Abstract
Background
People experiencing homelessness (PEH) may be at risk for COVID19. We synthesised evidence on SARS-Cov-2 infection, transmission, outcomes of disease, effects of non-pharmaceutical interventions (NPI), and the effectiveness of strategies for infection prevention and control (IPC).
Methods
Systematic review of articles, indexed in electronic databases (EMBASE, WHO—Covid19, Web of Science), institutional websites and the Norwegian Institute of Public Health's live map of COVID-19 evidence, and published from December 1st, 2019, to March 3rd, 2021. Empirical papers of any study design addressing Covid-19 and health(-related) outcomes in PEH or shelters’ staff were included. (PROSPERO-2020-CRD42020187033)
Findings
Of 536 publications, 37 studies were included (two modelling, 31 observational, four qualitative studies). Random-effect meta-analysis yields a baseline SARS-Cov-2 prevalence of 2•32% (95% Confidence-Interval, 95%CI=1•30–3•34) in PEH and 1•55% (95%CI=0•79–2•31) in staff. In outbreaks, the pooled prevalence increases to 31•59% (95%CI=20•48–42•71) in PEH and 14•80% (95%CI=10•73–18•87) in staff. Main IPC strategies were universal rapid testing, expansion of non-congregate housing, and in-shelter measures (bed spacing, limited staff rotation, reduction in number of residents).
Interpretation
32% of PEH and 15% staff are infected during outbreaks of SARS-Cov-2 in homeless shelters. Most studies were conducted in the USA. No studies were found quantifying health-related outcomes of NPI. Overview and evaluation of IPC strategies for PEH, a better understanding of disease transmission, and reliable data on PEH within Covid-19 notification systems are needed. Qualitative studies may serve to voice PEH and shelter staff experiences, and guide future evaluations and IPC strategies.
Funding
None.
Keywords: people experiencing homelessness, homeless shelters, SARS-CoV-2, COVID-19, systematic review, Meta-analysis
Research in context.
Evidence before this study
People experiencing homelessness (PEH) are at increased risk of infectious, chronic, and mental health adverse conditions. Due to the risk of transmission in shared accommodations, PEH may be particularly vulnerable to SARS-Cov-2 infection and worse clinical outcomes. Non-pharmaceutical interventions (NPIs) taken to mitigate the SARS-Cov-2 outbreak may have further aggravated health and social conditions. However, there is no evidence synthesis on the SARS-Cov-2 epidemiology among PEH, the correspondent clinical and other health-related outcomes as well as health effects of NPIs on these groups.
Added value of this study
We reviewed and synthesized existent evidence on the risk of infection and transmission, risk of severe course of disease, effect of NPIs on health outcomes and the effectiveness of implemented measures to avert risks and negative outcomes among PEH. Results of the identified studies suggest that both PEH and shelter staff are at high risk of SARS-Cov-2 infection, especially in case of a local outbreak. Due to the low prevalence of symptoms at the time of a positive SARS-Cov-2 test among PEH, symptom screening alone may not be efficient to control outbreaks. Instead, universal and rapid testing conjugated with expansion of non-congregate housing support, and individual measures in shelters, are discussed as sensible strategies.
Implications of all the available evidence
A comprehensive overview of NPIs and shelter strategies targeting PEH and evaluation of their effectiveness and unintended health consequences is needed. Further qualitative research considering living realities of PEH can facilitate understanding of their specific needs during the pandemic.
Alt-text: Unlabelled box
1. Introduction
Homelessness is associated with mental health problems [1,2], infectious diseases [3], cardiovascular and respiratory diseases, and several long-term conditions (e.g., asthma, COPD, epilepsy) [4]. People experiencing homelessness (PEH) are a diverse group categorized according to their living situation: (a) Rooflessness, people without shelter sleeping rough in the streets or in public spaces, (b) houselessness, with accommodation of temporary nature, (c) living in insecure housing, shaped by threats of insecure tenancy, eviction or domestic violence, and (d) living in inadequate housing, e.g. in caravans on illegal campsites or in extreme overcrowding [5,6].
The policy measures taken to mitigate the spread of the SARS-Cov-2 pandemic, such as physical distancing and national lockdowns, may have aggravated their health and social conditions, adding to their already marginalised situation [7], [8], [9].
Typical means of survival in daily life of PEH were disrupted, e.g. food banks and other basic aid facilities were shut-down, temporary housing projects were halted [8]. It is unclear how PEH dealt with the generalised closure of institutions, restrictions in movements and public transports, or how they perceived and adhered to the measures imposed, such as the use of nose and mouth protection.
PEH are vulnerable to SARS-Cov-2 infection due to the risk of transmission in shared accommodations, comorbidities and lower immune-response because of poor nutrition and food insecurity. Yet, evidence is scarce about the risk of infection among PEH, their differential susceptibility and health outcomes observed during the pandemic, to inform the design of infection protection and control (IPC) strategies.
We aimed to synthesize the evidence on the risk of infection and transmission, risk of severe course of disease, effect of non-pharmaceutical interventions (NPI) on health outcomes and IPC strategies to avert risks and negative outcomes among PEH.
2. Methods
2.1. Search strategy and selection criteria
The systematic review was guided by five specific research questions:
-
1.
What is the prevalence or incidence of infection with SARS-CoV-2 in homeless shelters?
-
2.
What is the evidence on transmission among PEH in different settings (e. g. in homeless shelters, at outreach events, when sleeping rough on the street)?
-
3.
What are clinical and other health-related outcomes of the disease among PEH (e. g. measured by hospitalisation, ICU, ventilation, mortality)?
-
4.
What is the evidence on the effects of lockdown measures and other non-pharmacological interventions on the health status of PEH?
-
5.
What is the evidence on the effects of policies/strategies specifically enacted for PEH?
We developed and registered a review protocol in PROSPERO (PROSPERO registration 2020 CRD42020187033) [10], and followed the recommendations of the taskforce on guidelines for systematic reviews in health promotion and public health [11]. To answer these questions, we conducted a systematic search of scientific databases for peer-reviewed articles in EMBASE, the WHO Covid19 database, and Web of Science Core Collection, the Norwegian Institute of Public Health's live map of COVID-19 evidence, websites of relevant institutions and two not-indexed journals, including reference snowballing (search strategy details presented in supplementary material 1).
A PICO framework was used to help define the inclusion/exclusion criteria: population was defined as PEH fitting the FEANTSA-ETHOS definitions; empirical quantitative, qualitative and mixed-methods studies, documenting exposure to SARS-Cov-2 or any policy measure or specific IPC strategies for PEH during the Pandemic were included; the general population was considered the standard for comparison, although a comparison group was not a criteria for inclusion; outcomes could be any health-related effect of SARS-Cov-2, including prevalence, incidence, evidence on transmission, development of disease, risk of severe course of disease, hospitalisation, ICU utilization, ventilation, or mortality. Effects of lockdown and other NPIs on the health status of PEH were also considered.
Empirical studies in English published in peer-reviewed journals, indexed from December 1st 2019 onwards, were included, and the searches were conducted on March 3rd 2021.
2.2. Data analysis
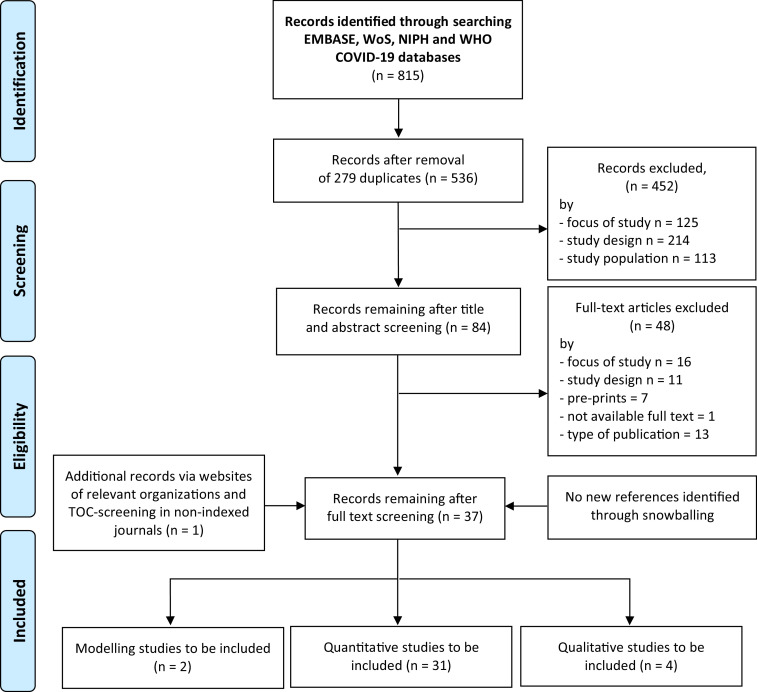
Titles and abstracts were screened by two independent researchers (AM, DC) applying pre-defined inclusion/exclusion criteria. After screening 20%, the review team refined the criteria for the remaining articles, namely by deciding to not include studies focused on PEH's health but not explicitly considering any Covid-19/SARS-Cov-2 related issue. Full texts were obtained for all included studies and for those where no agreement could be established based on title/abstracts alone. For all included full text articles, backward and forward citation search was conducted (PRISMA Flowchart Fig. 1).
Fig. 1.
PRISMA 2009 flow diagrams.
We extracted relevant parameters from cross-sectional quantitative studies to synthesise a pooled measure of SARS-Cov-2 prevalence. For this meta-analysis, we assumed that prevalences are randomly sampled from a distribution and thus applied a random-effects approach in place of a fixed-effect model which would have assumed one fixed population prevalence [12].
In addition to the Q-statistic, assessing the null hypothesis that all studies share a common prevalence, we calculated I², assessing the proportion of variance due to heterogeneity rather than chance, τ2, assessing dispersion between studies, and the 95% prediction intervals of the true prevalences to explore heterogeneity [12], [13], [14].
As some studies may report prevalence estimates of people experiencing rooflessness and staff, we conducted a second meta-analysis including these.
