Abstract
Introduction
Spinal cord stimulation (SCS) is an established strategy for pain reduction used in whole world including Japan to treat chronic intractable pain. Pain is a frequent comorbidity of Parkinson's disease (PD), leading to poorer quality of life. SCS has been reported to effectively reduce pain in PD and may also improve motor function, but most studies have employed the modality of tonic stimulation. As such, the effects of SCS using the newly developed paradigm of burst stimulation in PD remain relatively unexplored.
Methods
This case series reviewed PD patients who underwent SCS using BurstDR stimulation to treat intractable lower back pain (LBP). Pain and motor outcomes were assessed before and at several timepoints after implantation over a 24-week observation period.
Results
Pain indices (visual analogue scale [VAS] and short-form McGill Pain Questionnaire 2 [SF-MPQ-2] scores) improved in nearly all patients. Improvements were especially notable in the dimension of affective pain (SF-MPQ-2). Functional motor improvements were evident in the Unified Parkinson's Disease Rating Scale (UPDRS), especially walking-related items, and timed-up-and-go (TUG) test performance, which generally persisted through week 24 of observation.
Conclusion
Burst SCS improved pain (especially the affective component) in PD patients with LBP, with effects generally lasting for at least 24 weeks. Neither paresthesia nor obvious adverse events were experienced in any case. Motor symptoms as scored of UPDRS Part III had the trends of improvement in lower limb akinesia at week 24 and gait at week 4. These findings suggest that burst SCS may be an effective treatment option for LBP and may be influenced to gait-related motor symptoms in PD.
Keywords: Spinal cord stimulation, Parkinson's disease, Burst stimulation, Pain, Motor symptom
Highlights
-
•
Burst spinal cord stimulation (SCS) is used for chronic refractory pain in Parkinson's disease (PD).
-
•
We discussed in this paper about five PD patients receiving burst SCS for lower back pain.
-
•
Burst SCS improved affective pain in PD and shortened TUG score.
-
•
These effects persisted for at least 24 weeks.
-
•
Burst SCS may be an effective treatment option for pain and motor disturbance in PD.
1. Introduction
Pain, such as lower back pain (LBP), which is one of the non-motor symptoms of Parkinson's disease (PD), is seen relatively early and gradually worsens [1], significantly reducing activities of daily living and their quality of life. Drug treatment is successful in the early stages of PD, but if spinal deformity and gait and posture disturbance occur with the progress of the condition, the pain changes to symptoms of drug resistance and becomes intractable. In 2012, Agari et al. reported first a series of cases in which spinal cord stimulation (SCS) therapy underwent, established for chronic intractable pain treatment, showed not only improved pain but also reduced posture and gait disturbance in PD [2]. Since then, SCS has been tried as one of the therapeutic methods for these refractory symptoms.
SCS was invented based on the gate control theory of pain proposed by Melzack and Wall. In conventional SCS, a tonic stimulation (TS) is applied at the affected area, masking pain by evoking paresthesia. Evidence is still less available for the efficacy of SCS for pain in PD, but at least some of the case series demonstrated that TS-SCS reduced pain and improved some motor symptoms [3].
However, there were some disadvantages to conventional SCS for PD patients. At first due to posture fluctuation in PD, enough paresthesia was not obtained in the preferred position due to migration of the electrode or the difference in position. Moreover, for PD patients with impaired pain sensitivity, TS feels negatively discomfort so that it is difficult to continue treatment and there were cases where device was removed. For these reasons, it may still be considered difficult to progress spreading SCS clinically for unstable outcomes, even if these are not published in papers.
Recently, a new high frequency, paresthesia free stimulation modality has been developed, called burst stimulation (BS) [4]. A few reports about BS-SCS for pain in PD can be already seen. Kobayashi et al. detailed the pain relief of BS-SCS in a single case of PD [5], and Mazzone et al. reported about the BS for pain and motor deficit of PD [6]. Their data showed that the BS-SCS relieved pain and improved motor symptoms, too.
