Abstract
Background
Essential Tremor (ET) is one of the most common movement disorders but many controversies still exist in regards to its definition and pathophysiology. In view of the recent published criteria by the Tremor Task Force of the International Parkinson's and Movement Disorders Society (IPMDS), we intended to analyze if this has changed our view of ET and if new developments have arisen since.
Methods
A Medline search for English-written articles was done on June 15, 2019 using the keyword “Essential Tremor”. Publications from November 2017 (publication date of the new tremor classification) were taken into account. Reviews, letters and original studies relevant to the subject were selected and reviewed according to the following themes: clinical characteristics, epidemiology, genetics, pathology, biomarkers and treatment.
Results
Out of 132 publications the most relevant articles were selected and reviewed (total of 65 articles). The great majority of these studies focused on surgical treatments (new targets, new technologies) while relatively few articles addressed epidemiology, pathology and pathophysiology.
Conclusions
The use of the new classification is not commonly used still, excepting more recent studies on therapeutics. This is in keeping with diverse opinions and criticisms reported by the IPMDS task force members themselves. One important change has been validating ET as a heterogeneous condition and defining the ET-plus category. We propose a further sub-group classification derived from the new definition of ET-plus.
Keywords: Essential tremor, Tremor
Highlights
-
•
ET is an isolated tremor syndrome characterized by bilateral upper limb action tremor.
-
•
Boundaries between ET and ET-plus are not defined, making the latter much more common.
-
•
No causative gene, no precise pathology or biomarker for ET has been isolated.
-
•
Medication is effective only in half of patients.
-
•
Surgical treatment (new targets and technologies) is highly effective.
1. Introduction
Essential tremor (ET) is one of the most common movement disorders. In November 2017, the Tremor Task Force of the International Parkinson's and Movement Disorders Society (IPMDS) published a new tremor classification. In it, ET was defined as an isolated tremor syndrome involving both upper limbs during action for a minimum time frame of 3 years, with or without tremor in other body segments (Table 1) [1]. Furthermore, the construct of “ET-plus” was defined for those ET patients also presenting with other subtle signs, which are not evident enough to change the diagnosis to another clinical entity. Examples of these ‘soft signs’ are difficulties in tandem gait, questionable dystonic posturing, rest tremor, other non-motor symptoms such as mild memory impairment or any other mild neurological signs of unknown significance [1].
Table 1.
ET diagnostic criteria by the IPMDS Tremor Task Force (from [1]).
| Essential tremor diagnostic criteria |
|---|
| • Isolated tremor syndrome of bilateral upper limb action tremor |
| • At least 3 years duration |
| • With or without tremor in other segments (head, voice, lower limbs) |
| • Absence of other neurological signs (dystonia, ataxia, parkinsonism) |
| ET-plus |
| ET with additional neurological signs of uncertain significance (e.g. impaired tandem gait, questionable dystonic posturing, memory impairment, others) |
| Exclusion criteria |
| • Isolated focal tremor (voice, head) |
| • Orthostatic tremor with a frequency >12 Hz |
| • Task- or position-specific tremors |
| • Sudden onset and stepwise deterioration |
Abbreviations: ET: Essential Tremor; IPMDS: International Parkinson and Movement Disorders Society.
In the following paragraphs we will focus on the main publications after the release of this new tremor classification in order to further discuss how it has changed our view of ET.
2. Methods
A Medline search for English-written articles was done on June 15, 2019 using the keyword “Essential Tremor”. Publications from November 2017 onwards were taken into account. The search yielded 132 publications, of which a total of 65 reviews, letters and original studies relevant to the subject were selected and reviewed according to the following themes: clinical characteristics, epidemiology, genetics, pathology, biomarkers and treatment. These articles were selected based on the personal opinion of the authors, according to relevance identified by screening the title and abstract. Particular emphasis was given to studies discussing novel findings in any of the selected themes. In case relevant references were mentioned in any of these articles which were not identified by our first search, these were also included for review.
3. Clinical characteristics
Although the new IPMDS classification finally validates the well-known heterogeneity in ET, it also adds complexity and doubts of what ET truly is [2]. A frequent critique to the new definition is the difficulty in defining the accuracy and clinical meaning of soft signs, as these features are susceptible to a wide range of inter-observer variability [3]. Cerebellar signs (subclinical ataxia, eye movement abnormalities) and other soft signs have been described so frequently in these patients that ET-plus has been argued to be much more common than “pure ET” [4].
Resting tremor in ET is not an uncommon finding, and has been reported in up to 18–30% of patients [5,6]. Some authors state that resting tremor is more common in severe and longstanding cases. Lower limb tremor can also be found in ET and ET-plus patients, although it is rare (respectively seen in 28.6% and 51.4% of patients) [7]. Lower limb tremor is more common in dystonia, functional disorders, cerebellar tremors and Parkinson's disease (PD) but – when found in ET – it denotes longer and more severe disease than patients without it [7].
Differentiating ET from dystonic tremors can be specially challenging in patients with “ET-like tremors”. Dystonic tremor aside (i.e., when tremor affects the body region also presenting dystonia), tremor involving a different body segment from the one affected by dystonia should be labelled as ‘tremor associated with dystonia’, as proposed in the first tremor classification, two decades ago [8]. Likewise, ‘tremor associated with a dystonia gene’ (i.e., when no dystonia is seen in an ET-like tremor patient but dystonia is present among their family members) is also a useful construct especially for clinico-genetic studies [8]. Interestingly, neither tremor associated with dystonia nor tremor associated with a dystonia gene are mentioned in the new classification [1]. This probably reflects some intrinsic problems with these definitions. For example, the recognition of dystonia in family members is very low when patients are simply asked and no formal evaluation of the family is carried out [9].
