Abstract
Cerebellar ataxic syndrome is a heterogenous class of disorders which can result from a miscellany of causes- genetic or acquired. There are a few metabolic, immune mediated, inflammatory and hereditary causes of ataxia which can be diagnosed from the gamut of possibilities, offering great relief to the ailing patient, their family and the treating physician. A pragmatic algorithm for diagnosing treatable causes of ataxia includes a thorough clinical history, meticulous examination for associated signs and an investigative mind to clinch the diagnosis. With novel diagnostic techniques and targeted therapies, early diagnosis and treatment can lead to favourable outcomes. In this review, diseases presenting predominantly as cerebellar ataxia and are treatable by targeted therapies are discussed.
Keywords: Treatable ataxia, Cerebellar ataxia, Acute ataxia, Genetic ataxia
1. Introduction
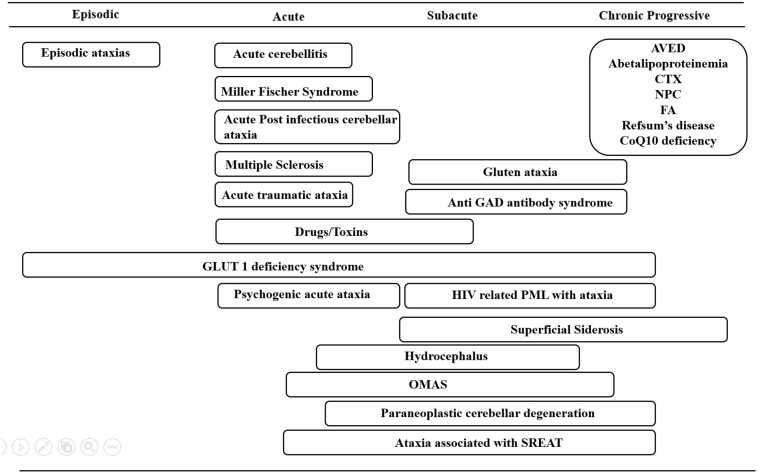
The advances in gene discovery have improved our understanding of the pathophysiology of cerebellar ataxia. However treatment remains supportive and symptomatic. Still, a limited group of heterogeneous progressive ataxic conditions may improve with disease-specific treatments, if instituted early. A comprehensive assessment is critical in elucidating important clinical keys, including nature of onset and progression (Fig.1), family history, specific diagnostic signs (Table 1), and brain magnetic resonance imaging (MRI). Recognizing the causes of cerebellar ataxia which are amenable to treatment is critical, not only to implement targeted treatment (Table 2) , but also to institute it as early as possible to halt neurological deterioration. Here we focus on disorders in which ataxia is a prominent clinical sign amenable to disease-specific treatment.
Fig. 1.
Temporal profile for differential diagnosis of treatable ataxias
Table 1.
Clues to the diagnosis of treatable ataxias
| Disease | Neurological features | Systemic features |
|---|---|---|
| Cerebrotendinous Xanthamatosis | Peripheral neuropathy ,seizures ,cognitive disturbances, spastic paraparesis | Tendon xanthomas , congenital/juvenile cataracts, premature atherosclerosis, osteoarthritis, skeletal fractures, pes cavus, pulmonary insufficiency, endocrinopathies, renal and hepatic calculi, and chronic diarrhea |
| Neimann Pick Disease -C | Vertical gaze palsy , chorea, dystonia, cataplexy,psychosis | Splenomegaly |
| Anti GAD antibody associated disease | Early cognitive impairment , diplopia, dysarthria, vertigo | |
| GLUT 1 deficiency | Chorea , dystonia (especially facial and limb) | |
| Ataxic variant of SREAT | Early cognitive impairment ,psychiatric symptoms, tremor, myoclonus | |
| Ataxia with Vitamin E deficiency | Visual loss, retinitis pigmentosa, sensory neuronopathy ,dystonia | |
| Wilson’s disease | Tremor , dysarthria, psychosis | KF ring |
| Co Q 10 deficiency | Seizures | |
| Whipples disease | Oculomotor apraxia | |
| Refsum’s disease | Sensory neuronopathy, retinitis pigmentosa, sensorineural hearing loss,anosmia, raised CSF protein without pleocytosis | Ichthyosis, cardiomyopathy, arrhythmias, skeletal abnormalities, renal failure |
| Superficial siderosis | Myelopathy , sensorineural hearing loss,dementia, palatal tremor | |
| Gluten ataxia | Gastrointenstinal symptoms | |
| Friedreich’s ataxia | Myelopathy, Sensory neuronopathy dysarthria | Cardiomyopathy |
Table 2.
