Abstract
Purpose
We aimed to investigate the effect of metal ions from oral prostheses (OPs) released into the saliva of patients with oral lichenoid lesions (OLLs).
Materials and Methods
Subjects (n=183) were divided into four groups according to the presence or absence of OLL and OP. Concentrations of the metal ions titanium, chromium (Cr), cobalt (Co), nickel (Ni), palladium (Pd), silver (Ag), platinum (Pt), gold (Au), and zirconium (Zr) were measured using a laser-ablation microprobe inductively coupled to a plasma mass spectrometer. Saliva levels of interleukin (IL)-6, IL-1β, IL-8, and tumor necrosis factor-α were detected using an enzyme-linked immunosorbent assay. The reticulation/keratosis, erythema, and ulceration (REU) scoring system was used to assess the severity of OLL.
Results
Mean concentrations of IL-6 and IL-8 were statistically higher in OLL patients with OPs. The concentration of Ni was high in OLL groups. The concentrations of Cr, Ni, and Au ions in the saliva were positively correlated with IL-8. REU scores were positively correlated with salivary concentrations of IL-6 and IL-8, as well as with concentrations of Cr, Ni, and Au.
Conclusion
Increased concentrations of metal ions, especially Ni, in saliva were positively correlated with IL-8 and showed positive correlations with the severity of OLL.
Keywords: Oral lichenoid lesion, metal ion, saliva, cytokine, oral prosthesis
INTRODUCTION
As patient desires for esthetics has increased, so has the use of ceramic and zirconium (Zr) dental prostheses also increased. However, the use of porcelain-fused metal (PFM) and gold crowns is still preferred. Although oral prostheses (OPs) are exposed to various environmental conditions, such as temperature and pH changes, toothpaste, drugs, food, bacteria, mechanical load, and galvanic reactions, they can last on average for more than 10 years. Previous studies, however, have demonstrated that these environmental factors increase the amount of metal ions released by corroding OPs, and many researchers have raised questions about adverse reactions between metal ions released from OPs in the mouth and the oral mucosa.1,2
Patient complaints of burning mouth syndrome, recurrent aphthous stomatitis, and gingivitis can be caused by contact allergic reactions between the oral mucosa and the OP. These allergic reactions are presumed to be an etiology of oral lichenoid lesions (OLLs), a T-cell-mediated chronic inflammatory disorder.3,4 Although it is clear that T cells are involved in OLL pathogenesis, it is still unclear which antigens elicit an immune response mediated by CD4+ and CD8+ T cells.
Dental materials are considered to be one etiologic factor of OLLs,5,6 and OLLs are considered to be either a disease or a consequence of OPs. They are sometimes regarded as both, with OPs considered an aggravating factor of existing OLLs.2 Hypersensitivity reactions in patients with OLLs caused by OPs usually resolve upon removal of the trigger, and a chronic inflammatory state is not maintained, as is characteristic of OLLs. However, when there are many OPs in the mouth, it can be difficult to decide exactly which OP should be removed if an adverse reaction occurs and to confirm whether the lesion was in fact caused by the OP.
Levels of cobalt (Co) and chromium (Cr) ions in serum have been measured in metal-on-metal hip resurfacing arthroplasties to evaluate adverse reactions.7 As in these studies, we also aimed to determine if concentrations of metal ions in saliva could affect OLL severity. The metal ions titanium (Ti), Cr, Co, nickel (Ni), palladium (Pd), silver (Ag), platinum (Pt), gold (Au), and Zr are widely used in prostheses, and the relationship between these ions, oral lesions, and other symptoms has been studied.8,9 The reason saliva was used in these studies is because collecting it is noninvasive, and it is readily collectible and relatively inexpensive. Moreover, previous studies on cytokine concentrations have shown that although greater levels of cytokines are detected in both serum and saliva of patients with OLL than in those of healthy controls (HCs), the average concentration of cytokines detected in saliva is higher than that found in the serum of patients with OLL.10,11,12 For this reason, saliva is believed to be more effective than serum when studying OLLs. Although various kinds of cytokines have been studied in patients with OLLs, NF-κB-dependent interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α were selected in this study as they have been verified as OLL biomarkers. They have also returned consistent results in terms of concentrations in saliva in many studies, and they are important for studying inflammatory reactions.10,11,12,13 The reticulation/keratosis, erythema, and ulceration (REU) scoring system was used to assess the severity of OLL.14,15
The aim of this study was to compare metal ion and cytokine concentrations in the saliva of patients with OLLs to those in the saliva of HCs and to investigate correlations between metal ion and cytokine concentrations in patients with OLL. A second aim of this study was to investigate the effects of OPs on salivary metal ion concentrations.
MATERIALS AND METHODS
Subjective
Subjects were divided into four groups: 1) HCs with no OPs (HCwoOP, n=30); 2) HCs with OPs (HCwOP, n=41); 3) patients diagnosed with OLL, clinically or histologically, and with OPs (OLLwOP, n=107); and 4) OLL and no OPs (OLLwoOP, n=5). Almost all patients had dental restorations, and the number of patients in the OLLwoOPs group was limited. Patients with OLLs were recruited from those who first visited the Department of Oral Medicine at the Pusan National University Dental Hospital from March 2015 to February 2019. Subjects were all South Korean. Subjects with other OLs were excluded, and those who were taking corticosteroids or immunosuppressive medications at that time due to OLL or other systemic diseases or had a record of taking them within 6 months were also excluded. The HC group consisted of volunteer participants. In the OLLwOP group, the visual analog scale (VAS) was used to assess pain scores, and the salivary flow rate was evaluated. The type and number of OPs were examined in the group with OPs. Written informed consent was obtained from all patients at the first visit. This study was approved by the Institutional Review Board of Pusan National University Dental Hospital (IRB No. PNUDH-2017-028).