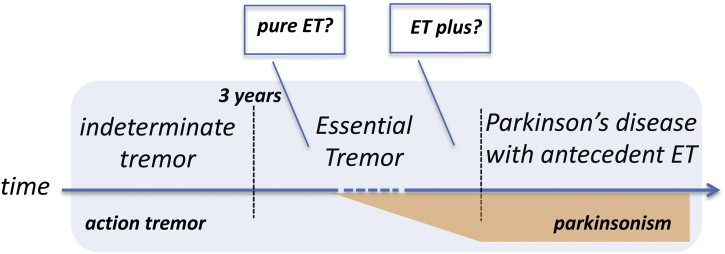
Another critical issue is the arbitrary time frame of 3 years to classify an action hand tremor as ET. “Indeterminate tremor” is the term used for patients whose disease duration is shorter than this proposed cut-off (Fig. 1) [1]. Three years is probably too short considering that most ET patients seek medical attention many years after tremor onset. Nevertheless, this cut-off might favor the differentiation between ET and medication/toxin-related tremor or enhancement of physiological tremor, which normally resolves when the causing factor is withdrawn. Accordingly, a recent study addressing the clinical differences between valproate-induced tremor versus ET found that the former is seen in younger patients and with a shorter disease duration by the time they consulted medical attention (1.8 years versus 15.2 years for ET patients) [10].
Fig. 1.
The possible evolution of a patient with action tremor later developing a neurodegenerative disease (PD in this example) based on the new tremor classification by the IPMDS Tremor Task Force.
Although ET patients present with a longer disease duration compared to PD or dystonic tremor [11], the differential diagnosis can still be difficult in some cases. Another layer of complexity is represented by those ET patients that are later diagnosed with PD, a condition labelled as “PD with antecedent ET” (Fig. 1) [1]. This clinical scenario is theoretically possible for many other conditions (e.g. “dystonia with antecedent-ET”), which are however not considered in the new classification.
Non-motor symptoms have been reported with increasing frequency in ET [12]. Cognitive impairment, hearing loss, dysautonomic features, neuropsychological comorbidities like depression and anxiety as well as sleep disorders like REM behavioural disorder (RBD) have been described. A recent Portuguese study reported that 98% of ET patients had at least one symptom of autonomic dysfunction (urinary being the most frequent). Authors also described a frequency of 27% of probable RBD (questionnaire-based) in ET patients who also happened to have higher autonomic dysfunction especially in the thermoregulatory sphere [13]. Unfortunately, this study used the old criteria for ET and included patients that would be disregarded according to the new criteria (e.g. isolated head tremor).
4. Epidemiology
Clinical manifestations and epidemiology seem to be connected in ET. For instance, a study found that ET incidence has two peaks: young-onset (<25 years) and late-onset (>65 years) [14]. Young-onset ET is more often characterized by a positive family history and alcohol responsiveness. Late-onset ET is linked to a greater frequency of dementia [12]. This bimodal distribution of ET cases might explain some of the inconsistencies seen in the past, e.g. the variable mortality rates [15].
A metanalysis found an overall ET prevalence of 0.4–0.9% (all ages) and of 4.6–6.3% in populations over 65 years [16]. The issue of multiple definitions of ET was taken into account by Eliasen et al. [17], although they used definitions derived from a population-based genetic study rather than the new IPMDS criteria. These authors studied the very enclosed population of the Faroe Island. During the first phase of the study, a questionnaire was randomly sent to subjects over 39 years. The second phase was a medical evaluation by a movement disorders specialist of the selected candidates according to their responses. A total prevalence of 2.9%, 3.1% and 4.8% was respectively found for the entire sample, for individuals over 40 and over 69 [17].
As for the incidence, there are no new studies using the new IPMDS criteria within the timeframe studied. A previous study reported an incidence of 0.6% in the Spanish population over 65, although – again –the authors acknowledged the difficulties in estimating prevalence and incidence for ET given so many different definitions [12].
5. Genetics
On an etiologic level, ET is considered to have a strong genetic component. Indeed, first-degree relatives of ET patients are 4–5 times more likely than first-degree relatives of controls to develop ET [12]. It is estimated that between 20% and 90% of ET patients have a positive family history, which is more frequent in young-onset cases [18,19]. There is a concordance between 60 and 77% in monozygotic twins [12,20].
In spite of these observations, identifying causal genes in mono or polygenic forms has proven challenging. Three loci were identified in linkage studies (ETM 1, 2 and 3), but no causal gene was found [21]. Genes associated with the risk of developing ET have been described: LINGO1, SLC1A2/EAAT2, SORT1, SCN4A, NOS3, KCN52, among others [12,21]. Recently, FUS mutation was described in one family affected by ET [18]. Another separate family, presenting with ET and PD in different family members, described mutations in the HTRA2 gene, with an earlier onset and more severe presentation (including PD) in the recessive dominant cases versus the autosomal ones [22]. The role of FUS and HTRA2 mutations have not been confirmed in other cohorts of patients.
5.1. The relationship between Essential tremor and Parkinson's disease
The discovery of HTRA2 mutated cases emphasized the well-known debate on whether a relationship between ET and PD really exists [23]. These are very common movement disorders, especially in elderly populations, and several studies have shown a significant overlap between them [23,25]. ET has been seen as a risk factor for the future development of PD, with some studies reporting up to a 4-fold increase [12]. Also, ET is more common in relatives of PD patients and, as already mentioned, ET and PD can co-exist among family members, possibly indicating ET-related genes might predispose to the future development of PD. A recent Canadian study searched for a potential genetic overlap between ET and PD in 717 PD patients and 595 controls from French and French-Canadian descent [26]. The authors tested 26 loci derived from genome-wide association studies in ET patients and found no role for them in PD patients. Similarly, a Chinese group studied LRRK1 and LRKK2 variants (a common cause of monogenic PD) in a cohort of ET patients, again finding no association [20].
6. Pathology
The pathology background is filled with inconsistent findings and only descriptive analyses with no controls were published before 2004 [12]. Subsequent controlled studies were performed showing inconsistent results. Louis et al. have described two types of mutually exclusive pathology: ET with brainstem Lewy Bodies (within the locus coeruleus in particular) and ET with loss of Purkinje cells (PC), presence of Torpedos (i.e., ovoid enlargement of the proximal part of PC's axon) and PC heterotopias (i.e., misplacement of the PC soma in the molecular layer of the cerebellum) [12,[27], [28], [29]]. The same research group recently compared 43 brains of ET patients with 22 age-matched controls and 31 spino-cerebellar ataxia (SCA) patients (SCA1, 2, 3 and 6). ET patients had moderate loss of PC and number of heterotopias, an intermediate degree between controls and SCA patients that had the greatest PC loss (except for SCA3) and heterotopias. Overall, their hypothesis is that ET is a neurodegenerative condition at an intermediate stage compared to cerebellar degeneration seen in SCAs [29]. Other researchers were not able to replicate the aforementioned pathological findings [27]. In fact, some authors have seen PC loss as a product of aging and torpedos as an unspecific finding of many degenerative ataxias.
