Abstract
Background
Huntington's disease (HD) causes dysphagia and dementia, both of which are risk factors for malnutrition. Gastrostomy is used to sustain enteral intake in neurodegenerative diseases and specifically improves outcomes in ALS, but its indications and outcomes in HD are understudied.
Objective
To explore the indications and outcomes for gastrostomy for HD.
Methods
We performed a retrospective cross-sectional analysis of all HD admissions in the National Inpatient Sample. Logistic regression models compared the patient- and hospital-level characteristics associated with gastrostomy placement in HD and the prevalence of associated diagnoses in HD vs. ALS gastrostomy patients. We also examined in-hospital mortality, length of stay (LOS), and discharge status.
Results
Between 2000 and 2010, 5.12% (n = 1614) of HD admissions included gastrostomy tube placement. Gastrostomy patients were more likely to be Black (adjusted odds ratio [AOR] 1.55, 95% CI: 1.09–2.21) and have Medicare coverage (AOR 1.43, 95% CI: 1.0–2.05). The most common comorbidities were aspiration pneumonia (34.1%), dementia (31.3%), malnutrition (30.3%), and dysphagia (29.5%). Dementia and delirium were associated with discharge type but not LOS. Aspiration pneumonia, sepsis, and Elixhauser comorbidity index were associated with LOS but not discharge type. Compared to 7908 ALS gastrostomy patients, those with HD more frequently had aspiration pneumonia (34.1% vs. 20.5%, p < 0.0001), sepsis (28.1% vs. 13.7%, p < 0.0001), prolonged LOS (OR 1.14, 95% CI: 1.02–1.28), and skilled nursing facility discharge (p < 0.0001, Wald chi square test).
Conclusions
Gastrostomy is frequently performed in HD patients with dementia and aspiration pneumonia who are at increased risk for negative hospitalization outcomes.
Keywords: Huntington's disease, Health services research, Outcome research, Gastrostomy
Highlights
-
•
More than 1,600 inpatient gastrostomies were performed for Huntington’s disease from 2000-2010 in the United States.
-
•
Gastrostomy is associated with aspiration pneumonia, dementia, prolonged hospitalization, hizationospital and non-routine discharge.
-
•
Negative outcomes after gastrostomy are more common in Huntingtons disease than motor neuron disease.
1. Introduction
Huntington's disease (HD) causes dysphagia and cognitive impairment in addition to typical motor manifestations of chorea and parkinsonism [1]. In neurologic disease, both dysphagia and dementia are risk factors for malnutrition and other downstream health consequences, and therefore many patients with stroke, motor neuron disease, or other neurodegenerative disorders undergo gastrostomy placement for enteral support [2]. However, the risks, benefits, and indications for gastrostomy vary dramatically depending on the neurologic disease in question. For example, in amyotrophic lateral sclerosis (ALS), there is evidence that gastrostomy prolongs survival and improves quality of life, resulting in its inclusion as part of best-practice guidelines [3]. However, in advanced dementia, gastrostomy placement has not been shown to prevent aspiration pneumonia or improve survival or quality of life, and the American Geriatrics Society, American Academy of Hospice and Palliative Medicine, and American Board of Internal Medicine therefore recommend against its use in this population [4].
Dysphagia is common in HD [5], and gastrostomy is performed, but its frequency, timing, indications, and outcomes are unknown, as there are no data on current utilization patterns of enteral support in HD. A central question is whether feeding tube placement in HD occurs primarily in the setting of neuromuscular dysphagia (similar to ALS, which is associated with favorable outcomes following gastrostomy) or advanced dementia (which is not associated with improved outcomes). In this study, we examine the clinical and demographic characteristics and discharge outcomes of HD patients undergoing gastrostomy in the United States using the National Inpatient Sample (NIS), a national database of hospital discharge data.
2. Methods
2.1. Standard protocol approvals, registrations, and patient consents
This study was approved by the University of Pennsylvania Institutional Review Board.
2.2. Data
We used data from the National Inpatient Sample (NIS), which is the largest all-payer inpatient healthcare database in the United States and is made available through the Healthcare Cost and Utilization Project (HCUP) by the Agency for Healthcare Research and Quality (AHRQ) [6]. Before 2012, the NIS included all discharges from a 20% sample of U.S. hospitals. Beginning in 2012, the NIS estimates a 20% sample (>7 million hospitalizations annually) of discharges from all U.S. hospitals. The database contains deidentified encounter-level information on patient and hospital demographics, diagnoses and comorbidities, inpatient procedures, and healthcare costs and payer information. For our analysis, we pooled data from the years 2000–2010.
2.3. Data availability policy
The full dataset is publicly available through HCUP.