OLL scoring system
REU scores were obtained for the OLL group. Based on the REU criteria, the oral cavity was divided into 10 sites (upper/lower labial mucosa, right buccal mucosa, left buccal mucosa, dorsal tongue, ventral tongue, floor of the mouth, hard palate mucosa, soft palate/tonsillar pillars, maxillary gingiva, and mandibular gingiva), and 0 or 1 point was given to each part for the existence or nonexistence of a reticular/hyperkeratotic form. In addition, if there was no erythema or ulcer, 0 points were given. One point was given for lesions smaller than 1 cm2, 2 points for 1–3-cm2 lesions, and 3 points for lesions greater than 3 cm2. The final score was calculated by summing the total scores of reticular, erythema, and ulcer lesions, with weights of 1, 1.5, and 2 given to them, respectively.14,15 For example, if a patient had lesions with a reticular lesion, with a 4-cm2 patch of erythema and a 0.5-cm2 ulcer on the right buccal mucosa, as well as a reticular lesion with 2-cm2 erythema patch on the left buccal mucosa, the final score was 11.5 points:
| Right buccal mucosa: (1×1)+(3×1.5)+(1×2)=7.5 |
| Left buccal mucosa: (1×1)+(2×1.5)=4 |
| Final score: 7.5+4=11.5 |
Saliva collection
Unstimulated whole saliva was collected from subjects at their first visit. The subjects were advised to avoid eating, drinking, smoking, and brushing their teeth for at least 1 hour before the collection. They were asked to rinse their mouths with water and wait at least 10 minutes after rinsing before giving a sample. A 2-mL polypropylene tube was used to collect saliva by passive drooling for 3 min. Collected saliva samples were immediately stored in a −80℃ refrigerator. The saliva samples were kept at −80℃ for later analysis.
Analysis of metal ions in saliva
Ti, Cr, Co, Ni, Pd, Ag, Pt, Au, and Zr, which are widely used in replacement prostheses, were investigated. Metal ions were measured using a laser-ablation microprobe inductively coupled to a plasma mass spectrometer (Optima 3000; Perkin Elmer, Norwalk, CT, USA). To assess the concentration of metal ions in saliva, samples were diluted directly with nitric acid, as described by Kim, et al.16 The manufacturer's recommended operating procedures for the instruments were followed.
Detection of cytokines by enzyme-linked immunosorbent assay (ELISA)
For enzyme-linked immunosorbent assay (ELISA), saliva specimens were thawed on ice. Following centrifugation at 6000×g at 4℃ for 20 min, cellular debris was removed, and the supernatant was used for cytokine analysis. Manufacturer's instructions (Human Uncoated ELISA kit; Invitrogen, Carlsbad, CA, USA) were followed to detect saliva levels of IL-6, IL-1β, IL-8, and TNF-α by ELISA.
Statistical analysis
All statistical analyses were performed using IBM Statistical Package for the Social Sciences version (SPSS) 22.0 (IBM Corp., Armonk, NY, USA). The independent t-test was used to compare the distribution of OPs between the HCwOP and OLLwOP groups. In addition, Kruskal-Wallis was used to compare the average concentrations of metal ions and cytokines in saliva and salivary flow rate among the four groups. Pearson's correlation test was used to verify the correlation between REU scores and metal ion concentrations and REU scores and cytokine concentrations. Pearson's correlation test was also used to analyze correlations between OPs and metal ion concentrations and between cytokines and metal ion concentrations in the OLLwOP group. Multiple linear regression was used to investigate the effects of independent variables on REU score. p<0.05 was considered statistically significant.
RESULTS
Higher number of OPs with high non-precious metal alloy content in the OLLwOP group than in the HCwOP group
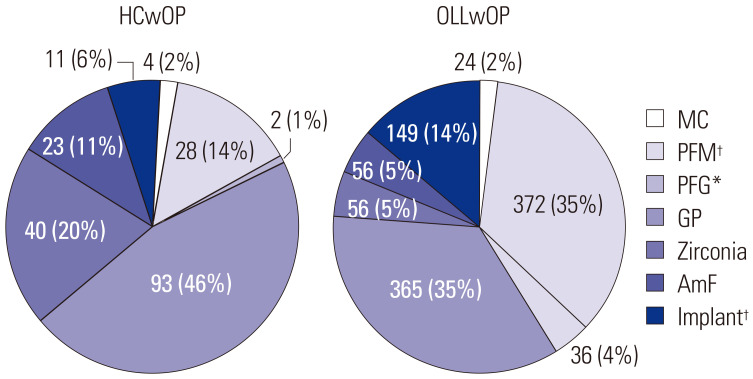
The ratio of females to males exceeded 1 in all groups except the HCwoOP group. The average non-stimulated salivary flow rate was not statistically different among the four groups, and neither was stimulated salivary flow, both of which suggest that the amount of saliva was not altered in OLL.17 The average age of the OLL group was higher than that of the HC group (Table 1). Most patients with OLL were in their 40s or older. An age-matched control group would have been desirable in the present study. However, older patients predominated made up the OLL group, and no age-matched control group without metal restorations was available. The mean REU scores of the OLLwOP and OLLwoOP groups were 7.58 [interquartile range (IQR), 6.50] and 5.10 (IQR, 4.50), respectively (Table 1). When the distributions of the type of OPs were examined, in the HCwOP group, gold prosthesis (GP) and Zr prostheses were most common, and PFMs and GP were most common in the OLLwOP group. In the OLLwOP group, there were statistically more PFMs (p<0.001) and porcelain-fused gold (PFG) (p<0.01) OPs, which are OPs with a high non-precious metal alloy content. In addition, the number of implants in patients of the OLLwOP group was also statistically higher than that in the HCwOP groups (p<0.001) (Fig. 1).