The extracted data were stratified based on the presence of an outbreak situation during data collection, either marked as such by authors or based on a prevalence above a pre-defined outbreak threshold, i.e. the proportion of infected individuals after which an outbreak is unlikely to go extinct without an external intervention or before infecting the full population [15]. As these thresholds are based on previous data, we chose the lowest reported prevalence measure (9% [16]) and decided on rounding down to an even lower threshold of 5% to be conservative. Further, risk of bias and publication bias were assessed through funnel plots and regression-based statistical testing by Egger et al. [17,18].
Stata V16 [19] with commands metaprop [20] and meta [21] were used for the analysis.
2.3. Role of the funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
3. Results
After duplicates removal, 536 title and abstracts were eligible for screening. 84 studies were included for full-text screening and 36 included in analysis. The manual screening of relevant websites and non-indexed journals resulted in one additional study to be included.
Information was thus extracted from 31 quantitative studies (two cohorts, one interrupted time series and 28 cross-sectional), two modelling studies and four qualitative studies (PRISMA Flowchart Fig. 1) [22].
19 quantitative studies were conducted in the USA [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], three in Canada [42], [43], [44],three in France [16,45,46], two in Italy [47,48], and one in Belgium [49], Spain [50], Slovakia [51], and Denmark [52], respectively. The data collection period considering all studies ranged from January 2020 to October 2020.
All studies focused on people experiencing houselessness (i.e. with a place to sleep but temporary in institutions or shelter according to FEANTSA-ETHOS definitions [5,6]), though not always clearly defined, with two studies [35,53] additionally covering people experiencing rooflessness (i.e. sleeping rough). 21 articles reported PCR-based estimates of SARS-Cov-2 prevalence with some offering additional data on other health or living conditions of homeless people.
A total of ten articles reported prevalence estimates for staff working at the homeless facilities [16,23,25,31,[35], [36], [37],42].
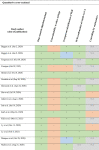
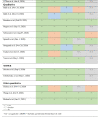
All study characteristics are presented in Table 1. Risk of bias assessment is presented in table 2.
Table 1.
Summary of studies included.
| Type of publication/ study | First author (date of publication) | ETHOS category of homelessness | City (Country), study period, indication for SARS-CoV-2 testing | Research objectives | Main conclusions as reported | Limitations as reported |
|---|---|---|---|---|---|---|
| Peer-reviewed, quantitative-cross-sectional |
Bagget et al. (Jun 2, 2020) | Houselessness | Boston (USA), April 1–2, 2020, outbreak suspicion |
To test for SARS-CoV-2 among all residents of a single large homeless shelter in Boston, USA | Universal SARS-CoV-2 PCR testing of an adult homeless shelter population in Boston shortly after the identification of a COVID-19 case cluster yielded a 36% positivity rate. The majority of individuals with newly identified infections had no symptoms and no fever at the time of diagnosis, suggesting that symptom screening in homeless shelters may not adequately capture the extent of disease transmission in this high-risk setting. These results support PCR testing of asymptomatic shelter residents if a symptomatic individual with COVID-19 is identified in the same shelter |
Cross-sectional study at a single shelter in Boston where several symptomatic individuals had been removed through prior symptom screening or self-referrals to outside care |
| Peer-reviewed, quantitative-cross-sectional |
Bagget et al. (Jun 9, 2020) |
Houselessness and rooflessness | Boston (USA), March 12-April 18, 2020, no explicit outbreak suspicion |
To describe the first 6 weeks of functioning of a comprehensive response model for homeless and marginally housed people in Boston | Universal testing, as resources permit, is a focal point of ongoing efforts to mitigate the effect of COVID-19 on this vulnerable group of people | N/A |
| Peer-reviewed, quantitative-cross-sectional |
Ferguson et al. (Oct 30, 2020) |
Unclear. “Homelessness was defined using outpatient stop codes reflecting use of homeless services and Veterans Affairs (VA) diagnosis codes” | National (USA Veteran patient data records), March 11-June 6, 2020, N/A |
To describe the shift from in-person to virtual care within Veterans Affairs (VA) during the early phase of the COVID-19 pandemic and to identify at-risk patient populations who require greater resources to overcome access barriers to virtual care | By June, 58% of VA care was provided virtually compared to only 14% prior. Rural and homeless Veterans were 12% and 11% less likely to use video care compared to urban (0.88 [95% CI 0.86, 0.90]) and non-homeless Veterans (0.89 [95% CI 0.86, 0.92]) | Evaluation focused on virtual care encounters and did not explore quality of care or clinical outcomes; did not account for patients’ preferences; captured rural and urban dwelling status, which may be correlated with, but is not a surrogate for, virtual care barriers; only examined potential interactions between rural and urban status |
| Peer-reviewed, quantitative-cross-sectional |
Finnigan (Feb 02, 2021) |
Mostly rooflessness | Sacramento (USA), October 26–28, 2020, N/A |
To study the impacts of the COVID-19 pandemic for people experiencing homelessness in Sacramento, California. The study also compares these self-reported economic impacts to a sample of low-income housed Californians. | Most PEH in Sacramento had limited self-reported exposure to COVID-19. Most PEH in Sacramento had access to testing. More than one-in-four respondents avoided shelters because of the coronavirus | Survey sample likely did not include PEH in temporary housing, potentially biasing estimates of perceived exposure downward. Bias may be small as Sacramento County reported few positive cases among PEH. Survey sampling through a homelessness service provider could have missed PEH who have more resources. E.g, the survey included more older people and people with disabilities or mental health challenges than the 2019 point in time survey for Sacramento. Survey also included a higher fraction of people experiencing homelessness for at least one year compared to the point in time survey 2019 for Sacramento. |
| Peer-reviewed, quantitative-cross-sectional |
Ghinai et al. (Oct 8, 2020) |
Houselessness | Chicago (USA), March 1-May 1, 2020, no explicit outbreak suspicion |
To describe the findings of point-prevalence surveys at 21 homeless shelters across Chicago; identify individual-level and facility-level risk factors for SARS-CoV-2 infection in homeless shelters | We identified a high prevalence of SARS-CoV-2 infections in homeless shelters. Reducing the number of residents sharing dormitories might reduce the likelihood of SARS-CoV-2 infection. When community transmission is high, limiting movement of persons experiencing homelessness into and out of shelters might also be beneficial. | PCR testing only detects current infections – the data presented here were collected several weeks into the epidemic in Chicago, and so they may underestimate factors that increased risk of infection early in the epidemic; most clinical and epidemiologic data were self-reported; some facility-level factors were ascertained during follow-up telephone calls several weeks after testing and, in some instances, data were estimated; results may not be generalizable to other cities or people living in different types of congregate settings. |
| Peer-reviewed, quantitative-cross-sectional |
Gombita et al. (Sep 30, 2020) |
Houselessness | Three shelters located in the border of Slovakia/Hungary, Slovakia/Austria, Slovakia/Poland, March 30-June 30, 2020, no explicit outbreak suspicion |
To show three examples in different senior/elderly shelters for homeless which remained completely disease free during the March to June periods of first and second waves of the Covid-19 pandemics, and describe modus vivendi (way of life) and modus operandi (way of working) in those facilities |
During the major peak of the pandemics, all clients remained Covid-19 free due to the life island policy characterized by semi-quarantine, due to incentive and social policies as well as initial testing |
– |
| Peer-reviewed, quantitative-cross-sectional |
Henwood et al. (Apr 24, 2020) |
Houselessness | Los Angeles (USA), March 20–27, 2020, N/A |
To examine permanent supportive housing (PSH) tenants’ knowledge of COVID-19; perceived risk; pre-existing condition risk factors; consistency of handwashing and social distancing since the outbreak began; recent experiences of flu-like symptoms, and tenants’ ability to shelter in place | PSH tenants are aware of the pandemic and many consider it to be a very serious health threat, which was found to be a strong predictor of taking protective measures as is the case in the general population. Targeted outreach may be needed to further reduce risk. Tenants with mental health diagnosis had lower odds of washing their hands consistently, which may speak to the need for increased mental health support and interventions that target daily functioning. Tenants in single rooms that have shared bathroom facilities had lower odds of social distancing. A lack of access to food, hygiene products, and medication delivery were common barriers to sheltering in place | Self-reported results. Further, lack of information about how tenants first learned of COVID, whether they had accurate information about pre-existing conditions that puts them at risk or flulike symptoms (as well as lack of specific mental health diagnoses), or whether they are exposed to updated information as knowledge of the pandemic increases. Unclear to which extent the sample is representative of most tenants in supportive housing |
| Peer-reviewed, quantitative-cross-sectional |
Hsu et al. (Jul 10, 2020) |
Houselessness and rooflessness | Boston (USA), March 1–May 18, 2020, N/A |
To describe the characteristics and clinical outcomes of adult patients with laboratory-confirmed COVID-19 treated at Boston Medical Center (BMC). | Hospitalized patients were more likely to be Hispanic or to be experiencing homelessness. COVID-19 patient characteristics, including age, race/ ethnicity, and homelessness could inform tailored strategies that might improve patient outcomes and mitigate strain on health care systems | Results from a single clinic and thus might not be generalizable to other institutions or locations. No causality can be inferred from the results. Comprehensive vital statistics were not available. Shortages of testing supplies changes BMC testing criteria within the study period. |
