Abstract
Objective
Metabolism management plays an essential role during cardiopulmonary bypass (CPB). There are different metabolic management devices integrated to heart–lung machines; the most commonly used and accepted metabolic target is indexed oxygen delivery (DO2i) (280 mL/min/m2) and cardiac index (CI) (2.4 L/min/m2), which can be managed independently or according to other metabolic parameters. Our objective was to compare lactate production during CPB procedures using different metabolic management: DO2i in relation to indexed oxygen extraction ratio (O2ERi) and CI in relation to mixed venous oxygen saturation (SvO2).
Methods
Data on 500 CPB procedures were retrospectively collected in a specialized regional tertiary cardiac surgery center in Italy between September and 2012 and November 2019. In group A, the DO2i with 280 mL/min/m2 target in relation to O2ERi 25% was used; in group B, CI with 2.4 L/min/m2 target in relation to SvO2 75% was used. During CPB, serial arterial blood gas analyses with blood lactate and glucose determinations were obtained. Hyperlactatemia (HL) was defined as a peak arterial blood lactate concentration >3 mmol/L. The postoperative outcome of patients with or without HL was compared.
Results
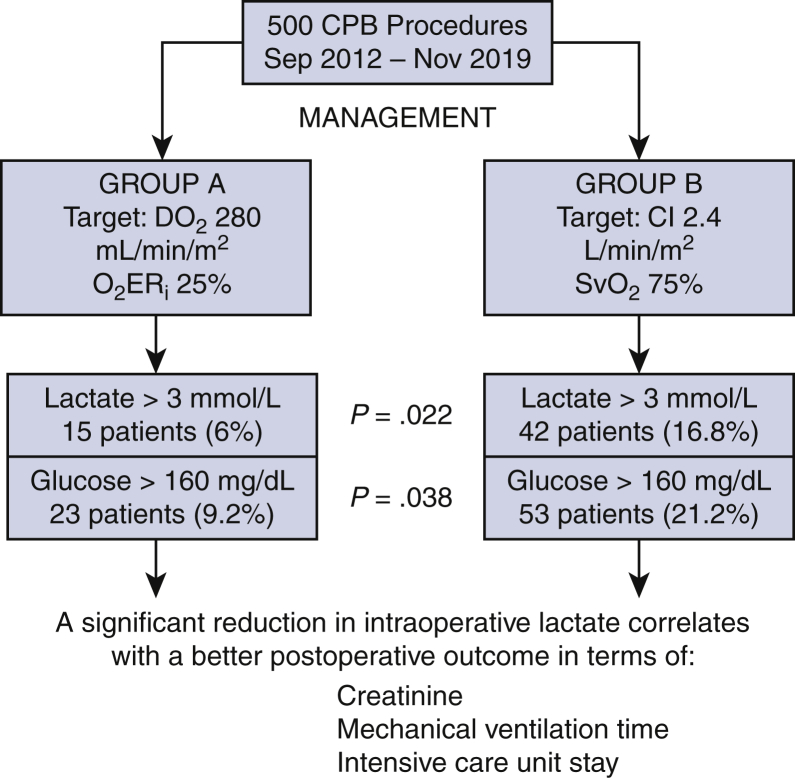
Eight pre- and intraoperative factors were found to be significantly associated with peak blood lactate level during CPB at univariate analysis. HL (>3 mmol/L) was detected in 15 (6%) patients of group A and in 42 (16.8%) patients of group B (P = .022); hyperglycemia (>160 mg/dL) was found in 23 (9.2%) patients of group A and in 53 (21.2%) patients of group B (P = .038). Patients with HL during CPB had a significant increase in serum creatinine value, higher rate of prolonged mechanical ventilation time and intensive care unit stay. A cutoff of DO2i <270 mL/min/m2 in relation to O2ERi >35% in group A and a cutoff of CI <2.4 L/min/m2 in relation to SvO2 <65% in group B were found to have a positive predictive value of 80% and 75% for HL, respectively. A cutoff of DO2i >290 mL/min/m2 in relation to O2ERi 24% in group A and a cutoff of CI >2.4 L/min/m2 in relation to SvO2 >75% in group B were found to have a negative predictive value of 78% and 62% for HL, respectively.