Table 1. Characteristics of the Samples.
| Groups | HCwoOP (n=30) | HCwOP (n=41) | OLLwOP (n=107) | OLLwoOP (n=5) | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 19 (63.3) | 18 (43.9) | 32 (29.9) | 2 (40) | |
| Female | 11 (36.7) | 23 (56.1) | 75 (70.1) | 3 (60) | |
| Salivary flow rate (mL/min)* | |||||
| Unstimulated | 0.35±0.32 | 0.50±0.38 | 0.53±0.55 | 0.16±0.11 | |
| Stimulated | 1.32±0.84 | 1.54±0.79 | 1.42±1.07 | 1.24±0.60 | |
| Age (mean±SD) | 30.6±9.27 | 39.93±13.97 | 60.11±11.75 | 51.80±18.02 | |
| REU score (IQR) | 7.58 (6.50) | 5.10 (4.50) | |||
HCwoOP, healthy control without oral prosthesis; HCwOP, healthy control with oral prosthesis; OLLwOP, oral lichenoid lesion with oral prosthesis; OLLwoOP, oral lichenoid lesion without oral prosthesis; IQR, interquartile range; REU, reticulation/keratosis, erythema, and ulceration.
p values were obtained by Kruskal-Wallis.
*p>0.05.
Fig. 1. Distribution of OPs in HCwOP and patients with OLLwOP. p values were obtained by independent t-test. *p<0.01, †p<0.001. HCwOP, healthy control with oral prosthesis; OLLwOP, oral lichen lesion with oral prosthesis; MC, metal crown; GP, gold prosthesis; PFM, porcelain-fused metal; PFG, porcelain-fused gold; AmF, amalgam filling; OP, oral prosthesis.
In the OLL group, the concentration of Ni in saliva was statistically higher than that in HCs
The mean concentrations of the various salivary metal ions in the four groups are shown in Table 2. Ni was observed at higher concentrations in the OLL group (p<0.01), while Pd (p<0.01) and Au (p<0.01) were observed at higher concentrations in the HC group. Ag was observed at a higher concentrations in HCwOP and OLLwOP groups. The only statistically significant differences in the concentrations of metal ions among the four groups were in Ni, Pd, Ag, and Au, not Ti and Zr (Table 2). There was a positive correlation between the number of metal crowns (p<0.01) and PFMs (p<0.05) and the concentration of Ni. Also, the number of PFG OPs (p<0.05) and the concentration of Ag were also positively correlated in the OLLwOP group (Table 3).
Table 2. Concentrations of Metal Ions [Parts Per Billion (ppb)] in Saliva.
| Metal ion | HCwoOP (n=30) | HCwOP (n=41) | OLLwOP (n=107) | OLLwoOP (n=5) | p value |
|---|---|---|---|---|---|
| Ti | 444.37 (375.04) | 607.10 (450.16) | 628.99 (507.08) | 1154.20 (469.70) | 0.484 |
| Cr | 7.96 (7.68) | 8.36 (7.28) | 8.16 (7.18) | 10.25 (10.40) | 0.556 |
| Co | 0.31 (0.00) | 0.24 (0.00) | 0.51 (0.00) | 0.84 (1.01) | 0.161 |
| Ni‡ | 7.89 (7.95) | 7.55 (6.53) | 14.11 (8.33) | 25.50 (16.61) | 0.023* |
| Zr | 0.28 (0.00) | 0.03 (0.00) | 0.38 (0.00) | 0.00 (0.00) | 0.437 |
| Pd | 3.18 (1.77) | 5.73 (2.13) | 1.97 (0.00) | 0.27 (0.00) | 0.014* |
| Ag | 0.00 (0.00) | 1.22 (0.00) | 1.36 (0.00) | 0.00 (0.00) | 0.008† |
| Pt | 0.00 (0.00) | 0.17 (0.00) | 0.28 (0.00) | 0.00 (0.00) | 0.775 |
| Au | 25.53 (17.38) | 65.50 (5.36) | 13.34 (3.81) | 0.00 (0.00) | <0.001† |
HCwoOP, healthy control without oral prosthesis; HCwOP, healthy control with oral prosthesis; OLLwOP, oral lichenoid lesion with oral prosthesis; OLLwoOP, oral lichenoid lesion without oral prosthesis; Ti, titanium; Cr, chromium; Co, cobalt; Ni, nickel; Zr, zirconium; Pd, palladium; Ag, silver; Pt, platinum; Au, gold; IQR, interquartile range.
Data are shown as the median (IQR); p values were obtained by one-way Kruskal-Wallis.
*p<0.05, †p<0.01, ‡Ni was investigated in 97 out of 107 OLLwOP patients, and 10 patients were not analyzed.
Table 3. Correlations between OPs and Metal Ions in Patients with OLLwOP.
| OP | Ti | Cr | Co | Ni | Zr | Pd | Ag | Pt | Au | |
|---|---|---|---|---|---|---|---|---|---|---|
| MC | ||||||||||
| r | −0.062 | 0.021 | 0.081 | 0.265 | 0.052 | −0.113 | −0.111 | −0.029 | −0.122 | |
| p | 0.524 | 0.830 | 0.406 | 0.009† | 0.598 | 0.247 | 0.256 | 0.767 | 0.210 | |
| PFM | ||||||||||
| r | −0.099 | 0.011 | −0.001 | 0.210 | −0.045 | −0.105 | 0.104 | 0.035 | −0.060 | |
| p | 0.311 | 0.908 | 0.992 | 0.039* | 0.644 | 0.282 | 0.286 | 0.718 | 0.539 | |
| PFG | ||||||||||
| r | 0.012 | 0.155 | 0.035 | 0.193 | 0.069 | −0.069 | 0.224 | −0.032 | 0.151 | |
| p | 0.900 | 0.111 | 0.719 | 0.058 | 0.478 | 0.480 | 0.021* | 0.742 | 0.121 | |
| GP | ||||||||||
| r | −0.012 | −0.106 | 0.008 | 0.053 | −0.069 | −0.189 | 0.133 | 0.118 | 0.034 | |
| p | 0.905 | 0.279 | 0.932 | 0.606 | 0.477 | 0.052 | 0.172 | 0.227 | 0.729 | |
| Zirconia | ||||||||||
| r | −0.143 | −0.102 | −0.165 | −0.149 | 0.005 | −0.127 | −0.132 | −0.037 | −0.160 | |
| p | 0.141 | 0.298 | 0.089 | 0.145 | 0.961 | 0.192 | 0.175 | 0.701 | 0.099 | |
| AmF | ||||||||||
| r | 0.081 | 0.009 | −0.106 | −0.140 | 0.078 | −0.038 | −0.059 | −0.047 | −0.038 | |
| p | 0.405 | 0.927 | 0.275 | 0.172 | 0.423 | 0.699 | 0.548 | 0.629 | 0.696 | |
| Implant | ||||||||||
| r | −0.053 | 0.037 | −0.181 | −0.024 | 0.034 | 0.112 | 0.094 | 0.023 | 0.163 | |
| p | 0.587 | 0.701 | 0.062 | 0.819 | 0.726 | 0.252 | 0.337 | 0.813 | 0.093 | |
OP, oral prosthesis; OLLwOP, oral lichenoid lesion with oral prosthesis; Ti, titanium; Cr, chromium; Co, cobalt; Ni, nickel; Zr, zirconium; Pd, palladium; Ag, silver; Pt, platinum; Au, gold; MC, metal crown; GP, gold prosthesis; PFM, porcelain-fused metal; PFG, porcelain-fused gold; AmF, amalgam filling. r=Pearson's correlation coefficient; p values were obtained by Pearson's correlation
*p<0.05, †p<0.01.