Diagnostic and treatment modalities for the medically amenable ataxias
| Disease | Diagnostic tests | Treatment |
|---|---|---|
| Ataxia with Vitamin E Deficiency | Vitamin E levels | Vitamin E (800 mg/d) in divided doses |
| Abetalipoproteinemia | Peripheral blood acanthocytes, vitamin E levels, LDL-C,TGL,apolipo B, MTTP gene sequencing | Low fat diet with vitamin E (100-300 mg/kg/d) and Vitamin A replacement (100-400 IU/ kg/d) |
| Cerebro tendinous xanthomatosis | Serum cholestanol and urinary bile alcohol levels | Oral chenodeoxycholic acid 250 mg thrice a day |
| Niemann-Pick disease type C | Serum oxysterol, NPC gene testing | Miglustat 200mg tid, Cyclodextrin* |
| Gluten Ataxia | IgA deposits against TG 2 in small bowel biopsy | Gluten-free diet |
| Anti GAD antibody associated Ataxia | Anti GAD antibody | Immunosuppressive treatments |
| Ataxic variant of SREAT | Serum thyroperoxidase, thyroglobulin antibodies | Immunotherapy |
| Opsoclonus myoclonus ataxia syndrome | Malignancy screen | Immunotherapy , removal of primary tumor |
| Acute post-infectious cerebellar ataxia | Viral PCR | Immunotherapy |
| Coenzyme Q10 (CoQ10) deficiencies | CoQ10 measurement in skeletal muscle | CoQ10 30 mg /kg/d orally tid |
| Friedreich’s ataxia | Frataxin gene testing | Idebenone |
| Paraneoplastic cerebellar degeneration | Paraneoplastic antibody panel | Immunotherapy , removal of primary tumour |
| Refsum’s disease | Serum phytanic acid levels | Dietary restriction of phytanic acid, Acute worsening– Plasma exchange |
| Glucose transporter type 1 (GLUT1) deficiency | Erythrocyte glucose uptake assay, low CSF glucose | Ketogenic diet |
| Episodic ataxia 2 | EA2 gene mutation | Acetazolamide , 4- aminopyridine |
| Superficial siderosis | MRI Brain | Deferiprone 30 mg/kg/d |
| Drug induced ataxia | Serum drug levels | Withold the offending drug |
Glossary : LDL-C-low-density lipoprotein C , TGL-triglycerides, apolipo B- apolipoprotein B, IgA – immunoglobulin A , TG2 – transglutaminase 2 , GAD-Glutamic Acid Decarboxylase, SREAT- Steroid--responsive encephalopathy associated with autoimmune thyroiditis,MRI -Magnetic resonance imaging *- trial ongoing
2. Treatable genetic causes of ataxia
2.1. Ataxia with vitamin E deficiency
Ataxia with vitamin E deficiency (AVED) is an autosomal recessive (AR) disease due to mutations in the alpha tocopherol transfer protein (TTPA gene) on chromosome 8q13. It presents as a slowly progressive spinocerebellar ataxia syndrome resembling Friedreich’s ataxia (FA). Some of the shared features of AVED and FA include ataxia, loss of deep tendon reflexes, vibratory and sensory disturbances, muscle weakness, dysarthria, and upper motor neuron signs [1]. Cardiomyopathy is less common in AVED, whereas head titubation and dystonia are more specific for AVED. Patients with AVED have a more protracted course, with mild neuropathy. Age at onset of AVED is usually before age 20. Daily high doses of vitamin E (800 mg/d) in divided doses (good practice point) [2] typically lead to neurological improvement, although recovery may be moderate and incomplete. The results of vitamin E supplementation is most beneficial if started with disease duration of less than 15 years [3].
2.2. Abetalipoproteinemia
Abetalipoproteinemia is caused by mutations in the gene for the large subunit of microsomal triglyceride transfer protein (MTTP), located on chromosome 4q22-24. When MTTP is mutated, plasma apolipoprotein B containing lipoproteins are absent, which leads to the compromise of fat-soluble vitamins especially vitamin E. Symptoms begin before age 20.These patients have hyporeflexia, reduced proprioception and vibratory sense, muscle weakness, and spino-cerebellar ataxia. Specific laboratory investigations include blood smear showing acanthocytosis, and a lipid profile revealing nearly absent low-density lipoprotein C (<0.1 mmol/L), triglycerides (<0.2 mmol/L), and apolipoprotein B (<0.1 g/L). Diagnosis is confirmed by molecular testing, by sequencing the MTTP. Treatment involves dietary modification and vitamin replacement, which will prevent neurological complications if begun early. Dietary modification consists of a low-fat diet and replacement of vitamin E and A, which are thought to slow retinal degeneration and neurological complications. Large doses, of vitamin E (100-300 mg/kg/d) ,are needed to prevent neurological deterioration along with vitamin A supplementation (100-400 IU/ kg/d) that can normalize serum levels (good practice point) [2,4].
2.3. Cerebrotendinous xanthomatosis
Cerebrotendinous xanthomatosis (CTX) is a autosomal recessive lipid storage disorder caused by a mutation of the mitochondrial enzyme 27-sterol hydroxylase (CYP27 gene) on chromosome 2, which is a part of the hepatic bile-acid synthesis pathway. Reduction in synthesis of these acids leads to an increase in serum cholestanol and urinary bile alcohols and deposition of these metabolites as xanthomatous lesions in various tissues, particularly the brain, tendons, and ocular lenses. Neurological symptoms generally start by 20 years of age and include cerebellar ataxia, spastic paraparesis, seizures , sensorimotor peripheral neuropathy, extrapyramidal signs, psychiatric issues, cognitive impairment [5].
Associated non-neurological features include congenital/juvenile cataracts, tendon xanthomas (particularly over Achilles tendon), premature atherosclerosis, osteoarthritis, skeletal fractures, pes cavus, pulmonary insufficiency, endocrinopathies, chronic diarrhea, renal and hepatic calculi. Diagnosis can be confirmed by testing serum cholestanol levels and urinary bile alcohol levels. Global atrophy and parenchymal lesions on MRI, axonal neuropathy on nerve conduction studies, delayed central conduction times on evoked visual, brainstem auditory, and somatosensory evoked potentials and diffuse slowing with paroxysmal discharges on electroencephalography are typically seen. It is easily treatable with oral chenodeoxycholic acid supplementation in a dose of 250 mg three times per day (level C evidence) [2]. Initiating treatment at the earliest is imperative to prevent neurological deterioration [6,7].
2.4. Niemann–Pick disease (type C)
Niemann-Pick disease type C (NPC) is a lipid storage disorder marked by abnormalities of intracellular transport of endocytosed cholesterol resulting in sequestration of unesterified cholesterol in lysosomes and late endosomes. It has an autosomal-recessive inheritance. A vast majority of the patients (about 95%) have mutations in the NPC1 gene (mapped at 18q11) which encodes a large membrane glycoprotein with late endosomal localization. The rest of the patients have mutations in the NPC2 gene (mapped at 14q24.3). Cumulation of glucosylceramide, lactosylceramide, GM2 and GM3 gangliosides in the brain may be responsible for at least some of the neurological manifestations of NPC [8]. Age of onset range from the neonatal period to late adulthood. Involvement of other body systems – spleen, liver and occasionally lungs, apart from neurological or psychiatric symptoms manifest at varied times and follow unrelated courses. In juvenile and adult presentations, the commonest presenting feature is cerebellar ataxia (76%). Other findings include vertical supranuclear ophthalmoplegia (75%), dysarthria (63%), cognitive impairment (61%), movement disorders (58%), splenomegaly (54%), psychiatric disorders (45%), and dysphagia (37%) [9].
