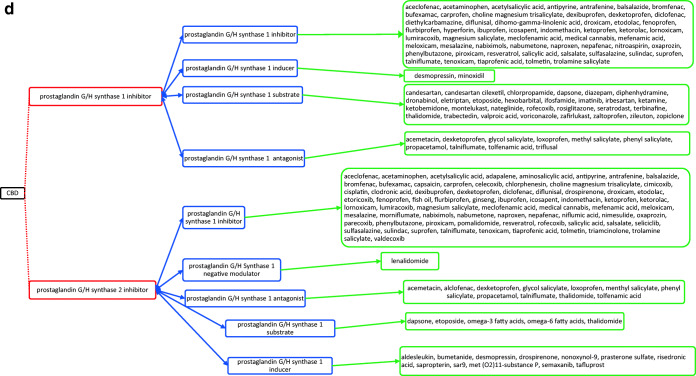
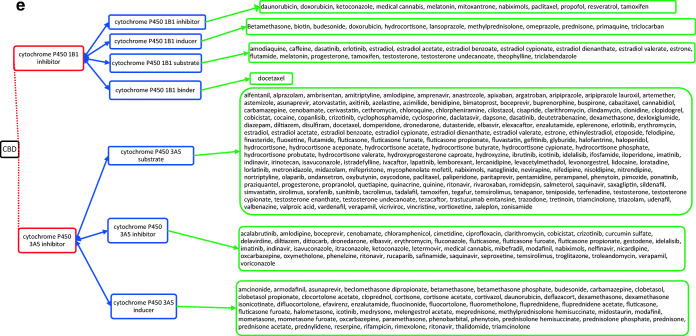
CBD acts through several different targets and acts as cannabinoid receptor 1 and 2 antagonist (Fig. 1a), G-protein-coupled receptor 12 inverse agonist (Fig. 1a), glycine receptor subunit alpha-3 potentiator, 5-hydroxytryptamine receptor 1A (Fig. 1a) and 2A agonist (Fig. 1b), 3A antagonist (Fig. 1c), prostaglandin G/H synthase 1 and 2 inhibitor (Fig. 1d), and cytochrome P450 1B1 (Fig. 1e)/3A5 (Fig. 1e)/2D6 (Fig. 1f)/3A7 (Fig. 1f)/1A2 (Fig. 1g) inhibitor as well. The drugs that act on these targets as agonists, partial agonists, antagonists, negative modulators, inducers, binders, activators, blockers, and substrates could have the potential to interact as they work on the same target and mechanisms27. The possible drug–drug interactions of CBD based on these known targets against potential medications are collectively listed as flow chart figures that could have high clinical significance and relevance. The double-headed arrows indicate that the interactions are possible on either side.
Figure 1.
a Target-mediated drug–drug interactions of cannabidiol with cannabinoid and 5-hydroxytryptamine 1A receptors27. b Target-mediated drug–drug interactions of cannabidiol with 5-hydroxytryptamine 2A receptors27. c Target-mediated drug–drug interactions of cannabidiol with 5-hydroxytryptamine 3A receptors27. d Target-mediated drug–drug interactions of cannabidiol with prostaglandin G/H synthase 1 and 2 inhibitors27. e Target-mediated drug–drug interactions of cannabidiol with Cytochrome P450 1B1 and 3A5 inhibitor27. f Target-mediated drug–drug interactions of cannabidiol with Cytochrome P450 2D6 and 3A7 inhibitor27. g Target-mediated drug–drug interactions of cannabidiol with Cytochrome P450 1A2 inhibitor27. The red dotted lines indicate CBD’s mechanism/actions as listed in red boxes. The blue double-headed arrows indicate the possible targets and interactions of CBD with other targets/mechanisms as listed in blue boxes. Green single-headed arrows indicate the drugs that act on these targets, as listed in green boxes. Such drugs may have additive/synergistic or antagonistic effects if given concomitantly with CBD.