Although both concentrations of IL-6 and IL-8 were high in patients with OLL, only concentrations of IL-8 were highly correlated with specific metal ions
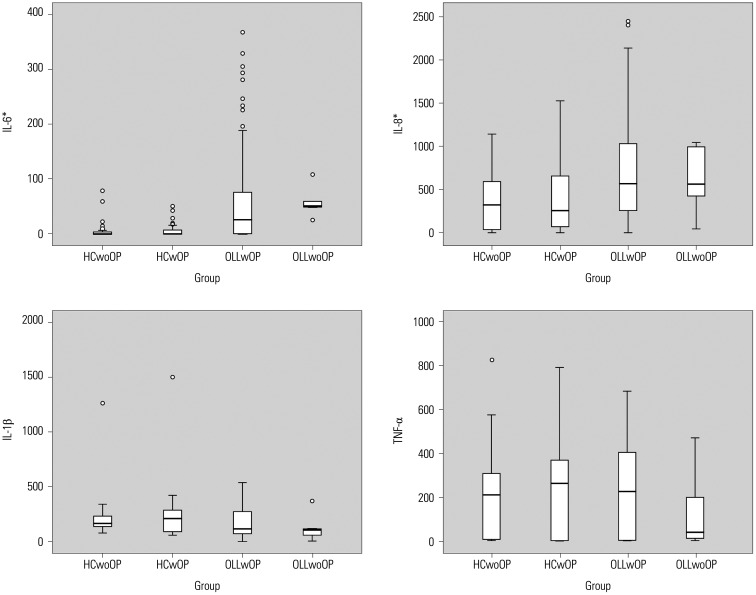
The mean concentrations of IL-6 (p<0.001) and IL-8 (p<0.001) were statistically higher in the OLL group; the salivary levels of IL-1β and TNF-α were not statistically different among the four groups (Fig. 2). The concentration of IL-6 in the saliva of patients with OLLwOP was not correlated with that of metal ions, while IL-8 concentrations were highly correlated with Cr (p<0.001), Co (p<0.01), Ni (p<0.01), Pd (p<0.001), and Au (p<0.001) concentrations. There was no correlation between metal ions Ti, Zr, Ag, and Pt and an increase in IL-8 (p>0.05). In addition, there was a positive correlation between IL-1β and Cr (p<0.05) and Au (p<0.05), whereas IL-1β was negatively correlated with Ti (p<0.05) (Table 4). In the HC group, there was no correlation between metal ions and IL-6, between metal ions and IL-8, or between metal ions and IL-1β. However, in the HCwOP group, an increase in TNF-α concentrations was negatively correlated with Cr (p<0.01) and Ni (p<0.01). In addition, in the HCwoOP group, an increase in TNF-α concentrations had a strong negative correlation with Co (p<0.001) and Ni (p<0.001). As for the correlation between cytokines, IL-8 increased when IL-1β increased, although IL-1β (p<0.001) did not have any statistical relationship with IL-6 (p>0.05) (Supplementary Table 1, only online). There was a correlation between an increase in IL-6 and that in IL-8 (p<0.05) (Supplementary Table 1, only online).
Fig. 2. Concentration of cytokines in saliva. *p<0.001. p values were obtained by Kruskal-Wallis. HCwoOP, healthy control without oral prosthesis; HCwOP, healthy control with oral prosthesis; OLLwOP, oral lichen lesion with oral prosthesis; OLLwoOP, oral lichen lesion without oral prosthesis.
Table 4. Correlations between Metal Ions and Cytokines.
| Group | Ti | Cr | Co | Ni | Zr | Pd | Ag | Pt | Au | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OLLwOP | |||||||||||
| IL-6 | |||||||||||
| r | 0.074 | 0.136 | 0.111 | 0.144 | 0.046 | 0.120 | −0.45 | 0.055 | 0.003 | ||
| p | 0.450 | 0.162 | 0.251 | 0.159 | 0.639 | 0.218 | 0.641 | 0.575 | 0.975 | ||
| IL-8 | |||||||||||
| r | −0.016 | 0.652 | 0.266 | 0.275 | −0.009 | 0.410 | 0.244 | 0.059 | 0.472 | ||
| p | 0.869 | <0.001‡ | 0.006† | 0.007† | 0.924 | <0.001‡ | 0.011* | 0.548 | <0.001‡ | ||
| IL-1β | |||||||||||
| r | −0.203 | 0.235 | 0.033 | −0.021 | 0.132 | −0.015 | −0.141 | −0.022 | 0.273 | ||
| p | 0.036* | 0.015* | 0.737 | 0.840 | 0.174 | 0.882 | 0.146 | 0.825 | 0.004† | ||
| TNF-α | |||||||||||
| r | −0.130 | 0.043 | 0.011 | −0.138 | 0.067 | 0.131 | 0.128 | 0.165 | 0.066 | ||
| p | 0.183 | 0.664 | 0.911 | 0.179 | 0.491 | 0.177 | 0.190 | 0.089 | 0.498 | ||
| HCwOP | |||||||||||
| TNF-α | |||||||||||
| r | 0.048 | −0.454 | −0.270 | −0.491 | −0.182 | 0.220 | −0.033 | −0.003 | 0.041 | ||
| p | 0.769 | 0.003† | 0.092 | 0.005† | 0.373 | 0.172 | 0.841 | 0.985 | 0.802 | ||
| HCwoOP | |||||||||||
| TNF-α | |||||||||||
| r | −0.155 | −0.346 | −0.765 | −0.788 | 0.061 | −0.052 | . | . | −0.301 | ||
| p | 0.414 | 0.061 | <0.001‡ | <0.001‡ | 0.747 | 0.785 | . | . | 0.105 | ||
HCwoOP, healthy control without oral prosthesis; HCwOP, healthy control with oral prosthesis; OLLwOP, oral lichenoid lesion with oral prosthesis; Ti, titanium; Cr, chromium; Co, cobalt; Ni, nickel; Zr, zirconium; Pd, palladium; Ag, silver; Pt, platinum; Au, gold; IL, interleukin; TNF, tumor necrosis factor.