Despite these conflicting pathologic findings, there are other types of studies supporting a cerebellar origin of ET. For example, volumetric studies by means of magnetic resonance imaging (MRI) have consistently found cerebellar atrophy in ET patients relative to controls or PD patients [28,30].
7. Biomarkers
7.1. Biochemical and other systemic biomarkers
In the search of a valid biomarker for ET, uric acid has been studied recently. This metabolite is known as a natural antioxidant and could have a protective role in neurodegeneration. It has been reported to be low in several neurodegenerative diseases, such as PD, Alzheimer's disease and amyotrophic lateral sclerosis [31]. A prospective study, dividing cases of sporadic-ET and hereditary-ET, compared their levels of uric acid with controls. No significant differences were found, thus not supporting a neuroprotective role of uric acid in ET. However, it is interesting to note that there was a correlation between lower levels of uric acid and higher age of onset in sporadic cases that could point to its role as a marker of neurodegeneration in those patients [32].
Retinal thickness measured through optical coherence tomography has been discovered to be low in several neurodegenerative diseases such as PD, Alzheimer's Disease and others. A group studied this phenomenon in young-onset ET patients as a surrogate measure of neurodegeneration. In keeping with previous studies, they found a bilateral decrement of retinal nerve fiber layer thickness only in the nasal area in ET patients compared to controls. Furthermore, this study also found an increase of the global thickness of the choroid layer, which indicates more vascularization and possible inflammation, features supposed to occur prior to neurodegeneration [33].
7.2. Brain imaging
Dopamine transporter (DAT) imaging has been proposed as a useful tool to differentiate tremor dominant-PD from ET with 84.4% sensitivity and 96.2% specificity, but this technique is expensive and involves use of radiation [34]. As an alternative, brain MRI has been studied in the differential diagnosis between PD and ET. Neuromelanin inside the dopaminergic neurons of the substantia nigra has paramagnetic properties that makes it visible with high resolution T1-weighted fast spin echo imaging, the so-called neuromelanin-sensitive MRI (NM-MRI). This signal has been shown to be decreased in PD patients, while it remains unchanged in ET [34]. The absence of nigrosome-1 (N1) in T2*-weighted or susceptibility weighted imaging (SWI) sequences is described as the “swallow-tail sign” and has been described in PD patients, but not in ET [34]. A study used NM-MRI combined with N1 imaging using quantitative susceptibility mapping (QSM) in 68 PD patients, 25 ET and 34 controls and found that the combination of both images can differentiate de novo PD from non-treated ET or controls with 85.3% sensitivity and 92% specificity [34].
In regards to functional neuroimaging, a recent study examined the effects of visual feedback on blood-oxygen-level-dependent activation and connectivity during the completion of a grip-force task in dystonic tremor versus ET patients. The results showed that dystonic tremor and ET are characterized by distinct functional activation signatures in higher-level cortical and visual regions, but common cerebellar impairments [35].
7.3. Neurophysiology
There are also accelerometric differences between ET tremor, tremor dominant-PD and dystonic tremor that have been recently described. A study by Bove et al. proposed that ET could be diagnosed with a 95% sensitivity and 90% specificity if at least 4 of five criteria are fulfilled. These criteria are: 1. tremor frequency of 5-15 Hz, 2. a peak dispersion equal or below 2.5 Hz, 3. spectral coherence higher than 80%, 4. no unilateral tremor, and 5. action amplitude greater than resting amplitude [11].
An interesting work published by di Biase et al. presented another promising neurophysiological parameter that was shown to differentiate ET from PD-tremor with high accuracy: the tremor stability index (TSI). TSI is a quantitative measure taken by accelerometry, independent of the condition in which the tremor is registered (rest or postural) and operator-independent. TSI derives from measuring the changes of varying tremor frequency during 10 s. This index shows a higher value in patients with ET, while in PD tremor the index is lower, reflecting lesser frequency changes over time. TSI showed a high sensibility and specificity (~90–95%) and was validated in another cohort of heterogeneous published patients with ET and PD with tremor, with the limitation of clinical diagnoses (formulated using the old consensus criteria) [36].
8. Treatment
ET is a burden for the patients and their caregivers. This is determined not only by the functional impairment brought by the tremor itself, but also from the social stigma that this visible condition brings. A recent study found that patient's embarrassment as perceived by the caregiver is a stronger predictor of caregiver burden, more than the cognitive and physical impairment of the patient himself [37].
A recent systematic review by the IPMDS Tremor Task Force analyzed the medical and surgical treatments for ET [38]. Primidone, propranolol and topiramate were found to be clinically useful, particularly primidone and propranolol, which can improve tremor in 30–70% of patients by a significant, but modest, extent [39]. Unfortunately, about half the patients started on these medications eventually stop them due to side effects [40,41]. Botulinum toxin type A injection and alprazolam were found possibly useful with less quality evidence or conflicting results in different studies [38].
Two main surgical treatments are currently available: non-lesional (neuromodulation) or lesional (thalamotomy). Although surgery is more effective than medical treatments, it is reasonable to perform a trial of at least two first-line drugs before considering a surgical procedure due to the irreversible adverse events that can be caused by the latter.