With the availability of genetic testing , detection of mutations in NPC 1 and NPC2 genes is used in diagnosis. In a minority of patients (about 10%), heterozygous mutation in the gene or new mutations representing variants of uncertain significance may be found. Heterozygous mutations also result in cellular trafficking of cholesterol, and detection of oxidative cholesterol metabolites like serum oxysterol which can be used as initial means of diagnosis followed by genetic testing. This gives a positive predictive value of over ninety seven percent [10]. However, higher levels of serum oxysterol may also be seen in deficiency of acid sphingomyelinase and lysosomal acid lipase and occasionally CTX.
Miglustat (level B evidence) [2], at doses of 200 mg three times daily, stabilized disease progression in 72% of patients treated for 1 year or more as demonstrated by a composite assessment of horizontal saccadic eye movement velocity, ambulation, swallowing and cognition [11]. Benefits are generally modest, suggesting that miglustat may slow, but not prevent, the progression of neurological abnormalities. Cyclodextrin, a cholesterol-sequestering agent, has also shown possible therapeutic value in NPC in preliminary studies, and clinical trials are underway.
2.5. Autosomal recessive cerebellar ataxia due to coenzyme Q10 deficiency
Coenzyme Q10 (CoQ10) deficiencies are characterized by a primary deficiency of CoQ10 due to mutations in genes encoding CoQ10 biosynthesis enzymes which are autosomal recessive. Secondary CoQ10 deficiency are related to mutations that indirectly affect CoQ10 biosynthesis [12]. The initial identified causative mutations in the ataxic form of CoQ10 deficiency were detected in APTX gene, which encodes aprataxin - the causative gene for ataxia with oculomotor apraxia 1.
CoQ10 deficiency is associated with overlapping neurological features broadly divided into five major clinical phenotypes, including encephalomyopathy, severe infantile multisystemic disease, nephropathy, isolated myopathy, and cerebellar ataxia [12]. A slowly progressive cerebellar syndrome is the most common phenotype related to CoQ deficiency. Patients manifest with gait ataxia, and over time, develop prominent dysarthria, limb ataxia, brisk lower extremity muscle stretch reflexes, and minor abnormalities in saccade and smooth pursuit eye movements.
Direct measurement of CoQ10 in skeletal muscle by high-performance liquid chromatography is the most reliable test for the diagnosis. The response to replacement therapy in the ataxic form of CoQ10 deficiency is variable, ranging from good clinical outcome to negligible benefit after CoQ10 supplementation in several small reports. The authors used CoQ10 30 mg/kg/d orally three times per day in primary CoQ10 deficiency (good practice point) [2,13].
2.6. Friedreich's ataxia
FA is an autosomal recessive degenerative disorder caused by an unstable GAA triplet repeat expansion on the gene encoding frataxin a mitochondrial protein involved in iron–sulfur cluster biosynthesis.Reduced frataxin synthesis leads to disrupted iron–sulfur biosynthesis, mitochondrial iron overload and an increased sensitivity to oxidative stress [14]. Onset of symptoms is typically before 25 years, with early limb and truncal ataxia and absent muscle stretch reflexes. Loss of joint position and vibration sense, upper motor neuron dysfunction, and dysarthria eventually occur in all patients. Cardiomyopathy is seen in two thirds of patients.
Therapeutic strategies tried are 1) increase frataxin levels on the transcriptional level by histone deacetylase (HDAC) inhibitors or the protein level by recombinant human erythropoietin; 2) use antioxidants such as co-enzyme Q10, its homoloidebenone, and vitamin E; 3) lower mitochondrial iron stores with deferiprone; and 4) improve energy metabolism by L-carnitine supplementation. However, controlled studies failed to show modification of disease progression with these approaches. Favorable effects of idebenone on cardiac hypertrophy associated with FRDA has been documented [15]. Treatment should be individualized and FA patients with severe hypertrophic cardiomyopathy might benefit from this treatment [16]. However, there is no sufficient evidence to recommend idebenone for the treatment of FA (level A evidence).
2.7. Refsum's disease
Refsum's disease is a rare autosomal recessive disorder of fatty acid metabolism, caused in most cases by mutations of the peroxisomal enzyme phytanoyl-CoA hydroxylase gene on chromosome 10 [17]. The peroxisomal protein catalyses the first step in the α-oxidation of phytanic acid. A diagnostic tetrad of retinitis pigmentosa, cerebellar ataxia, polyneuropathy and high cerebrospinal fluid (CSF) protein content without pleocytosis has been found in almost all patients. It is characterized by sensorineural deafness, anosmia, skeletal abnormalities, ichthyosis, renal failure, cardiomyopathy or arrhythmias as associated features [18].
Onset is typically by 20-30 years of age, with night blindness as the earliest manifestation. Symptoms relate to failing vision, weak extremities, or unsteady gait. Because of impaired branched chain fatty acid α-oxidation, phytanic acid, found primarily in dairy products, meat, and fish, accumulates to high levels in body fat. Analysis of phytanic acid concentration in plasma/serum is used to confirm the diagnosis. Dietary restriction halts disease progression in most cases (good practice point) [2]. The goal of treatment is reduction of phytanic acid intake to less than 10mg/day (normal daily intake-50-100 mg) [19]. Stressful conditions, such as rapid weight loss and illness, can result in mobilization of phytanic acid from fat stores, causing sudden worsening of symptoms or even an acute Guillain-Barre syndrome-like presentation [20]. Plasma exchange or chronic lipid apheresis can be tried in patients with acute symptoms or progression despite dietary changes.
2.8. Glucose transporter type 1 deficiency
Glucose transporter type 1 (GLUT1) deficiency syndrome is an autosomal dominant neurometabolic disorder caused by disturbed glucose transport over the BBB . The disease is caused by mutations in the gene encoding the GLUT1 transporter (SLC2A1; 138140) on chromosome 1p35-p31.3.
GLUT1 deficiency has diverse phenotypical manifestations ranging from intellectual disability and epilepsy to motor impairment [21]. It can present as complex movement disorders or as a dominating ataxia syndrome [22]. Patients with the ataxic phenotype typically present with limb ataxia, dysarthria, subtle cognitive difficulties, and other movements disorders, primarily limb and facial dystonia. Ataxic gait may occur in 70% of patients (35% have purely ataxic gait and 35% have ataxic spastic gait). Movement disorders are paroxysmal in some patients because of exacerbated states of deficiency induced by physical activity, fatigue, fasting, anxiety, excitement, low ketones, and poor dietary compliance to treatment [23].