| Peer-reviewed, quantitative-cross-sectional |
Imbert et al. (Aug 2, 2020) |
Houselessness | San Francisco (USA), March 29-April 11,2020, outbreak suspicion |
To describe the lessons learned from the public health response to a COVID-19 outbreak that occurred | This outbreak demonstrates high risk of transmission of COVID-19 in homeless shelters and limited utility of a public health response that focused solely on identifying bed mates and close contacts; Location-based contact tracing among PEH should be preferred compared to person-based contact tracing; Cases widely distributed throughout shelter reinforce the risk of congregate living and highly populated shelters without capacity for social distancing | Cross-sectional study at a single shelter in San Francisco; poor case interview completion rate and limited number of close contacts identified; at-risk population not completely represented |
| Peer-reviewed, quantitative-cross-sectional |
Jatt et al. (Jun 16, 2020) |
Houselessness and rooflessness | Los Angeles (USA), March 11-April 29, 2020, no explicit outbreak suspicion |
To describe a widespread laboratory surveillance program for SARS-CoV-2 at an integrated medical campus that includes a tertiary-care center, a skilled nursing facility, a rehabilitation treatment center, and temporary shelter units | As testing capacity increased in early April and the importance of asymptomatic transmission was recognized, we transitioned to a more comprehensive program. Two key components enabled the success of this widespread laboratory surveillance program: (1) close collaboration with laboratory to secure access to high-volume molecular testing and (2) strong coordination of staff from multiple disciplines to implement testing. Implementation of widespread surveillance testing strategy likely prevented asymptomatic transmission of SARS CoV-2, preventing potential outbreaks. | Not enough info provided |
| Peer-reviewed, quantitative-cross-sectional |
Karb et al. (May 24, 2020) |
Houselessness | Providence (USA), April 19–24, 2020, no explicit outbreak suspicion |
To describe the varying prevalence of asymptomatic SARS-CoV-2 infection in congregate shelters and associated shelter characteristics and practices | Shelters with more transient residents had higher prevalence rates. Shelters in locations with lower population density and who limited new residents during the outbreak had zero prevalence in our sample. Results add to growing evidence that symptom screening and temperature monitoring are insufficient means to mitigate transmission of SARS-CoV-2 in congregate settings |
At the time of study, many shelter residents who had tested positive were already housed in a hotel, which likely led to an underestimate of true prevalence in the unhoused population; Testing done at the shelters with transient residents only reflects those staying there on the night of testing, and not intermittent users. |
| Peer-reviewed, quantitative-cross-sectional |
Kelly et al. (Mar 16, 2021) |
Houselessness | Michigan (USA), March 13-April 30, 2020, no explicit outbreak suspicion |
To describe COVID-19 infection prevention strategies at a shelter with universal testing results and outcomes. | An early and comprehensive COVID-19 preparedness plan may effectively protect a vulnerable homeless population: Symptom screening before entry, conducted multiple times daily, identified the only 2 COVID-19 cases at our facility before widespread transmission could occur. Maintaining the warming shelter and expanding our capacity to shelter all “in-need” early minimized the flow of clients through public places. Onsite medical and psychiatric assessment identified high-risk individuals to prioritize for isolation. We optimized communication within our site with phone meetings 3 times daily and had daily communication with the local public health team. | Success of our implementation was challenged by innate health risks faced by the population served, including mental health conditions and substance abuse. The sensitivity of our screening decreased by clients presenting intoxicated. Intoxicated clients were less adherent to social distancing and more likely to have another comorbid medical condition. |
| Peer-reviewed, quantitative-cross-sectional |
Ly et al. (Dec 13, 2020) |
Houselessness | Marseille (France), March 31-April 6, April 22–23, July 16, 2020, outbreak suspicion |
To compare clinical respiratory symptoms and respiratory viral and bacterial carriage during three different time periods (in early period of lockdown, in late period of lockdown, in summer) in the same population of sheltered homeless people in Marseille, France | High carriage rates of SARS-CoV-2 were observed, confirming that homeless people are at high risk for COVID-19. Measures aiming at mitigating SARS-CoV-2 transmission were effective and also impacted bacterial carriage |
Population was not randomly and homogenously recruited. The proportion of paired samples was very low. The medical histories of participants and individual adherence to preventive measures were not documented |
| Peer-reviewed, quantitative-cross-sectional |
Ly et al. (Feb 5, 2021) |
Houselessness | Marseille (France). March 26-April 17, 2020, outbreak suspicion |
To conduct a screening campaign among sheltered homeless individuals and compare them with asylum-seekers, other people living in precarious conditions and employees | Homeless people and professionals in contact with homeless people are therefore at a high risk of COVID-19. Symptom screening alone is insufficient to prevent SARS-CoV-2 transmission in vulnerable sheltered people. Systematic testing should be promoted |
Study population was not randomly and homogeneously recruited. Participants’ medical histories and use of individual preventive measures were not documented. Individuals were not asked about anosmia and ageusia. No information was available regarding possible interactions of populations at other facilities (soup kitchens and day shelters) before lockdown. |
| Peer-reviewed, quantitative-cross-sectional |
Marquez et al. (Oct 28, 2020) |
Houselessness | San Diego (USA), April 16-August 5, 2020, no explicit outbreak suspicion |
To identify potential asymptomatic residents, staff, or volunteers by pre-emptive testing with the goal of preventing a potential community outbreak. | Findings suggest that a pre-emptive testing strategy in congregant living settings, combined with accessible isolation of individuals found to be positive and consistent symptom screening of individuals found to be negative, may be sufficient to avoid large outbreaks among PEH | N/A |
| Peer-reviewed, quantitative-cross-sectional |
Martin et al. (Aug 21, 2020) |
Houselessness | Salamanca (Spain), March-May 2020, no explicit outbreak suspicion |
To describe the health care and treatment process of mental health problems among homeless people (HP) in the city of Salamanca during the crisis caused by the COVID 19 pandemic. | Homeless population received direct assistance during the pandemic and their contagion was avoided. More than 60% of them presented mental disorders and within 8 weeks they were visited in person 2–3 times. There are differences between treatments prior to and after the intervention, and the contact with the emergency services in the hospital was avoided, which could have contributed to none of them getting infected. | N/A |
| Peer-reviewed, quantitative-cross-sectional |
O'Shea et al. (Jun 8, 2020) |
Houselessness | Hamilton (Canada), March 17-April 30, 2020, no explicit outbreak suspicion |
To describe experience with shelter facility restructuring, daily symptom screening, and rapid testing to mitigate the risk of COVID-19 in the homeless shelter setting in Hamilton, Ontario, Canada | Results emphasize the importance of taking a proactive, aggressive approach to outbreak mitigation in high-risk settings. Four factors important: 1) increased capacity of shelter space by opening surge shelters and hotel rooms, allowing for more effective physical distancing; 2) access to rapid assessment and testing on site when symptomatic residents or staff are identified through active screening; 3) restructuring of physical spaces to accommodate isolation of residents with confirmed COVID-19 and those awaiting test results; 4) rapid turnaround of test results through collaboration with regional laboratory program | Our testing program provided evaluation of those staff and residents who were identified as symptomatic through active screening within the shelters. We are aware of instances where shelter residents and staff presented to other settings where testing was performed, and this is not captured in our data. Second, the test characteristics of an NPS can be influenced by testing technique; the sensitivity of our test in the real-world setting of a mobile testing unit has not been clearly established. However, the lack of large-scale outbreaks in area shelters suggests that we have not had a large number of false-negative tests thus far. |
| Peer-reviewed, quantitative-cross-sectional |
Ralli et al. (Nov 29, 2020) |
Houselessness | Rome (Italy), April-July 2020, no explicit outbreak suspicion |
To evaluate through rapid serology-based testing the prevalence of SARS-CoV-2 infection in the homeless population in the city of Rome, Italy. | Study is first to report data on people experiencing homelessness in the city of Rome, Italy Additional studies to evaluate the prevalence of COVID-19 infection in fragile populations, including more testing methods such as nasopharyngeal swab or quantitative analysis on peripheral blood, as well as symptomatic patients, are needed to evaluate the effectiveness of public health interventions against the spread of COVID-19 in these communities and to prevent and intercept new clusters of infection in the upcoming months. | The first is the small number of patients evaluated that may have limited the exact representation of virus diffusion among the target population. Furthermore, mental and physical comorbidities have not been investigated. Secondly, the exclusion from the screening of symptomatic patients may have affected the number of positive patients found. The third is that this study relied exclusively on rapid serological tests, while additional screening tests such as rapid antigen or PCR testing from nasopharyngeal swabs and antibody testing for the qualitative detection of antibodies against SARS-CoV-2 in the blood were not used and would have been more indicative of infection especially in the initial phase of the infection and in asymptomatic patients |
