Abstract
Ipilimumab and nivolumab for melanoma induced smoldering myocarditis remitting with steroids. Rechallenge with nivolumab produced steroid-refractory myocarditis confirmed by electron microscopy. Tacrolimus and mycophenolate transiently reduced inflammation, but antithymocyte globulin induced remission. Cardiomyopathy with fatty infiltration ensued, but the patient succumbed to rampant melanoma progression after lymphocyte depletion. (Level of Difficulty: Advanced.)
Key Words: antithymocyte globulin, ipilimumab, melanoma, myocarditis, nivolumab
Abbreviations and Acronyms: ATG, antithymocyte globulin; CK-MB, creatine kinase–myocardial band; CPB, checkpoint blockade; cTnI, cardiac troponin I; ECG, electrocardiography; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; ULN, upper limit of normal
Graphical abstract
Ipilimumab and nivolumab for melanoma induced smoldering myocarditis remitting with steroids. Rechallenge with nivolumab produced steroid-refractory…
History of Presentation
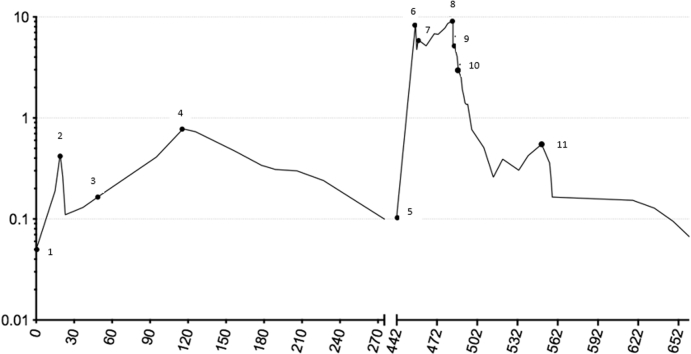
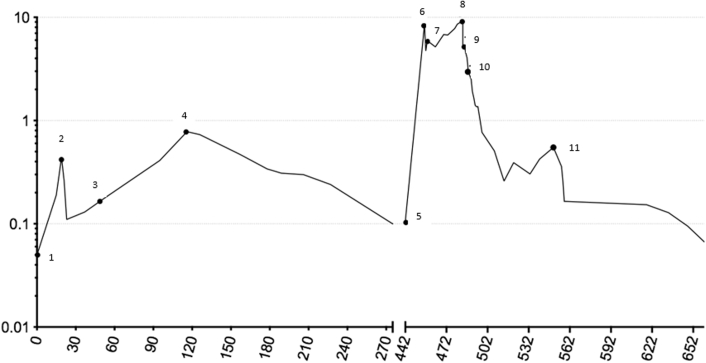
A 57-year-old Caucasian woman with no cardiac risk factors developed biopsy-confirmed pulmonary metastases from a BRAF wild-type cutaneous melanoma. Serum cardiac troponin I (cTnI) level was normal immediately preceding immune checkpoint blockade (CPB) with ipilimumab 3 mg/kg and nivolumab 1 mg/kg. Two days later, she experienced self-limited thyroiditis with no clinically apparent cardiac effects. Two weeks thereafter, she developed nausea, and cTnI was elevated to 3 times the upper limit of normal (ULN), which doubled during the next 4 days (Figure 1). Creatine kinase–myocardial band (CK-MB) became mildly elevated to 8.4 ng/ml (normal range <5 ng/ml). Total creatine kinase was normal, indicating no generalized myositis.
Learning Objectives
-
•
Vigilant monitoring and early intervention are important for CPB myocarditis.
-
•
CPB rechallenge may induce treatment-refractory exacerbation of myocarditis.
-
•
Immune suppression to treat CPB myocarditis can promote tumor growth and metastasis.
Figure 1.
Serial Troponins From Dose 1 of Immunotherapy
Logarithmic representation of serial troponin levels, with numeric markings indicating responses to various therapeutic agents over nearly 2 years.
Differential Diagnosis
The differential diagnosis included myocardial ischemia, viral myocarditis, and CPB-induced myocarditis.