2.4. Population
We identified all inpatient admissions with HD using ICD-9 code 333.4. Gastrostomy placement was identified using AHRQ Clinical Classification Software (CCS) code 71, a validated algorithm developed by HCUP that aggregates multiple diagnostic and procedural codes to identify incident gastrostomy placement during an admission. This code only identifies new gastrostomy tube placement and is different from the code identifying the presence of a gastrostomy tube from a previous admission. However, the timing of gastrostomy placement within a given hospitalization cannot be determined. In addition to describing and comparing the clinical and demographic characteristics and outcomes of HD inpatients with and without gastrostomy as detailed below, we also compared inpatient gastrostomy for HD to amyotrophic lateral sclerosis (ALS), which was identified using ICD-9 codes 335.20 or 335.21. We selected ALS as a comparator because it is a neurodegenerative disease with evidence and best-practice guidelines supporting the use of gastrostomy to improve outcomes in this population [3], in contrast to other neurodegenerative diseases where evidence is either lacking or to the contrary.
2.5. Outcomes and covariates
Our primary outcomes were inpatient mortality, hospital length of stay (LOS), and disposition status. We also considered patient-level factors such as age group (18–39, 40–49, 50–59. 60–69, 70–79, ≥80 years), race/ethnicity (White, Black, Hispanic, Asian or Pacific Islander, Native American, or other), gender, insurance provider (Medicare, Medicaid, private, self-pay, or no charge/other), and median income by ZIP code (≤$37,999, $38,000–$47,999, $48,000–$63,999, ≥$64,000), as well as hospital-level factors such as size (small, medium, or large as defined by HCUP), teaching status, and geographic region (Northeast, South, Midwest, or West). We recorded whether hospital admission was electively planned (as opposed to occurring via presentation to the emergency department or transfer from another hospital), though this not identify whether gastrostomy itself was elective or not. Severity of comorbid medical illness was quantified using the Elixhauser comorbidity index [7], which incorporates a number of different diagnoses including diabetes, hypertension, and cardiopulmonary disease. Specific associated diagnoses were defined using diagnostic and procedural codes (Supplemental Table 1).
2.6. Statistical analysis
We summarized the patient and hospital-level characteristics of HD admissions with and without gastrostomy using descriptive statistics and compared them using single-variable and multivariable logistic regression (adjusted for age, race, gender, elective admission status, median income by ZIP, insurance payer, hospital size, hospital location, and teaching status). We also summarized the Elixhauser comorbidity index, frequency of associated diagnoses, hospital length of stay, and in-hospital mortality for inpatient HD gastrostomies and compared them to inpatient ALS gastrostomies using chi-square tests. We constructed separate logistic regression models for HD gastrostomies and ALS gastrostomies to determine the patient and hospital characteristics and comorbidities associated with non-routine discharge (defined as discharge to a short-term hospital, skilled nursing facility, or other institution). LOS was not normally distributed due to ceiling effects (specifically, all observations in the NIS dataset are terminated at 365 days such that individuals cannot be tracked across years), so LOS was log-transformed for linear regression, and regression coefficients were exponentiated and interpreted as the ratio of geometric mean LOS. Sampling weights were applied to account for the complex survey design of the NIS and ensure proper confidence intervals for both descriptive statistics and regression models. Statistical significance was defined at the p < 0.05 level.
3. Results
3.1. Clinical characteristics of HD gastrostomy patients
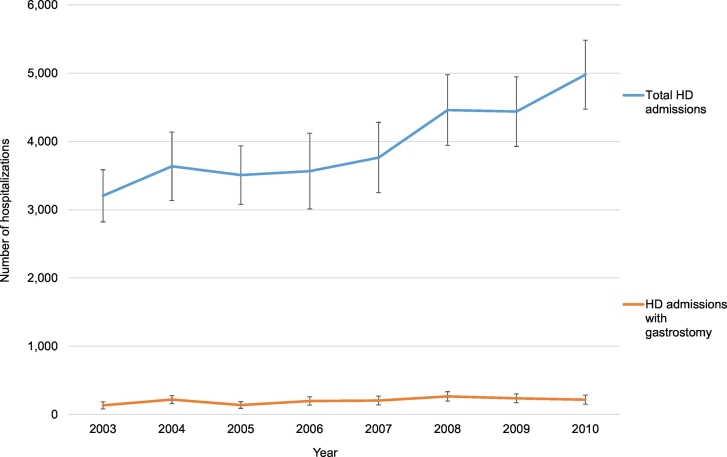
We identified 31,551 inpatient admissions with a diagnosis of HD during the study period, of which 1614 (5.12%) included gastrostomy tube placement. While we pooled data from 2000 to 2010, complete information on HD admissions was only available in NIS beginning in 2003. Fig. 1 illustrates the total number of HD admissions and the number of HD admissions with gastrostomy by year. The patient- and hospital-level characteristics of HD admissions with vs. without inpatient gastrostomy tube placement are summarized in Table 1. In both unadjusted and adjusted analyses, Native American (AOR 3.47, 95% CI: 1.21–9.95) or Black (adjusted odds ratio [AOR] 1.55, 95% CI: 1.09–2.21) ancestry was associated with gastrostomy, as compared to White. After adjusting for age, race, gender, elective admission status, median income by ZIP, and hospital characteristics, Medicare insurance was associated with gastrostomy compared to private insurance (AOR 1.43, 95% CI: 1.0–2.05). A similar proportion (12.5%) of hospitalizations for HD, either with or without gastrostomy, was elective. There were no significant differences between gastrostomy and non-gastrostomy HD admissions in terms of age, gender, median income by ZIP, or hospital size, location, or teaching status.