The hallmark of this disorder is low CSF glucose concentration in the setting of normoglycemia with a CSF/blood glucose ratio of 0.4. Diagnosis encompasses analysis of an erythrocyte glucose uptake assay which is a sensitive test for GLUT-1 deficiency [24]. Ketogenic diet can be effective in treating the manifestations of the disease and halting progression by 40% to 70% in most patients [25].
2.9. Episodic ataxia type 2
The episodic ataxias are diverse neurological conditions characterized by paroxysmal episodes of incoordination and imbalance, often with associated progressive ataxia .There are at least six well-defined subtypes (EA 1-6). Episodic ataxia (EA2) type 2 is an autosomal dominant disorder caused by mutations of the CACNA1A gene encoding the alpha-subunit of P/Q-type calcium channel on chromosome 19 [26]. Most of these are nonsense loss of function mutations resulting in an abnormally truncated protein. Sporadic cases have also been reported.
EA2 is characterized by episodes of ataxia, commonly triggered by emotional or physical stress, with interictal nystagmus lasting for hours to days, ranging in frequency from a few times a year to three to four episodes per week. Symptoms vary from a pure ataxia to symptoms suggesting involvement of the brainstem and rarely the cerebral cortex. Symptoms during an attack include nausea, vertigo, dysarthria, and truncal ataxia. Nearly 50% of patients may also acknowledge a headache reminiscent of basilar migraine. Spontaneous vertical nystagmus, particularly downbeat nystagmus, is seen in approximately one third of cases [27]. Midline anterior cerebellar vermis atrophy has been described in patients with long-standing EA2.
EA2, familial hemiplegic migraine 1 and spinocerebellar ataxia 6 are allelic disorders caused by mutations in CACNA1A, with clinical overlap among them. Approximately two thirds of EA2 patients respond to treatment with acetazolamide in doses between 250-1,000 mg/day. A randomized, double-blind, crossover trial of the potassium channel blocker 4-aminopyridine, 5 mg three times daily versus placebo showed significant reduction in frequency of attacks and improved the quality of life.
3. Acquired, immune-mediated cerebellar ataxias
3.1. Gluten ataxia
Gluten ataxia (GA) is an insidious onset sporadic ataxia with positive serological markers for gluten sensitivity [28]. Patients present in adulthood with insidious-onset, progressive, pure cerebellar ataxia syndrome. Ocular findings of cerebellar dysfunction including gaze evoked nystagmus are seen in majority of patients. More than 90% of patients with gluten ataxia may not have gastrointestinal symptoms. One third of patients have histopathologic findings consistent with enteropathy. There is evidence of cerebellar atrophy in MRI in most cases.
The identification of immunoglobulin A deposits against transglutaminase (TG) 2 in small bowel biopsy specimens helps in the identification of gluten related disease especially in patients with extraintestinal manifestations, including patients with GA as they may not have obvious enteropathy. Analogous to TG2, transglutaminase primarily expressed in neural tissue (TG6) appears to be a marker of GA in up to 32% in idiopathic sporadic ataxia and 73% in patients with GA [29]. However, these antibodies have not been reproduced in other research laboratories. Other markers include antigliadin, endomysial antibodies and antibodies directed to surface cell transglutaminase.
Strict adherence to gluten-free diet is the cornerstone of therapy. Significant improvement in ataxia scores and subjective global clinical impression scale have been found when patients are on a gluten-free diet [30].
3.2. Anti GAD (glutamic acid decarboxylase) antibody associated ataxia
Glutamic acid decarboxylase (GAD) is a major enzyme of the CNS that catalyzes the conversion of glutamate to γ-aminobutyric acid, the major CNS inhibitory neurotransmitter. High levels of anti GAD antibody has been found in patients with cerebellar ataxia, supporting an autoimmune pathogenesis of the cerebellar syndrome [31]. In two large series of patients with elevated GAD antibodies titers and multifocal neurological deficits, cerebellar ataxia accounted for 28% and 63% of neurological presentations [32,33].
Patients with GAD-associated ataxia develop symptoms insidiously over weeks to years. Gait ataxia appears to be the most common feature, though limb ataxia, dysarthria, and nystagmus also may be present. Patients may develop episodes of diplopia, dysarthria, or vertigo of unclear etiology months before the development of full-blown cerebellar ataxia. Those with subacute onset and progression are more likely to benefit from immunotherapy and achieve long-term response [34]. Immunosuppressive treatments primarily plasma exchange with methyl prednisolone pulse therapy (20-25 mg /kg /week) as single bolus dose have been used in several case series with marked benefit.
3.3. Ataxic variant of steroid-responsive encephalopathy associated with autoimmune thyroiditis
Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT), often referred to as Hashimoto’s encephalopathy, is an autoimmune syndrome characterized by subacute onset of confusion with altered level of consciousness, seizures, and myoclonus .Elevated serum levels of thyroperoxidase (TPO) and thyroglobulin antibodies with variable thyroid profiles (often euthyroid) have been described. A slowly progressive, sporadic, adult-onset cerebellar ataxia mimicking spino-cerebellar degeneration responsive to steroids has been reported [ 35,36].
Serum autoantibodies against the amino (NH2) terminal region of neuronal alpha-enolase have been reported as a specific diagnostic marker of the ataxic variant of SREAT [37]. 50% of patients can harbor other neurological symptoms namely, altered level of consciousness, mild cognitive impairment, psychiatric symptoms, tremors, and myoclonus. MRI demonstrate no or only mild cerebellar atrophy. High-dose intravenous methylprednisolone followed by oral prednisone taper and steroid sparing immunomodulators is the most commonly used strategy.