r=Pearson's correlation coefficient; p values were obtained by Pearson's correlation.
*p<0.05, †p<0.01, ‡p<0.001.
Correlation between clinical severity and levels of inflammatory cytokines and metal ions
REU scores were well correlated with subjective pain scores on VAS (p<0.01). Interestingly, REU scores were positively correlated with salivary concentrations of IL-6, IL-8, and IL-1β (all p<0.001, respectively) and three metal ions [Cr (p<0.01), Ni (p<0.01), and Au (p<0.001)] (Table 5). A multiple regression analysis a stepwise regression method were utilized to investigate what variables have important effects on increased REU scores. Among 107 subjects, 95 were targeted, excluding those with missing values. Two variables (IL-6 and Ni) were able to account for 26.9% of the REU scores (25.3% according to the correction factor), which was statistically significant (p<0.001). As a result of the analysis, we found that IL-6 and Ni variables had statistically significant positive effects on REU scores (p<0.05). When β values were compared with one another to determine the level of relative importance among factors affecting REU scores, IL-6 (β=0.457) had the strongest effect, followed by Ni (β=0.190). Overall these results suggested that the higher the concentrations of IL-6 and Ni are, the higher REU scores are (Table 6).
Table 5. Correlations among Metal Ions, Cytokines, and REU Score.
| Ti | Cr | Co | Ni | Zr | Pd | Ag | Pt | Au | IL-6 | IL-8 | IL-1β | TNF-α | VAS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REU score | |||||||||||||||
| r | 0.043 | 0.253 | 0.095 | 0.286 | −0.062 | 0.119 | 0.093 | −0.036 | 0.343 | 0.439 | 0.380 | 0.416 | 0.114 | 0.282 | |
| p | 0.657 | 0.008† | 0.330 | 0.005† | 0.528 | 0.224 | 0.343 | 0.716 | <0.001‡ | <0.001‡ | <0.001‡ | <0.001‡ | 0.242 | 0.003† | |
Ti, titanium; Cr, chromium; Co, cobalt; Ni, nickel; Zr, zirconium; Pd, palladium; Ag, silver; Pt, platinum; Au, gold; IL, interleukin; TNF, tumor necrosis factor; REU, reticulation/keratosis, erythema, and ulceration; VAS, visual analog scale.
r=Pearson's correlation coefficient; p values were obtained by Pearson's correlation.
†p<0.01, ‡p<0.001.
Table 6. Associations for REU Score with Cytokines and Metal Ions by Multiple Linear Regression Analysis.
| Unstandardized coefficients | Standardized coefficients | t | p value | Collinearity statistics | ||||
|---|---|---|---|---|---|---|---|---|
| B | SE | β | Tolerance | VIF | ||||
| Constant | 4.680 | 0.564 | 8.300 | <0.001† | ||||
| IL-6 | 0.024 | 0.005 | 0.457 | 5.106 | <0.001† | 0.981 | 1.019 | |
| Ni | 0.057 | 0.027 | 0.190 | 2.126 | 0.036* | 0.981 | 1.019 | |
Variables: Ti, titanium; Cr, chromium; Co, cobalt; Ni, nickel; Zr, zirconium; Pd, palladium; Ag, silver; Pt, platinum; Au, gold; IL, interleukin; TNF, tumor necrosis factor; dependent variable: REU, reticulation/keratosis, erythema, and ulceration score; B, regression coefficient; SE, standard error; β, standardized coefficient; VIF, variation inflation factor.
R2 (0.269), adjusted R2 (0.253), F=17.100, p=0.000, Durbin-Watson (1.925). A stepwise regression method was utilized. A multiple regression analysis was performed in the OLL group.
*p<0.05, †p<0.001.