Fig. 1.
Trend in hospitalization for Huntington's disease and gastrostomy utilization by year, National Inpatient Sample 2003–2010.
Vertical bars denote 95% confidence intervals.
Table 1.
Patient- and hospital-level characteristics of hospital admissions for Huntington's disease patients with vs. without inpatient gastrostomy tube placement.
| Gastrostomy (n = 1614) | No gastrostomy (n = 29,937) | OR, unadjusted (95% CI) | OR, adjusteda (95% CI) | |
|---|---|---|---|---|
| Age | ||||
| 18–39 years | 188 (12%) | 3666 (12.4%) | REF | REF |
| 40–49 years | 405 (25.8%) | 6203 (20.9%) | 1.28 (0.86–1.89) | 1.2 (0.81–1.79) |
| 50–59 years | 383 (24.4%) | 7971 (26.9%) | 0.94 (0.64–1.37) | 0.88 (0.6–1.3) |
| 60–69 years | 332 (21.1%) | 6233 (21%) | 1.04 (0.7–1.55) | 0.95 (0.63–1.43) |
| 70–79 years | 191 (12.2%) | 3927 (13.3%) | 0.95 (0.61–1.49) | 0.82 (0.51–1.33) |
| ≥80 years | 73 (4.6%) | 1632 (5.5%) | 0.87 (0.48–1.59) | 0.75 (0.4–1.38) |
| Race | ||||
| White | 1156 (71.6%) | 23,766 (79.4%) | REF | REF |
| Black | 232 (14.4%) | 2967 (9.9%) | 1.61 (1.16–2.23) | 1.55 (1.09–2.21) |
| Hispanic | 138 (8.6%) | 1998 (6.7%) | 1.42 (0.95–2.13) | 1.32 (0.85–2.03) |
| Asian or Pacific Islander | 20 (1.3%) | 301 (1%) | 1.39 (0.51–3.84) | 1.35 (0.49–3.69) |
| Native American | 12 (0.7%) | 70 (0.2%) | 3.45 (1.24–9.58) | 3.47 (1.21–9.95) |
| Other | 55 (3.4%) | 840 (2.8%) | 1.36 (0.75–2.46) | 1.32 (0.7–2.47) |
| Gender | ||||
| Male | 769 (47.7%) | 13,680 (45.7%) | REF | REF |
| Female | 845 (52.3%) | 16,262 (54.3%) | 0.92 (0.74–1.15) | 0.94 (0.75–1.17) |
| Elective admission (no, %) | ||||
| No | 1412 (87.5%) | 26,199 (87.5%) | REF | REF |
| Yes | 202 (12.5%) | 3743 (12.5%) | 1 (0.71–1.41) | 0.99 (0.69–1.41) |
| Insurance | ||||
| Medicare | 1055 (65.4%) | 18,223 (60.9%) | 1.29 (0.92–1.81) | 1.43 (1–2.05) |
| Medicaid | 318 (19.7%) | 6034 (20.2%) | 1.18 (0.8–1.73) | 1.11 (0.73–1.69) |
| Private insurance | 196 (12.2%) | 4371 (14.6%) | REF | REF |
| Self-pay | 13 (0.8%) | 614 (2.1%) | 0.48 (0.14–1.59) | 0.48 (0.14–1.61) |
| No-charge/other | 31 (1.9%) | 700 (2.3%) | 0.99 (0.43–2.27) | 0.88 (0.36–2.19) |
| Median income by ZIP | ||||
| Lowest $1–$37,999 | 485 (30%) | 9085 (30.3%) | 1 (0.72–1.41) | 0.89 (0.63–1.27) |
| $38,000–$47,999 | 414 (25.7%) | 8469 (28.3%) | 0.92 (0.66–1.29) | 0.89 (0.63–1.26) |
| $48,000–$63,999 | 418 (25.9%) | 6815 (22.8%) | 1.15 (0.82–1.62) | 1.15 (0.82–1.62) |
| Highest $64,000+ | 297 (18.4%) | 5574 (18.6%) | REF | REF |
| Hospital size | ||||
| Small | 171 (10.6%) | 4401 (14.7%) | REF | REF |
| Medium | 453 (28.1%) | 7944 (26.5%) | 1.47 (0.98–2.22) | 1.49 (0.97–2.29) |
| Large | 990 (61.3%) | 17,598 (58.8%) | 1.45 (0.99–2.14) | 1.48 (0.99–2.21) |
| Teaching status | ||||
| No | 951 (58.9%) | 18,489 (61.8%) | REF | REF |
| Yes | 663 (41.1%) | 11,453 (38.2%) | 0.89 (0.7–1.12) | 0.92 (0.73–1.17) |
| Location | ||||
| Northeast | 435 (27%) | 7768 (25.9%) | REF | REF |