Conclusions
This retrospective observational analysis showed that management of DO2i in relation to O2ERi was 16% more specific in terms of negative predictive value for HL during CPB compared with the use of CI in relation to SvO2. Group A reported a significant reduction in the incidence of intraoperative lactate peak, correlated with postoperative reduction of serum creatinine value, mechanical ventilation time, and intensive care unit stay, compared with group B.
Key Words: hyperlactatemia, oxygen delivery, cardiac index, cardiopulmonary bypass
Abbreviations and Acronyms: CI, cardiac index; CPB, cardiopulmonary bypass; DO2i, indexed oxygen delivery; Hb, hemoglobin; Hct, hematocrit; HL, hyperlactatemia; ICU, intensive care unit; O2ERi, indexed oxygen extraction ratio; SvO2, venous oxygen saturation
Graphical abstract


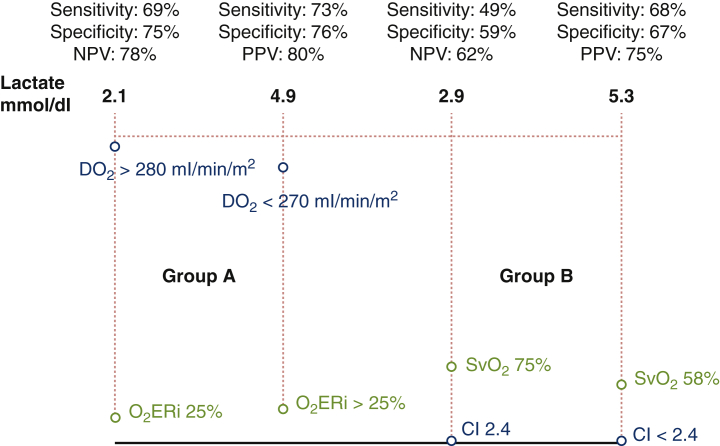
Sensitivity/specificity curves for cardiac index, indexed oxygen delivery, indexed oxygen extraction ratio, and mixed venous oxygen saturation.
Central Message.
This study showed that management of indexed oxygen delivery in relation to indexed oxygen extraction ratio was more specific in terms of negative predictive value for hyperlactatemia during cardiopulmonary bypass compared with cardiac index and mixed venous oxygen saturation.
Perspective.
The management of indexed oxygen delivery in relation to indexed oxygen extraction ratio follow the cardiopulmonary bypass was more specific in terms of negative predictive value for hyperlactatemia compared to the use of cardiac index in relation to mixed venous oxygen saturation. That management can correlate with postoperative better outcome especially in terms of serum creatinine, mechanical ventilation time, and intensive care unit stay.
During cardiac surgery with cardiopulmonary bypass (CPB) in adult patients, hyperlactatemia (HL) is detectable at a considerable rate (10%-20%)1,2 and is associated with postoperative morbidity and mortality.1 At present, the nature of HL during and after cardiac operations is not totally clear, but the majority of authors3, 4, 5, 6 tend to attribute this finding to tissue hypoxia (type A HL) even if type B HL (without tissue hypoxia) has been advocated in some cases.7, 8, 9 The main factors leading to a possible organ dysoxia during CPB are the hemodilution degree10 and a low peripheral oxygen delivery.1,2,4, 5, 6,10, 11, 12 In the state of perfusion, there are different metabolic management devices integrated to the heart–lung machine (eg, Quantum Spectrum [Spectrum Medical, Cheltenham, England], Connect Livanova [London, England], CDI Terumo Medical, Vaughan, Ontario, Canada], Landing Eurosets [Medolla, Italy]), with multiple measured and calculated parameters; the most commonly used and accepted metabolic target for the scientific community is the value of indexed oxygen delivery (DO2i) (280 mL/min/m2) and the cardiac index (CI) (2.4 L/min/m2). These parameters can be managed independently or according to other metabolic parameters (eg, hemoglobin [Hb], vascular resistance, temperature, and diuresis), resulting in wide variability in CPB management of each center.
This study has the objective to compare lactate production during CPB procedures using different metabolic management: DO2i in relation to indexed oxygen extraction ratio (O2ERi) (group A), and CI in relation to mixed venous oxygen saturation (SvO2) (group B) (Video 1).