However, the interactions presented in these figures are predicted from in vitro evidence, preclinical animal data or from their reported mechanism of actions, and their translation into clinical activities have not been established. These interactions could be concentration dependent and may require very high concentration of CBD and the other drug for any interaction to occur. Complexities in drug bioavailability, bio-absorption, pharmacokinetics in humans may also play a major role in CBD–drug interactions. Therefore, these reported interactions warrant further detailed research in human trials for accuracy and clinical significance.
CANNABIDIOL INTERACTIONS
CBD’s interaction with AEDs and antidepressants is a topic of interest for physicians because of the possibility of simultaneous consumption of both. CBD has been reported to interact with several anticonvulsants, including diazepam, lamotrigine, and phenytoin28,29; sedative drugs including barbiturates such as phenobarbital and hexobarbital30; and narcotics such as codeine and morphine.
Cannabidiol’s Interaction with Anti-epileptic Drugs
CBD has clear interactions with multiple AEDs, including clobazam, stiripentol, and valproate. CBD inhibits CYP2C19 and CYP3A4, which catalyze the metabolism of N-desmethylclobazam (nCLB), an active metabolite of clobazam11,31–33. The inhibition of these enzymes by CBD leads to the accumulation of nCLB, which is about 20–100% as potent as clobazam34; therefore, monitoring of clobazam and nCLB levels is necessary when these medications are used concomitantly14. A highly purified CBD oral solution has been approved in the USA for seizures associated with Lennox-Gastaut and Dravet syndromes in patients aged ≥ 2 years, for which AEDs are commonly used. A recent trial investigated the impact of CBD on steady-state pharmacokinetics of clobazam (and nCLB), stiripentol, and valproate35. The study also examined the reciprocal effect of these drugs on CBD’s safety and tolerability and its major metabolites (7-hydroxy-cannabidiol [7-OH-CBD] and 7-carboxy-cannabidiol [7-COOH-CBD]) when co-administered. Concomitant CBD had significant effect on nCLB exposure (with 3.4-fold Cmax (maximum concentration) and AUC (area under the concentration-time curve)), and little effect on clobazam or stiripentol exposure, while no clinically relevant effect on valproate exposure was observed. Stiripentol decreased 7-OH-CBD exposure by 29% and 7-COOH-CBD exposure by 13%. CBD was moderately well-tolerated when co-administered with AEDs35. The most common side effects of CBD are diarrhea and sedation36. There was also an increased incidence of aspartate aminotransferase and alanine aminotransferase elevations while taking CBD, with concomitant valproate37.
A pharmacodynamic animal study using maximal electroshock and audiogenic seizure models showed that CBD potentiated the anticonvulsant effects of phenytoin by twofold and discreetly potentiated the effect of phenobarbital. CBD also reduced the anticonvulsant properties of chlordiazepoxide, clonazepam, and ethosuximide29,38,39. A pharmacokinetic interaction between CBD and clobazam was reported with decreased clobazam serum levels noted after increasing CBD doses40. Another study suggests that CBD is effective in reducing seizure frequency and severity from baseline in adults and children with treatment-resistant epilepsy. According to this study, CBD has its own seizure-reducing efficacy and not affected by pharmacokinetic drug–drug interactions with other AEDs. The efficacy of AEDs can be modulated by CBD but CBD’s anti-epileptic efficacy is unaffected by AEDs41.
Socala et al.42 observed that CBD increased the activity of topiramate, oxcarbazepine, pregabalin, tiagabine, and gabapentin, but did not affect the anticonvulsant effect of lamotrigine and lacosamide. Increased anticonvulsant activity of AEDs was partly related to pharmacokinetic interactions with CBD because CBD increased serum and brain concentrations of these AEDs. Although CBD did not affect the anticonvulsant activity of lacosamide, pharmacokinetic interactions between these two drugs cannot be excluded as CBD increased the brain concentration of lacosamide and vice versa. Interestingly, cannabidiol attenuated the anticonvulsant activity of levetiracetam and this interaction is pharmacodynamic in nature because no changes in serum and brain concentrations of either levetiracetam or CBD were observed.