Though, the common understanding about SCS for PD remains relatively unexplored. Here, we report the scored outcomes about pain and motor symptoms for five PD patients administered BS-SCS to treat refractory low back pain (LBP).
2. Methods
2.1. Subjects
Five idiopathic PD patients, 2 males and 3 females, aged 66-81 years old (mean 74.0), Hoen & Yarh stage 2–4 (mean 3.0), underwent BS-SCS implantation surgery to treat refractory LBP in our hospital between April and December 2018 were reviewed. The duration of PD was 5-31 years (mean 12.4). Refractory LBP was defined as pain in the lumbar region which was poorly responsive to anti-Parkinson's drug regimen changes, analgesic administration, nerve blocks. None of them had severe neuropathic pain due to spondylosis. They were possible to walk >10 m without walking device, and there were few problems with their cognitive function. PD was confirmed using the clinical diagnostic criteria of the International Parkinson's and Movement Disorder Society.
2.2. Spinal cord stimulation
SCS device implantation was performed according to the standard technique. Percutaneous insertion of a single electrode was done into the epidural space under local anesthesia, with the tip placed at the thoracic level of T8–9. Patients initially underwent a trial stimulation using the external pulse generator. The electrode placement was established by applying TS, adjusted to cover with paresthesia on the low back and bilateral lower limbs, along the dermatome at the level from L1 to sacral area. As soon as once confirmed, stimulation was switched to BS then it was continued. The intensity of BS was set at 60% of paresthesia level. Since the pain reduced, the implantable pulse generator (IPG) was placed at the buttock 3 to 7 days after first operation. During about 12 weeks after the implantation, the modification of the stimulation site was done to be covered whole lumbar region. We used the devices, Lamitrode™ S-8 Lead, Burst DR™ (pulse width: 1000 μs, inter-burst rate: 40 Hz, intra-burst rate: 500 Hz), Proclaim™ Elite IPG system, distributed in Japan by Abbott Medical Japan.
2.3. Pain and motor assessments
Pain indices were assessed using a visual analogue scale (VAS) and the short-form McGill Pain Questionnaire 2 (SF-MPQ-2). Motor function metrics were assessed using Part III of the Unified Parkinson's Disease Rating Scale (UPDRS-III) and timed-up-and-go (TUG) test. All the assessments were performed at pre-trial period (baseline), 4, 12, and 24 weeks continuing with BS. Pain etiology was categorized according to Ford's classification [1]. Evaluation of pain and motor function, UPDRS was performed by the same evaluator, clinical psychologist, physiotherapist, and doctor for all patients. Patients with wearing-off were assessed in the ‘on’ state. Adverse events were monitored throughout the evaluation period.
2.4. Statistical analysis
Assessment data are presented as mean ± standard deviation (SD). Rating differences at each post-treatment time point with respect to baseline were tested using the Wilcoxon signed-rank test. Statistical significance was defined as p < .05.
2.5. Ethical considerations
This study was approved by the ethics committee of the National Center of Neurology and Psychiatry (NCNP), and informed consent was obtained from all patients.
3. Results
Five patients underwent BS-SCS for the refractory LBP. No obvious adverse events were observed throughout the evaluation period. Wearing off was experienced in two patients but the changes in the drug did not affect the extent of the pain.