Investigations
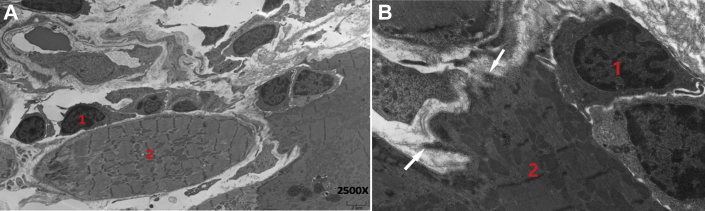
Results of electrocardiography (ECG), echocardiography, and cardiac magnetic resonance imaging (MRI) were normal. There was no evidence of ischemia or antecedent viral illness (1). CPB was discontinued because of myocarditis, and methylprednisolone was initiated at 125 mg/day for 4 days, followed by prednisone 1 mg/kg daily tapered over 1 month. Troponin nadired at 1.8 times ULN but climbed again to 13 times ULN 2 months after prednisone was discontinued (Figure 1). The patient remained asymptomatic, with normal results on ECG, echocardiography, and cardiac MRI. Right heart catheterization with endomyocardial biopsies demonstrated in 1 of 5 fragments early collagen deposition and inflammatory cells, with CD8+ granzyme B+ cytotoxic T cells predominating. Electron microscopy demonstrated multiple T lymphocytes attacking individual cardiac myocytes (Figure 2A) with extensive pore formation and cytoplasmic extrusion but preservation of sarcomeres and mitochondria (Figure 2B). Results of exercise single-photon emission computed tomographic myocardial perfusion were normal.
Figure 2.
Electron Micrographs From Endomyocardial Biopsy 3 Months Following Initial Dose of CPB
(A) Multiple T lymphocytes (1) attacking the surface of a cardiac myocyte (2). (B) Myocyte cytoplasmic extrusion (arrows) through membrane pores with preservation of sarcomeres and mitochondria.
Management
For myocarditis smoldering for 4 months with preserved myocardial function and stable metastases, the patient received low-dose prednisone 10 mg/day for 5 months, and cTnI levels steadily declined to 1.7 times ULN and remained near normal for another 6 months off steroids (Figure 1). Pulmonary metastases progressed 15 months after a single dose of ipilimumab and nivolumab, and resumption of immune CPB was the only available therapy known to influence survival. Rechallenge with single-agent nivolumab was considered reasonable because the patient was asymptomatic without changes on ECG, echocardiography, or cardiac MRI, and only approximately 30% of serious adverse events following ipilimumab and nivolumab are reinduced by nivolumab alone. Nivolumab 240 mg fixed dose was administered once, and within 2 weeks CK-MB was 24 ng/ml with cTnI 300 times ULN, 17-fold higher than the peak following the first dose of CPB (Figure 1). Results of cardiac MRI (Figure 3A), ECG, and left heart catheterization were normal. She received methylprednisolone 125 mg/day for 12 days, followed by prednisone 1 mg/kg/day. A single dose of infliximab produced no improvement or clinical deterioration. Cardiac enzymes were not improved 2 weeks later, and a second right heart catheterization with biopsies revealed in 1 of 5 fragments marked CD8+ cytolytic T cell infiltration of the myocardium with scattered CD68+ macrophages and myocyte necrosis (Figure 4). Immunostaining demonstrated effector molecules granzyme B and perforin, with no evidence of complement activation by C4d immunostaining.
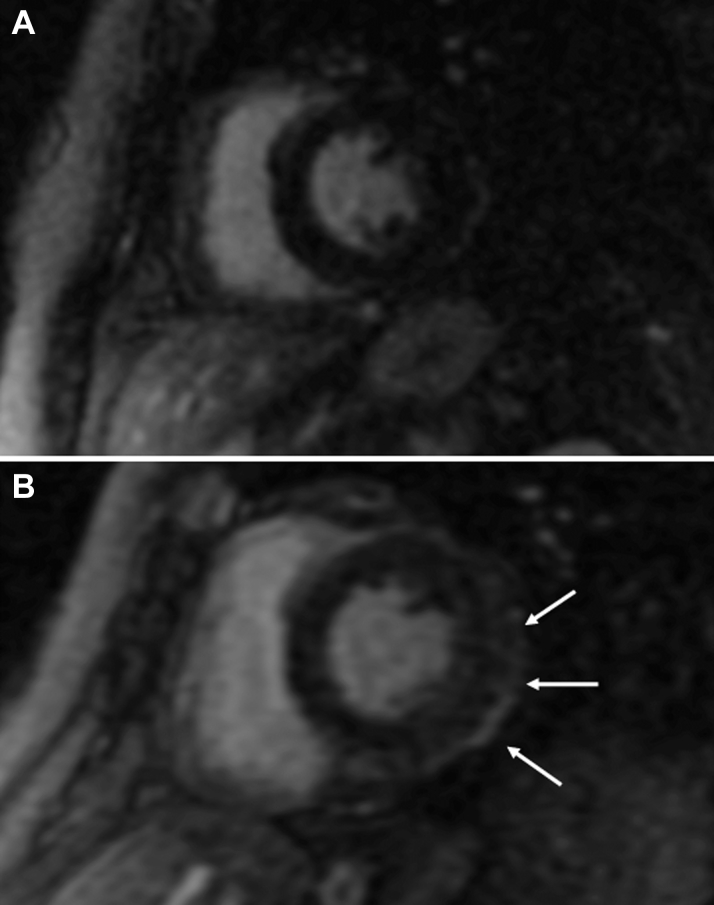
Figure 3.