DISCUSSION
Studies on metal ions released from removable and fixed OPs have been conducted both in vitro and in vivo. In studies on metal ions released from Cr-Co alloy, the concentration of metal ions was higher in patients with metal alloy OPs than in patients without it.18 In addition, studies on saliva concentrations of metal ions in patients with fixed orthodontic appliances have found that the concentrations of Ni and Cr in the saliva of these patients were higher than those in the saliva of HCs.19,20 Similar results were also found in an artificial saliva environment in vitro.21 In studies on serum metal ion concentrations following metalon-metal hip replacement arthroplasty, the amount of metal ion peaked 3–4 years following surgery, and either remained at that same level or increased in some patients thereafter, based on long-term research continuing for 5–10 years after surgery.22 Unlike serum ion concentrations, previous studies on saliva have found that the highest amount of salivary metal ions appear initially following insertion of OPs in the mouth and then gradually decrease within the next 10 weeks.19,20 In our study, as the subjects had OPs for at least 5 years, the difference in metal ion concentration in saliva occurred with Ni, Au, and Pd (Table 2). In the OLL group, the concentration of Ni appeared to be statistically high, which may be because PFMs have a relatively higher content of Ni-Cr alloy and Co-Cr alloys, which are non-precious alloys. The data in Table 3 also show a similar tendency: more specifically, in the OLLwOP group, the number of metal crowns and PFMs in the oral cavity had a positive correlation with Ni concentrations in saliva. On the other hand, the concentrations of Au and Pd were higher in HC. Au was also highly correlated with REU scores and IL-8 concentration. Au is a stable metal, but clinical experience has revealed many patients with lesions around GPs. This correlation between GPs and an increase in the concentration of Au seems to arise from the fact that GP is not pure Au and is mixed with non-precious metals in the form of an alloy. However, there are also reports that Au itself causes an allergic reaction, and this is why further study on Au is necessary.23
Increased concentrations of metal ions in the body have been shown to be associated with chronic inflammation, carcinogenesis, mutagenesis, and cellular death.24 In Co-Cr based metal-on-metal hip joint replacement, it has been reported that metal ions, both at toxic and subtoxic levels, induced antigen-presenting cells and T-lymphocyte-mediated hypersensitivity. Moreover, metal ions released by corrosion can induce bone resorption cytokines (IL-1β, IL-6, and TNF-α), which originate from macrophages.25 In addition, other research has confirmed that metal ions of Cr and Ni could elicit the release of IL-1β through oxidative stress and the NF-κB-dependent signaling pathway.26 In another study, researchers showed that Ni2+ induces the expression of inflammatory genes, including IL-8, in human primary monocytes.27 Meanwhile, in another study of biological interactions between oral tissue and dental cast alloy, Ni-containing alloys repressed the proliferation rate of gingival fibroblasts and caused cell toxic reactions. In addition, it has also been reported that Ni, Au, Pd, and Co cause allergic reactions to dental cast alloy.2 Our study also showed that an increase in the concentrations of Cr and Au was correlated with IL-1β and that increases in the concentrations of Cr, Co, Ni, Pd, and Au had strong positive correlations with IL-8, an inflammatory cytokine (Table 4). However, in the HC group, there was a high negative correlation between the concentrations of Ni, Co, and Cr and TNF-α. In previous research, it has been shown that TNF-α is upregulated with metal ions like Ni and that Ni induces an inflammatory reaction. However, research has indicated that Ni selectively reduces the production of lipopolysaccharideinduced IL-6 by lowering the stability of mRNA, and some research has claimed that Ni suppresses the activation of NF-κB in human oral squamous cell carcinoma.28,29 There is not a meaningful reference that can explain the negative correlation between Ni, Co, Cr, and TNF-α in healthy people and thus consequentially support the results of this research. Additional research is needed on why metal ions have little effect on cytokines and why metal ions do not suppress cytokines in HCs, but do in patients with OLL.
In a previous study, the concentrations of TNF-α, IL-1α, IL-6, and IL-8, which are NF-κB-dependent cytokines, all increased more in the patients with OLL than in HCs, and it was noted that TNF-α, IL-1α, IL-6, and IL-8 had diagnostic and prognostic potential for monitoring disease activity and assisting in therapeutic decision-making in patients with OLL.30 Another study on TNF-α, IL-6, and IL-8 also showed that the concentration of all three increased more in patients with OLL than in HCs and suggested that IL-8 could be used as a biomarker for OLL severity.31 In our study, only the concentrations of IL-6 and IL-8 showed significant differences among the study groups, with those in the OLL group highest (Fig. 2), and OLL severity was correlated with both IL-6 and IL-8. In conventional cases, a scoring system has been used to objectively measure lesion changes.
There have been frequent attempts to find objective monitoring biomarkers for OLL. In Rhodus, et al.'s32 study, in which noninvasive monitoring was used, when patients with OLL were treated with 0.1% dexamethasone, cytokines in saliva decreased. The measurement of cytokines in saliva has the potential to be an objective monitoring biomarker. However, further studies on OLL monitoring using cytokines in saliva need to be conducted in the form of controlled treatment with a large number of samples.
In patients with OLLwOP, increases in metal ions in saliva were correlated with IL-8, and Cr and Au were correlated with IL-1β. In previous studies, the signaling of reactive oxygen species (ROS) was found to induce generation of IL-8 via IL-1β.33 It has also been reported that IL-1β induces the production of IL-8 during an inflammatory reaction of dental pulp cells.34,35 In our study, although IL-1β amounts were not significantly different among the four groups, IL-8 increased as did IL-1β, although IL-1β did not have any statistical relationship with IL-6 (Supplementary Table 1, only online).
Based on previous studies, metal haptens could induce ROS, which controls the release of IL-1β. ROS stimulates toll like receptor (TLR) 4 and is involved in innate immune signaling.36,37 Moreover, Ni, Co, and Pd are often bound directly to TLR4 and induce an inflammatory response.38 In Salem, et al.'s39 study, when comparing patients with OLL with HCs, the transcription of TLR4 was upregulated in the oral epithelium of patients with OLL, and the reactivity of TLR4 was reinforced by the recruitment of T lymphocytes, giving rise to a pro-inflammatory loop cycle. Based on the results of this and other studies, it is possible to hypothesize about the effects of metal ions in saliva on OLL. In patients with OLL who have an inflammatory reaction, specific metal ions stimulate the signaling of T cells, leading to the production of IL-1β, which in turn leads to the production of IL-8, which aggravates OLL. However, in HCs, an increase in IL-1β was not related to that of IL-8, and further research is needed to investigate the reason underlying the difference.
Currently, for the diagnosis and treatment of OLL, a skin-patch test is conducted if a dental-fixed OP is suspected as the etiology, and the OP is removed if the result is positive. The disease is considered confirmed if the lesion resolves. Although a skin-patch test is useful to confirm contact allergy, it cannot be implemented currently as the import of dental kits for this test has been temporarily discontinued in South Korea. Additionally, because microenvironments and the permeability of tissues of the skin and oral mucosa are different, it is difficult to be certain that a skin contact allergen always corresponds to an oral contact allergen. It is also possible that adverse reactions might occur even though the result of a skin test is negative.2,40 Consequently, this study, utilizing the measurement of metal ions and cytokines in saliva, is a starting point for further investigations into biomarkers that, instead of a skin-patch test, can diagnose metal-induced OLL by saliva in a noninvasive simple way and for discovering more objective criteria for the diagnosis of OLL.