| Peer-reviewed, quantitative-cross-sectional |
Ralli et al. (Dec 10, 2020) |
Houselessness | Rome (Italy), until October 31, 2020, no explicit outbreak suspicion |
To report experience on residents and staff of homeless shelters in the City of Rome, Italy, with a particular focus on asymptomatic transmission, and compare it with the available evidence. | Asymptomatic carriers must always be considered, especially in vulnerable settings and congregate living conditions. Prevention measures including routine surveillance with molecular and nasopharyngeal antigen swabs and serological tests, in addition to other measures such as strict hygiene rules inside and outside the shelter, adequate distancing protocols, continuous symptom screening, and health education programs, should be implemented in all homeless shelters to intercept new clusters of infection and prevent outbreaks. | N/A |
| Peer-reviewed, quantitative-cross-sectional |
Roederer et al. (Feb 05, 2021) |
Houselessness | Ile-de-France (France), June 23-July 2, 2020, no explicit outbreak suspicion |
To assess SARS-CoV-2 antibody seropositivity prevalence and risk factors of exposure. | These results show high exposure to SARS-CoV-2 with important variations between those at different study sites. Living in crowded conditions was the strongest factor associated with exposure level. This study underscores the importance of providing safe, uncrowded accommodation, alongside adequate testing and public health information. | Cross-sectional design makes it extremely difficult to determine when or where participants became seropositive. Some studies have reported stable antibody concentrations within the first 3 months of recovery, whereas others have shown a rapid decrease regardless of disease severity after 3 or 6 months. Thus, some participants could potentially have tested seronegative despite having been infected before the survey. Study sites were not randomly selected but were a convenience sample from locations where Médecins Sans Frontières provided medical services during the first wave of the pandemic generalising these results to other similar populations (in France or elsewhere) is therefore inappropriate. Participant selection within study sites could have been biased by the relatively high replacement rate (up to a third of individuals) due to absence or refusal to participate. Social desirability bias could also have affected answers (especially when discussing lockdown adherence and prevention measures) |
| Peer-reviewed, quantitative-cross-sectional |
Rogers et al. (Sep 15, 2020) |
Houselessness | Washington County (USA), January 1-April 24, 2020, most testing without explicit outbreak suspicion |
To investigate SARS-CoV-2 case counts across several adult and family homeless shelters in a major metropolitan area | Active surveillance and surge testing were used to detect multiple cases of asymptomatic and symptomatic SARS CoV-2 infection in homeless shelters. The findings suggest an unmet need for routine viral testing outside of clinical settings for homeless populations. | This study's findings may be subject to selection bias because all participation was voluntary. In addition, reducing onsite testing from 6 to 3 days per week (during the study period) may have decreased our ability to detect additional positive cases at participating sites. Lack of robust follow-up data on participants. Very low response rates to a follow-up survey sent via text message or e-mail to asymptomatic participants 7 days after onsite study. Participation to evaluate for new or worsening symptoms, thus excluded from analyses. Small numbers of SARS-CoV-2 cases and unmeasured shelter-level covariates limit the extent to which we can draw conclusions about how sleeping arrangements may mitigate transmission. This study was not able to track unique participants and could not reliably identify encounters in the same participant. The sensitivity of self-sampling for SARS-CoV-2 (in April) detection may also be a problem |
| Peer-reviewed, quantitative-cross-sectional |
Schrooyen et al. (Aug 07, 2020) |
Houselessness | Brussels (Belgium), n = 14 PEH, n = 42 non-homeless (age- and sex-matched 1:3 out of a total of March 3-May 26, 2020, N/A |
To assess the prevalence, incidence and outcome of homeless patients hospitalized with COVID-19 in affiliated hospital | In conclusion, we found a high incidence of hospitalization for COVID-19 among homeless patients in Brussels. They had a high but similar proportion of comorbidities as compared to non-homeless patients. | The main limitation of our study is the small sample size of the homeless group and the monocentric design. Larger studies are required to properly assess the outcome of COVID-19 in homeless patients. |
| Peer-reviewed, quantitative-cross-sectional |
Spinelli et al. (Dec 1, 2020) |
Unclear | San Francisco (USA), December 2019-April 2020, N/A |
To examine trends in viral non-suppression and retention-in-care for people with HIV after the San Francisco shelter-in-place ordinance in large urban clinic | Homeless individuals had higher odds of unsuppressed viral loads post-COVID-19 vs. pre-COVID-19, despite higher visit attendance. The disproportionate economic impact of the shutdown on those with housing instability, coupled with depopulation of San Francisco shelters with COVID-19 outbreaks, are expected to destabilize viral suppression, despite ongoing or increased healthcare utilization by this group. | Limitations of our study included its non-randomized observational pre/post design and the short time intervals analysed. |
| Peer-reviewed, quantitative-cross-sectional |
Storgaard et al. (Nov 20, 2020) |
Mixed population | Aarhus (Denmark), six days in April and four days in June (2020), no explicit outbreak suspicion |
To assess the prevalence of COVID-19 infection, antibody response to COVID-19 and self-reported symptoms in the homeless and vulnerable population of Aarhus | The results from our study indicate that the COVID-19 burden in Aarhus is so small that the homeless population, just like the majority of Aarhus, has avoided the disease by coincidence. Alternatively, the homeless population may be so isolated in society that this produces a protective effect against the COVID-19 pandemic. | A limitation of this study is that it presents a snapshot. This is a limitation introduced by the cross-sectional study design with no follow-up and by the use of oropharyngeal swab tests, which only indicate if COVID-19 RNA is present at the time of testing. This makes it possible to have been infected in-between testing rounds despite testing PCR-negative in our study. |
| Peer-reviewed, quantitative-cross-sectional |
Tucker et al. (Jul 13, 2020) |
18–25 years with previous or current experience of homelessness | Los Angeles (USA) April 10-July 9, 2020, N/A |
To understand sources of information about COVID-19, perceived susceptibility, engagement in protective strategies, and outbreak effects on mental health, substance use, and ability to meet their basic needs and access services | Overall, the results are encouraging in suggesting that knowledge of COVID-19 and engagement in protective strategies is widespread among emerging adults with experiences of homelessness. However, many report increased behavioural health problems, combined with greater difficulty in accessing services. Many are also having difficulty meeting their basic needs for food, safe shelter, and hygiene. Innovative strategies are needed to address the increased behavioural health needs of young people experiencing homelessness during events such as the COVID-19 outbreak. | The results should be interpreted with caution, given that they are based on self-report data from a small sample of YEH in the Los Angeles area who participated in a clinical trial at a drop-in center. |
| Peer-reviewed, quantitative-cross-sectional |
Yoon et al. (May 1, 2020) |
Houselessness | Atlanta (USA), April 7-May 6, 2020, no explicit outbreak suspicion |
To (1) determine the SARS-CoV-2 prevalence among clients living sheltered and unsheltered and homelessness service staff through viral testing; (2) describe the clinical statuses of PEH and staff at the time of testing; 3) evaluate the sensitivity and specificity of symptom screening for COVID-19 detection; (4) review shelter infection prevention and control (IPC) policies and provide recommendations to mitigate SARS-CoV-2 transmission | PEH living in shelters experienced a higher SARS-CoV-2 prevalence compared with PEH living unsheltered. Facility-wide testing in congregate settings allowed for the identification and isolation of COVID-19 cases and is an important strategy to interrupt SARS-CoV-2 transmission. | Cross-sectional of selected shelters in Atlanta; different specimen collection methods used; underestimation of people reporting symptoms. |
| Not peer-reviewed (MMWR*), quantitative- cross-sectional |
Mosites et al. (Sep 8, 2020) |
Houselessness | Seattle, Boston, San Francisco, Atlanta (USA) March 27-April 15, 2020, mixed |
To test all shelter residents and staff members at each assessed facility, irrespective of symptoms | Given the high proportion of positive tests in the shelters with identified clusters and evidence for pre-symptomatic and asymptomatic transmission of SARS-CoV-2, testing of all residents and staff members regardless of symptoms at shelters where clusters have been detected should be considered. If testing is easily accessible, regular testing in shelters before identifying clusters should also be considered. Testing all persons can facilitate isolation of those who are infected to minimize ongoing transmission in these settings. | First, testing represented a single time point. Second, although testing all residents and staff members at each shelter was the objective, some were not available or declined (e.g., in San Francisco 143 of an estimated 255 residents at risk were tested). Finally, symptom information for persons tested was not consistently available and thus not included, although symptom information from Boston is available elsewhere. |
| Not peer-reviewed (MMWR*), quantitative- cross-sectional |
Tobolowsky, et al. (May 1, 2020) |
Houselessness | Seattle (USA), March 30-April 8, 2020, outbreak suspicion |
None explicitly stated. | This COVID-19 outbreak involved transmission among residents and staff members of three affiliated homeless shelters in Seattle, Washington. Conditions that might have contributed to SARS-CoV-2 transmission in these sites include 1) the mobile nature of the community and use of multiple homeless service sites among residents; 2) crowding and use of congregate sleeping arrangements; 3) challenges enforcing physical distancing; 4) possible asymptomatic transmission; and 5) unavailability of face coverings for residents before public health intervention. |