8.1. Deep brain stimulation
Deep brain stimulation (DBS) of the intermedius ventralis nucleus (VIM) of the thalamus is a known effective treatment for medication refractory-ET. Studies show a reduction of 50–90% in tremor scores and significant improvement in functionality [42]. Good results in cases of ET with dystonic features (ET-plus) have also been reported [43]. In spite of its success, VIM-DBS has two main caveats: side effects and loss of benefit in the long run. Bilateral procedures have been related to a higher risk of side effects (up to 76%), gait/balance abnormalities and dysarthria being the most common [44]. A five-year follow-up study reported 25% of gait abnormalities in bilateral VIM-DBS compared to 0% in unilateral procedure [45]. In another recent study involving unilateral or bilateral VIM-DBS for ET with axial involvement, 25% of patients with unilateral VIM-DBS developed dysarthria and/or gait abnormalities, which could be resolved by re-programming in a third of the cases. The percentage of these side effects in the cohort that underwent staged bilateral VIM-DBS rose to 40% and could be resolved with reprogramming only in 14% of the cases [46]. Interestingly, this study also shows that even unilateral VIM-DBS may have a significant efficacy in the control of axial tremor (by 58–65%).
Although gait and speech problems have been more frequently reported in bilateral VIM-DBS procedures, unilateral procedure is not free of these risks. A recent publication studied gait before and after unilateral VIM-DBS in 24 subjects with ET and assessed patients on and off DBS after a washout period of 1 h. Results showed that there was a significant decrease in walking speed (−10–30%) after DBS in 25% of the patients. This change was not dependent on stimulation, as it was present with or without ongoing stimulation, thus suggesting a detrimental effect of the surgery itself. Coincidentally, all these patients reported a subjective worsening of gait after DBS. These authors also suggest that a diagnosis of ET-plus could predict worsening of gait after VIM-DBS, since in their cohort, patients who worsened their gait after DBS had worse tandem gait performance pre-DBS (slower velocity and more missteps) [47].
Other possibly underreported side effects of VIM-DBS have been described, such as dysgeusia, which was found in 15% of a 52-patient series [48].
New technological advances have been adopted to overcome the development of side effects. Recently an ET-plus patient with head and hands tremor who had bilateral VIM-DBS with a directional lead was reported: steered stimulation avoiding medial and posterior areas of the thalamus did not worsen baseline ataxia, as compared to non-steered stimulation, which worsened it [49]. Shortening the pulse width from the standard 60 microseconds is another way to minimize side effects, as this expands the therapeutic window without compromising tremor control [50]. Square biphasic pulse stimulation has been tried to enhance DBS effectiveness in ET. A study using it unilaterally for 3 h showed better or as good tremor control as conventional stimulation, with no side effects [51].
The long-term effectiveness of VIM-DBS has been reported to be lost in 15–40% of patients in one study [52], but up to 73% in another one [53]. The variable and individual combination of disease progression with habituation (historically labelled as “tolerance”) has been pointed as culprits. Since ET is not constant (i.e. it is an action tremor) delivering stimulation only as needed (closed loop DBS) might mitigate tremor habituation over time. In order to detect action tremor, local field potentials from the thalamic electrodes have been proven to be able to differentiate voluntary movement from tremor [54]. Further prospective studies are needed to prove that closed loop DBS reduces the risk of habituation in the long term.
Finally, new DBS targets have been the focus of interest for patients in whom VIM-DBS has not been successful (Table 2) [40,43,[55], [56], [57], [58]].
Table 2.
DBS targets for tremor control in ET.
| Motor thalamus |
|---|
| • Ventro-Intermediate (VIM) |
| • Ventralis oralis anterior (VOA) |
| • Ventralis oralis posterior (VOP) |
| Posterior subthalamic area* |
| • Caudal Zona Incerta (cZi) |
| • Prelemniscal radiation (Raprl) |
| • Cerebello-thalamic tract (or Dentato-rubro-thalamic tract) |
| Others |
| • Globus pallidus pars interna (GPi) |
| • Subthalamus (STN) |
8.2. Lesional therapies
There are various techniques to perform a thalamotomy. Currently, there are invasive (radio-frequency) and non-invasive (gamma knife radiosurgery and MRI-guided focused ultrasound, MRgFUS) ones. Unfortunately, there are no randomized controlled trials comparing these different techniques or VIM-DBS with the newer MRg-FUS thalamotomy directly. But it is interesting to note that some indirect comparison studies show similar clinical efficacy between DBS and thalamotomy [59,60]. Others have shown less absolute reduction in tremor control with MRg-FUS, but it has shown to be significant enough to improve functionality and quality of life [41,59]. One hypothesis is that the effect is technique-independent because targeting VIM might only be able to reduce tremor to that certain extent (ceiling effect) [61].
MRg-FUS thalamotomy is the most recent approved treatment for ET and it has been reported to reduce tremor scores and disability by about 35–56% and 60–80%, respectively [62]. The longest reported follow-up is 4 years in a prospective study showing significant and sustained tremor improvement without permanent adverse events [62]. By contrast, another study has reported a 23% worsening in tremor scores after the first year post-MRIgFUS thalamotomy [52]. Indeed, some MRgFUS patients also lose completely the benefit initially seen and VIM-DBS [52], radio-frequency thalamotomy or even a second MRIg-FUS thalamotomy have been performed successfully [52].
In a safety analysis of MRg-FUS thalamotomy (186 patients reported in 5 studies with variable follow-up duration), a total rate of 1.6% procedure-related serious adverse events was reported. Milder side effects varied between 9 and 49% and were mostly sensory and gait disturbances, transient or mild (79%) or moderate causing some impact in daily living (20%). Severe side effects (incapacitating) were only seen in 1% of patients [63]. Unilateral MRIgFUS thalamotomy had been shown to be safe from a cognitive standpoint. According to a 1-year follow up, there was no decline in cognitive tests of various domains [64].
MRg-FUS is a fairly new technique with some unknowns. For example, a recent study showed that there is a loss of energy-temperature efficacy during the process of serial sonications as the surrounding tissue (skull mainly) heats not uniformly, among other variables. This could produce a non-linear relationship between the energy delivered and the temperature reached, resulting in greater diffusion and focus dispersion, producing a larger lesion in the end [65]. Besides, targeting is not directly seen by the MRI, and there is no microrecording to assist in targeting precision. The optimal site of the lesion within the VIM is still under investigation. A recent study suggests this site is within the posterior part of the VIM. Interestingly, this study also relates side effects with the total volume of the lesion as these were more common with lesions over 170mm3 [66].