Cardiac Magnetic Resonance Imaging
(A) Cardiac magnetic resonance imaging (MRI) 2 weeks following nivolumab rechallenge with no significant late gadolinium enhancement (LGE). (B) Cardiac MRI 6 weeks following nivolumab rechallenge with mild subepicardial LGE along the inferior and lateral walls of the left ventricle.
Figure 4.
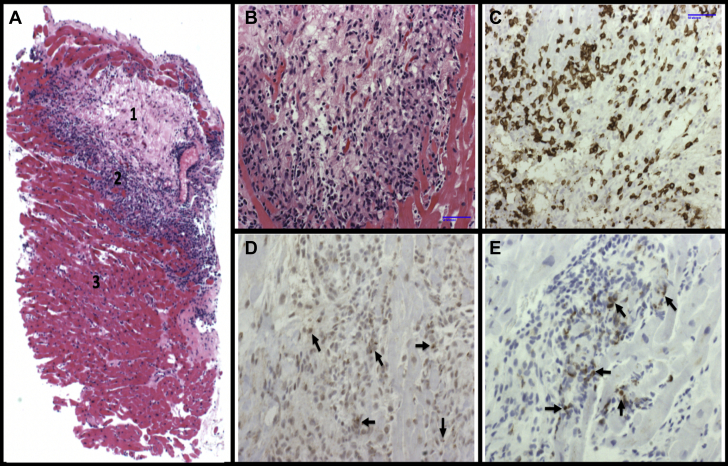
Endomyocardial Biopsy Following Immunotherapy Rechallenge
(A) Hematoxylin and eosin stain of endomyocardial tissue demonstrating areas of myocardial necrosis (1), leading edge of inflammation with mononuclear invasion (2) of normal myocardium (3). (B) Higher power view of leading edge of mononuclear infiltration. (C to E) Immunohistochemical stains characterizing infiltrating cytotoxic T cells that stain positive for CD8, granzyme (arrows), and perforin (arrows), respectively.
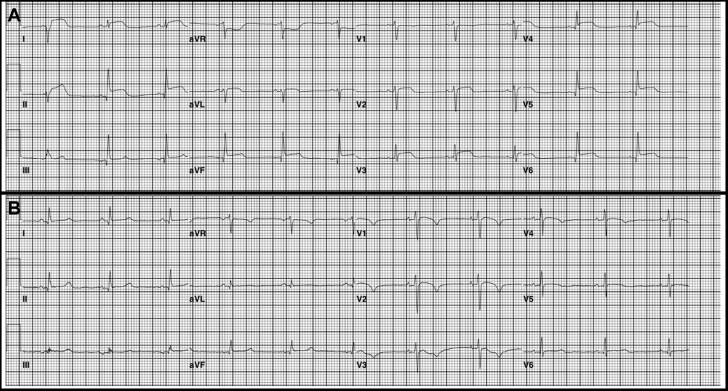
Tacrolimus was initiated at 0.08 mg/kg divided twice daily, and 1 week later, ECG demonstrated diffuse ST-segment elevation (Figure 5A) and cTnI remained >300 times ULN, with CK-MB of 38 ng/ml. Cardiac MRI (Figure 3B) revealed subepicardial gadolinium enhancement along the inferolateral left ventricle with mild corresponding hypokinesis but normal left ventricular ejection fraction (LVEF). Echocardiographic global longitudinal strain, a sensitive method for detecting subtle changes in left ventricular function, was abnormal (−16.8%). Methylprednisolone 500 mg/day added to tacrolimus produced little improvement, so the patient received mycophenolate 800 mg/m2 twice daily, tacrolimus, and prednisone 1 mg/kg/day. Ten days later, CK-MB had improved to 11 ng/ml and cTnI to 50 times ULN (Figure 1). After 2 months, findings on ECG improved (Figure 5B), and cTnI nadired at 9 times ULN but subsequently doubled despite 3 months of triple immunosuppression that was causing significant gastrointestinal toxicity. Out of concern over long-term cardiac sequelae due to poor control of chronic myocarditis, the patient received rabbit antithymocyte globulin (ATG) 1 mg/kg/day for 2 days, and the peripheral lymphocyte count immediately dropped 100-fold to <1% of circulating leukocytes (Figure 1). Tacrolimus, mycophenolate, and high-dose prednisone were continued. CK-MB and cTnI gradually normalized such that mycophenolate and tacrolimus were discontinued, with prednisone tapered to 30 mg daily.