Video 1.
Ignazio Condello, PhD, summarizes the results of the study and explains the relevance of the research for the readers of the Journal. Video available at: https://www.jtcvs.org/article/S2666-2507(20)30189-9/fulltext.
Materials and Methods
Population and Study Design
This study presents a comparative retrospective analysis that has been carried out between 2 historical times: the first historical period (2012-2015) used conventional extracorporeal circulation with blood gas test for metabolic management during CPB; the second historical period (2016-2019) used conventional extracorporeal circulation with blood gas test and the integration with metabolic parameter monitoring system. Between September 2012 and November 2019, 500 adults aged >28 to 80 years were collected for elective cardiac surgery procedures, without chronic kidney failure and with calculated European System for Cardiac Operative Risk Evaluation II score (mean value, 4.1%-4.5%) at our institution (Department of Cardiothoracic Surgery, Anthea Hospital, Bari, Italy). The study protocol was approved by the local ethics committee and all patients provided written consent to scientific treatment of their data. Patients were divided into 2 groups for CPB metabolic management: in group A (study group, n = 250), the DO2i target with a target of 280 mL/min/m2 was used in relation to O2ERi; in group B (control group, n = 250), the CI target with a target of 2.4 L/min/m2 was used in relation to SvO2.
Data Collection
Patients were selected according to the following criteria:
-
•
Elective, primary cardiac surgery: complete CPB and cardioplegic arrest had to be foreseen with an expected CPB duration >90 minutes.
-
•
Patients were excluded if they presented abnormal plasma lactate levels (>2 mmol/L) before entering CPB, renal or liver failure, obesity, uncompensated diabetes, autoimmune disease, active infection, any immunosuppressant therapy, or coagulation disorder. Patients undergoing surgery with circulatory arrest or having preoperative hematocrit (Hct) <27% were also excluded.
The cardiac surgery procedures that were analyzed for this study are coronary artery bypass graft (n = 200), isolated aortic valve replacement (n = 100) and mitral valve repair with minimally invasive approach (n = 200).
Preoperative data included patient demographic characteristics, baseline serum creatinine levels, ventricular ejection fraction, comorbidities (eg, chronic obstructive pulmonary disease or previous cerebrovascular accident), baseline Hb, logistic European System for Cardiac Operative Risk Evaluation II score and New York Heart Association functional class.2
Perioperative data included type of operation, CPB duration, nadir body temperature during CPB, nadir Hct and Hb values (measured at the start of the CPB operation and every 20 minutes thereafter), nadir DO2i, nadir DO2i/O2ERi ratio during CPB, nadir CI, nadir CI/SvO2, peak serum lactate, and glucose during CPB. Postoperative data included peak serum creatinine, mechanical ventilation time, and days spent in the intensive care unit (ICU).
The primary end points were specificity and sensitivity, positive and negative predictive value for HL between target DO2i in relation to O2ERi during CPB compared with the control group in terms of intraoperative lactate and glycemia trends. Secondary end points were peak postoperative serum creatinine level,13,14 mechanical ventilation time, and length of ICU stay.
Anesthetics and Surgical Procedures
Patients were monitored with 5-lead electrocardiography, a left radial artery catheter, capnography, pulse oximetry, and rectal/urine bladder temperature sensors. Transesophageal echocardiography was performed in all patients. Anticoagulant therapy consisted of heparin sodium before CPB at 300 IU/kg to give an activated clotting time of >480 seconds (ACT PLUS; Medtronic, Minneapolis, Minn); for antagonization of heparin, 0.5 to 0.75 mg protamine was applied for every 100 U heparin. Anesthesia was induced with intravenous sufentanil (0.5-1 μg/kg) and midazolam (0.08-0.2 mg/kg), and tracheal intubation was facilitated with intravenous rocuronium (0.6-1 mg/kg). Anesthesia was maintained with propofol (2-5 mg/kg) and sufentanil (0.5-2.0 μg/kg), and the depth of anesthesia was monitored using bispectral index values (BIS XP; Aspect Medical System, Newton, Mass). The dose of propofol was titrated to maintain bispectral index values between 40 and 60. Aortic valve replacement and coronary artery bypass graft procedures were performed in median sternotomy with central cannulation, MVR in right minithoracotomy approach with peripheral cannulation, and surgical procedures were performed as routine by 2 surgeons. Concentrated red blood cells were transfused whenever Hb concentrations fell below 6 g/dL during surgery or below 8 g/dL during ICU stay.