9. Discussion and unmet needs
In this review we gathered the main publications after the release of the new tremor classification by the IPMDS Tremor Task Force. In recent years our view of ET has changed. For example, the construct of ET-plus has forced clinicians to develop the term “Pure ET”, which is a less common and unclear entity. Patients and their family members rarely present with just tremor. More often we encounter hand tremor along with tremor in other body segments, or subtle parkinsonism, mild ataxia, cognitive impairment or dystonia. If not present during the initial evaluations, frequently some of these features appear with disease evolution or are present in other family members, thus forcing clinicians to a constant monitoring of patients' features (and diagnosis).
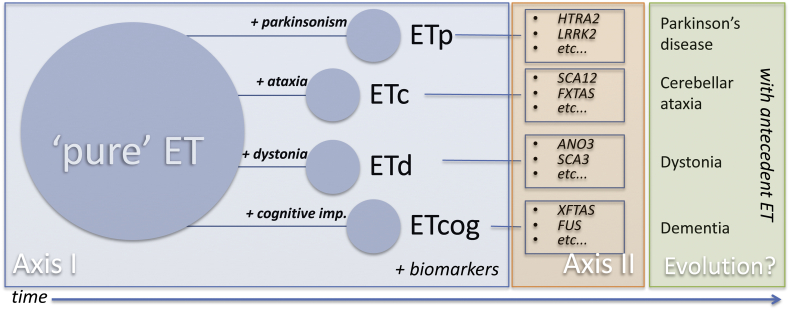
The new IPMDS tremor classification does not allow for more detailed classification among the ET-plus group. This scenario might argue for a new paradigm, which could subclassify ET-plus according to its accompanying symptoms and signs, as Axis I could divide ET into subtypes: ETc (as in cerebellum) if ataxia is present, ETp if mild parkinsonism is present, ETd if mild dystonia is present, ETcog if cognitive impairment is present (Fig. 2). This model accepts that each syndromic category can, with time, change to overt parkinsonism or cerebellar ataxia, dystonia or dementia, keeping the fact that it presented with antecedent ET. Future studies are needed to clarify if an evolution from pure ET to ET-plus and then to another overt neurological condition has biological plausibility under one mechanism. Alternately, concurrent dual (or more) pathology is to be acknowledged.
Fig. 2.
A proposal to further subclassify ET based on the new tremor classification by the IPMDS Tremor Task Force. According to the accompanying symptoms and signs, axis I would divide ET-plus into ETc (as in cerebellum) if ataxia is present, ETp if mild parkinsonism is present, ETd if mild dystonia is present, ETcog if cognitive impairment is present. Each syndromic category can, with time, change to overt parkinsonism or cerebellar ataxia, dystonia or dementia (and therefore these conditions presented with ‘antecedent ET’).
In conclusion, some progress has been achieved in the uncertain terrain of ET, but perhaps much more challenges stand ahead of us, as we still struggle to find biomarkers allowing deep phenotyping (e.g. genetic mutation in Fragile X-associated tremor/ataxia syndrome or DAT imaging in ‘monosymptomatic tremor at rest’ caused by PD pathology).
It has to be recognized that most studies quoted in this review did not embrace the new classification nor used it to analyze their findings. For example, is Lewy body pathology more common in ET-plus with parkinsonian signs (ETp)? Although very recent studies are now adopting the IPMDS criteria, the great majority of ET studies focus on surgical treatments (new targets, new technologies) whereas the least address epidemiology, pathology and etiology, without any consistent finding in most cases. Clearly, we are still far from understanding what ET (or its complex) really is. Validating ET as a heterogeneous condition, and not the monosymptomatic disease it once was thought to be, has certainly been an important step forward.
Authors contributions
CS: search of publications (acquisition of data) and drafting of the manuscript.
AF: conception and design of the review, selection of publications included, critical revision of the manuscript for intellectual content.
Study funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Full financial disclosure
CS reports no financial disclosures.
AF received consultancies from Abbvie, Medtronic,Boston Scientific, Sunovion, Chiesi farmaceutici, UCB, Insightec and Ipsen; honoraria from Abbvie, Medtronic, Boston Scientific, Sunovion, Chiesifarmaceutici, UCB, and Ipsen; grants from University of Toronto, Weston Foundation, Abbvie, Medtronic and Boston Scientific. Hes erveson the advisory board for Abbvie, Boston Scientific and Ipsen.
Declaration of competing interest
CS: none.
AF received honoraria and research funding from Medtronic, Boston Scientific and Insightec.
References
- 1.Bhatia K.P., Bain P., Bajaj N., Elble R.J., Hallett M., Louis E.D., Raethjen J., Stamelou M., Testa C.M., Deuschl G., The Tremor Task Force of the International Parkinson and Movement Disorder Society Consensus statement on the classification of tremors, from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018;33(1):75–87. doi: 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fasano A., Lang A.E., Espay A.J. What is “essential” about essential tremor? A diagnostic placeholder. Mov. Disord. 2018;33(1):58–61. doi: 10.1002/mds.27288. [DOI] [PubMed] [Google Scholar]
- 3.Fearon C., Espay A.J., Lang A.E., Lynch T., Martino D., Morgante F., Quinn N.P., Vidailhet M., Fasano A. Soft signs in movement disorders: friends or foes? J. Neurol. Neurosurg. Psychiatry. 2019;90(8):961–962. doi: 10.1136/jnnp-2018-318455. [DOI] [PubMed] [Google Scholar]
- 4.Rajalingam R., Breen D.P., Lang A.E., Fasano A. Essential tremor plus is more common than essential tremor: insights from the reclassification of a cohort of patients with lower limb tremor. Parkinsonism Relat. Disord. 2018;56:109–110. doi: 10.1016/j.parkreldis.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Rajput A., Robinson C.A., Rajput A.H. Essential tremor course and disability: a clinicopathologic study of 20 cases. Neurology. 2004;62(6):932–936. doi: 10.1212/01.wnl.0000115145.18830.1a. [DOI] [PubMed] [Google Scholar]