OPs can increase the amounts of metal ions, particularly Ni, in saliva and exacerbate OLL. Therefore, the replacement of Nireleasing OPs with other types of OPs is strongly recommended in patients with refractory OLL, particularly if the OPs are suspected as aggravating factors.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017032298).
The biospecimens and data used for this study were provided by the Biobank of Pusan National University Dental Hospital.
Footnotes
- Conceptualization: Sung-Hee Jeong.
- Data curation: Hye-Min Ju, Yong-Woo Ahn, and Soo-Min Ok.
- Formal analysis: Hye-Min Ju.
- Investigation: Hye-Min Ju, Yong-Woo Ahn, and Soo-Min Ok.
- Methodology: Sung-Hee Jeong.
- Project administration: Sung-Hee Jeong.
- Resources: Hye-Min Ju, Sun-Nyoung Yu, and Soon-Cheol Ahn.
- Supervision: Sung-Hee Jeong.
- Validation: Sun-Nyoung Yu and Soon-Cheol Ahn.
- Visualization: Hye-Min Ju, Yong-Woo Ahn, and Soo-Min Ok.
- Writing—original draft: Hye-Min Ju.
- Writing—review & editing: Sung-Hee Jeong.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIAL
Correlations among Cytokine Concentrations.
References
- 1.Andrei M, Tovaru S, Parlatescu I, Gheorghe C, Pirvu C. Correlation of corrosion resistance of dental alloy restorations with oral lichen planus pathology. Mater Corros. 2016;67:882–887. [Google Scholar]
- 2.Schmalz G, Garhammer P. Biological interactions of dental cast alloys with oral tissues. Dent Mater. 2002;18:396–406. doi: 10.1016/s0109-5641(01)00063-x. [DOI] [PubMed] [Google Scholar]
- 3.Torgerson RR, Davis MD, Bruce AJ, Farmer SA, Rogers RS., 3rd Contact allergy in oral disease. J Am Acad Dermatol. 2007;57:315–321. doi: 10.1016/j.jaad.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Bakula A, Lugovic´-Mihic´ L, Situm M, Turcin J, Sinkovic´ A. Contact allergy in the mouth: diversity of clinical presentations and diagnosis of common allergens relevant to dental practice. Acta Clin Croat. 2011;50:553–561. [PubMed] [Google Scholar]
- 5.Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222–229. doi: 10.4103/0019-5154.156315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson MA, Rogers RS, 3rd, Bruce AJ. Oral lichen planus. Clin Dermatol. 2016;34:495–504. doi: 10.1016/j.clindermatol.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Grammatopoulos G, Munemoto M, Pollalis A, Athanasou NA. Correlation of serum metal ion levels with pathological changes of ARMD in failed metal-on-metal-hip-resurfacing arthroplasties. Arch Orthop Trauma Surg. 2017;137:1129–1137. doi: 10.1007/s00402-017-2723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park YM, Kim KH, Lee S, Jeon HM, Heo JY, Ahn YW, et al. Titanium Ions released from oral casting alloys may contribute to the symptom of burning mouth syndrome. J Oral Med Pain. 2017;42:102–108. [Google Scholar]
- 9.Syed M, Chopra R, Sachdev V. Allergic reactions to dental materials-a systematic review. J Clin Diagn Res. 2015;9:ZE04–ZE09. doi: 10.7860/JCDR/2015/15640.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozaffari HR, Sharifi R, Mirbahari S, Montazerian S, Sadeghi M, Rostami S. A systematic review and meta-analysis study of salivary and serum interleukin-8 levels in oral lichen planus. Postepy Dermatol Alergol. 2018;35:599–604. doi: 10.5114/ada.2018.77611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mozaffari HR, Sharifi R, Sadeghi M. Interleukin-6 levels in the serum and saliva of patients with oral lichen planus compared with healthy controls: a meta-analysis study. Cent Eur J Immunol. 2018;43:103–108. doi: 10.5114/ceji.2018.74880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humberto JSM, Pavanin JV, Rocha MJAD, Motta ACF. Cytokines, cortisol, and nitric oxide as salivary biomarkers in oral lichen planus: a systematic review. Braz Oral Res. 2018;32:e82. doi: 10.1590/1807-3107bor-2018.vol32.0082. [DOI] [PubMed] [Google Scholar]
- 13.Mozaffari HR, Ramezani M, Mahmoudiahmadabadi M, Omidpanah N, Sadeghi M. Salivary and serum levels of tumor necrosis factor-alpha in oral lichen planus: a systematic review and meta-analysis study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124:e183–e189. doi: 10.1016/j.oooo.2017.06.117. [DOI] [PubMed] [Google Scholar]
- 14.Piboonniyom SO, Treister N, Pitiphat W, Woo SB. Scoring system for monitoring oral lichenoid lesions: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:696–703. doi: 10.1016/j.tripleo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Park HK, Hurwitz S, Woo SB. Oral lichen planus: REU scoring system correlates with pain. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:75–82. doi: 10.1016/j.oooo.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Kim YJ, Kim YK, Kho HS. Effects of smoking on trace metal levels in saliva. Oral Dis. 2010;16:823–830. doi: 10.1111/j.1601-0825.2010.01698.x. [DOI] [PubMed] [Google Scholar]
- 17.Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2015;11:45–51. doi: 10.2147/TCRM.S76282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucchetti MC, Fratto G, Valeriani F, De Vittori E, Giampaoli S, Papetti P, et al. Cobalt-chromium alloys in dentistry: an evaluation of metal ion release. J Prosthet Dent. 2015;114:602–608. doi: 10.1016/j.prosdent.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Matos de Souza R, Macedo de Menezes L. Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod. 2008;78:345–350. doi: 10.2319/111806-466.1. [DOI] [PubMed] [Google Scholar]