Not all residents were present during the site visits. Thus, residents with SARS-CoV-2 infection could have been missed during the testing events or symptom screening. |
| Peer-reviewed, quantitative- interrupted time-series |
Hickey et al. (Nov 14, 2020) |
Houselessness and rooflessness | San Francisco (USA), October 17, 2019-August 16, 2020, N/A |
To analyse care engagement and viral suppression among unhoused individuals in the “POP-UP” low-barrier, high intensity HIV primary care program during COVID-19. | Care engagement and viral suppression did not worsen during COVID-19 among people experiencing homelessness engaged in a supportive program to provide low-barrier, comprehensive HIV primary care, in contrast to worsening viral suppression rates observed among patients accessing traditional care models | Nonrandomized pre-post design and limited sample size. Limited sample size reduced the detection of smaller changes in the time series. |
| Peer-reviewed, quantitative- prospective-cohort |
Wang et al. (Oct 9, 2020) |
Houselessness | Greater Toronto Area (Canada), January 23-May 20, 2020, N/A (using health administrative data) |
To compare testing for, diagnosis of and death after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection across 3 settings (residents of long-term care homes, people living in shelters and the rest of the population) | Long-term care homes and shelters had disproportionate diagnosed cases per capita, and residents of long-term care homes diagnosed with COVID-19 had higher case fatality than the rest of the population. Heterogeneity across micro-epidemics among specific populations and settings may reflect underlying heterogeneity in transmission risks, necessitating setting-specific COVID-19 prevention and mitigation strategies. | Analyses were limited to subpopulations by availability of population size denominators. Our case-fatality estimates could be underestimated, as 4.3% of cases had an unknown outcome by the end of follow-up. Test positivity and case fatality proportions are limited to individuals with at least 1 test and thus may not generalize to those never tested, who may have lower test positivity and case-fatality proportions. |
| Peer-reviewed, quantitative- retrospective-cohort |
Richard et al. (Jan 11, 2021) |
People with a recent history of homelessness | Ontario (Canada), January 23-July 31, 2020, N/A (using health administrative data) |
To describe and compare testing for SARS-CoV-2, test positivity and hospital admission, receipt of intensive care and mortality rates related to COVID-19 for people with a recent history of homelessness versus community-dwelling people | In Ontario, people with a recent history of homelessness were significantly more likely to be tested for SARS-CoV-2, to have a positive test result, to be admitted to hospital for COVID-19, to receive intensive care for COVID-19 and to die of COVID-19 compared with community-dwelling people. People with a recent history of homelessness should continue to be considered particularly vulnerable to SARS-CoV-2 infection and its complications. | Ontario Health Insurance Plan eligibility does not extend to certain subgroups, in particular Indigenous people on reserves and certain refugee claimants who do not meet the refugee definition in the 1951 Geneva Convention. As both groups are overrepresented in Canada's homeless population, our counts of people with a recent history of homelessness are underestimates, particularly in the Greater Toronto Area, where refugees comprise one-third of shelter users. Thus, our results should be generalized only to people with Ontario health care coverage |
| Peer-reviewed, modeling |
Bagget et al. (Dec 22, 2020) |
Houselessness | Boston (USA), April-August 2020 | To assess the estimated clinical outcomes, costs, and cost-effectiveness associated with strategies for COVID-19 management among adults experiencing sheltered homelessness | Across all epidemic scenarios, daily symptom screening with PCR testing of individuals who had positive screening results and alternative care site based COVID-19 management was the most efficient strategy and was cost-saving relative to no intervention | Findings specific to adults and excluded those experiencing homelessness as part of a family and individuals experiencing unsheltered homelessness; assumed homogeneous mixing of adults experiencing sheltered homelessness; focused analysis on Boston, which has a 29.7% higher cost of living than the US mean. |
| Peer-reviewed, modeling |
Lewer et al. (Sep 23, 2020) |
Mixed | England, February-March 2020 |
To estimate the avoided deaths and health-care use among people experiencing homelessness during the so-called first wave of COVID-19 in England and the potential impact of COVID-19 on this population in the future |
Outbreaks of SARS-CoV-2 in homeless settings can lead to a high attack rate among people experiencing homelessness, even if incidence remains low in the general population. Avoidance of deaths depends on prevention of transmission within settings such as hostels and night shelters | Uncertainty about the size and structure of homeless population; assumed no mixing between subgroups of homeless; did not vary the degree of infectiousness or duration of disease states by severity in models; treated homeless population as static |
| Peer-reviewed, qualitative |
De Paula et al. (Aug 22, 2020) |
Houselessness and rooflessness | Rio de Janeiro (Brazil), end March-early April 2020 |
To analyse how homeless people live in Rio de Janeiro during COVID-19 using ethnography | Isolation led to empty streets and less passer-by's, damaging PEH's ways of living and their survival tactics. Hunger, thirst, absence of places for bathing and for fulfilling physiological needs became part of daily lives. Final considerations: given the impossibility of having a place to shelter, acquiring food and water and the limitations in carrying out preventive measures, care actions offered by managers to limit the virus to spread, even in this population, are ineffective | Study carried out in a single setting/neighbourhood |
| Peer-reviewed, qualitative |
Marcus et al. (Aug 24, 2020) |
Houselessness | Tshwane (South Africa), March 24-April 6, 2020 |
To provide a qualitative account of the response to COVID-19 lockdown, describing the adaption of the Caledonian Stadium, the main mass temporary shelter created for homeless people in the City of Tshwane that emerged from the announcement of the COVID-19 lockdown |
The Caledonian shelter is an account of organisational resilience in the face of homelessness and substance use emergencies triggered by lockdown. Through community-oriented, bottom-up self-organisation, a clinically led team navigated a response to the immediate needs of people who are homeless and/or use drugs that evolved into a more sustainable intervention. Key lessons learnt were the importance of communicating with people directly affected by emergencies, the value of using methadone to reduce harms during emergencies and the imperative of including opioid substitution therapy in essential primary healthcare. | This account of the first 2 weeks of work has several limitations. By definition, it is partial both in terms of perspective and in time. The voices of other responders as well as the people coming in and outside the shelter would enrich the narrative. The story, too is still unfolding as the lockdown and the pandemic are far from over. Lastly, in the immediacy of the response data inevitably are partial and in places inconsistent, but the experience, threads and lessons override these gaps. |
| Peer-reviewed, qualitative |
Ramaswamy et al. (May 8, 2020) |
Mixed | USA, March-April 2020 |
To describe the experiences of women living in the community who simultaneously negotiate criminal justice involvement and COVID-19 in three urban areas | Despite many barriers to staying clear of COVID-19, most women we talked to were doing the best they could to follow recommendations about staying home, social distancing, handwashing, and wearing masks | N/A |
| Peer-reviewed, qualitative |
Redondo-Sama et al. (Oct 16, 2020) |
Houselessness | Barcelona (Spain), March 20–27, 2020 |
To analyse the responses in social work to vulnerable groups in the first 15 days of the pandemic | To sum up, this study shows the role of social work to overcome difficulties of vulnerable groups in the context of the COVID-19, integrating transformative practices in collaboration with other disciplines. dialogue and communication with vulnerable groups as well as the collaboration of civil society have emerged as some of the most transformative aspects underpinning the findings. | N/A |
MMWR = Morbidity and Mortality Weekly Report by CDC.
Table 2.
Risk of bias assessment of included studies
 |
 |
 |
3.1. Prevalence of SARS-Cov2 in homeless shelters
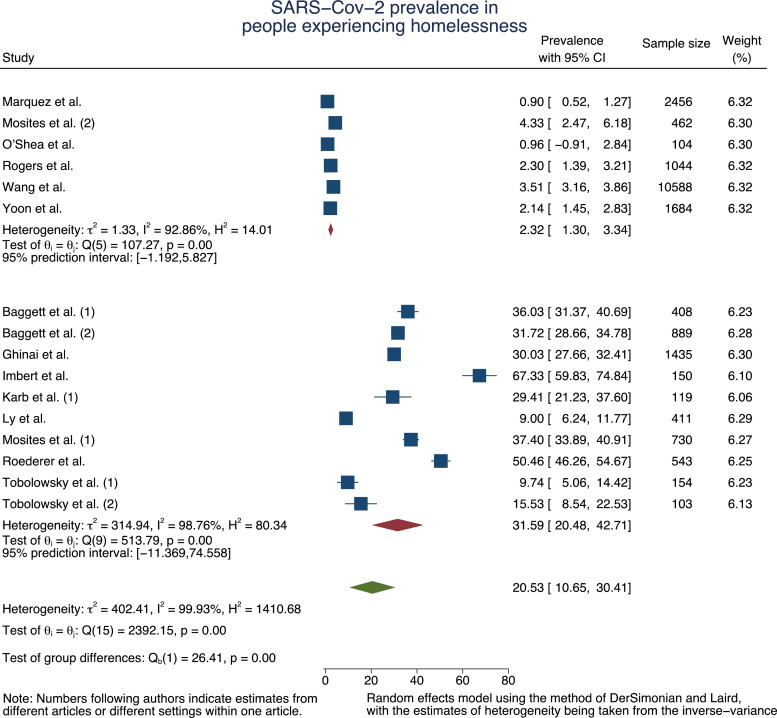
We found 16 quantitative articles reporting PCR-based SARS-Cov-2 prevalence estimates in PEH and ten studies reporting prevalence estimates for shelter staff members. To adjust for the varying settings and contexts, we pooled the measures in a random-effects meta-analysis model for PEH (n = 21 280) and staff (n = 1 662). To consider the difference in nature of investigating an outbreak [16,23,24,36,37,39] and testing for estimation of a baseline prevalence [16,23,34,35,42] in our meta-analysis, we sub-pooled the measures accordingly.
For PEH, the pooled SARS-Cov-2 prevalence estimate was 31·59% (95% Confidence Interval, 95% CI=20·48%−42·71%, τ2 = 314·94, I2 = 98·76%) in the context of outbreak situations. In case of no acute outbreak, the pooled baseline prevalence was 2·32% (95% CI=1·30%−3·34%, τ2 = 1·33, I2 = 92·86%) (Fig. 2a).
Fig. 2.
a: Forest plot of SARS-Cov-2 prevalence pooled by outbreak situation and in total for PEH
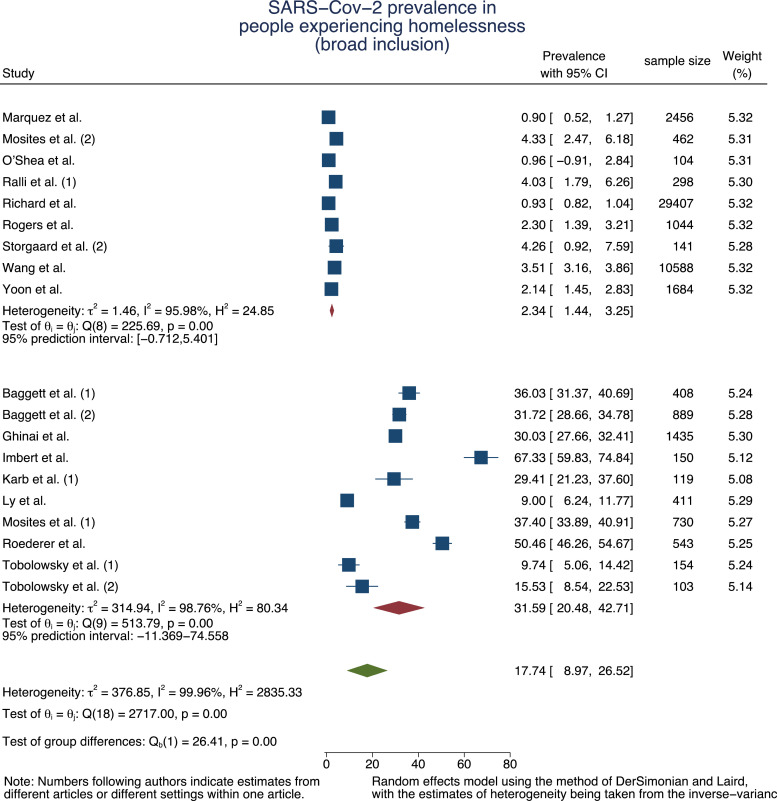
b: Forest plot of SARS-Cov-2 prevalence pooled by outbreak situation and in total for PEH – broader inclusion.
The second meta-analysis (n = 51 226) including articles where staff [47], people with recent history of homelessness [43] and other vulnerable populations (such as refugees or substance users) [52] may have been included resulted in a pooled base prevalence of 2·34% (95% CI=1·44%−3·25%, τ2 = 1·46, I2 = 95·98%) which increased 31·59% (95% CI=20·48%−42·71%, τ2 = 314·94, I2 = 98·76%) in case of an outbreak (Fig. 2b).
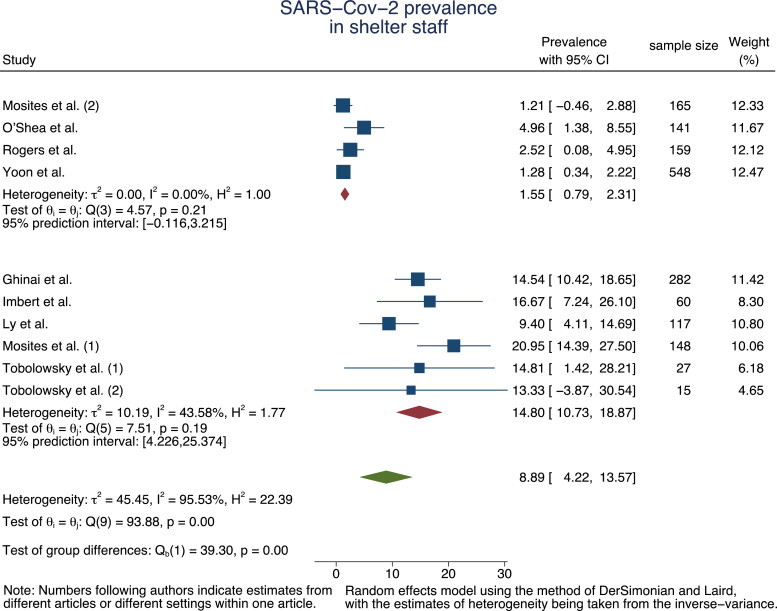
For staff members, the random-effect meta-analysis resulted in a pooled prevalence of 14·80% (95% CI=4·22%−13·57%, τ2 = 10·19, I2 = 43·58%) during an outbreak situation and 1·55% (95% CI=0·79%−2·31%, τ2 = 0·00, I2 = 0·00%) for baseline estimation (Fig. 3).