3.4. Opsoclonus myoclonus ataxia syndrome
Opsoclonus myoclonus ataxia (OMA) syndrome can manifest from infancy.It is marked by chaotic conjugate high-amplitude eye movements (opsoclonus) , myoclonus,axial/appendicular ataxia and irritability. It commonly occurs as a paraneoplastic autoimmune phenomenon and an underlying neuroblastoma or ganglioneuroblastoma is often found. Identification of the syndrome is simplified when the triad of symptoms present in contiguity. When ataxia presents in isolation without eye findings , the diagnosis becomes challenging. Children should be evaluated with meta-iodo benzyl guanidine scintigraphy (MIBG scan) which has fairly high sensitivity. If negative, a high-resolution computerized tomography (CT) or MRI of the chest and abdomen should be done [38].
3.5. Acute post-infectious cerebellar ataxia
Acute post-infectious cerebellar ataxia (APCA) is commonly seen after immunizations or a febrile illness, most often after varicella infection. Other viral etiologies plausible include coxsackie B, Epstein-Barr virus , mumps, echoviruses and influenza A/B. The pathology usually is a cross reaction of antibodies to epitopes in the cerebellum causing acute demyelination [39]. The onset of symptoms can be up to 3 weeks after the systemic illness has resolved. Symptoms develop acutely over few hours with relatively prompt resolution over the next few days. The sensorium usually remains normal and the presence of extreme irritability should raise the suspicion of an acute infective cerebellitis.
Examination shows gait ataxia with significant truncal ataxia. Recovery occurs in less than 2 weeks after disease onset. It is often a self-limited condition and a short course of intravenous methyl prednisolone pulse therapy (20-25 mg /kg) for five days can produce marked benefit in children. Lumbar puncture with analysis of cerebrospinal fluid usually shows mild pleocytosis.MRI brain is often normal.
3.6. Paraneoplastic cerebellar degeneration
Paraneoplastic cerebellar degeneration (PCD) represents a clinical syndrome characterized by progressive ataxia and cerebellar findings (nystagmus, vertigo, opsoclonus, dysarthria) caused by antineuronal antibodies in response to an immunologic trigger to tumor antigens that are similar to intracellular neuronal proteins. The most common associated malignancies are cancer from the ovaries, breast, uterus, or small cell carcinoma of the lung. Several small series reveal improvement with immunosuppressive therapy, however, treatment response is variable, with a considerable number of patients not responding to treatment. Management should be individualized and must include cancer treatment when identified, followed by immunotherapy. Treatments used include immunoglobulin G, corticosteroids, cyclophosphamide, rituximab, mycophenolate, and plasma exchange, in conjunction with sequential or associated chemotherapy and resection of identified tumors
3.7. Multiple sclerosis (MS)
Ataxia is a relatively common presentation of children with MS. About 5% to 15% of adolescents and a half of children aged less than 5 years with MS have ataxia [40]. In comparison to adults, children with MS have greater number of disease relapses and have more severe disability in their disease course. A short course of intravenous methyl prednisolone pulse therapy have been used with marked benefit in children followed by disease modifying therapy as indicated .
3.8. Miller Fisher syndrome
Miller Fisher syndrome (MFS) often manifests after a preceding viral infection or gastroenteritis in at least half of the cases. Weakness and ataxia reach a nadir within a few hours or days. Less often , patients may progress to quadriparesis and have respiratory muscle involvement. Diagnosis is mostly clinical when patients presents with a triad of ataxia, areflexia and ophthalmoplegia . Eye muscles may so severly be affected to manifest as frozen eyes; however pupillary reflexes will be retained.Nerve conduction studies must be performed to confirm the diagnosis. CSF analysis shows albumin-cytological dissociation and treatment is by plasma exchange.
4. Infectious and toxin mediated causes of treatable ataxias
4.1. Drugs and toxins
The Purkinje cells of the cerebellum are exquisitely sensitive to toxic injury. They are the largest cells in the CNS visible with the naked eyes and are metabolically very demanding. Therefore, mild changes in metabolic milieu will affect Purkinje cells initially. Classic toxin mediated acute ataxia is ethyl alcohol consumption. Binge drinking can cause an acute ataxia which resolves with time. However, chronic alcohol use leads to vermian atrophy and an ataxic gait. Antiepileptic drug overdose, especially phenytoin, carbamazepine, oxcarbazepine , lamotrigine can cause acute or subacute ataxia which improves with drug dose modification . Use of thiamine high dose (300 mg / day) for three days is advised in all cases of acute ataxia as thiamine deficiency may often coexist. Always rule out subclinical hypothyroidism if a patient manifests with gait ataxia with drug levels in the normal range.
Accidental ingestion of drugs may account for a third of cases of acute ataxia in toddlers. Anticonvulsants, dextromethorphan, insecticides such as paraquat and phosphine, lead, eucalyptus oil and shellfish poisoning may present with disabling cerebellar features [41]. Clinical features include depressed mentation or agitation, seizures, and cerebellar signs. A urine and serum drug screen apart from reviewing the medication prescription of all household members may be suggestive.
Teenagers and young adults presenting with acute ataxia should be queried for drugs – therapeutic or recreational. Antineoplastics such as fluorouracil (5-FU) , cytarabine (ara- C), and methotrexate have potential to cause acute cerebellar dysfunction. Cocaine, heroin, toluene and phencyclidine are drugs of abuse known to produce ataxia [42]. Scorpion sting envenomation and cocaine use may also lead to cerebellar infarctions [43].
4.2. Acute cerebellitis
Acute cerebellitis may result from a direct infection of the cerebellum or following a systemic illness. Mycoplasma, rotavirus and human herpesvirus are the usual pathogens implicated in direct infection [44]. Presentation is with features of raised intracranial pressure, altered sensorium , irritability and cerebellar dysfunction. A lumbar puncture at the time of active infection may be dangerous in view of an impending risk of coning. MRI Brain shows features of cerebellar oedema. Death may occur due to brain edema and tonsillar herniation [45]. Early therapy with parenteral acyclovir at 10 mg / kg / dose three times a day for 21 days is advised.
4.3. HIV-related progressive multifocal leukoencephalopathy and ataxia
A subacute onset ataxia of months duration was described in patients with retroviral illness which on evaluation was found to have progressive multifocal leukoencephalopathy (PML). Hot cross bun sign, a cruciate hyperintensity in the pons, best seen on axial T2-weighted and FLAIR sequences of MRI has been described.This sign is classically described in degenerative diseases like multiple system atrophy (MSA) , Spinocerebellar Ataxia 2 and 3 , variant Creutzfeldt-Jacob disease and rarely with infectious diseases of the CNS. Treatment is by anti retroviral therapy [46].