- 6.Cohen O., Pullman S., Jurewicz E., Watner D., Louis E.D. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch. Neurol. 2003;60(3):405–410. doi: 10.1001/archneur.60.3.405. [DOI] [PubMed] [Google Scholar]
- 7.Rajalingam R., Breen D.P., Chen R., Fox S., Kalia L.V., Munhoz R.P., Slow E., Strafella A.P., Lang A.E., Fasano A. Parkinsonism Relat Disord. 2019. The clinical significance of lower limb tremors. [DOI] [PubMed] [Google Scholar]
- 8.Deuschl G., Bain P., Brin M., an IIAd Hoc Scientific Committee Consensus statement of the movement disorder society on tremor. Mov. Disord. 1998;13(S3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 9.Martino D., Aniello M.S., Masi G., Lamberti P., Lucchese V., Lamberti S., Livrea P., Berardelli A., Defazio G. Validity of family history data on primary adult-onset dystonia. Arch. Neurol. 2004;61(10):1569–1573. doi: 10.1001/archneur.61.10.1569. [DOI] [PubMed] [Google Scholar]
- 10.Alonso-Juarez M., Baizabal-Carvallo J.F. Distinguishing features between valproate-induced tremor and essential tremor. Acta Neurol. Scand. 2018;138(2):177–181. doi: 10.1111/ane.12953. [DOI] [PubMed] [Google Scholar]
- 11.Bove F., Di Lazzaro G., Mulas D., Cocciolillo F., Di Giuda D., Bentivoglio A.R. A role for accelerometry in the differential diagnosis of tremor syndromes. Funct. Neurol. 2018;33(1):45–49. doi: 10.11138/FNeur/2018.33.1.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis E.D. Chapter 16: essential tremor and the cerebellum. Handb Clinical Neurol. 2018;155(3rd series):245–258. doi: 10.1016/B978-0-444-64189-2.00016-0. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa R., Mendonca M., Ladeira F., Miguel R., Bugalho P. Probable REM-sleep behavior disorder and dysautonomic symptoms in essential tremor. Tremor Other Hyperkinet Mov. 2017:7. doi: 10.7916/D8Z61VW5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou J.S., Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology. 1991;41(2):234–238. doi: 10.1212/wnl.41.2_part_1.234. [DOI] [PubMed] [Google Scholar]
- 15.Louis E.D., Benito-León J., Ottman R., Bermejo-Pareja F. A population-based study of mortality in essential tremor. Neurology. 2007;69(21):1982–1989. doi: 10.1212/01.wnl.0000279339.87987.d7. [DOI] [PubMed] [Google Scholar]
- 16.Louis E.D., Ferreira J.J. How common is the Most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov. Disord. 2010;25(5):534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 17.Eliasen E.H., Ferrer M., Gaini S., Louis E.D., Petersen M.S. Prevalence of essential tremor in the Faroe Islands: a population-based study. Neuroepidemiology. 2019;52:227–235. doi: 10.1159/000499070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopfner F., Helmich R.C. The etiology of essential tremor: genes versus environment. Parkinsonism Relat. Disord. 2018;46(S1):S92–S96. doi: 10.1016/j.parkreldis.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Naranjo K.V., Park J., Chen K.P., Hernandez N., Clark L.N., Ottman R., Louis E.D. 27(8) 2018. Genetic testing preferences of individuals in families with essential tremor, Tremor Other Hyperkinet Mov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H., Yuan L., Song Z., Deng X., Yang Z., Gong L., Zi X., Deng H. Genetic analysis of LRRK1 and LRRK2 variants in essential tremor patients. Genet Test Mol Biomarkers. 2018;22(6):398–402. doi: 10.1089/gtmb.2017.0277. [DOI] [PubMed] [Google Scholar]
- 21.Hopfner F., Deuschl G. Is essential tremor a single entity? Eur. J. Neurol. 2018;25(1):71–82. doi: 10.1111/ene.13454. [DOI] [PubMed] [Google Scholar]
- 22.Tzoulis C., Zayats T., Knappskog P.M., Müller B., Larsen J.P., Tysnes O.B., Bindoff L.A., Johansson S., Haugarvoll K. HTRA2 p.G399S in Parkinson disease, essential tremor, and tremulous cervical dystonia. Proc. Natl. Acad. Sci. U. S. A. 2015;112(18):E2268. doi: 10.1073/pnas.1503105112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Algarni M., Fasano A. The overlap between Essential tremor and Parkinson disease. Parkinsonism Relat Disord. 2018;46 doi: 10.1016/j.parkreldis.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Tarakad A., Jankovic J. Essential tremor and Parkinson’s disease: exploring the relationship. Tremor Other Hyperkinet Mov. 2019;8:589. doi: 10.7916/D8MD0GVR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross J.P., Mohtashami S., Leveille E., Johnson A.M., Xiong L., Dion P.A., Fon E., Dauvilliers Y., Dupré N., Rouleau G.A., Gan-Or Z. Association study of essential tremor genetic loci in Parkinson’s disease. Neurobiol. Aging. 2018;66(178):e13–178. doi: 10.1016/j.neurobiolaging.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Shill H.A., Adler C.H., Sabbagh M.N., Connor D.J., Hentz J.G., Beach T.G. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70:1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 28.Mavroudis I., Petridis F., Kazis D. Neuroimaging and neuropathological findings in essential tremor. Acta Neurol. Scand. 2019;139:491–496. doi: 10.1111/ane.13101. [DOI] [PubMed] [Google Scholar]