- 20.Petoumenou E, Arndt M, Keilig L, Reimann S, Hoederath H, Eliades T, et al. Nickel concentration in the saliva of patients with nickel-titanium orthodontic appliances. Am J Orthod Dentofacial Orthop. 2009;135:59–65. doi: 10.1016/j.ajodo.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Kuhta M, Pavlin D, Slaj M, Varga S, Lapter-Varga M, Slaj M. Type of archwire and level of acidity: effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009;79:102–110. doi: 10.2319/083007-401.1. [DOI] [PubMed] [Google Scholar]
- 22.Kim CH, Ryu JJ, Jeong MY, Kim JW, Chang JS, Yoon PW. Serum metal ion levels in cementless metal-on-metal total hip arthroplasty: long-term follow-up trends. J Arthroplasty. 2019;34:534–537. doi: 10.1016/j.arth.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Ahlgren C, Bruze M, Möller H, Gruvberger B, Axéll T, Liedholm R, et al. Contact allergy to gold in patients with oral lichen lesions. Acta Derm Venereol. 2012;92:138–143. doi: 10.2340/00015555-1247. [DOI] [PubMed] [Google Scholar]
- 24.Au A, Ha J, Hernandez M, Polotsky A, Hungerford DS, Frondoza CG. Nickel and vanadium metal ions induce apoptosis of T-lymphocyte Jurkat cells. J Biomed Mater Res A. 2006;79:512–521. doi: 10.1002/jbm.a.30811. [DOI] [PubMed] [Google Scholar]
- 25.Niki Y, Matsumoto H, Suda Y, Otani T, Fujikawa K, Toyama Y, et al. Metal ions induce bone-resorbing cytokine production through the redox pathway in synoviocytes and bone marrow macrophages. Biomaterials. 2003;24:1447–1457. doi: 10.1016/s0142-9612(02)00531-8. [DOI] [PubMed] [Google Scholar]
- 26.Ferko MA, Catelas I. Effects of metal ions on caspase-1 activation and interleukin-1β release in murine bone marrow-derived macrophages. PLoS One. 2018;13:e0199936. doi: 10.1371/journal.pone.0199936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das S, Reddy RC, Chadchan KS, Patil AJ, Biradar MS, Das KK. Nickel and oxidative stress: cell signaling mechanisms and protective role of vitamin C. Endocr Metab Immune Disord Drug Targets. 2020;20:1024–1031. doi: 10.2174/1871530319666191205122249. [DOI] [PubMed] [Google Scholar]
- 28.Asakawa S, Kishimoto Y, Takano T, Okita K, Takakuwa S, Sato T, et al. Nickel ions selectively inhibit lipopolysaccharide-induced interleukin-6 production by decreasing its mRNA stability. PLoS One. 2015;10:e0119428. doi: 10.1371/journal.pone.0119428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shionome T, Endo S, Omagari D, Asano M, Toyoma H, Ishigami T, et al. Nickel ion inhibits nuclear factor-kappa B activity in human oral squamous cell carcinoma. PLoS One. 2013;8:e68257. doi: 10.1371/journal.pone.0068257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhodus NL, Cheng B, Myers S, Bowles W, Ho V, Ondrey F. A comparison of the pro-inflammatory, NF-kappaB-dependent cytokines: TNF-alpha, IL-1-alpha, IL-6, and IL-8 in different oral fluids from oral lichen planus patients. Clin Immunol. 2005;114:278–283. doi: 10.1016/j.clim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Lin M, Zhang S, Wang Z, Jiang L, Shen J, et al. NF-kappaB-dependent cytokines in saliva and serum from patients with oral lichen planus: a study in an ethnic Chinese population. Cytokine. 2008;41:144–149. doi: 10.1016/j.cyto.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Rhodus NL, Cheng B, Bowles W, Myers S, Miller L, Ondrey F. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112–116. doi: 10.1111/j.1601-0825.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim GY, Lee JW, Ryu HC, Wei JD, Seong CM, Kim JH. Proinflammatory cytokine IL-1beta stimulates IL-8 synthesis in mast cells via a leukotriene B4 receptor 2-linked pathway, contributing to angiogenesis. J Immunol. 2010;184:3946–3954. doi: 10.4049/jimmunol.0901735. [DOI] [PubMed] [Google Scholar]
- 34.Chang MC, Chang HH, Lee MY, Lin CC, Yeh HW, Yang TT, et al. Prostaglandin F(2alpha)-induced interleukin-8 production in human dental pulp cells is associated with MEK/ERK signaling. J Endod. 2009;35:508–512. doi: 10.1016/j.joen.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Lin SI, Lin LD, Chang HH, Chang MC, Wang YL, Pan YH, et al. IL-1β induced IL-8 and uPA expression/production of dental pulp cells: role of TAK1 and MEK/ERK signaling. J Formos Med Assoc. 2018;117:697–704. doi: 10.1016/j.jfma.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Alsousi AA, Igwe OJ. Redox-active trace metal-induced release of high mobility group box 1(HMGB1) and inflammatory cytokines in fibroblast-like synovial cells is Toll-like receptor 4 (TLR4) dependent. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3847–3858. doi: 10.1016/j.bbadis.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt M, Goebeler M. Innate immune system response in metal allergy: toll-like receptors. In: Chen J, Thyssen J, editors. Metal allergy. Cham: Springer; 2018. pp. 75–84. [Google Scholar]
- 38.Rachmawati D, Bontkes HJ, Verstege MI, Muris J, von Blomberg BM, Scheper RJ, et al. Transition metal sensing by Toll-like receptor-4: next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermatitis. 2013;68:331–338. doi: 10.1111/cod.12042. [DOI] [PubMed] [Google Scholar]
- 39.Salem A, Mustafa R, Listyarifah D, Al-Samadi A, Barreto G, Nordström D, et al. Altered expression of toll-like receptors in human oral epithelium in oral lichenoid reactions. Am J Dermatopathol. 2017;39:811–818. doi: 10.1097/DAD.0000000000000807. [DOI] [PubMed] [Google Scholar]
- 40.Suter VG, Warnakulasuriya S. The role of patch testing in the management of oral lichenoid reactions. J Oral Pathol Med. 2016;45:48–57. doi: 10.1111/jop.12328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlations among Cytokine Concentrations.