CPB Setting
Both open (Admiral; Remo-well Eurosets; EOS Dideco; Mirandola, Italy; Inspire 6F; LivaNova) and closed circuits (Closed Eurosets) were used for CPB. Pericardial blood was collected separately and could be processed or reinjected, if needed. The hard shell and soft shell reservoir, oxygenating module and circuits were treated with phosphorylcholine (Agile Eurosets; P.hisio. LivaNova). All patients were treated with mild hypothermic CPB (34°C-36°C); a volume of 1250 mL crystalloid Ringer acetate solution was used for priming. The surgical procedures selected for this study do not justify the use of moderate hypothermia by falling below 34°C. For this reason, in the event of an initial increase in anaerobic metabolism, the first compensation approach was not to lower the temperature but possibly liquids or red blood cells were integrated.
The hardware consisted of a Stöckert S5 heart-lung machine and a Stöckert Heater Cooler System 3T (LivaNova) and the same cannulae were employed in both groups. For the administration of myocardial protection, a closed circuit for cardioplegia with heat exchanger, with an infusion syringe pump in series and Saint Thomas solution with procaine were used and repeated every 30 minutes. Group A used the Landing monitoring system (Eurosets) for DO2 management during CPB. In both groups, blood gas analyses were performed using alpha-stat management with a blood-gas analyzer (GEM Premier 3000 IQM; Instrumentation Laboratory, Werfen Group IVD company, Munich, Germany) set to measure at 37°C.4 On the basis of arterial blood data, we assessed the lowest Hct (percentage) on CPB; every 20 minutes, an arterial blood gas analysis, including blood glucose and lactate determination, was obtained. An Hb value <6 to 7 g/dL during CPB was considered the trigger point for red blood cell transfusion. All patients received tranexamic acid according to the routine protocol. Mean arterial pressure during CPB procedures was managed for values between 55 and 70 mm Hg.
Metabolic Management During CPB in Group A
In group A, DO2i with a target of 280 mL/min/m2 was managed in relation to O2ERi (the cutoff for increase in DO2i was >25% O2ERi, the cutoff for decrease in DO2i was <25% O2ERi). DO2i and O2ERi-related measurements were performed using a Landing system provided by Eurosets. Data were collected every 5 seconds during CPB. Data required to calculate DO2i and O2ERi were arterial Hb; measured parameters included arterial saturation, SvO2, blood pump flow, Hb, arterial and venous temperature, mean arterial pressure, body surface area, and CI.
DO2i was calculated using the following equation:
where arterial O2 content was calculated as follows: (CaO2) arterial O2 content (mL/100 mL) = Hb (mg/dL) × 1.34 × Hb saturation (%) + 0.003 × O2 tension (mm Hg).
O2ERi (%) was calculated using the following equation:2,15
Metabolic Management During CPB in Group B
In group B, CI with a target of 2.4 L/min/m2 was managed in relation to SvO2 (the cutoff for increase in CI was <75% SvO2, the cutoff for decrease in CI was >75% SvO2). Related measurements were performed using a Flowmeter probe in arterial line to measure the real flow of the roller pump and the blood gas analyzer (GEM Premier 3000 IQM; Instrumentation Laboratory, Werfen Group IVD company) set to measure at 37°C4 for measurement of SvO2 during CPB. Data were collected every 20 minutes during CPB.
CI was calculated using the following formula:
where pump flow (L/min) = body surface area (m2) × CI (L/min/m2).
SvO2, in the clinical and intensivist practice, was a true reflection of the global balance between oxygen delivery and consumption because it is measured through the venous drainage line during CPB where venous blood returning to the right heart from the superior vena cava, inferior vena cava, and the coronary sinus have mixed. SvO2 has been extensively studied and used clinically to monitor the global balance between DO2 and oxygen consumption. In the literature, for patients with multiple injuries, normal SvO2 values between 65% and 70% and increasing DO2 are more relevant for survival.