Figure 5.
Serial Electrograms
Electrocardiograms demonstrating diffuse ST-segment changes 1 month following rechallenge with single-agent nivolumab (A) with subsequent partial normalization 1 month later (B).
Follow-Up
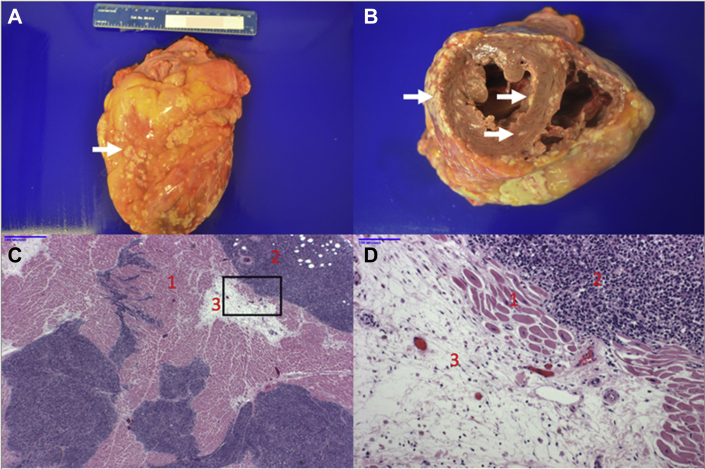
Four months following ATG, the patient presented with fatigue, and echocardiography revealed an LVEF of 25% to 30% with moderate mitral regurgitation, indicating delayed nonischemic cardiomyopathy despite essentially normal CK-MB and cTnI levels (Figure 1). Seven months following ATG, melanoma dramatically progressed, with new metastases in the bones, liver, spleen, and adrenal glands. Despite chemotherapy, the patient rapidly succumbed to melanoma with essentially normal cardiac enzymes (Figure 1) and LVEF stable at 25% to 30%. Post-mortem examination revealed no myocarditis but miliary melanoma metastases to the myocardium and pericardium at the end stage of her malignancy (Figure 6A). Fatty infiltration of the myocardium was thought to represent a late sequela of myocarditis (Figures 6B and 6C). Cardiomyopathy presenting 6 months before death was thought unrelated to melanoma cardiac metastases, which paralleled diffuse spread to other organs 4 months before death with fulminant progression in the face of a stably depressed LVEF.
Figure 6.
Post-Mortem Heart
(A) Anterior wall of heart covered by multiple metastatic nodules (white arrow). (B) Short-axis section demonstrates multiple epicardial, myocardial, and endocardial metastases (white arrows). (C) Low-power magnification of hematoxylin and eosin–stained myocardial section shows normal myocardium (1), melanoma nodules (2), and adipose tissue (3). (D) Higher power magnification of boxed area in C.
Discussion
A multicenter review of CPB-induced myocarditis reported electrocardiographic abnormalities and late gadolinium enhancement on cardiac MRI in most patients, but T cell infiltration of the myocardium was often patchy, and LVEF was more often preserved compared with myocarditis of other etiologies. Adverse cardiac outcomes correlate with cTnI ≥1.5 ng/ml rather than LVEF decrease (2). We hypothesize that marked elevation of serum cTnI and frequent arrhythmias with preservation of LVEF relates to the mechanism of cytolytic T cell attack in which perforin polymerizes in myocyte and conduction cell membranes to promote leakage of troponin and ions necessary for electric conduction. Relatively massive cardiac myocytes 10-fold larger than average cells may sustain substantial pore formation without lethality, as shown on electron micrography with cytoplasmic extrusion but maintenance of sarcomeres and mitochondria (Figure 2B). Myocarditis is the second leading cause of fatal toxicity following combination CPB (25% of deaths) and had the highest mortality rate (40%) of any immune-related adverse event, followed by closely related myositis (17%) (3).