Cannabidiol’s Interaction with Antidepressants
CBD inhibits hepatic enzyme CYP2D6, and because of this inhibition, the serum concentrations of selective serotonin reuptake inhibitor (SSRIs), tricyclic antidepressants, antipsychotics, beta-blockers, and opioids may be increased as these antidepressants are metabolized by this enzyme. CBD can also affect metabolism of omeprazole and risperidone by CYP2D6 interactions43. CBD also interacts with monoamine oxidase inhibitors (MAOIs) like tranylcypromine, phenelzine, and isocarboxazid by inhibiting their metabolism and causing these substances to remain in the circulatory system for longer periods of time leading to unpleasant side effects44.
When sertraline, a SSRI, was administered in combination with CBD in mouse model of post-traumatic stress disorder, the combination produced synergistic action on cognitive and emotional disturbances (severe anxiety and aggressive behavior)45. The noradrenergic antidepressant, desipramine, when administered concurrently with CBD, at subtherapeutic doses of both, resulted in significant antidepressant like effects, thus implicating a synergistic or additive mechanism46.
Amitriptyline, a tricyclic antidepressant, is metabolized by cytochrome P450 isozymes CYP2D6, CYP2C19, CYP3A4, CYP1A2 and CYP2C9, and CBD inhibits these enzymes, which may increase adverse effects simultaneously (e.g., anticholinergic syndrome, drowsiness, and QT interval prolongation)47.
Additionally, gabapentin, pregabalin, citalopram, paroxetine, and mirtazapine are all metabolized by cytochrome enzymes that are known to be inhibited by CBD and co-administration of CBD with these medications may have adverse effects47.
Cannabidiol’s Interaction with Opioids
CBD has been shown to have divergent effects when co-administered with opioids. CBD’s interaction with morphine varied in different behavior models. For example, when the acetic acid stimulated stretching assay model was used, the combination showed synergistic effects. In the hot plate thermal nociceptive assay model, acetic acid decreased operant responding for palatable food model and sub-additive effects (an effect that is less than additive) were observed. These results suggest that distinct mechanisms of action underlie the interactions between CBD and morphine. Thus, the choice of appropriate combination therapies for the treatment of acute pain conditions may depend on the underlying pain type and stimulus modality48.
CBD is shown to inhibit heroin (diamorphine) metabolism and 6-monoacetylmorphine hydrolysis in in vitro conditions, which may be of clinical relevance49. A double-blind, placebo-controlled, crossover study in healthy volunteers with concomitant use of CBD and fentanyl showed that CBD does not exacerbate adverse effects associated with fentanyl and co-administration was well tolerated50.
Cannabidiol’s Interactions with -Δ9 -Tetrahydrocannabinol (THC)
There are 565 chemical compounds and 120 phytocannabinoids (as of 2017) isolated from cannabis, including THC and CBD51. THC produces the main psychoactive effects of cannabis, while CBD does not appear to have similar effects. Studies conflict as to whether CBD attenuates or exacerbates the behavioral and cognitive effects of THC. This includes the effects of CBD on THC-induced anxiety52, psychosis53, and cognitive deficits54. In a mouse model of paclitaxel-induced neuropathic pain, CBD synergized the effects of THC in attenuating mechanical allodynia, pain from usually non-painful stimuli. Also, CBD attenuated oxaliplatin- but not vincristine-induced mechanical sensitivity55. CBD inhibited the acute effects of THC and decreased THC effects on brain regions involved in memory, anxiety, and body temperature regulation56.
On the basis of CBD:THC ratios in cannabis, individuals from different populations were directly compared on indices of the reinforcing effects of drugs, explicit liking, and implicit attentional bias to drug stimuli. When intoxicated, smokers of high CBD:THC strains showed reduced attentional bias to drug and food stimuli compared with smokers of low CBD:THC. Those smoking higher CBD:THC strains also showed lower self-rated liking of cannabis stimuli on both test days. These reports suggest that CBD has potential as a treatment for cannabis use disorder57.