Statistical Analysis
All data are expressed as mean ± standard error of the mean or as absolute numbers and percentage, as appropriate. Statistical analysis was performed using SPSS version 11.0 software (SPSS Inc, Chicago, Ill). Univariate association with peak blood lactate was tested with a correlation matrix. Factors significantly (P < .05) associated with peak blood lactate at this preliminary step were entered into a stepwise forward multivariable linear regression analysis, with adequate corrections to avoid multicollinearity within the model. The multivariable approach was applied to assess the independent association between the variables tested and peak blood lactate. Subsequently, the population was explored in terms of HL (>3 mmol/L) incidence. Normally distributed continuous variables are expressed as means ± standard deviation, and categorical variables as frequencies and percentages. DO2i in relation to target O2ERi vs CI in relation to SvO2 during CPB were tested for association with peak lactate and peak glucose blood. Intraoperative variables were tested for predictive ability of HL by using a receiver operating characteristic analysis. Postoperative outcome was firstly analyzed in the population with or without HL during CPB using a univariate approach (Student t test for unpaired data or relative risk analysis) and was subsequently corrected for other covariates.
Results
Demographic, preoperative, and operative details of the patient population are shown in Tables 1 and 2. Eight pre- and intraoperative factors were found to be significantly associated with peak blood lactate level during CPB at univariate analysis (Table 3): age, isolated coronary operation, lowest pump flow, lowest temperature, Hct, and DO2i were negatively correlated with peak blood lactate value during CPB, whereas CPB duration and peak blood glucose were positively correlated with peak blood lactate value during CPB. The same intraoperative factors were tested for predictivity of HL with receiver operating characteristic analysis (Figure 1). The area under the curve was significant for all factors. We therefore decided to explore the adequate cutoff values for target DO2i in relation to O2ERi versus CI in relation to SvO2 ratio during CPB as possible predictors of HL. A cutoff of DO2i <270 mL/min/m2 in relation to O2ERi >35% during CPB in group A (Table 4 and Figure 2) and a cutoff of CI <2.4 L/min/m2 in relation to SvO2 <65% in group B (Table 5 and Figure 3) were found to have a positive predictive value of 80% (sensitivity 73%, specificity 76%) and 75% (sensitivity 68%, specificity 67%), respectively (Figure 4). A cutoff of 155 mg/dL for peak blood on CPB showed a positive predictive value of 85% (sensitivity 84%, specificity 83%) (Table 6). A cutoff of DO2i >290 mL/min/m2 in relation to O2ERi 24% during CPB in group A (Figure 2) and a cutoff of CI >2.4 L/min/m2 in relation to SvO2 >75% during CPB in group B (Figure 3) were found to have a negative predictive value of 78% (sensitivity 69%, specificity 75%) and 62% (sensitivity 49%, specificity 59%), respectively (Figure 4). A cutoff of 128 mg/dL for peak blood glucose on CPB showed a negative predictive value of 74% (sensitivity 79%, specificity 80%) (Table 7). HL (>3 mmol/L) was detected in 15 (6%) patients of group A and in 42 (16.8%) patients of group B (P = .001); hyperglycemia (>160 mg/dL) was found in 23 (9.2%) patients of group A and in 53 (21.2%) patients of group B (P = .001) (Table 8). Patients without HL or hyperglycemia had significantly lower values of peak blood lactate; patients with both HL and hyperglycemia had significantly higher peak blood lactate values than patients with only HL or hyperglycemia. Only patients with associated HL and hyperglycemia had significantly lower values of DO2i with higher value of O2ERi for group A and lower CI with low SvO2 for group B on CPB. Group A patients with higher values of DO2i and O2ERi showed a lower incidence of HL and hyperglycemia, which was 14.4% less than in group B patients for CI and SvO2 target. Patients with HL during CPB had a significant increase in serum creatinine value,13 higher rate of prolonged mechanical ventilation time and ICU stay (Table 9 and Figure 5). Patients with hyperglycemia not associated with HL were separately investigated for the outcome variables. No significant differences in terms of morbidity or mortality were detected in association with this isolated condition.