Fig. 3.
Forest plot of SARS-Cov-2 prevalence pooled by outbreak situation and in total for staff.
Funnel plots of studies reporting SARS-Cov-2 prevalence and corresponding standard errors are presented in supplementary material 2. The Egger test suggests absence of significant publication bias in all strata except for the overall prevalence among PEH and staff, which both disappears after stratification by outbreak situation (supplementary material 2), supporting the differentiation between baseline estimation and outbreak investigation.
3.2. Transmission among PEH
In their modelling study for England, Lewer et al. [54] conclude that SARS-Cov-2 outbreaks in homeless shelters can lead to a high attack rate if unmitigated, despite low incidence in the general population. Four studies assessed the proportion of pre-/asymptomatic but seropositive cases in homeless shelters and found no symptoms at time of testing in 68–85% of the cases [31,46,47,55].
Additionally, while most studies tested PEH in homeless shelters, one study by Yoon et al. [35] conducted additional testing of clients living unsheltered during homeless outreach service events (e.g. meal services). This resulted in a prevalence of 0·5% (3 out of 636) in the unsheltered PEH.
3.3. Clinical and health-related outcomes among PEH
Twelve articles [16,[24], [25], [26],33,35,38,39,41,50,52,56] presented data on clinical outcomes of PEH, ranging from respiratory symptoms to previous mental health diagnosis and other somatic issues (e.g. chronic kidney or liver disease), and one article [36] provided data on emergency department visits, hospitalization and mortality. One article [49] identified homeless within hospitalized symptomatic SARS-COV-2 positive patients in Belgium and found increased odds of smoking, alcoholism, methadone use, and neurological diseases for homeless (n = 14) compared with non-homeless patients (n = 42). Seven articles [24,31,35,36,39,47,48,52] provided data specifically for PEH testing positive: specific symptoms (cough, shortness of breath, fever) were either uncommon or did not differ significantly from the overall tested population of PEH. Neither did comorbidities [39]. Imbert et al. [36] used a city-wide administrative database and reported 12% (n = 12) of those tested positive to have had a treatment and release emergency department visit, 8% (n = 8) a hospitalization and 1% (n = 1) died.
An analysis conducted at the Boston Medical Centre[28] with patients treated for Covid-19 found that one in five patients hospitalized were experiencing homelessness. When classified by clinical severity the share of PEH among all admitted patients was highest among non-intensive care unit (ICU) with 24.3%, compared to 15.9% among ICU hospitalizations without mechanical ventilation, 15.1% among ICU hospitalizations with mechanical ventilation, and 15.3% among those who died.
A retrospective registry-based analysis conducted in Ontario, Canada [43] (from March to June 2020) identified 30,000 people with a recent history of homelessness and found that SARS-CoV-2 testing, test positivity and hospital admission, intensive care and mortality rates related to Covid-19 were all higher among this group compared to the community dwelling population.
Yoon et al. calculated sensitivity and specificity of symptom screening compared to RT-PCR, stating that fever, cough, or shortness of breath were 14% sensitive and 89% specific for identifying COVID-19 cases when reported in the previous day, changing to 24% and 85% when reported in the previous week. An expansion to any symptom reported resulted in no improvement of sensitivity, but a loss in specificity.
Two studies modelled different health-related outcomes among PEH with one conducted for England [54] and one for the USA [57].
3.4. Non-pharmaceutical interventions (NPI)
One study [41] looked at the shift from in-person to virtual care within Veterans Affairs (VA) records in the US during the pandemic. The authors characterized 4% of their sample (out of approx. 5·4 million records) as homeless, and identified this group as being 11% less likely to use video care compared to non-homeless Veterans.
One qualitative study [58] conducted unstructured interviews through telephone or social media conversations with 35 participants from a US cohort study of criminal justice-involved women to explore how they were faring during this period. Thirteen of the interviewees reported some form of homelessness, and for some of the women their unstable living situations prevented them from being able to follow the social distancing recommended.
Another qualitative study conducted in South Africa described the adaption to a Stadium to serve as the main mass temporary shelter for homeless people during the lockdown in the City of Tshwane [59]. The authors conducted interviews with healthcare professionals and provided a description of the arising needs (such as substitution therapy and harm reduction services for people with opioid dependence, and ensuring withdrawal is not confused with or masked by a SARS-Cov-2 infection) and information given to more than 1000 sheltered and relocated people.
An ethnographic study conducted in Rio de Janeiro, Brazil, looked at how homeless people lived during the pandemic [60]. Using interviews and document analysis, the authors describe how isolation led to emptying the streets and reducing passers-by, negatively impacting homeless’ ways of living survival tactics. Hunger, thirst, absence of places for bathing and for fulfilling physiological needs became apparent and that the actions offered to limit virus dissemination were largely ineffective.
In Barcelona, Spain, Redondo-Sama et al. [61] performed qualitative interviews with social workers in different fields including PEH, and identified the following main difficulties brought about by the pandemic to social work directed at vulnerable groups: general lack of preparedness of social workers, scarcity of PPE, increase in basic demands (food, housing, etc.) for vulnerable groups, closure of dedicated services, and changes in official guidelines and protocols to work in the Covid-19 context.
3.5. Infection protection and control (IPC) strategies in homeless shelters
We identified no study quantifying the effectiveness of strategies for IPC among PEH. 11 papers [25,29,37,39,42,47,51,54,56,57,62] discussed strategies for IPC more generally, and one study [35] actively investigated these at 9 shelters. Yoon et al. [35] describe different pandemic preparation measures, e.g., 55·6% of shelters (5 in 9) discontinued taking in new clients, 77·8% (7 in 9) created separate isolation areas for PEH with suspected infection and 55·6% (5 in 9) increased the spacing between individual beds. Accordingly, Roederer et al. [46] found that medium and high crowding, a composite score based on the number of people sharing the room, a sanitary facility, a kitchen and the number of close contacts per day, had the strongest correlation with seropositivity among PEH.
Across all studies, the major strategies discussed as important and necessary were comprehensive and rapid testing, expansion of non-congregate supportive housing for PEH and, specific strategies for IPC to be taken in congregate settings, e.g. increased spacing between beds, staggered showering and meal schedules, and/or limited staff rotation. O'Shea et al. [63] and Baggett et al.[56] further discussed potential benefits in administrative measures, e.g. collaborating with regional laboratories for quick turnaround of test results which enable the isolation of the infected.
Furthermore, Henwood et al. [38] reported that 39% (207 of 532) of the tenants of supportive housing in Los Angeles considered themselves to be at serious risk for COVID-19 due to a pre-existing condition, 59% (316 of 532) had a registered mental health diagnosis and 55·3% (294 of 532) believed they can shelter in place if needed.
One study reporting about the results of testing 331 senior homeless residents of three shelters in Slovakia[51] did not find any positive case and attributed this result to the implementation of a life island policy, characterized by semi-quarantine, made possible due to incentive and social policies as well as initial testing.
Similarly, Jatt et al. [29], described that the implementation of a widespread surveillance testing strategy has likely prevented asymptomatic transmission of SARS-CoV-2, thereby preventing potential outbreaks of Covid-19 in their study, which included 121 homeless (out of 1781 participants), at an integrated medical campus with tertiary-care center, skilled nursing facility, rehabilitation treatment center, and temporary shelter units.
4. Discussion
In this systematic review including a total of 37 empirical studies, we identified a baseline SARS-Cov-2 prevalence of 2·32% (95% CI=1·30–3·34%) among PEH in homeless shelters, and 1·55% (95%CI=0·79–2·31%) among staff. In case of an outbreak situation, these estimates increase to 31·59% (95% CI=20·48–42·71%) among PEH and 14·80% (95%CI= 10·73–18·87%) among staff. For unsheltered PEH, we only identified one study which reported a prevalence of 0·5%.
Specific symptoms, e.g. cough, shortness of breath or fever, were either uncommon or did not differ significantly from the overall tested population of PEH [16,24,35,36,38,39], neither did comorbidities [39] (except for a low-powered study conducted in Belgium [49]). The reviewed evidence, despite being scarce, suggests that hospitalisation, intensive care utilization and mortality rates related to Covid-19 were higher among PEH compared to community dwelling populations [43], and that a considerable proportion of PEH who were tested positive used emergency services (12%), were hospitalised (8%) or died (1%) [36].
Evidence on living realities was scarce but suggests that unstable living situations may impede adherence to social distancing practices [64], and that stay at home measures led to empty streets and less passer-bys menacing exisiting survical tactics of PEH [60].
Evidence quantifying the effectiveness of IPC strategies among PEH was only available from modelling studies, indicating high number of infections (cumulative incidence of 49.3%) if no interventions were taken, even with low SARS-Cov-2 incidence in the general population [54]. Daily symptom screening with PCR testing of individuals who had positive screening results and the provision of alternative care sites for Covid-19 management were modelled to be the most efficient and cost-saving strategy to tackle the pandemic among PEH [65].
In terms of IPC strategies, 11 studies [29,31,35,37,39,42, 47,48,54,56,57] discussed comprehensive and rapid testing including collaboration with regional laboratories for quick turnaround of positive tests, expansion of non-congregate housing and individual strategies in the shelters, e.g. spacing between beds and limited staff rotation, as critical measures to be taken. PEH living in overcrowded shelters were found to have higher odds of seropositivity compared to those in low density shelters (OR: 3·4; 95% CI: 1·7–6·9, p<0·0001) [46].