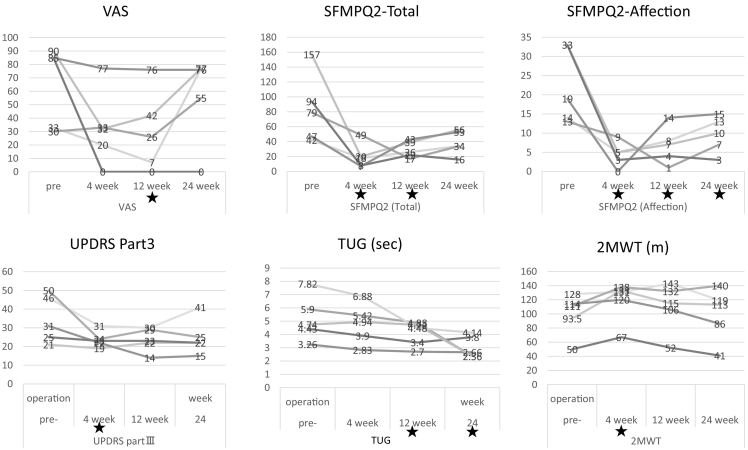
Fig. 1 shows the changes in pain and motor function scores before and after BS. VAS scores at baseline (BL), week 4 (W4), week 12 (W12), and week 24 (W24), respectively, are 64.6 ± 30.3,32.4 ± 28.3(P = .079),30.2 ± 30.4(P < .05),57.0 ± 33.2 and respectively it decreased at W4, W12, but increased again at W24. Total pain score (SF-MPQ-2) in turn, 83.8 ± 46.3, 20.4 ± 17.0, 29.4 ± 11.1, 38.6 ± 16.3, showed lower than BL at W4 and W12 for all cases (p < .05) and at W24 for four cases(P = .079). Notably, affective pain score (SF-MPQ-2), 22.4 ± 9.9, 4.4 ± 3.3, 6.8 ± 4.8, 9.6 ± 4.8, showed lower than BL for all patients at all assessment points (p < .05).
Fig. 1.
Changes in pain and motor function scores before and after burst spinal cord stimulation.★:P < 0.05
Severity of motor symptoms (UPDRS items) demonstrated positive trends (Fig. 1, Fig. 2). The score 34.6 ± 12.8, 23.8 ± 4.4, 23.6 ± 6.4, 25.0 ± 9.7, showed significantly lower than BL at W4 in all cases (p < .05). Total UPDRS-III reduced score was maintained at W24 in four cases(P = .079). TUG time, 23.1 ± 11.9, 21.3 ± 10.9, 17.7 ± 7.9, 12.7 ± 4.7, tended to improve throughout the evaluation period in all cases; a significant reduction relative to BL was observed at W12 and W24.
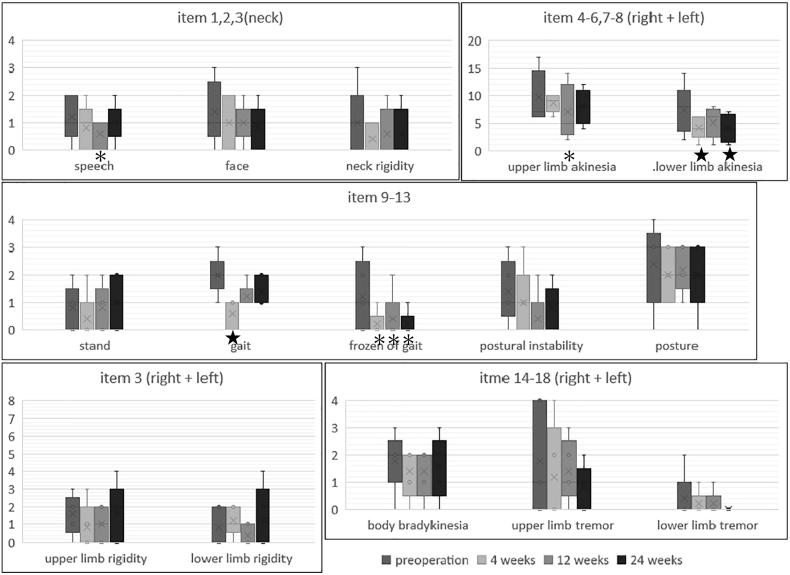
Fig. 2.
Changes in the details of MDS-UPDRS PartIII ★:P<or ≓0.05, *:P ≓ 0.1
Changes in the details of MDS-UPDRS Part III are shown in Fig. 2. For individual UPDRS-III items, improvements in lower limb akinesia (item7 & 8, right and left totals) and gait (item 10) were observed at W4 in all cases (p < .05), which were maintained through W24 only in lower limb akinesia. Freezing of gait (FOG, item 11) were seen in three patients at BL, one of them was improved from the score 3 to 1, and two of them extinguished the score 1 and 2 to zero at W24, but it was not significant(P = .11). Tremor (item 12) had the slight tendency but had no significance. There was no change in the score on the posture, body bradykinesia in our cases.