Table 1.
Preoperative profile and operative data
| Characteristic | Group A (n = 250) |
Group B (n = 250) |
|---|---|---|
| Mean age (y) | 69.6 | 71.3 |
| Male sex | 110 (44) | 121 (48) |
| Mean body surface area (m2) | 1.75 | 1.79 |
| Mean left ventricular ejection fraction (%) | 46 | 48 |
| Median NYHA functional class | 2 | 2 |
| EuroSCORE II (mean) | 4.1 | 4.7 |
| Pre-CPB hematocrit (%) | 32.4 ± 1.2 | 32.6 ± 1.9 |
| Pre-CPB Hb (g/dL) | 10.4 ± 1.1 | 10.8 ± 1.2 |
| No. of chronic obstructive pulmonary disease cases (mean) | 23 | 24 |
| Creatinine (mg/dL) | 1.09 ± 0.6 | 1.06 ± 0.9 |
| Obstructive coronary artery disease (%) | 23 | 24 |
Values are presented as n (%), or mean ± standard deviation. NYHA, New York Heart Association; EuroSCORE, European System for Cardiac Operative Risk Evaluation; CPB, cardiopulmonary bypass; Hb, hemoglobin.
Table 2.
Operative data
| Parameter | Group A (n = 250) |
Group B (n = 250) |
P value |
|---|---|---|---|
| CPB time (min) | 125 ± 13.2 | 120 ± 8.37 | .92 |
| Aortic crossclamp time (min) | 61 ± 4 | 68 ± 7 | .75 |
| Nadir temperature (°C) during CPB | 34.9 ± 1.1 | 34.7 ± 2.1 | .75 |
| Nadir hemoglobin value (mg/dL) during CPB | 8.73 ± 1.53 | 8.89 ± 1.25 | .88 |
| Nadir hematocrit (%) during CPB | 25.6 ± 3.8 | 25.9 ± 3.1 | .89 |
| Nadir DO2i (mL/min/m2) during CPB | 290 ± 29 | 278 ± 14 | .039 |
| O2ERi (%) during CPB | 24 ± 1 | 29 ± 5 | .0029 |
| Nadir CI (L/min/m2) during CPB | 2.6 ± 0.2 | 2.4 ± 0.1 | .0032 |
| Nadir SvO2 (%) | 81 ± 2 | 70 ± 5 | .0029 |
Values are presented as mean ± standard deviation. CPB, Cardiopulmonary bypass; DO2i, indexed oxygen delivery; O2ERi, indexed oxygen extraction ratio; CI, cardiac index; SvO2, mixed venous oxygen saturation.
Table 3.
Univariate analysis (correlation matrix)
| Factor | Correlation coefficient | P value |
|---|---|---|
| Age (y) | −0.079 | .029 |
| Isolated coronary operation | −0.075 | .039 |
| Lowest temperature on CPB | −0.219 | .001 |
| Lowest hematocrit on CPB | −0.149 | .001 |
| CPB duration | 0.049 | .001 |
| Lowest pump flow | −0.239 | .001 |
| CPB lowest DO2i | −0.254 | .001 |
| CPB peak blood glucose | 0.497 | .001 |
CPB, Cardiopulmonary bypass; DO2i, indexed oxygen delivery.
Figure 1.
Receiver operating characteristic curves for lactate peak prediction based on target indexed oxygen delivery (DO2i), indexed oxygen extraction ratio (O2ERi), cardiac index (CI), and mixed venous oxygen saturation (SvO2).
Table 4.
Subgroup analysis for peak blood lactate and lowest indexed oxygen delivery (DO2i) in relation to indexed oxygen extraction ratio (O2ERi) on cardiopulmonary bypass for group A (n = 250)
| Variable | No HL or HG | HL alone | HG alone | HL and HG |
|---|---|---|---|---|
| No. of patients | 223 | 4 | 12 | 11 |
| Peak blood lactate (mmol/L) | 1.28 ± 0.45 | 3.68 ± 0.35 | 1.82 ± 0.65 | 4.91 ± 3.21 |
| Lowest DO2i (mL/min/m2) | 304 ± 21 | 287 ± 13 | 289 ± 21 | 195 ± 40 |
| Highest O2ERi (%) | 20 ± 3 | 25 ± 2 | 25 ± 3 | 38 ± 4 |
Values are presented as mean ± standard deviation. HL, Hyperlactatemia; HG, hyperglycemia; DO2i, indexed oxygen delivery; O2ERi, indexed oxygen extraction ratio.