| South | 240 (14.9%) | 5673 (18.9%) | 0.76 (0.51–1.12) | 0.74 (0.49–1.11) |
| Midwest | 610 (37.8%) | 10,846 (36.2%) | 1 (0.75–1.34) | 1.01 (0.75–1.35) |
| West | 329 (20.4%) | 5655 (18.9%) | 1.04 (0.74–1.45) | 0.95 (0.68–1.33) |
Adjusted for all variables in this table.
Next, we examined the medical comorbidities and associated diagnoses for HD patients who underwent inpatient gastrostomy (Table 2). The distribution of inpatient HD gastrostomy patients was skewed towards higher Elixhauser comorbidity scores. The most common associated diagnoses were aspiration pneumonia (34.1%), dementia (31.3%), malnutrition (30.3%), and dysphagia (29.5%). Dementia and delirium were both more common among HD patients undergoing gastrostomy than those who did not receive gastrostomy (p < 0.0001 and p = 0.003, respectively). Of those with dementia (weighted sample size = 515), 243 (47.2%) also had dysphagia, whereas 272 (52.8%) did not.
Table 2.
Diagnoses and comorbidities associated with inpatient gastrostomy for Huntington's disease vs. amyotrophic lateral sclerosis.
| HD |
ALS |
||||
|---|---|---|---|---|---|
| Number (%) with gastrostomy | Number (%) without gastrostomy | Number (%)with gastrostomy | Number (%) without gastrostomy | p-Value (HD gastrostomy vs. ALS gastrostomy) | |
| Elixhauser comorbidity index | 0.01 | ||||
| 0–1 | 195 (12.1%) | 5229 (17.5%) | 876 (11.1%) | 8861 (15.3%) | |
| 2 | 399 (24.7%) | 8935 (29.8%) | 1761 (22.3%) | 14,080 (24.3%) | |
| 3 | 512 (31.7%) | 7601 (25.4%) | 2019 (25.5%) | 14,947 (25.8%) | |
| 4+ | 507 (31.4%) | 8172 (27.3%) | 3253 (41.1%) | 20,152 (34.7%) | |
| Aspiration pneumonia | 550 (34.1%) | 3463 (11.6%) | 1620 (20.5%) | 6778 (11.7%) | <0.0001 |
| Dementia | 505 (31.3%) | 7613 (25.4%) | 374 (4.7%) | 2527 (4.4%) | <0.0001 |
| Malnutrition | 489 (30.3%) | 1817 (6.1%) | 2226 (28.2%) | 3944 (6.8%) | 0.48 |
| Dysphagia | 477 (29.6%) | 1726 (5.8%) | 2470 (31.2%) | 5037 (8.7%) | 0.59 |
| Infection, sepsis | 453 (28.1%) | 5479 (18.3%) | 1085 (13.7%) | 10,388 (17.9%) | <0.0001 |
| Delirium, dementia, and amnestic and other cognitive disorders | 277 (17.2%) | 4329 (14.5%) | 244 (3.1%) | 1633 (2.8%) | <0.0001 |
| Mechanical ventilation | 234 (14.5%) | 1369 (4.6%) | 2553 (32.3%) | 16,525 (28.5%) | <0.0001 |
| Anorexia | 33 (2.1%) | 208 (0.7%) | 133 (1.7%) | 264 (0.5%) | 0.67 |
3.2. Outcomes associated with gastrostomy in HD
The median hospital length of stay for HD patients who underwent inpatient gastrostomy tube placement was 8.04 days (interquartile range: 4.34–14.32 days). Thirty five patients (2.25%) died during hospitalization. Of the remaining 1574 patients who were discharged, the majority (n = 1201, 69.9%) were discharged to a skilled nursing facility or other facility. Of the 473 patients who were discharged home, 228 (48.2%) required home health services, and only 245 (15.2% of all discharges) were considered routine.