As both THC and CBD are hepatically metabolized, the potential exists for pharmacokinetic drug interactions via inhibition or induction of enzymes or transporters. In a study on the co-administration of CBD with THC in 5:1 dose ratio, CBD did not alter the trajectory of enduring THC-induced anxiety nor tolerance to the pharmacological effects of THC. There was no evidence of CBD potentiation of the behavioral effects of THC whereas CBD:THC in 1:1 co-administration increased histone 3 acetylation (H3K9/14ac) in the VTA (ventral tegmental area are group of neurons in the mid-brain) and ΔFosB, a transcription factor expression in the nucleus accumbens. Increased histone 3 acetylation in the VTA region associated with addictive properties of drug abuse. These changes suggest that CBD might have some protective effects over THC’s adverse effects over these brain regions and the process of memory58.
Cannabidiol’s Interaction with Other Drugs
Pharmacodynamic interactions may occur if CBD is administered with other central nervous system depressant drugs and cardiac toxicity may occur via additive hypertension and tachycardia with sympathomimetic agents. More vulnerable populations, such as older patients, may benefit from the potential symptomatic and palliative benefits of cannabinoids but are at increased risk of adverse effects59.
Warfarin
A case study described a patient with CBD treatment for the management of epilepsy, ultimately necessitating a 30% reduction in warfarin dose to maintain therapeutic international normalized ratio (INR) values60,37 with excessive bleeding as side effects.
Cyclosporine and Tacrolimus
CBD has the potential to affect the immunosuppressant cyclosporine’s metabolism which may result in increased cyclosporine blood levels and an increase in its toxic side effects. Another study reported CBD’s interaction with the immunosuppressant tacrolimus with 3-fold increase in dose-normalized tacrolimus concentrations61.
Acetaminophen
Caution should be taken when CBD is used with medications with the potential to cause hepatic injury, such as acetaminophen. CBD carries a recommendation for lowered doses in patients with hepatic impairment62. In a recent animal study, co-administration of CBD at the dose of 116 mg/kg (human dose of CBD is 10 mg/kg) with acetaminophen (400 mg/kg) resulted in 37.5% mortality associated with liver injury. No mortality was observed in the CBD-alone or acetaminophen-alone groups. The co-administration led to greater activation of c-Jun N-terminal kinase (JNK). Surprisingly, these effects were not observed in mice with a higher dose of CBD (290 mg/kg CBD with mouse equivalent dose of 25 mg/kg). This shows an interesting paradoxical effect of CBD/APAP-induced hepatotoxicity63.
Capsazepine
Co-administration of CBD, together with a TRPV-1 antagonist, capsazepine, reduces L-DOPA-induced dyskinesia (LID) by acting on CB1 and PPARγ receptors and reducing the expression of the inflammatory markers cyclooxygenase-2 and nuclear factor-kappa B64. These interactions could play a significant role as L-DOPA remains the most effective pharmacotherapy for Parkinson’s disease.
Other reports also demonstrate the possible interactions of CBD with rufinamide, zonisamide, and eslicarbazepine—increased accumulation of these drugs in the blood with concomitant use of CBD and their levels should be closely monitored37.
In a recent publication, Wilson-Morkeh et al.47 listed the interactions of CBD with corticosteroids (CS), commonly used drugs in the field of rheumatology. Hydrocortisone and prednisolone, commonly used CS, are both metabolized by the cytochrome P450 enzyme CYP3A65. CBD, as potent inhibitor of CYP3A when with these steroids may decrease glucocorticoid clearance and increase risk of systemic CS-induced side effects as well66. In addition, CBD could potentially interact with naproxen, tramadol, celecoxib, etoricoxib, fluoxetine, and tofacitinib as these are metabolized in the liver by cytochrome P450 enzymes. Another widely used RA drug, baricitinib, could be an exception as it was cleared by kidneys with minimal mediation by CYP3A4, and thus, the CBD interaction could be minimal. Methotrexate (MTX), hydroxychloroquine (HCQ), sulfasalazine (SSZ), mycophenolate mofetil (MMF), mesalazine, adalimumab, etanercept, abatacept, infliximab, and rituximab belong to the class of DMARDs (“disease-modifying anti-rheumatic drugs”), and these drugs have not been shown to produce interactions with CBD47.