Figure 2.
Lactate and glucose trend according to the distribution of target indexed oxygen delivery (DO2i) level and indexed oxygen extraction ratio (O2ERi) during cardiopulmonary bypass.
Table 5.
Subgroup analysis for peak blood lactate and lowest cardiac index (CI) in relation to mixed venous oxygen saturation (SvO2) on cardiopulmonary bypass for group B (n = 250)
| Variable | No HL or HG | HL alone | HG alone | HL and HG |
|---|---|---|---|---|
| No. of patients | 187 | 10 | 21 | 32 |
| Peak blood lactate (mmol/L) | 1.39 ± 0.69 | 3.48 ± 0.38 | 1.79 ± 0.55 | 5.31 ± 3.83 |
| Lowest CI (L/min/m2) | 2.4 ± 0.2 | 2.4 ± 0.1 | 2.4 ± 0.1 | 1.8 ± 0.4 |
| Lowest SvO2 (%) | 80 ± 3 | 73 ± 1 | 72 ± 1 | 55 ± 12 |
Values are presented as mean ± standard deviation. HL, Hyperlactatemia; HG, hyperglycemia; CI, cardiac index; SvO2, mixed venous oxygen saturation.
Figure 3.
Lactate and glucose trend according to the distribution of cardiac index (CI) level and mixed venous oxygen saturation (SvO2) during cardiopulmonary bypass.
Figure 4.
Negative predictive value (NPV) and positive predictive value (PPV) of hyperlactatemia. The receiver operating characteristic curve analysis shows that oxygen delivery (DO2) >280 mL/min/m2 in relation to indexed oxygen extraction ratio (O2ERi) 25% is more specific and sensitive than the calculated cardiac index (CI), and is 16 times higher as a predictive value for values <3 mmol/dL lactates. SvO2, Mixed venous oxygen saturation.
Table 6.
Receiver operating characteristic analysis for the 5 intraoperative positive predictive value (PPV) of hyperlactatemia
| Factor | AUC | 95% confidence interval | P value | Cutoff value | Sensitivity, % | Specificity, % | PPV, % |
|---|---|---|---|---|---|---|---|
| Lowest DO2i on CPB | 0.71 | 0.58-0.81 | .001 | 180 mL/min/m2 | 73 | 74 | 75 |
| High O2ERi on CPB | 0.77 | 0.73-0.85 | .001 | 40% | 73 | 76 | 78 |
| Peak blood glucose on CPB | 0.92 | 0.82-0.97 | .001 | 160 mg/dL | 81 | 80 | 85 |
| Low CI on CPB | 0.67 | 0.62-0.80 | .009 | 1.8 L/min/m2 | 65 | 69 | 74 |
| Low SvO2 on CPB | 0.65 | 0.60-0.78 | .007 | 55% | 68 | 67 | 77 |
AUC, Area under the curve; PPV, positive predictive value; DO2i, indexed oxygen delivery; CPB, cardiopulmonary bypass; O2ERi, indexed oxygen extraction ratio; CI, cardiac index; SvO2, mixed venous oxygen saturation.
Table 7.
Receiver operating characteristic analysis for the 5 intraoperative negative predictive value (NPV) of hyperlactatemia
| Factor | AUC | 95% confidence interval | P value | Cutoff value | Sensitivity, % | Specificity, % | NPV, % |
|---|---|---|---|---|---|---|---|
| High DO2i on CPB | 0.75 | 0.70-0.83 | .001 | 299 mL/min/m2 | 73 | 74 | 77 |
| Low O2ERi on CPB | 0.79 | 0.73-0.85 | .001 | 24% | 73 | 76 | 79 |
| Low blood glucose on CPB | 0.89 | 0.82-0.93 | .001 | 128 mg/dL | 79 | 80 | 74 |
| High CI on CPB | 0.68 | 0.65-0.79 | .039 | 2.4 L/min/m2 | 64 | 69 | 63 |
| High SvO2 on CPB | 0.63 | 0.60-0.78 | .035 | 85% | 62 | 67 | 62 |
AUC, Area under the curve; NPV, negative predictive value; DO2i, indexed oxygen delivery; CPB, cardiopulmonary bypass; O2ERi, indexed oxygen extraction ratio; CI, cardiac index; SvO2, mixed venous oxygen saturation.