- 29.Louis E.D., Kuo S.H., Tate W.J., Kelly G.C., Gutierrez J., Cortes E.P., Vonsattel J.G., Faust P.L. Heterotopic Purkinje cells: a comparative postmortem study of essential tremor and spinocerebellar ataxias 1, 2, 3, and 6. Cerebellum. 2018;17:104–110. doi: 10.1007/s12311-017-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao H., Wang R., Luo X., Li X., Hallet M., Thompson-Westra J., Yang J., Qu Q., Yang X. A voxel-based magnetic resonance imaging morphometric study of cerebral and cerebellar gray matter in patients under 65 years with essential tremor. Med. Sci. Monit. 2018;24:3127–3135. doi: 10.12659/MSM.906437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham A., Drory V.E. Influence of serum uric acid levels on prognosis and survival in amyotrophic lateral sclerosis: a meta-analysis. J. Neurol. 2014;261:1133–1138. doi: 10.1007/s00415-014-7331-x. [DOI] [PubMed] [Google Scholar]
- 32.Lee C.Y., Yoon J.H., Park J.H., Hong J.Y., Sunwoo M.K., Kang S.Y. Uric acid level may not be reduced in essential tremor. Int J Neurosci. 2018;128(12):1163–1167. doi: 10.1080/00207454.2018.1492574. [DOI] [PubMed] [Google Scholar]
- 33.Tak A.Z.A., Sengul Y., Karadag A.S. Evaluation of thickness of retinal nerve fiber layer, ganglion cell layer, and choroidal thickness in essential tremor: can eyes be a clue for neurodegeneration? Acta Neurol. Belg. 2018;118(2):235–241. doi: 10.1007/s13760-017-0852-1. [DOI] [PubMed] [Google Scholar]
- 34.Jin L., Wang J., Wang C., Lian D., Zhou Y., Zhang Y., Lv M., Li Y., Huang Z., Cheng X., Fei G., Liu K., Zeng M., Zhong C. Combined visualization of nigrosome-1 and neuromelanin in the substantia nigra using 3T MRI for the differential diagnosis of essential tremor and de novo Parkinson’s disease. Front. Neurol. 2019;10:100. doi: 10.3389/fneur.2019.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeSimone J.C., Archer D.B., Vaillancourt D.E., Wagle Shukla A. Network-level connectivity is a critical feature distinguishing dystonic tremor and essential tremor. Brain. 2019;142:1644–1659. doi: 10.1093/brain/awz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.di Biase L., Brittain J.S., Shah S.A., Pedrosa D.J., Cagnan H., Mathy A., Chu Chen C., Martín-Rodríguez J.F., Mir P., Timmerman L., Schwingenschuh P., Bhatia K., Di Lazzaro V., Brown P. Tremor stability index: a new tool for differential diagnosis in tremor syndromes. Brain. 2017;140(7):1977–1986. doi: 10.1093/brain/awx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kellner S., Morgan S., Gutierrez J., Collins K., Rohl B., Migliore F., Cosentino S., Huey E.D., Louis E.D., Monin J.K. Perceived embarrassment and caregiver burden in essential tremor caregivers. J. Neurol. Sci. 2017;383:205–210. doi: 10.1016/j.jns.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira J.J., Mestre T.A., Lyons K.E., Benito-León J., Tan E.K., Abbruzzese G., Hallet M., Haubenberger D., Elble R., Deuschl G. MDS evidence-based review of treatments for essential tremor. Mov. Disord. 2019;34(7):950–958. doi: 10.1002/mds.27700. [DOI] [PubMed] [Google Scholar]
- 39.Schaefer S.M., Vives Rodriguez A., Louis E.D. Brain circuits and neurochemical systems in essential tremor: insights into current and future pharmacotherapeutic approaches. Expert. Rev. Neurother. 2018;18(2):101–110. doi: 10.1080/14737175.2018.1413353. [DOI] [PubMed] [Google Scholar]
- 40.Haubenberger D., Hallet M. Essential tremor. N. Engl. J. Med. 2018;378:1802–1810. doi: 10.1056/NEJMcp1707928. [DOI] [PubMed] [Google Scholar]
- 41.Rohani M., Fasano A. Focused ultrasound for essential tremor: review of the evidence and discussion of current hurdles. Tremor Other Hyperkinet Mov. 2017:7. doi: 10.7916/D8Z89JN1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nazzaro J.M., Lyons K.E., Pahwa R. Deep brain stimulation for essential tremor. Handb. Clin. Neurol. 2013;116:155–166. doi: 10.1016/B978-0-444-53497-2.00013-9. [DOI] [PubMed] [Google Scholar]
- 43.Patel A., Deeb W., Okun M.S. Deep brain stimulation management of essential tremor with dystonic features. Tremor Other Hyperkinet Mov. 2018;8:557. doi: 10.7916/D8P85VBQ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paschen S., Forstenpointner J., Becktepe J., Heinzel S., Hellriegel H., Witt K., Helmers A.K., Deuschl G. Long-term efficacy of deep brain stimulation in essential tremor, an observer-blinded study. Neurology. 2019;92(12):e1378–e1386. doi: 10.1212/WNL.0000000000007134. [DOI] [PubMed] [Google Scholar]
- 45.Pahwa R., Lyons K.E., Wilkinson S.B., Simpson R.K., Ondo W.G., Tarsy D., Norregaard T., Hubble J.P., Smith D.A., Hauser R.A., Jankovic J. Long-term evaluation of deep brain stimulation of the thalamus. J. Neurosurg. 2006;104(4):506–512. doi: 10.3171/jns.2006.104.4.506. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell K., Larson P., Starr P.A., Okun M.S., Wharen R.E., Uitti R.J., Guthrie B.L., Peichel D., Pahwa R., Walker H.C., Foote K., Marshall F.J., Jankovic J., Simpson R., Phibbs F., Neimat J.S., Stewart R.M., Dashtipur K., Ostrem J.L. Benefits and risks of unilateral and bilateral ventral intermediate nucleus deep brain stimulation for axial essential tremor symptoms. Parkinsonism Relat. Disord. 2019;60:126–132. doi: 10.1016/j.parkreldis.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Roemmich R., Roper J.A., Eisinger R.S., Cagle J.N., Maine L., Deeb W., Wagle Shukla A., Hess C.W., Gunduz A., KD Foote M.S., Okun C.J. Hass. Gait worsening and the microlesion effect following deep brain stimulation for essential tremor. J Neurol Neurosurg Psychiatry. 2019;90(8):913–919. doi: 10.1136/jnnp-2018-319723. [DOI] [PubMed] [Google Scholar]