Cannabidiol’s Interactions with Alcohol
Although CBD’s effects on alcohol consumption are poorly understood, CBD is known to act as an agonist of the 5-HT1A receptors and results suggest that CBD can attenuate alcohol consumption and potentially protect against certain harmful effects of alcohol, such as liver and brain damage67. When CBD was injected 30 min prior to each alcohol binge episode, it protected against hepatic injury, as measured by an attenuation in multiple markers of liver injury and oxidative stress68. Similarly, when CBD was co-administered with ethanol to rats, CBD was able to attenuate alcohol-induced brain damage in the hippocampal and entorhinal cortices. Alcohol-induced cell death was reduced by approximately 60% in both hippocampal granular cells and the entorhinal cortical pyramidal cells69. In a clinical study, CBD, when consumed with alcohol, produced significant impairments of motor and psychomotor performances, overestimations of time production (estimation of alcohol content over various time periods) and subjective responses indicating some protective effects, including an accurate self-perception of one’s intoxication and deficits. CBD did not prevent enhanced locomotor response once alcohol sensitization had developed70. CBD also lowered blood alcohol levels71. The timing and dosage of CBD administration could influence alcohol pharmacokinetics. Long-term effects of CBD on alcohol-induced anxiety and impulsivity need further exploration67.
CONCLUSION
This review provides an insight into the possible and potential interactions of CBD with other classes of commonly used drugs based on the evidence and knowledge currently available. Despite the increased popularity of CBD as a medication for myriad medical conditions, the limited availability of applicable pharmacokinetic and pharmacodynamic information highlights the need to initiate prescribing CBD using a “start low and go slow” approach, carefully observing the patient for desired and adverse effects. Further clinical studies in the patient populations for whom prescribing may be considered are needed to derive a better understanding of these drugs and enhance safe and optimal prescribing. Given few existing case reports or clinical trials available on CBD’s interactions with other drugs, future research should address and characterize the mechanisms of these interactions. The growing popularity of CBD use and the lack of sufficient information on CBD drug–drug interactions make it imperative that we investigate the impact of CBD upon concomitant drug use in future randomized, controlled trials.
Acknowledgments
The authors thank Dr. Larry A. Walker, Emeritus Director, National Center for Natural Products Research, School of Pharmacy, University of Mississippi, for reviewing the manuscript and providing insightful suggestions.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ElSohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78(5):539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Joy JE, Watson S, Benson J. Marijuana and Medicine. Assessing the Science Base. 1999:1999. [PubMed]
- 3.Mikulic M. Global Pharmaceutical Industry–Statistics & Facts. In: Statista; 2019.
- 4.Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisanti S, Malfitano AM, Ciaglia E, et al. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–150. doi: 10.1016/j.pharmthera.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 6.Campos AC, Fogaça MV, Sonego AB, Guimarães FS. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119–127. doi: 10.1016/j.phrs.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Consroe P, Benedito MA, Leite JR, Carlini EA, Mechoulam R. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur J Pharmacol. 1982;83(3-4):293–298. doi: 10.1016/0014-2999(82)90264-3. [DOI] [PubMed] [Google Scholar]
- 8.Coimbra N, Mendes-Gomes J, Da Silva J, Dos Anjos-Garcia T, Ullah F, Almada R. New Ethological and Morphological Perspectives for the Investigation of Panicolytic-Like Effects of Cannabidiol. In: Handbook of Cannabis and Related Pathologies. Elsevier; 2017:e140-e149.