5. Other treatable causes of ataxia
5.1. Superficial siderosis
Superficial siderosis (SS) manifests with the classic triad of cerebellar ataxia, sensorineural deafness, and myelopathy. Dementia and palatal tremor, in addition to other brainstem findings, have been reported [47]. SS results from recurrent hemorrhages into the subarachnoid space, with hemosiderin deposition in the subpial layers of the cranial nerves, cerebellum, brainstem, and spinal cord leading to neurological dysfunction. Hemorrhages are often due to dural vascular abnormalities, trauma, parenchymal vascular lesions, tumors, or neurosurgical procedures. However, the source of bleeding has only been found in approximately 50% of known cases.
The MRI findings in superficial siderosis include suseptibilty weighted images (SWI) showing dense hypointensities surrounding brain parenchyma consistent with hemosiderin deposition in the sulcal spaces and meningeal layers. Cerebellar hemispheric or cerebellar peduncles atrophy, inferior olivary hypertrophy, T2 olivary hyperintensity are typically encountered.
Several reports have evaluated the use of the iron chelator deferiprone in SS, as the drug crosses the blood–brain barrier, potentially removing hemosiderin from the CNS space [48]. Prominent subjective improvement in ataxia and other neurological symptoms have been documented using 30 mg/kg/d deferiprone [49].
5.2. Hydrocephalus
Children and young adults may present with ataxia arising from hydrocephalus owing to mass lesions or hemorrhage from vascular lesions and is usually accompanied by other symptoms such as headache and vomiting. Clinical signs can include papilledema and paresis of lateral gaze. Emergent third ventriculostomy or external ventricular drainage combined with the definitive procedure depending on the lesion with anti-cerebral edema measures helps to alleviate the ataxia.
5.3. Acute traumatic cerebellar ataxia
Isolated involvement of cerebellum, sparing the more commonly affected supratentorial structures and cervical cord , in trauma is rare [50]. Unsteady gait is a usual symptom post-concussion [51] and limb dysmetria is conspicuously absent. Less often , trauma can also produce dissection of the vertebral arteries [52] especially at the level where it pierces the dura, at the origin of intracranial V4 segment. Acute ataxia, headache, neck pain and vomiting are the most common symptoms. Diffusion-weighted MRI, including T1-weighted images with fat suppression and MR angiography are noninvasive imaging tools that are useful. Digital substraction angiography confirms the diagnosis. Treatment is with antiplatelet drugs. Trauma may also cause acute worsening of ataxia in certain conditions like vanishing white matter disease [53] and episodic ataxia type 2.
5.4. Functional or psychogenic acute ataxia
Acute gait disturbance mimicking ataxia may also be a feature of psychogenic or functional movement disorder especially in teenaged girls [54]. Psychogenic limb tremor mimicking appendicular ataxia is a common accompaniment [55] which may further complicate the diagnosis. It typically has a rapid onset and severity varies. Functional limb tremor or functional appendicular ataxia maybe present irrespective of rest, posture or during action, in contrast to organic disease. Noteworthy features of functional gait disturbance include patients maladaptation to the gait problem in the expected way like a lack of wide-based gait, absence of falls and injuries [56].
6. Conclusion
The primary concern in a patient with ataxia is to exclude treatable causes, including CNS infections, mass lesions, demyelination and immune mediated diseases. Ataxia can be diagnostically approached by considering the temporal course, most acute and subacute ataxias have a reversible etiology .The presence or absence of associated neurologic abnormalities gives clinical insight to the etiology. Ancillary testing should be individualized, but strong consideration should be given to assessing for potentially treatable causes. Recognizing treatable causes of ataxia is essential to implement targeted treatments, and also to institute definitive long term management as early as possible to check neurological deterioration.
CRediT authorship contribution statement
K.P. Divya:Writing - original draft.Asha Kishore:Writing - review & editing.
Footnotes
Financial disclosures: None.
References
- 1.Gotoda T., Arita M., Arai H. Adult-onset spinocerebellar dysfunction caused by a mutation in the gene for the alphatocopherol-transfer protein. N Engl J Med. 1995;333:1313–1318. doi: 10.1056/NEJM199511163332003. [DOI] [PubMed] [Google Scholar]
- 2.van de Warrenburga B.P.C., van Gaalena J., Boeschb S. EFNS/ENS Consensus on the diagnosis and management of chronic ataxias in adulthood. European Journal of Neurology. 2014;21:552–562. doi: 10.1111/ene.12341. [DOI] [PubMed] [Google Scholar]
- 3.Gabsi S., Gouider-Khouja N., Belal S. Effect of vitamin E supplementation in patients with ataxia with vitamin E deficiency. Eur J Neurol. 2001;8:477–481. doi: 10.1046/j.1468-1331.2001.00273.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee J., Hegele R.A. Abetalipoproteinemia and homozygous hypobetalipoproteinemia:a framework for diagnosis and management. J Inherit Metab Dis. 2014;37:333–339. doi: 10.1007/s10545-013-9665-4. [DOI] [PubMed] [Google Scholar]
- 5.Moghadasian M.H., Salen G., Frohlich J.J., Scudamore C.H. Cerebrotendinous xanthomatosis: a rare disease with diverse manifestations. Arch Neurol. 2002;59:527–529. doi: 10.1001/archneur.59.4.527. [DOI] [PubMed] [Google Scholar]