The patient- and hospital-level features associated with non-routine discharge and prolonged length of stay for HD inpatient gastrostomy are shown in in Table 3. Women had a lower odds of prolonged length of stay compared to men (OR 0.85, 95% CI: 0.73–1.0), as did those from the Midwest compared to the Northeast (OR 0.29, 95% CI: 0.09–0.97). Otherwise, there were no differences in outcome after gastrostomy between age, race, insurance payer, median income by ZIP, or hospital characteristics. Dementia (OR 3.27, 95% CI: 1.31–8.20) and delirium (OR 8.18, 95% CI: 1.35–49.66) were both strongly associated with non-routine discharge but not prolonged length of stay. Aspiration pneumonia (OR 1.39, 95% CI: 1.2–1.62), sepsis (OR 1.76, 95% CI: 1.81–2.05), and mechanical ventilation (OR 2.17, 95% CI: 1.72–2.73), and higher Elixhauser comorbidity scores were associated with prolonged length of stay but not with non-routine discharge.
Table 3.
Adjusteda odds ratios for discharge outcomes as a function of patient- and hospital-level characteristics and comorbidities for inpatient gastrostomy in Huntington's disease.
| Non-routine discharge | Prolonged length of stay | |
|---|---|---|
| Race | ||
| White | REF | REF |
| Black | 1.33 (0.47–3.72) | 1.1 (0.87–1.4) |
| Hispanic | 0.43 (0.13–1.43) | 1.08 (0.79–1.46) |
| Other | 0.25 (0.06–1.05) | 1.37 (0.87–2.14) |
| Gender | ||
| Male | REF | REF |
| Female | 0.69 (0.34–1.42) | 0.85 (0.73–1) |
| Age | ||
| 18–39 years | REF | REF |
| 40–49 years | 1.79 (0.67–4.81) | 0.98 (0.7–1.38) |
| 50–59 years | 2.11 (0.81–5.5) | 1.04 (0.76–1.43) |
| 60–69 years | 2.88 (0.79–10.52) | 0.85 (0.61–1.17) |
| 70–79 years | 3.06 (0.7–13.45) | 1.07 (0.74–1.56) |
| ≥80 years | 0.95 (0.16–5.65) | 1.1 (0.7–1.72) |
| Insurance | ||
| Medicare | 1.45 (0.55–3.82) | 0.85 (0.65–1.12) |
| Medicaid | 1.28 (0.43–3.79) | 0.82 (0.59–1.13) |
| Private insurance | REF | REF |
| Self-pay, no-charge, or other | 1.31 (0.2–8.7) | 1.06 (0.63–1.78) |
| Median income by ZIP | ||
| $1–$37,999 | 0.95 (0.33–2.75) | 0.91 (0.71–1.18) |
| $38,000–$47,999 | 1 (0.37–2.7) | 0.95 (0.75–1.21) |
| $48,000–$63,999 | 2.37 (0.74–7.56) | 0.97 (0.77–1.21) |
| $64,000+ | REF | REF |
| Hospital size | ||
| Small | REF | REF |
| Medium | 1.7 (0.56–5.14) | 1.04 (0.81–1.34) |
| Large | 0.94 (0.36–2.46) | 1 (0.8–1.25) |
| Teaching status | ||
| No | 1.44 (0.72–2.89) | 0.96 (0.81–1.14) |
| Yes | REF | REF |
| Location | ||
| Northeast | REF | REF |
| South | 0.45 (0.13–1.53) | 0.88 (0.68–1.14) |
| Midwest | 0.29 (0.09–0.96) | 0.94 (0.76–1.17) |
| West | 1.06 (0.25–4.44) | 0.88 (0.68–1.15) |
| Elective admission (yes versus no) | 0.42 (0.17–1.04) | 0.58 (0.45–0.75) |
| Elixhauser comorbidity index (quartiles) | ||
| 0–1 | REF | REF |
| 2 | 1.49 (0.55–4.04) | 0.87 (0.64–1.16) |
| 3 | 1.23 (0.48–3.16) | 1.03 (0.77–1.37) |
| 4+ | 1.59 (0.55–4.59) | 1.19 (0.88–1.59) |
| Diagnoses | ||
| Aspiration pneumonia | 0.71 (0.32–1.60) | 1.39 (1.2–1.62) |
| Dementia | 3.27 (1.31–8.20) | 0.98 (0.82–1.16) |
| Malnutrition | 0.94 (0.41–2.15) | 0.84 (0.7–1.01) |
| Dysphagia | 0.53 (0.20–1.39) | 0.84 (0.68–1.03) |
| Infection, sepsis | 0.75 (0.31–1.80) | 1.76 (1.51–2.05) |
| Delirium, dementia, and amnestic and other cognitive disorders | 8.18 (1.35–49.66) | 0.92 (0.74–1.15) |
| Mechanical ventilation | 1.81 (0.39–8.46) | 2.17 (1.72–2.73) |
Adjusted for age, race, gender, insurance, median income by ZIP, hospital size and teaching status, geographic location, elective admission, Elixhauser comorbidity index, and year.