Although excluded from this review, we found several reports retrieved from the relevant websites searched providing IPC strategies specifically for staff working in homeless shelters (supplementary material 3). These strategies should be adapted to the respective context and enacted to mitigate the spread of SARS-Cov-2 infection among this vulnerable population group. We further identified important research gaps to be addressed by future research (Table 3).
Table 3.
Research gaps identified.
| (A) Lack of data for evaluation of NPIs effectiveness and (un)intended health consequences | ||
| (1) lack of reliable and separate data on PEH within Covid-19 notification systems | (2) lack of a comprehensive overview of NPIs and shelter strategies targeting PEH | (3) lack of understanding of infectious disease spread among PEH, e.g. in homeless shelters |
| (B) Targeted research into people experiencing rooflessness | ||
| While PEH may be deemed as a hard-to-reach population in general, it is essential to differentiate those living in shelters from those sleeping rough. People experiencing rooflessness may be at a less risk of SARS-Cov-2 infection as long as they do not seek congregate shelter but at the same time, they may be the ones experiencing more heavily the challenges of social distancing to mental and social wellbeing associated with the imposed lockdowns. | ||
| (C) Lack of qualitative research into living realities of PEH | ||
| More qualitative research is needed to better understand living realities and particular needs of PEH during the pandemic. This can further kick-start the participation of PEH in the research process. | ||
| (D) Timely updates of the review | ||
| With the current infection dynamics and speed of research, timely updates of synthesised information are essential. | ||
Studies providing prevalence estimates were based on PCR testing and some included testing of staff for a complete picture of SARS-Cov-2 infection in shelters. Overall, the quality of evidence was moderate. Most articles provide little detail on how and why shelters were sampled. Cross-sectional studies may have missed infected patients who do not have enough viral particles yet to be detected in PCR. Only two articles conducted follow-up testing [35,37]. The recruitment of participants was based on accommodation in homeless shelters with little systematic surveying of housing situation and residence type in shelter. This may be of special importance considering the diverse background of PEH and their everyday life, because not all shelters offer accommodation during the day. Most identified studies were monocentric and based in high-income countries raising questions of generalizability especially to middle- and lower-income countries that differ in population demographics, cultural and social dimensions as well as definitions of homelessness.
The qualitative studies offered sufficient insight into their methodology and data analysis. One qualitative article [64] offered little details on study design (including questions on how conversations were guided), details on interviewers and implications for the way interviews progress as well as ethical concerns. Further, most studies did not discuss the relationship between researchers and participants.
Both modelling studies offered insights into their structural and parameter assumptions and methods applied which enable potential replication.
Besides major databases, we searched websites of relevant institutions as well as table of contents of non-indexed journals and consulted the Covid-19 evidence live map of the Norwegian Institute of Public Health. Further, we have used forward and backward reference screening on all identified articles. All steps of the review have been conducted by two reviewers for quality assurance. Finally, we tested for publication bias, both visually through funnel plotting as well as statistically based on Egger et al. [17,18]. Additionally, risk of publication bias might be low as we are investigating prevalence estimates (compared to measures of effect) during the first year of a pandemic of global relevance.
We have used English search terms only, thus might have missed relevant studies in other languages. Nevertheless, we found 11 studies from non-English language countries. As most of the studies focus on North American and European countries, our findings may not be generalizable to other settings than the ones where the studies were conducted, as droplet or potentially airborne transmission may be different in other contexts of social and cultural communication and interaction. Even though we searched several databases and repositories, we might not have detected all relevant studies considering the amount of evidence being generated during the current pandemic. Additionally, we cannot rule out the possibility of publication bias, particularly regarding the baseline prevalence among staff since estimates for this group were not part of these studies’ initial objectives.
In order to explore heterogeneity underlying our meta-analyses, we have calculated 95% confidence intervals. For our baseline estimations for both PEH as well as staff, the heterogeneity was low and ranged 2% around the estimated mean. For estimations during an outbreak, the prediction intervals covered a broader range of 10% for staff and 45% for PEH around their respective estimated mean. This suggests large heterogeneity and caution discussing SARS-Cov-2 attack rates in homeless shelters. As a global systematic review, the shelters included may be defined by different characteristics, e.g. spacing between beds, meal or shower administration, resulting in different attack rates.
As the quality of included studies varies, findings should be considered with caution. Yet, they clearly suggest that PEH are at high risk of being infected with SARS-Cov-2, especially in case of outbreaks in shelters. Potential pathways for transmissions deserving further investigation are the “mobile nature of the community and use of multiple homeless service sites among residents” [37], crowded sleeping arrangements and difficulties in adhering to recommended behavioural interventions due to lack of resources and private space. Furthermore, PEH face serious mental health challenges [38,53] and economic difficulties that prevent them from accessing preventive material and maintaining physical distancing in general [58].
The nature and direction of the infectious disease spread is unclear but staff working at shelters are at increased risk as well, reinforcing the importance of adherence to strategies of IPC in congregate shelters and the expansion of provision of non-congregate housing for PEH.
Due to the absence or low prevalence of symptoms (e.g. fever) at the time of diagnosis of SARS-Cov-2 among PEH [16,24,35,39], symptom screening alone is not suitable, i.e. not sensitive enough for adequately capturing the extent of disease transmission in such high-risk settings. Instead, comprehensive and early testing for timely identification and isolation of the infected, combined with collaboration with regional laboratories for quick turnaround of positive test results might be important strategies to break the virus transmission [35,42].
Finally, against the backdrop of current debates on vaccination strategies it is essential to discuss and evaluate the most suitable vaccination prioritization strategies and outreach campaigns to ensure timely vaccination of people experiencing homelessness, particularly in light of their high mental health burden [66,67] and preventing PEH from avoiding shelter out of fear of infection [53].
Declaration of Competing Interest
The review has been conducted in the scope of the German Competence Net Public Health Covid-19. JS is volunteering (without financial compensation) for a German NGO which provides medical services free of charge for - among others - individuals living in homeless shelters. He further reports membership of the Social Democratic Party of Germany (SPD). The other authors state that they have no competing interests.
Acknowledgments
Data availability statement
Detailed review lists can be provided by the corresponding author upon reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
Conceptualisation: AM, DC, KB
Data curation / Search: Embase - SR; WHO Covid19 - DC; WoS - SR; websites - DC; NIPH live map - AM; Snowballing: JS
Title and abstract screening: AM, DC
Full-text screening: AM, DC
Quality appraisal: AM, DC, JS, SR
Data extraction: AM, DC
Data-synthesis: AM, DC
Writing of first and final draft: AM, DC
Revision for important intellectual content: KB, JS, SR
Footnotes
Funding No funding source.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101032.
Appendix. Supplementary materials
References
- 1.Ayano G., Ayano G., Shumet S., Tesfaw G., Tsegay L. A systematic review and meta-analysis of the prevalence of bipolar disorder among homeless people. BMC Public Health. 2020;20:731. doi: 10.1186/s12889-020-08819-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayano G., Solomon M., Tsegay L., Yohannes K., Abraha M. A systematic review and meta-analysis of the prevalence of post-traumatic stress disorder among homeless people. Psychiatr Q. 2020;91:949–963. doi: 10.1007/s11126-020-09746-1. [DOI] [PubMed] [Google Scholar]
- 3.Beijer U., Wolf A., Fazel S. Prevalence of tuberculosis, hepatitis C virus, and HIV in homeless people: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:859–870. doi: 10.1016/S1473-3099(12)70177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewer D., Aldridge R.W., Menezes D. Health-related quality of life and prevalence of six chronic diseases in homeless and housed people: a cross-sectional study in London and Birmingham, England. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-025192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FEANTSA . ETHOS - European Typology on Homelessness and housing exclusion; Brussels: 2005. European federation of national organisations working with the homeless. [Google Scholar]
- 6.Amore K., Baker M., Howden-Chapman P. The ETHOS definition and classification of homelessness: an analysis. Eur J Homelessness. 2011;5:19–37. [Google Scholar]
- 7.Tsai J., Wilson M. COVID-19: a potential public health problem for homeless populations. Lancet Public Heal. 2020;5:e186–e187. doi: 10.1016/S2468-2667(20)30053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maxmen A. Coronavirus is spreading under the radar in US homeless shelters. Nature. 2020;581:129–130. doi: 10.1038/d41586-020-01389-3. [DOI] [PubMed] [Google Scholar]
- 9.Conway B., Truong D., Wuerth K. COVID-19 in homeless populations: unique challenges and opportunities. Future Virol. 2020;15:331–334. [Google Scholar]
- 10.Mohsenpour A., Bozorgmehr K., Rohleder S., Stratil J., Costa D. Homelessness and COVID-19: a rapid systematic review. PROSPERO 2020 CRD420201807033. 2020. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020187033.