4. Discussion
4.1. Tonic SCS for pain in PD
Evidence of SCS for pain in PD has been reviewed by De Andrade et al. [3]. According to this, positive report cases of pain relief effects caused by TS exists. As the report of Agari et al. [2], Nishioka et al. showed that the UPDRS PartIII scores were improved even in motor symptoms of tremor and rigidity by TS for pain in PD [7]. Both suggest very meaningful that the original effect of SCS for neuropathic pain derived from the spine was obtained even in PD, then the motor symptoms of PD improved together. The LBP in our cases did not include such a neuropathic pain but included the body axis pain which is difficult to obtain the effect of TS-SCS. Although the types of pain are classified by Ford et al., more discussion is needed to define pain according to like this.
4.2. Burst SCS for pain in PD
Burst stimulation is a novel high frequency, paresthesia free technologies. De Ridder designed a new stimulation method named BurstDR™ (a patent from Abbott), which was resemble the physiological firing patterns of subthalamic neurons [4]. Clinical efficacy was investigated in the SUNBURST trial, compared TS versus BS in 100 individuals with neuropathic pain of the trunk and limbs using the VAS score [8]. Our investigation revealed significantly improvements in five PD patients with LBP scored by SF-MPQ2 (that is lower than BL, as the score of total pain at 12 W, and as it of affective pain further at 24 W) by BS-SCS. Thus, the SCS using BurstDR™ stimulation for pain in idiopathic PD might have positive effect, as same as TS, although a higher evidence-level design is required.
What is characteristic of our research is the difference between VAS score and SF-MPQ-2 score evaluation results (Fig. 1). This is considered that it is difficult to evaluate the degree of pain themselves, but it may be easier to be aware of relieving affliction associated with pain.
4.3. The mechanisms of the pain relief by BS
There are two ascending pathway of pain: “medial pathway” of the sensory identification system and the other is “lateral pathway” of the emotional system connecting at the dorsal Anterior Cingulate Cortex (dACC) which is responsible for encoding pain memories. An EEG-based comparison of BS and TS modes in SCS found BS to significantly increase neural synchronization in the dACC [9]. Our clinical data are consistent with these knowledges that may modulate the affective component of pain via the medial pathway. DACC is also involved in motor learning by linking it with areas related to motor output, associating with episodic memory through pain discomfort. Our data of TUG, that is said to correlate with cognitive function, decreased at W12 and W24 significantly. It is very interesting of thinking about the relation of affective pain and motor learning.
4.4. SCS and motor symptoms in PD
The matter currently unresolved is whether the effect for motor is the direct one of SCS or is the secondary. The study by Samotus et al. of SCS in PD without pain show the trends for improvements were observed in Freezing of Gait Questionnaire score (albeit non-significant) [10]. This is still controversial, but Fuentes et al., in 2009 [11] and Bryset et al. in 2017 [12] showed the direct effect of motor symptoms in animal model PD. It should be proposed that also the TS-SCS affect motor symptoms in PD aside pain, by concerning the central nervous system through the mediation of sensory tracts of spinal cord. It is natural to think that it is effective for both pain and motor symptoms when returning to the discussion of dACC. To argue about the superiority of BrustDR for motor function on PD, further studies like about the gait analysis or the motor performance are expected in the future.
4.5. Limitations
This is a case series of a retrospective study with a small number of patients. The long-term effects of SCS were not assessed since the observation period lasted only 24 weeks. Because the treatment was continued during the evaluation period, difference in the state where SCS was turned on and off was unknown. All cases were hospitalized until W4, and they underwent regular rehabilitation.