3.3. Gastrostomy for HD versus ALS
Compared to inpatient gastrostomy patients with ALS, those with HD had significantly greater odds of aspiration pneumonia (34.1% vs. 20.5%, p < 0.0001) and sepsis (28.1% vs. 13.7%, p < 0.0001). Dementia and delirium were both much more common in HD than ALS admissions with gastrostomy (p < 0.0001). Mechanical ventilation was more common in ALS than HD. Compared to inpatient ALS gastrostomies, HD patients had longer length of stay (OR 1.14, 95% CI: 1.02–1.28) and greater skilled nursing facility discharge (p < 0.0001 for Wald chi square test) but less in-hospital mortality (OR 0.35, 95% CI: 0.16–0.77).
4. Discussion
In this study of over 30,000 hospital admissions with HD in the U.S. over a 10-year period, 1614 (5.12%) were associated with gastrostomy tube placement. Black or Native American patients and Medicare beneficiaries were more likely to undergo gastrostomy placement during admission. Gastrostomy was associated with diagnoses of aspiration pneumonia, dementia, and delirium; prolonged hospital length of stay; and discharge to a skilled nursing facility. These outcomes were more frequent than a comparable group of ALS patients receiving inpatient gastrostomy.
The association between Medicare coverage and gastrostomy in HD patients is likely confounded by disease severity. Specifically, older adults in the U.S. automatically become eligible for Medicare at age 65, but individuals with disabling medical conditions can become eligible before age 65. Over 65% of admitted HD patients who underwent gastrostomy received Medicare coverage, yet <37% would have met eligibility criteria by age. We therefore suspect that the majority of Medicare beneficiaries with HD are eligible due to disability, and that even after adjusting for age, the greater disease duration and severity which result in disability increase the odds of gastrostomy.
The association between race or ethnicity and gastrostomy in HD is less clear. Previous studies have shown differences in HD incidence according to nationality or ethnic background [[8], [9], [10]], but these have not examined outcomes such as gastrostomy. It is possible that race is a proxy for socioeconomic status and that decreased access to resources such as speech therapy or home health aides for supervised oral feeding results in earlier initiation of enteral support. However, we did not find an association between median income by ZIP and gastrostomy in this population. Interestingly, an analysis of administrative claims data from the Veterans Health Administration also found a higher incidence of gastrostomy among Black veterans with dementia compared to White veterans with dementia, the reasons for which remain unclear but have been hypothesized to reflect differences in end-of-life healthcare decision-making [11]. Of note, the overall prevalence of Black and Hispanic HD patients in the National Inpatient Sample (10.1% and 6.8%, respectively) is lower than current U.S. Census estimates (13.4% and 18.3%, respectively) [12], especially for Hispanics. The reasons for this are also not clear. The prevalence of HD is generally reported to be higher in Northern European populations [13], possibly due to genetic founder effects, and multicenter HD cohorts have found even lower prevalences of Black and Hispanic patients of 1–2.5% [14,15]. However, the latter are limited by research recruitment and participation, and as referral for genetic testing is required for a diagnosis of HD, under-ascertainment among minority populations is also possible.
Over 30% of HD patients undergoing gastrostomy were diagnosed with dementia, an inevitable consequence of this disease, and over half of these patients did not carry a diagnosis of dysphagia. This suggests that dementia may have been the primary indication for gastrostomy placement in a significant proportion of the HD population. Current guidelines recommend against feeding tube placement for advanced dementia, suggesting that up to 30% of gastrostomies placed for HD are in conflict with best practice recommendations. However, these guidelines are based primarily on data from Alzheimer's disease and may not be generalizable to HD. Furthermore, “advanced dementia” is defined in these studies using scales such as the Functional Assessment staging tool or Clinical Dementia Rating scale, but the NIS does not contain information regarding dementia severity or associated defining features (e.g. incontinence, need for assistance with all activities of daily living), so it is difficult to tell whether the HD patients undergoing gastrostomy in this cohort fall into this category or not.