- 9.Schiavon AP, Bonato JM, Milani H, Guimarães FS, de Oliveira RMW. Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;64:27–34. doi: 10.1016/j.pnpbp.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011;89(5-6):165–170. doi: 10.1016/j.lfs.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Jiang R, Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metabolism and Pharmacokinetics. 2013:DMPK-12-RG-129. [DOI] [PubMed]
- 12.Zendulka O, Dovrtelová G, Nosková K, et al. Cannabinoids and cytochrome P450 interactions. Curr Drug Metab. 2016;17(3):206–226. doi: 10.2174/1389200217666151210142051. [DOI] [PubMed] [Google Scholar]
- 13.Bland TM, Haining RL, Tracy TS, Callery PS. CYP2C-catalyzed delta (9)-tetrahydrocannabinol metabolism: kinetics, pharmacogenetics and interaction with phenytoin. Biochem Pharmacol. 2005;70(7):1096–1103. doi: 10.1016/j.bcp.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246–1251. doi: 10.1111/epi.13060. [DOI] [PubMed] [Google Scholar]
- 15.Machado Bergamaschi M, Helena Costa Queiroz R, Waldo Zuardi A, Crippa AS. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6(4):237–249. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- 16.Monographie N. Cannabidiol. Deutscher Arzneimittel-Codex (DAC) inkl Neues Rezeptur-Formularium (NRF) DAC/NRF October. 2015;22.
- 17.Wedman-St Louis B. 14 Drug Interactions & Dosing Issues. Cannabis as Medicine. 2019.
- 18.Lauckner JE, Jensen JB, Chen H-Y, Lu H-C, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci. 2008;105(7):2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sylantyev S, Jensen TP, Ross RA, Rusakov DA. Cannabinoid-and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci. 2013;110(13):5193–5198. doi: 10.1073/pnas.1211204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan JS, Stella N, Catterall WA, Westenbroek RE. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci. 2017;114(42):11229–11234. doi: 10.1073/pnas.1711351114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feinshtein V, Erez O, Ben-Zvi Z, et al. Cannabidiol changes P-gp and BCRP expression in trophoblast cell lines. PeerJ. 2013;1:e153. doi: 10.7717/peerj.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Database MC. https://reference.medscape.com/drug-interactionchecker Accessed March 28 2020.
- 23.Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2(1):139–154. doi: 10.1089/can.2016.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, van Velzen M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160(4):860. doi: 10.1097/j.pain.0000000000001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoury JM, Neves MCL, Roque MAV, et al. Is there a role for cannabidiol in psychiatry? World J Biol Psychiatry. 2019;20(2):101–116. doi: 10.1080/15622975.2017.1285049. [DOI] [PubMed] [Google Scholar]
- 27.Database DB. https://www.drugbank.ca/drugs/DB09061/biointeractions#target-tab Accessed April 15 2020.
- 28.Chesher G, Jackson DM. Anticonvulsant effects of cannabinoids in mice: drug interactions within cannabinoids and cannabinoid interactions with phenytoin. Psychopharmacologia. 1974;37(3):255–264. doi: 10.1007/BF00421539. [DOI] [PubMed] [Google Scholar]
- 29.Consroe P, Wolkin A. Cannabidiol--antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther. 1977;201(1):26–32. [PubMed] [Google Scholar]
- 30.Benowitz NL, Nguyen TL, Jones RT, Herning RI, Bachman J. Metabolic and psychophysiologic studies of cannabidiol-hexobarbital interaction. Clin Pharmacol Ther. 1980;28(1):115–120. doi: 10.1038/clpt.1980.139. [DOI] [PubMed] [Google Scholar]
- 31.Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos. 2011;39(11):2049–2056. doi: 10.1124/dmd.111.041384. [DOI] [PubMed] [Google Scholar]
- 32.Naccarato M, Yoong D, Kovacs C, Gough K. Case report a case of a potential drug interaction between clobazam and etravirine-based antiretroviral therapy. Antivir Ther. 2012;17:589–592. doi: 10.3851/IMP1953. [DOI] [PubMed] [Google Scholar]
- 33.Giraud C, Tran A, Rey E, Vincent J, Tréluyer J-M, Pons G. In vitro characterization of clobazam metabolism by recombinant cytochrome P450 enzymes: importance of CYP2C19. Drug Metab Dispos. 2004;32(11):1279–1286. [PubMed] [Google Scholar]
- 34.Walzer M, Bekersky I, Blum RA, Tolbert D. Pharmacokinetic drug interactions between clobazam and drugs metabolized by cytochrome P450 isoenzymes. Pharmacotherapy. 2012;32(4):340–353. doi: 10.1002/j.1875-9114.2012.01028.x. [DOI] [PubMed] [Google Scholar]
- 35.Morrison G, Crockett J, Blakey G, Sommerville K. A phase 1, open-label, pharmacokinetic trial to investigate possible drug-drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol Drug Dev. 2019;8(8):1009–1031. doi: 10.1002/cpdd.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grotenhermen F, Gebhardt K, Berger M. Cannabidiol. Solothurn, Schweiz: Nachtschatten Verlag 2016:17-63.