- 11.Jackson N., Waters E. Criteria for the systematic review of health promotion and public health interventions. Health Promot Int. 2005;20:367–374. doi: 10.1093/heapro/dai022. [DOI] [PubMed] [Google Scholar]
- 12.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 13.IntHout J., Ioannidis J.P.A., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartfield M., Alizon S. Introducing the Outbreak Threshold in Epidemiology. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ly T.D.A., Hoang V.T., Goumballa N. Screening of SARS-CoV-2 among homeless people, asylum-seekers and other people living in precarious conditions in Marseille, France, March–April 2020. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2021.02.026. 2020.05.05.20091934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne J.A.C., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 19.StatCorp . 2001. Stata statistical. [Google Scholar]
- 20.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:1–10. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne J., Harbord R., White I. Stata Users Group; 2010. An overview of meta-analysis in stata. United Kingdom Stata users’ group meetings 2010 11. [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosites E., Parker E.M., Clarke K.E.N. Assessment of SARS-CoV-2 infection prevalence in homeless shelters — four U.S. Cities, March 27–April 15, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:521–522. doi: 10.15585/mmwr.mm6917e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baggett T.P., Keyes H., Sporn N., Gaeta J.M. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA J Am Med Assoc. 2020;323:2191–2192. doi: 10.1001/jama.2020.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghinai I., Davis E.S., Mayer S. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in homeless shelters in Chicago, Illinois - March-May 2020. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa477. ofaa477–ofaa477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hickey M.D., Imbert E., Glidden D.V. Viral suppression not decreased during COVID-19 among people with HIV and unstable housing enrolled in a low-barrier clinic-based program (POP-UP) AIDS. 2020 doi: 10.1097/qad.0000000000002793. Publish Ah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han E., Tan M.M.J., Turk E. Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet. 2020;396:1525–1534. doi: 10.1016/S0140-6736(20)32007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu H.E., Ashe E.M., Silverstein M. Race/ethnicity, underlying medical conditions, homelessness, and hospitalization status of adult patients with COVID-19 at an urban safety-net medical center — Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:864–869. doi: 10.15585/mmwr.mm6927a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jatt L.P., Winnett A., Graber C.J., Vallone J., Beenhouwer D.O., Goetz M.B. Widespread severe acute respiratory coronavirus virus 2 (SARS-CoV-2) laboratory surveillance program to minimize asymptomatic transmission in high-risk inpatient and congregate living settings. Infect Control Hosp Epidemiol. 2020;41:1331–1334. doi: 10.1017/ice.2020.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly D., Murphy H., Vadlamudi R. Successful public health measures preventing COVID-19 at a Michigan homeless shelter. Infect Control Hosp Epidemiol. 2020:1–2. doi: 10.1017/ice.2020.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers J.H., Link A.C., McCulloch D. Characteristics of COVID-19 in homeless shelters : a community-based surveillance study. Ann Intern Med. 2021;174:42–49. doi: 10.7326/M20-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spinelli M.A., Hickey M.D., Glidden D.V. Viral suppression rates in a safety-net HIV clinic in San Francisco destabilized during COVID-19. AIDS. 2020;34:2328–2331. doi: 10.1097/QAD.0000000000002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tucker J.S., D'Amico E.J., Pedersen E.R., Garvey R., Rodriguez A., Klein D.J. Behavioral health and service usage during the COVID-19 pandemic among emerging adults currently or recently experiencing homelessness. J Adolesc Health. 2020;67:603–605. doi: 10.1016/j.jadohealth.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marquez H., Ramers C., Northrup A. Response to the coronavirus disease 2019 pandemic among people experiencing homelessness in congregant living settings in San Diego, California. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1668. published online Oct 28. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon J.C., Montgomery M.P., Buff A.M. Coronavirus disease 2019 (COVID-19) prevalences among people experiencing homelessness and homelessness service staff during early community transmission in Atlanta, Georgia, April–May 2020. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1340. published online Sept 8. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imbert E., Kinley P.M., Scarborough A. Coronavirus disease 2019 outbreak in a San Francisco homeless shelter. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1071. published online Aug 3. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobolowsky F.A., Gonzales E., Self J.L. COVID-19 outbreak among three affiliated homeless service sites — King County, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:523–526. doi: 10.15585/mmwr.mm6917e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henwood B.F., Redline B., Lahey J. Surveying tenants of permanent supportive housing in skid row about COVID-19. J Health Care Poor Underserved. 2020;31:1587–1594. doi: 10.1353/hpu.2020.0120. [DOI] [PubMed] [Google Scholar]
- 39.Karb R., Samuels E., Vanjani R., Trimbur C., Napoli A. Homeless shelter characteristics and prevalence of SARS-CoV-2. West J Emerg Med. 2020;21 doi: 10.5811/westjem.2020.7.48725. 2020.05.21.20108985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baggett T.P., Racine M.W., Lewis E. Addressing COVID-19 among people experiencing homelessness: description, adaptation, and early findings of a multiagency response in Boston. Public Health Rep. 2020;135:435–441. doi: 10.1177/0033354920936227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferguson J.M., Jacobs J., Yefimova M., Greene L., Heyworth L., Zulman D.M. Virtual care expansion in the Veterans health administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2020 doi: 10.1093/jamia/ocaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Shea T., Bodkin C., Mokashi V. Pandemic planning in homeless shelters: a pilot study of a coronavirus disease 2019 (COVID-19) testing and support program to mitigate the risk of COVID-19 outbreaks in congregate settings. Clin Infect Dis. 2021;72:1639–1641. doi: 10.1093/cid/ciaa743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richard L., Booth R., Rayner J., Clemens K.K., Forchuk C., Shariff S.Z. Testing, infection and complication rates of COVID-19 among people with a recent history of homelessness in Ontario, Canada: a retrospective cohort study. CMAJ Open. 2021;9:E1–E9. doi: 10.9778/cmajo.20200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Ma H., Yiu K.C.Y. Heterogeneity in testing, diagnosis and outcome in SARS-CoV-2 infection across outbreak settings in the Greater Toronto Area, Canada: an observational study. CMAJ Open. 2020;8:E627–E636. doi: 10.9778/cmajo.20200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ly T.D.A., Hoang V.T., Goumballa N. Variations in respiratory pathogen carriage among a homeless population in a shelter for men in Marseille, France, March–July 2020: cross-sectional 1-day surveys. Eur J Clin Microbiol Infect Dis. 2021 doi: 10.1007/s10096-020-04127-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Roederer T., Mollo B., Vincent C. Seroprevalence and risk factors of exposure to COVID-19 in homeless people in Paris, France: a cross-sectional study. Lancet Public Health. 2021 doi: 10.1016/s2468-2667(21)00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ralli M., Morrone A., Arcangeli A., Ercoli L. Asymptomatic patients as a source of transmission of COVID-19 in homeless shelters. Int J Infect Dis. 2021;103:243–245. doi: 10.1016/j.ijid.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ralli M., Cedola C., Urbano S. Assessment of sars-cov-2 infection through rapid serology testing in the homeless population in the city of Rome, Italy. Preliminary results. J Public health Res. 2020;9:556–559. doi: 10.4081/jphr.2020.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schrooyen L., Delforge M., Lebout F., Vanbaelen T., Lecompte A., Dauby N. Homeless people hospitalized with COVID-19 in Brussels. Clin Microbiol Infect. 2021;27:151–152. doi: 10.1016/j.cmi.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin C., Andrés P., Bullón A. COVID pandemic as an opportunity for improving mental health treatments of the homeless people. Int J Soc Psychiatry. 2020 doi: 10.1177/0020764020950770. 20764020950770–20764020950770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gombita P., Olah M., Kovac R. Senior Homeless Population was Covid-19 Free in 3 shelter communities after adapting the Life Island model (Note) Clin Soc Work Heal Interv. 2020;11:78–79. [Google Scholar]
- 52.Storgaard S.F., Eiset A.H., Abdullahi F., Wejse C. First wave of COVID-19 did not reach the homeless population in Aarhus. Dan Med J. 2020;67 https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-1070419NS [PubMed] [Google Scholar]
- 53.Finnigan R. Self-reported impacts of the COVID-19 pandemic for people experiencing homelessness in Sacramento, California. J Soc Distress Homelessness. 2021:1–9. [Google Scholar]
- 54.Lewer D., Braithwaite I., Bullock M. COVID-19 among people experiencing homelessness in England: a modelling study. Lancet Respir Med. 2020;8:1181–1191. doi: 10.1016/S2213-2600(20)30396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karb R., Samuels E., Vanjani R., Trimbur C., Napoli A. Homeless shelter characteristics and prevalence of SARS-CoV-2. West J Emerg Med. 2020;21:1048–1053. doi: 10.5811/westjem.2020.7.48725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baggett T.P., Racine M.W., Lewis E. Addressing COVID-19 among people experiencing homelessness: description, adaptation, and early findings of a multiagency response in Boston. Public Health Rep. 2020;135:435–441. doi: 10.1177/0033354920936227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Culhane D., Treglia D., Steif K., et al. Estimated emergency and observational/quarantine capacity need for the US homeless population related to COVID-19 exposure by county; projected hospitalizations, intensive care units and mortality. 2020 https://escholarship.org/uc/item/9 g0992bm.
- 58.Ramaswamy M., Hemberg J., Faust A. Criminal Justice–involved women navigate COVID-19: notes from the field. Health Educ Behav. 2020;47:544–548. doi: 10.1177/1090198120927304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marcus T.S., Heese J., Scheibe A., Shelly S., Lalla S.X., Hugo J.F. Harm reduction in an emergency response to homelessness during South Africa's COVID-19 lockdown. Harm Reduct J. 2020;17:60. doi: 10.1186/s12954-020-00404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paula HC de, Daher D.V., Koopmans F.F., Faria MG de A, Lemos P.F.S., Moniz M de A. No place to shelter: ethnography of the homeless population in the COVID-19 pandemic. Rev Bras Enferm. 2020;73 doi: 10.1590/0034-7167-2020-0489. [DOI] [PubMed] [Google Scholar]
- 61.Redondo-Sama G., Matulic V., Munté-Pascual A., Vicente I de. Social work during the COVID-19 crisis: responding to urgent social needs. Sustainability. 2020;12:1–16. [Google Scholar]
- 62.Martin C., Andrés P., Bullón A. COVID pandemic as an opportunity for improving mental health treatments of the homeless people. Int J Soc Psychiatry. 2021;67:335–343. doi: 10.1177/0020764020950770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Shea T., Bodkin C., Mokashi V. Pandemic planning in homeless shelters: a pilot study of a coronavirus disease 2019 (COVID-19) testing and support program to mitigate the risk of COVID-19 outbreaks in congregate settings. Clin Infect Dis. 2020;08:8. doi: 10.1093/cid/ciaa743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramaswamy M., Hemberg J., Faust A. Criminal justice-involved women navigate COVID-19: notes from the field. Health Educ Behav. 2020 doi: 10.1177/1090198120927304. 1090198120927304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baggett T.P., Scott J.A., Le M.H. Clinical outcomes, costs, and cost-effectiveness of strategies for adults experiencing sheltered homelessness during the COVID-19 Pandemic. JAMA Netw open. 2020;3 doi: 10.1001/jamanetworkopen.2020.28195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazereel V., Van Assche K., Detraux J., De Hert M. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8:444–450. doi: 10.1016/S2215-0366(20)30564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Hert M., Mazereel V., Detraux J., Van Assche K. Prioritizing COVID-19 vaccination for people with severe mental illness. World Psychiatry. 2021;20:54–55. doi: 10.1002/wps.20826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Detailed review lists can be provided by the corresponding author upon reasonable request.