In a recent review of CPB-induced myocarditis describing 42 cases, presenting symptoms were often nonspecific dyspnea, fatigue, and myalgia with onset following 1 or 2 doses of CPB in 62% of cases and 93% of fatalities (4). Among patients who died, 64% developed complete heart block, but 50% had normal ejection fractions. MRI is the preferred noninvasive diagnostic test, with the ability to detect hyperemia, edema, and scar with sensitivity and specificity of 76% and 96%, respectively (4). In equivocal cases, endomyocardial biopsy is recommended, which typically demonstrates predominant CD8+ T cells, but multiple specimens are necessary because inflammation may be patchy.
The American Society of Clinical Oncology guidelines for management of CPB-induced myocarditis recommend immediate initiation of high-dose steroids with 1 mg/kg methylprednisolone intravenously (5). However, there are minimal data to guide additional therapy if steroids fail. Infliximab, which itself can precipitate heart failure, was associated with rapid death in 3 of 5 patients (4). ATG resulted in good intermediate-term control of myocarditis in 1 of 3 patients, but another died quickly of progressive malignancy (4). Alemtuzumab, a monoclonal antibody producing rapid cytolytic depletion of mature lymphocytes, monocytes, macrophages, and dendritic cells, led to resolution of myocarditis in a single patient with sustained tumor response for 4 months (6). Abatacept, a cytotoxic T lymphocyte–associated antigen 4 agonist, induced resolution of myocarditis in 1 patient, with tumor control persisting 1 month after the dose (7). No data exist regarding the pattern of cardiomyopathy occurring as a late consequence of CPB-induced myocarditis or the impact of requisite immune suppression on control of the underlying malignancy, as previous reports provide <6 months of follow-up.
Our patient is unique in providing a 15-month history of smoldering myocarditis following the first dose of ipilimumab and nivolumab, fulminant myocarditis following retreatment with single-agent nivolumab, partial control of myocardial inflammation over several months with triple oral immunosuppression, complete elimination of myocarditis by ATG, late-onset cardiomyopathy despite absence of inflammation, and the unleashing of aggressive melanoma by lymphocyte depletion. This singular case with 30 months of follow-up demonstrates the enigma that patients surviving the initial bout of myocarditis will typically require additional immunotherapy for their malignancy, which can exacerbate cardiac damage. Persistence of low-level cardiac inflammation during reversible immune suppression may have contributed to late development of cardiomyopathy, arguing for earlier use of ATG. However, this comes at the risk of rapidly fatal progression of malignancy. An international registry is urgently needed to provide structured longitudinal follow-up of CPB-induced myocarditis to guide treatment and provide better understanding of long-term consequences to the heart and control of the underlying malignancy.
Conclusions
CPB-induced myocarditis involves pore formation in cardiac myocytes, which promotes release of troponin and intracellular ions potentiating arrhythmias, while myocyte viability and LVEF are frequently maintained. Smoldering myocarditis can be patchy and subtle to diagnose but manageable with a protracted steroid taper. Rechallenge with CPB more than 1 year later can produce more fulminant, steroid-refractory relapse. Tacrolimus and mycophenolate are useful, and ATG may induce remission at the risk of fulminant cancer progression. Partial suppression of myocarditis over months to years can lead to fatty infiltration of the myocardium and cardiomyopathy.
Footnotes
Dr. Conry is on the Speakers Bureau for Bristol-Myers Squibb. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
References
- 1.Norwood T.G., Westbrook B.C., Johnson D.B. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer. 2017;5:91. doi: 10.1186/s40425-017-0296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D.Y., Cohen J.V., Chandra S. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atallah-Yunes S.A., Kadado A.J., Kaufman G.P. Immune checkpoint inhibitor therapy and myocarditis: a systematic review of reported cases. J Cancer Res Clin Oncol. 2019;145:1527–1557. doi: 10.1007/s00432-019-02927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J.R., Lacchetti C., Thompson J.A. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol Pract. 2018;14:247–249. doi: 10.1200/JOP.18.00005. [DOI] [PubMed] [Google Scholar]
- 6.Esfahani K., Buhlaiga N., Thebault P., Lapointe R., Johnson N.A., Miller W.H., Jr. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380:2375–2376. doi: 10.1056/NEJMc1903064. [DOI] [PubMed] [Google Scholar]
- 7.Salem J.E., Allenbach Y., Vozy A. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380:2377–2379. doi: 10.1056/NEJMc1901677. [DOI] [PubMed] [Google Scholar]