- 37.Gaston TE, Szaflarski JP. Cannabis for the treatment of epilepsy: an update. Curr Neurol Neurosci Rep. 2018;18(11):73. doi: 10.1007/s11910-018-0882-y. [DOI] [PubMed] [Google Scholar]
- 38.Consroe PF, Wolkin AL. Anticonvulsant interaction of cannabidiol and ethosuximide in rats. J Pharm Pharmacol. 1977;29(8):500–501. doi: 10.1111/j.2042-7158.1977.tb11378.x. [DOI] [PubMed] [Google Scholar]
- 39.Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G. Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytotherapy Res. 2009;23(5):597–602. doi: 10.1002/ptr.2625. [DOI] [PubMed] [Google Scholar]
- 40.Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP, Program UC. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58(9):1586–1592. doi: 10.1111/epi.13852. [DOI] [PubMed] [Google Scholar]
- 41.Gaston TE, Bebin EM, Cutter GR, et al. Drug–drug interactions with cannabidiol (CBD) appear to have no effect on treatment response in an open-label Expanded Access Program. Epilepsy Behav. 2019;98:201–206. doi: 10.1016/j.yebeh.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Socała K, Wyska E, Szafarz M, Nieoczym D, Wlaź P. Acute effect of cannabidiol on the activity of various novel antiepileptic drugs in the maximal electroshock-and 6 Hz-induced seizures in mice: Pharmacodynamic and pharmacokinetic studies. Neuropharmacology. 2019;158:107733. doi: 10.1016/j.neuropharm.2019.107733. [DOI] [PubMed] [Google Scholar]
- 43.Ujváry I, Hanuš L. Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res. 2016;1(1):90–101. doi: 10.1089/can.2015.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Păunescu H, Coman O, Coman L, et al. Cannabinoid system and cyclooxygenases inhibitors. J Med Life. 2011;4(1):11. [PMC free article] [PubMed] [Google Scholar]
- 45.Gasparyan A, Navarrete F, Manzanares J. P. 417 Effects of cannabidiol and sertraline on behavioral and neurochemical alterations induced by a new long-lasting animal model of PTSD. Eur Neuropsychopharmacol. 2019;29:S296. [Google Scholar]
- 46.Sales AJ, Crestani CC, Guimarães FS, Joca SR. Antidepressant-like effect induced by Cannabidiol is dependent on brain serotonin levels. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;86:255–261. doi: 10.1016/j.pnpbp.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Wilson-Morkeh H, Al-Abdulla A, Sien L, Mohamed H, Youngstein T. Important drug interactions exist between cannabidiol oil and commonly prescribed drugs in rheumatology practice. Rheumatology. 2020. [DOI] [PubMed]
- 48.Neelakantan H, Tallarida RJ, Reichenbach ZW, Tuma RF, Ward SJ, Walker EA. Distinct interactions of cannabidiol and morphine in three nociceptive behavioral models in mice. Behav Pharmacol. 2015;26(3):304–314. doi: 10.1097/FBP.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 49.Qian Y, Gilliland TK, Markowitz JS. The influence of carboxylesterase 1 polymorphism and cannabidiol on the hepatic metabolism of heroin. Chem Biol Interact. 2020;316:108914. doi: 10.1016/j.cbi.2019.108914. [DOI] [PubMed] [Google Scholar]
- 50.Manini AF, Yiannoulos G, Bergamaschi MM, et al. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med. 2015;9(3):204. doi: 10.1097/ADM.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. Phytochemistry of Cannabis sativa L. In: Phytocannabinoids. Springer; 2017:1-36. [DOI] [PubMed]
- 52.Zuardi AW, Shirakawa I, Finkelfarb E, Karniol I. Action of cannabidiol on the anxiety and other effects produced by Δ 9-THC in normal subjects. Psychopharmacology. 1982;76(3):245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]