Our primary outcome analyses revealed that gastrostomy among HD inpatients was associated with prolonged hospital length of stay and discharge to a skilled nursing facility or other facility rather than home. Previous NIS data have shown that inpatient admission for HD in general frequently results in discharge to a facility rather than home, regardless of gastrostomy [16]. This reflects the challenges in maintaining care at home in the presence of motor and cognitive disability.
In addition to providing national data on gastrostomy utilization and outcomes in HD, we also compared the indications and outcomes for gastrostomy between HD and ALS, a neuromuscular disorder for which gastrostomy has been demonstrated to improve survival and quality of life [3]. Inpatient gastrostomy for HD was frequently associated with diagnoses of dysphagia, malnutrition, and aspiration pneumonia, which are all common indications for enteral support in patients with neurologic disease. ALS inpatients receiving gastrostomy had a similar prevalence of dysphagia and malnutrition, but a significantly lower prevalence of aspiration pneumonia compared to HD. We believe this reflects the fact that gastrostomy is initiated earlier in ALS, often prior to an aspiration pneumonia or other sentinel decompensation event, than in HD, in accordance with clinical guidelines [3]. However, it is difficult to definitively ascertain from claims data whether aspiration pneumonia occurred before or after gastrostomy, and it is also possible that ALS patients receive earlier speech therapy and other non-surgical interventions to prevent aspiration compared to HD. Inpatient gastrostomy for HD was also associated with a greater odds of non-routine discharge than inpatient gastrostomy for ALS, again reflecting the fact that other variables besides gastrostomy affect discharge outcomes. Given that dementia is a key driver of nursing home placement in the older adult population, especially in the setting of neurodegenerative disease, we hypothesize that the increased prevalence of dementia in HD compared to ALS at least partly explains this difference in discharge outcome.
Hospital length of stay and discharge outcomes are frequently grouped together for analysis in health services research, but we observed an important difference in their risk factors among HD inpatients with gastrostomy: intrinsic HD-related comorbidities (e.g. dementia, delirium) were associated with discharge outcome but not length of stay, whereas infectious complications and other medical comorbidities (e.g. aspiration pneumonia, sepsis, Elixhauser comorbidity index) were associated with length of stay but not discharge outcome. This has important implications for the potential benefits of future healthcare interventions. Specifically, interventions to prevent aspiration pneumonia or other infectious complications may reduce length of stay but may not determine the ultimate hospitalization outcome at discharge. Likewise, the presence of dementia may limit measures designed to increase the probability of home discharge.
Limitations of this study include its reliance on administrative claims coding of HD, which has not previously been validated [17], though we estimate misclassification of HD to be low as it is a discrete disorder with a single confirmatory genetic test rather than a purely clinical diagnosis without defined confirmatory testing. As the NIS dataset consists solely of inpatient data, we were unable to obtain information regarding genetic testing, symptom duration and treatment, or precise nutritional status (e.g. weight or body mass index). As neurologic severity and malnutrition are associated with both gastrostomy utilization and health outcomes, these could have confounded the associations between gastrostomy and outcomes such as LOS and discharge status. We are also unable to capture outpatient surgical encounter data, and the proportion of gastrostomies performed for HD on an inpatient vs. outpatient basis is unknown. Additionally, because the NIS is an encounter-level rather than patient-level database, it is possible that the same patient was admitted and counted more than once. Nevertheless, we were able to demonstrate important clinical and demographic factors associated with gastrostomy utilization and outcomes in HD and key differences from ALS using a nationally representative patient sample.
In summary, gastrostomy tube placement for HD frequently co-occurs with aspiration pneumonia, delirium, and dementia and is associated with prolonged LOS and discharge to skilled nursing facilities. Future studies of gastrostomy in HD care should examine the timing of gastrostomy with respect to dysphagia vs. dementia, the use of palliative care services, and patient-reported outcomes such as quality of life.
The following is the supplementary data related to this article.
Diagnostic and procedural code definitions for associated medical conditions.
Funding sources
This work was supported by the National Institutes of Health [NINDS T32 NS061779-10 (AGH), NINDS R01 NS099129-01A1 (AWW)].
Financial disclosures for the previous 12 months
Dr. Hamedani reports speaking honoraria from Northwell Health. Dr. Gonzalez-Alegre reports consulting fees from Accorda Therapeutics and SAGE Therapeutics. Ms. Pauly, Mr. Thibault, and Dr. Willis report no financial disclosures.