- 48.Carlson J.D., McLeod K.E., Mark J.B., McLeod P.S., Bremer B.A. Dysgeusia in deep brain stimulation for essential tremor. J. Clin. Neurosci. 2018;50:242–246. doi: 10.1016/j.jocn.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Roque D.A. Segmented versus nonsegmented deep-brain stimulation for essential tremor differ in ataxic side effects. Tremor and other Hyperkinetic Movement Disorders. 2019 doi: 10.7916/d8-8vww-td18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moldovan A.S., Hartmann C.J., Trenado C., Meumertzheim N., Slotty P.J., Vesper J., Schnitzler A., Groiss S.J. Less is more pulse width dependent therapeutic window in deep brain stimulation for essential tremor. Brain Stimulation. 2018;11(5):1132–1139. doi: 10.1016/j.brs.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 51.De Jesus S., Almeida L., Shahgholi L., Martinez Ramirez D., Roper J., Hass C.J., Akbar U., Wagle Shukla A., Raike R.S., Okun M.S. Square biphasic pulse deep brain stimulation for essential tremor: the BiP tremor study. Parkinsonism Relat. Disord. 2017;46:41–46. doi: 10.1016/j.parkreldis.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 52.Wang T.R., Dellapiazza R.F., Moosa S., Huss D., Shah B.B., Elias W.J. Thalamic deep brain stimulation salvages failed focused ultrasound Thalamotomy for essential tremor: a case report. Stereotact. Funct. Neurosurg. 2018;96(1):60–64. doi: 10.1159/000486646. [DOI] [PubMed] [Google Scholar]
- 53.Sandoe C., Krishna V., Basha D., Sammartino F., Tatsch J., Picillo M., Di Biase L., Poon Y.Y., Hamani C., Reddy D., Munhoz R.P., Lozano A.M., Hutchison W.D., Fasano A. Predictors of deep brain stimulation outcome in tremor patients. Brain Stimulation. 2018;11(3):592–599. doi: 10.1016/j.brs.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Tan H., Debarros J., He S., Pogosyan A., Aziz T.Z., Huang Y., Wang S., Timmermann L., Visser-Vandewalle V., Pedrosa D.J., Green A.L., Brown P. Decoding voluntary movements and postural tremor based on thalamic LFPs as a basis for closed-loop stimulation for essential tremor. Brain Stimulation. 2019;12(4):858–867. doi: 10.1016/j.brs.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paschen S., Deuschl G. Patient evaluation and selection for movement disorders surgery: the changing spectrum of indications. Prog Neurol Surg. 2018;33:80–93. doi: 10.1159/000480910. [DOI] [PubMed] [Google Scholar]
- 56.Spears C.C., Almeida L., Okun M.S., Deeb W. An unusual case of essential tremor deep brain stimulation: where is the lead? Tremors Other Hyperkinet Mov. 2019;9 doi: 10.7916/d8-xj6w-cm53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samström L., Karlsson F., Blomstedt P. Unilateral left deep brain stimulation of the caudal Zona Incerta is equally effective on voice tremor as bilateral stimulation: evidence from 7 patients with essential tremor. Stereotact. Funct. Neurosurg. 2018;96(3):157–161. doi: 10.1159/000489938. [DOI] [PubMed] [Google Scholar]
- 58.Holslag J.A.H., Neef N., Beudel M., Drost G., Oterdoom D.L.M., Kremer N.I., Van Laar T., van Dijk J.M.C. Deep brain stimulation for essential tremor: a comparison of targets. World Neurosurg. 2018:e580–e584. doi: 10.1016/j.wneu.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 59.Kim M., Jung N.Y., Park C.K., Chang W.S., Jung H.H., Chang J.W. Comparative evaluation of magnetic resonance-guided focused ultrasound surgery for essential tremor. Stereotact. Funct. Neurosurg. 2017;95(4):279–286. doi: 10.1159/000478866. [DOI] [PubMed] [Google Scholar]
- 60.Tasker R.R., Munz M., Junn F.S., Kiss Z.H., Davis K., Dostrovsky J.O., Lozano A.M. Deep brain stimulation and thalamotomy for tremor compared. Acta Neurochir. Suppl. 1997;68:49–53. doi: 10.1007/978-3-7091-6513-3_9. [DOI] [PubMed] [Google Scholar]
- 61.Harary M., Segar D.J., Hayes M.T., Cosgrove G.R. Unilateral thalamic deep brain stimulation versus focused ultrasound thalamotomy for essential tremor. World Neurosurg. 2019;126:e144–e152. doi: 10.1016/j.wneu.2019.01.281. [DOI] [PubMed] [Google Scholar]
- 62.Park Y.S., Jung N.Y., Na Y.C., Chang J.W. Four-year follow-up results of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Mov Disord. 2019;34(5):727–734. doi: 10.1002/mds.27637. [DOI] [PubMed] [Google Scholar]
- 63.Fishman P.S., Elias W.J., Ghanouni P., Gwinn R., Lipsman N., Schwartz M., Chang J.W., Taira T., Krishna V., Rezai A., Yamada K., Igase K., Cosgrove R., Kashima H., Kaplitt M.G., Tierney T.S., Eisenberg H.M. Neurological adverse event profile of magnetic resonance imaging-guided focused ultrasound thalamotomy for essential tremor. Mov. Disord. 2018;33(5):843–847. doi: 10.1002/mds.27401. [DOI] [PubMed] [Google Scholar]
- 64.Gasca-Salas C., Guida P., Piredda R., Obeso I., Vela Desojo L., Martinez-Fernandez R., Hernandez-Fernandez F., Mañez-miro J., Pineda-Pardo J.A., Del Alamo M., Rodriguez-Rojas R., Mata-Marin D., Alonso-Frech F., De Luis E., Obeso J.A. Cognitive safety after unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor. J Neurol Neurosurg Psychiatry. 2019;90(7):830–831. doi: 10.1136/jnnp-2018-320129. [DOI] [PubMed] [Google Scholar]
- 65.Hughes A., Huang Y., Schwartz M.L., Hynynen K. The reduction in treatment efficiency at high acoustic powers during MR-guided transcranial focused ultrasound thalamotomy for essential tremor. Med. Phys. 2018;45(7):2925–2936. doi: 10.1002/mp.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boutet A., Ranjan M., Zhong J., Germann J., Xu D., Schwartz M.L., Lipsman N., Hynynen K., Devenyi G.A., Chakravarty M., Hlasny E., Llinas M., Lozano C.S., Elias G.J.B., Chan J., Coblentz A., Fasano A., Kucharczyk W., Hodaie M., Lozano A.M. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain. 2018;141(12):3405–3414. doi: 10.1093/brain/awy278. [DOI] [PubMed] [Google Scholar]