A prospective case control trial including more patients will be necessary to confirm the BS-SCS how reduce pain and improve motor symptoms in PD. Further studies using only PD patients without pain symptoms will be needed to substantiate its efficacy of BS-SCS in motor symptoms of PD.
Acknowledgments
Acknowledgement
Funding source
This work was supported in part by Intramural Research Grant for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry (NCNP), Japan.
Financial disclosure
Ayano Matsui has received honoraria for lectures and writing about SCS from Abbott Medical Japan. None of the other authors has any financial disclosure to report. This conflict of interest (COI) has been appropriately reviewed and approved by COI committee of NCNP.
References
- 1.Ford B. Pain in Parkinson’s disease. Mov. Disord. 2010;25(Suppl. 1):S98–103. doi: 10.1002/mds.22716. [DOI] [PubMed] [Google Scholar]
- 2.Agari T., Date I. Spinal cord stimulation for the treatment of abnormal posture and gait disorder in patients with Parkinson’s disease. Neurol. Med. Chir. 2012;52:470–474. doi: 10.2176/nmc.52.470. [DOI] [PubMed] [Google Scholar]
- 3.E.M. de Andrade, M.G. Ghilardi, R.G. Cury, E.R. Barbosa, R. Fuentes, M.J. Teixeira, E.T. Fonoff, Spinal cord stimulation for Parkinson's disease: a systematic review, Neurosurg Rev. 39 (2016) 27–35; discussion 35. 10.1007/s10143-015-0651-1. [DOI] [PubMed]
- 4.De Ridder D., Vanneste S., Plazier M., van der Loo E., Menovsky T. Burst spinal cord stimulation: toward paresthesia-free pain suppression. Neurosurgery. 2010;66:986–990. doi: 10.1227/01.NEU.0000368153.44883.B3. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi R., Kenji S., Taketomi A., Murakami H., Ono K., Otake H. New mode of burst spinal cord stimulation improved mental status as well as motor function in a patient with Parkinson’s disease. Parkinsonism Relat. Disord. 2018;57:82–83. doi: 10.1016/j.parkreldis.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Mazzone P., Viselli F., Ferraina S., Giamundo M., Marano M., Paoloni M., Masedu F., Capozzo A., Scarnati E. High cervical spinal cord stimulation: a one year follow-up study on motor and non-motor functions in Parkinson’s disease. Brain Sci. 2019:9. doi: 10.3390/brainsci9040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishioka K., Nakajima M. Beneficial therapeutic effects of spinal cord stimulation in advanced cases of Parkinson’s disease with intractable chronic pain: a case series. Neuromodulation. 2015;18:751–753. doi: 10.1111/ner.12315. [DOI] [PubMed] [Google Scholar]
- 8.Deer T., Slavin K.V., Amirdelfan K., North R.B., Burton A.W., Yearwood T.L., Tavel E., Staats P., Falowski S., Pope J., Justiz R., Fabi A.Y., Taghva A., Paicius R., Houden T., Wilson D. Success Using Neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21:56–66. doi: 10.1111/ner.12698. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarthy K., Fishman M.A., Zuidema X., Hunter C.W., Levy R. Mechanism of action in burst spinal cord stimulation: review and recent advances. Pain Med. 2019;20:S13–S22. doi: 10.1093/pm/pnz073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samotus O., Parrent A., Jog M. Spinal cord stimulation therapy for gait dysfunction in advanced Parkinson’s disease patients. Mov. Disord. 2018;33:783–792. doi: 10.1002/mds.27299. [DOI] [PubMed] [Google Scholar]
- 11.Fuentes R., Petersson P., Siesser W.B., Caron M.G., Nicolelis M.A. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science (80-) 2009;323:1578–1582. doi: 10.1126/science.1164901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brys I., Bobela W., Schneider B.L., Aebischer P., Fuentes R. Spinal cord stimulation improves forelimb use in an alpha-synuclein animal model of Parkinson’s disease. Int. J. Neurosci. 2017;127:28–36. doi: 10.3109/00207454.2016.1138296. [DOI] [PubMed] [Google Scholar]