- 6.Lorincz M.T., Rainier S., Thomas D., Fink J.K. Cerebrotendinous xanthomatosis: possible higher prevalence than previously recognized. Arch Neurol. 2005;62:1459–1463. doi: 10.1001/archneur.62.9.1459. [DOI] [PubMed] [Google Scholar]
- 7.Yahalom G., Tsabari R., Molshatzki N., Ephraty L., Cohen H., Hassin-Baer S. Neurological outcome in cerebrotendinous xanthomatosis treated with chenodeoxycholic acid: early versus late diagnosis. Clin Neuropharmacol. 2013;36:78–83. doi: 10.1097/WNF.0b013e318288076a. [DOI] [PubMed] [Google Scholar]
- 8.Vanier M.T. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anheim M., Lagha-Boukbiza O., Fleury-Lesaunier M.C. Heterogeneityand frequency of movement disorders in juvenile and adult-onset Niemann-Pick C disease. J Neurol. 2014;261:174–179. doi: 10.1007/s00415-013-7159-9. [DOI] [PubMed] [Google Scholar]
- 10.McKay Bounford K., Gissen P. Genetic and laboratory diagnostic approach in Niemann Pick disease type C. J Neurol. 2014;261(Suppl. 2):569–575. doi: 10.1007/s00415-014-7386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyseng-Williamson K.A. Miglustat: a review of its use in Niemann-Pick disease type C. Drugs. 2014;74:61–74. doi: 10.1007/s40265-013-0164-6. [DOI] [PubMed] [Google Scholar]
- 12.Emmanuele V., Lopez L.C., Berardo A. Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. ArchNeurol. 2012;69:978–983. doi: 10.1001/archneurol.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pineda M., Montero R., Aracil A. Coenzyme Q(10)-responsive ataxia: 2-year-treatment follow-up. Mov Disord. 2010;25:1262–1268. doi: 10.1002/mds.23129. [DOI] [PubMed] [Google Scholar]
- 14.Schmucker S., Puccio H. Understanding the molecular mechanisms of Friedreich’s ataxia to develop therapeutic approaches. Hum Mol Genet. 2010;19:R103–R110. doi: 10.1093/hmg/ddq165. [DOI] [PubMed] [Google Scholar]
- 15.Meier T., Perlman S.L., Rummey C., Coppard N.J., Lynch D.R. Assessment of neurological efficacy of idebenone in pediatric patients with Friedreich’s ataxia: data from a 6-month controlled study followedby a 12-month open-label extension study. J Neurol. 2012;259:284–291. doi: 10.1007/s00415-011-6174-y. [DOI] [PubMed] [Google Scholar]
- 16.Ilg W., Bastian A.J., Boesch S. Consensus paper: management of degenerative cerebellar disorders. Cerebellum. 2014;13:248–268. doi: 10.1007/s12311-013-0531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherji M., Chien W., Kershaw N.J. Structure-function analysisof phytanoyl-CoA 2-hydroxylase mutations causing Refsum’s disease. Hum Mol Genet. 2001;10:1971–1982. doi: 10.1093/hmg/10.18.1971. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein R. Phytanic acid storage disease (Refsum’s disease): clinical characteristics, pathophysiology and the role of therapeutic apheresis in its management. J Clin Apheresis. 1999;14:181–184. doi: 10.1002/(sici)1098-1101(1999)14:4<181::aid-jca5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Masters-Thomas A., Bailes J., Billimoria J.D., Clemens M.E., Gibberd F.B., Page N.G. Heredopathia atactica polyneuritiformis (Refsum’s disease): 1. Clinical features and dietary management. J Hum Nutr. 1980;34:245–250. doi: 10.3109/09637488009143444. [DOI] [PubMed] [Google Scholar]
- 20.Wills A.J., Manning N.J., Reilly M.M. Refsum’s disease. QJM. 2001;94:403–406. doi: 10.1093/qjmed/94.8.403. [DOI] [PubMed] [Google Scholar]
- 21.Leen W.G., Wevers R.A., Kamsteeg E.J., Scheffer H., Verbeek M.M., Willemsen M.A. Cerebrospinal fluid analysis in the workup of GLUT1 deficiency syndrome: a systematic review. JAMA Neurol. 2013;70:1440–1444. doi: 10.1001/jamaneurol.2013.3090. [DOI] [PubMed] [Google Scholar]
- 22.Leen W.G., Klepper J., Verbeek M.M. Glucose transporter-1 deficiency syndrome: the expanding clinical and genetic spectrum of a treatable disorder. Brain. 2010;133:655–670. doi: 10.1093/brain/awp336. [DOI] [PubMed] [Google Scholar]
- 23.Pons R., Collins A., Rotstein M., Engelstad K., De Vivo D.C. The spectrum of movement disorders in Glut-1 deficiency. Mov Disord. 2010;25:275–281. doi: 10.1002/mds.22808. [DOI] [PubMed] [Google Scholar]
- 24.Yang H., Wang D., Engelstad K. Glut1 deficiency syndrome and erythrocyte glucose uptake assay. Ann Neur Ol. 2011;70:996–1005. doi: 10.1002/ana.22640. [DOI] [PubMed] [Google Scholar]
- 25.Ramm-Pettersen A., Nakken K.O., Skogseid I.M. Good outcome in patients with early dietary treatment of GLUT-1 deficiency syndrome: results from a retrospective Norwegian study. Dev Med Child Neurol. 2013;55:440–447. doi: 10.1111/dmcn.12096. [DOI] [PubMed] [Google Scholar]
- 26.Jen J.C., Graves T.D., Hess E.J. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain. 2007;130:2484–2493. doi: 10.1093/brain/awm126. [DOI] [PubMed] [Google Scholar]
- 27.Jen J., Kim G.W., Baloh R.W. Clinical spectrum of episodic ataxia type 2. Neurology. 2004;62:17–22. doi: 10.1212/01.wnl.0000101675.61074.50. [DOI] [PubMed] [Google Scholar]
- 28.Hadjivassiliou M., Sanders D.S., Grunewald R.A., Woodroofe N., Boscolo S., Aeschlimann D. Gluten sensitivity: from gut to brain. Lancet Neurol. 2010;9:318–330. doi: 10.1016/S1474-4422(09)70290-X. [DOI] [PubMed] [Google Scholar]
- 29.Hadjivassiliou M., Aeschlimann P., Sanders D.S. Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology. 2013;80:1740–1745. doi: 10.1212/WNL.0b013e3182919070. [DOI] [PubMed] [Google Scholar]