CRediT authorship contribution statement
Ali G. Hamedani: Conceptualization, Funding acquisition, Methodology, Writing - original draft, Writing - review & editing. Meredith Pauly: Conceptualization, Writing - review & editing. Dylan P. Thibault: Data curation, Formal analysis, Methodology. Pedro Gonzalez-Alegre: Conceptualization, Writing - review & editing. Allison W. Willis: Conceptualization, Funding acquisition, Methodology, Writing - review & editing.
Declaration of competing interest
The authors report no conflicts of interest.
References
- 1.Kay C., Hayden M.R., Leavitt B.R. Epidemiology of Huntington disease. Handb. Clin. Neurol. 2017;144:31–46. doi: 10.1016/B978-0-12-801893-4.00003-1. [DOI] [PubMed] [Google Scholar]
- 2.Stavroulakis T., McDermott C.J. Enteral feeding in neurological disorders. Pract. Neurol. 2016;16(5):352–361. doi: 10.1136/practneurol-2016-001408. [DOI] [PubMed] [Google Scholar]
- 3.Miller R.G., Jackson C.E., Kasarskis E.J. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73(15):1218–1226. doi: 10.1212/WNL.0b013e3181bc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee American Geriatrics Society feeding tubes in advanced dementia position statement. J. Am. Geriatr. Soc. 2014;62(8):1590–1593. doi: 10.1111/jgs.12924. [DOI] [PubMed] [Google Scholar]
- 5.Heemskerk A.-W., Roos R.A.C. Dysphagia in Huntington’s disease: a review. Dysphagia. 2011;26(1):62–66. doi: 10.1007/s00455-010-9302-4. [DOI] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality R MD . Healthcare Cost and Utilization Project (HCUP) 2012. HCUP National Inpatient Sample (NIS)www.hcup-us.ahrq.gov/nisoverview.jsp [Google Scholar]
- 7.Moore B.J., White S., Washington R., Coenen N., Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity index. Med. Care. 2017;55(7):698–705. doi: 10.1097/MLR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 8.Bruzelius E., Scarpa J., Zhao Y., Basu S., Faghmous J.H., Baum A. Huntington’s disease in the United States: variation by demographic and socioeconomic factors. Mov Disord Off J Mov Disord Soc. 2019;34(6):858–865. doi: 10.1002/mds.27653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon P.H., Mehal J.M., Rowland A.S., Cheek J.E., Bartholomew M.L. Huntington disease among the Navajo: a population-based study in the Navajo Nation. Neurology. 2016;86(16):1552–1553. doi: 10.1212/WNL.0000000000002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pringsheim T., Wiltshire K., Day L., Dykeman J., Steeves T., Jette N. The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov Disord Off J Mov Disord Soc. 2012;27(9):1083–1091. doi: 10.1002/mds.25075. [DOI] [PubMed] [Google Scholar]
- 11.Braun U.K., Rabeneck L., McCullough L.B. Decreasing use of percutaneous endoscopic gastrostomy tube feeding for veterans with dementia-racial differences remain. J. Am. Geriatr. Soc. 2005;53(2):242–248. doi: 10.1111/j.1532-5415.2005.53109.x. [DOI] [PubMed] [Google Scholar]
- 12.U.S Census Bureau QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045218
- 13.Rawlins M.D., Wexler N.S., Wexler A.R. The prevalence of Huntington’s disease. Neuroepidemiology. 2016;46(2):144–153. doi: 10.1159/000443738. [DOI] [PubMed] [Google Scholar]
- 14.Huntington Study Group PHAROS Investigators At risk for Huntington disease: the PHAROS (Prospective Huntington At Risk Observational Study) cohort enrolled. Arch. Neurol. 2006;63(7):991–996. doi: 10.1001/archneur.63.7.991. [DOI] [PubMed] [Google Scholar]
- 15.Landwehrmeyer G.B., Fitzer-Attas C.J., Giuliano J.D. Data analytics from enroll-HD, a global clinical research platform for Huntington’s disease. Mov Disord Clin Pract. 2017;4(2):212–224. doi: 10.1002/mdc3.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubinsky R.M. No going home for hospitalized Huntington’s disease patients. Mov Disord Off J Mov Disord Soc. 2005;20(10):1316–1322. doi: 10.1002/mds.20589. [DOI] [PubMed] [Google Scholar]
- 17.St Germaine-Smith C., Metcalfe A., Pringsheim T. Recommendations for optimal ICD codes to study neurologic conditions: a systematic review. Neurology. 2012;79(10):1049–1055. doi: 10.1212/WNL.0b013e3182684707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagnostic and procedural code definitions for associated medical conditions.
Data Availability Statement
The full dataset is publicly available through HCUP.