- 53.Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M. Clinical and preclinical evidence for functional interactions of cannabidiol and Δ 9-tetrahydrocannabinol. Neuropsychopharmacology. 2018;43(1):142–154. doi: 10.1038/npp.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.King KM, Myers AM, Soroka-Monzo AJ, et al. Single and combined effects of Δ9-tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br J Pharmacol. 2017;174(17):2832–2841. doi: 10.1111/bph.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Todd S, Arnold J. Neural correlates of interactions between cannabidiol and Δ9-tetrahydrocannabinol in mice: implications for medical cannabis. Br J Pharmacol. 2016;173(1):53–65. doi: 10.1111/bph.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan CJ, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Δ 9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology. 2010;35(9):1879–1885. doi: 10.1038/npp.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Todd SM, Zhou C, Clarke DJ, Chohan TW, Bahceci D, Arnold JC. Interactions between cannabidiol and Δ9-THC following acute and repeated dosing: Rebound hyperactivity, sensorimotor gating and epigenetic and neuroadaptive changes in the mesolimbic pathway. Eur Neuropsychopharmacol. 2017;27(2):132–145. doi: 10.1016/j.euroneuro.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84(11):2477–2482. doi: 10.1111/bcp.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damkier P, Lassen D, Christensen MMH, Madsen KG, Hellfritzsch M, Pottegård A. Interaction between warfarin and cannabis. Basic Clin Pharmacol Toxicol. 2019;124(1):28–31. doi: 10.1111/bcpt.13152. [DOI] [PubMed] [Google Scholar]
- 61.Leino AD, Emoto C, Fukuda T, Privitera M, Vinks AA, Alloway RR. Evidence of a clinically significant drug-drug interaction between cannabidiol and tacrolimus. Am J Transplant. 2019;19(10):2944–2948. doi: 10.1111/ajt.15398. [DOI] [PubMed] [Google Scholar]
- 62.Brown JD, Winterstein AG. Potential adverse drug events and drug–drug interactions with medical and consumer Cannabidiol (CBD) use. J Clin Med. 2019;8(7):989. doi: 10.3390/jcm8070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ewing LE, McGill MR, Yee EU, et al. Paradoxical Patterns of Sinusoidal Obstruction Syndrome-Like Liver Injury in Aged Female CD-1 Mice Triggered by Cannabidiol-Rich Cannabis Extract and Acetaminophen Co-Administration. Molecules. 2019;24(12):2256. doi: 10.3390/molecules24122256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.dos-Santos-Pereira M, da-Silva CA, Guimaraes FS, Del-Bel E. Co-administration of cannabidiol and capsazepine reduces L-DOPA-induced dyskinesia in mice: possible mechanism of action. Neurobiol Dis. 2016;94:179–195. doi: 10.1016/j.nbd.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 65.Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Transact R Soc B: Biol Sci. 2013;368(1612):20120431. doi: 10.1098/rstb.2012.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamaori S, Ebisawa J, Okushima Y, Yamamoto I, Watanabe K. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011;88(15-16):730–736. doi: 10.1016/j.lfs.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 67.Nona CN, Hendershot CS, Le Foll B. Effects of cannabidiol on alcohol-related outcomes: A review of preclinical and human research. Exp Clin Psychopharmacol. 2019;27(4):359. doi: 10.1037/pha0000272. [DOI] [PubMed] [Google Scholar]
- 68.Yang L, Rozenfeld R, Wu D, Devi LA, Zhang Z, Cederbaum A. Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Radic Biol Med. 2014;68:260–267. doi: 10.1016/j.freeradbiomed.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther. 2005;314(2):780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 71.Consroe P, Carlini EA, Zwicker AP, Lacerda LA. Interaction of cannabidiol and alcohol in humans. Psychopharmacology. 1979;66(1):45–50. doi: 10.1007/BF00431988. [DOI] [PubMed] [Google Scholar]