- 30.Hadjivassiliou M., Davies-Jones G.A., Sanders D.S., Grunewald R.A. Dietary treatment of gluten ataxia. J Neurol Neurosurg Psychiatry. 2003;74:1221–1224. doi: 10.1136/jnnp.74.9.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honnorat J., Saiz A., Giometto B. Cerebellar ataxia with antiglutamic acid decarboxylase antibodies: study of 14 patients. Arch Neurol. 2001;58:225–230. doi: 10.1001/archneur.58.2.225. [DOI] [PubMed] [Google Scholar]
- 32.Pittock S.J., Lucchinetti C.F., Parisi J.E. Amphiphysin autoimmunity:paraneoplastic accompaniments. Ann Neurol. 2005;58:96–107. doi: 10.1002/ana.20529. [DOI] [PubMed] [Google Scholar]
- 33.Saiz A., Blanco Y., Sabater L. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131(Pt 10):2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 34.Arino H., Gresa-Arribas N., Blanco Y. Cerebellar ataxia and glutamic acid decarboxylase antibodies: immunologic profile and long-term effect of immunotherapy. JAMA Neurol. 2014;71:1009–1016. doi: 10.1001/jamaneurol.2014.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa H., Yoneda M., Fujii A., Kinomoto K., Kuriyama M. Hashimoto’s encephalopathy presenting with progressive cerebellar ataxia. J Neurol Neurosurg Psychiatry. 2007;78:196–197. doi: 10.1136/jnnp.2006.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selim M., Drachman D.A. Ataxia associated with Hashimoto’s disease:progressive non-familial adult onset cerebellar degeneration with autoimmune thyroiditis. J Neurol Neurosurg Psychiatry. 2001;71:81–87. doi: 10.1136/jnnp.71.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujii A., Yoneda M., Ito T. Autoantibodies against the amino terminal of alpha-enolase are a useful diagnostic marker of Hashimoto’s encephalopathy. J Neuroimmunol. 2005;162:130–136. doi: 10.1016/j.jneuroim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Desai J., Mitchell W.G. Acute cerebellar ataxia, acute cerebellitis, and opsclonusmyoclonus syndrome. J Child Neurol. 2012;27(11):1482–1488. doi: 10.1177/0883073812450318. [DOI] [PubMed] [Google Scholar]
- 39.Nussinovitch M., Prais D., Volovitz D., Shapiro R., Amir J. Post-infectious acute cerebellar ataxia in children. Clin Pediatr. 2003;42(7):581–584. doi: 10.1177/000992280304200702. [DOI] [PubMed] [Google Scholar]
- 40.Bigi S., Banwell B. Pediatric multiple sclerosis. J Child Neurol. 2012;27(11):1378–1383. doi: 10.1177/0883073812452784. [DOI] [PubMed] [Google Scholar]
- 41.Ryan M.M., Engle E.C. Acute ataxia in childhood. J Child Neurol. 2003;18(5):309–316. doi: 10.1177/08830738030180050901. [DOI] [PubMed] [Google Scholar]
- 42.King M.D. Neurological sequelae of toluene abuse. Hum Toxicol. 1982;1(3):281–287. doi: 10.1177/096032718200100311. [DOI] [PubMed] [Google Scholar]
- 43.Manto M. Toxic agents causing cerebellar ataxias. Handb Clin Neurol. 2012;103:201–213. doi: 10.1016/B978-0-444-51892-7.00012-7. [DOI] [PubMed] [Google Scholar]
- 44.Nussinovitch M., Prais D., Volovitz D., Shapiro R., Amir J. Post-infectious acute cerebellar ataxia in children. Clin Pediatr. 2003;42(7):581–584. doi: 10.1177/000992280304200702. [DOI] [PubMed] [Google Scholar]
- 45.Hacohen Y., Niotakis G., Aujla A. Acute life threatening cerebellitis presenting with no apparent cerebellar signs. Clin Neurol Neurosurg. 2011;113(10):928–930. doi: 10.1016/j.clineuro.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Padmanabhan S., Cherian A., Iype T., Mathew M., Smitha S. Hot cross bun sign in HIV-related progressive multifocal leukoencephalopathy. Ann Indian Acad Neurol. 2013;16:672–673. doi: 10.4103/0972-2327.120479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar N., Eggers S.D., Milone M., Keegan B.M. Acquired progressive ataxia and palatal tremor: importance of MRI evidence of hemosiderin deposition and vascular malformations. Parkinsonism Relat Disord. 2011;17:565–568. doi: 10.1016/j.parkreldis.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 48.Cummins G., Crundwell G., Baguley D., Lennox G. Treatment of superficial siderosis with iron chelation therapy. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-009916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy M., Llinas R.H. Update on a patient with superficial siderosis on deferiprone. Am J Neuroradiol. 2012;33:E99–100. doi: 10.3174/ajnr.A3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gohil J.R., Munshi S.S. Post concussion ataxia following minor head injury. Indian Pediatr. 2006;43(9):829. [PubMed] [Google Scholar]
- 51.Yeoh H.K., Lind C.R., Law A.J. Acute transient cerebellar dysfunction and stuttering following mild closed head injury. Childs Nerv Syst. 2006;22(3):310–313. doi: 10.1007/s00381-005-1154-0. [DOI] [PubMed] [Google Scholar]
- 52.Mortazavi M.M., Verma K., Tubbs R.S., Harrigan M. Pediatric traumatic carotid, vertebral and cerebral artery dissections: a review. Childs Nerv Syst. 2011;27(12):2045–2056. doi: 10.1007/s00381-011-1409-x. [DOI] [PubMed] [Google Scholar]
- 53.van der Knaap M.S., Pronk J.C., Scheper G.C. Vanishing white matter disease. Lancet Neurol. 2006;5(5):413–423. doi: 10.1016/S1474-4422(06)70440-9. [DOI] [PubMed] [Google Scholar]
- 54.Bisht J., Sankhyan N., Kaushal R.K., Sharma R.C., Grover N. Clinical profile of pediatric somatoform disorders. Indian Pediatr. 2008;45(2):111–115. [PubMed] [Google Scholar]
- 55.Schwingenschuh P., Pont-Sunyer C., Surtees R., Edwards M.J., Bhatia K.P. Psychogenic movement disorders in children: a report of 15 cases and a review of the literature. Mov Disord. 2008;23(13):1882–1888. doi: 10.1002/mds.22280. [DOI] [PubMed] [Google Scholar]
- 56.Edwards M.J., Bhatia K.P. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11(3):250–260. doi: 10.1016/S1474-4422(11)70310-6. [DOI] [PubMed] [Google Scholar]