Table 8.
Incidence of hyperlactatemia (HL) and hyperglycemia (HG) in the study population
| Variable | Group A | Group B | P value |
|---|---|---|---|
| No HL-HG | 223 | 187 | .035 |
| HL alone | 4 | 10 | .041 |
| HG alone | 12 | 21 | .029 |
| HL-HG | 11 | 32 | .032 |
| Total HG | 23 | 53 | .001 |
| Total HL | 15 | 42 | .001 |
HL, Hyperlactatemia; HG, hyperglycemia.
Table 9.
Hyperlactatemia (HL) during cardiopulmonary bypass and postoperative outcome
| Variable | Group A (n = 250) |
Group B (n = 250) |
||
|---|---|---|---|---|
| No HL (n = 235; 94%) | HL (n = 15; 6%) | No HL (n = 208; 83.2%) | HL (n = 42; 16.8%) | |
| Peak serum creatinine (mg/dL) | 1.1 ± 1.0 | 1.9 ± 1.5 | 1.2 ± 1.1 | 1.9 ± 1.5 |
| MV time (h) | 20.6 ± 45 | 54 ± 49 | 23.6 ± 55 | 54 ± 49 |
| ICU stay (d) | 2.8 ± 2.1 | 5.7 ± 4.9 | 3.1 ± 2.1 | 6.4 ± 3.9 |
Values are presented as mean ± standard deviation. HL, Hyperlactatemia; MV, mechanical ventilation; ICU, intensive care unit.
Figure 5.
Data on 500 cardiopulmonary bypass (CPB) procedures were retrospectively collected, the management of indexed oxygen delivery (DO2i) in relation to indexed oxygen extraction ratio (O2ERi) follow the CPB was more specific in terms of negative predictive value for hyperlactatemia compared with the use of cardiac index (CI) in relation to mixed venous oxygen saturation (SvO2). That management can correlate with postoperative better outcome especially in terms of serum creatinine, mechanical ventilation time and intensive care unit stay.
Discussion
In this analysis we tried to analyze the correlation of lactates and glycemia with the target managed in relation to the oxygen consumption variables, in a different way than in the previous studies, strengthening their conclusions.1, 2, 3,9,10
Our analysis demonstrates that the management of DO2i in relation to O2ERi was 16% more specific in terms of negative predictive value for HL during CPB compared with the use of CI in relation to SvO2. The group managed with DO2 and O2ERi reported a significant reduction in the incidence of intraoperative lactate peak, correlated with postoperative reduction of serum creatinine value, mechanical ventilation time, and ICU stay, compared with group managed with CI and SvO2.
The link between HL and hyperglycemia through the mechanism explained above was confirmed by Revelly and colleagues16 in an elegant study dealing with cardiogenic or septic shock. The role of adrenergic agonists in this setting is well defined: in cardiogenic shock, they are both endogenous or administered for cardiovascular therapy; in our model, they are endogenous in the majority of patients. None received epinephrine during CPB, and few received norepinephrine; however, unlike epinephrine, norepinephrine usually does not increase glucose production or induce an increase in plasma lactate concentration.6,17 The 2 mechanisms leading to HL in various clinical conditions are therefore anaerobic metabolism due to a poor DO2 and excess lactate production due to glucose failing to enter the oxidative pathway and being degraded to lactate by the glycolytic pathway.17 These mechanisms, if independently considered, lead to different acid–base balance conditions, the former being accompanied by metabolic acidosis and the latter not necessarily so. However, in the clinical conditions of this observational study, the acid-base balance is constantly maintained at a normal pH value by bicarbonate corrections applied by the perfusionist whenever the base excess starts decreasing. Therefore, we are unable to identify differences in HL related to different values of peak blood lactate. However, the evidence that only 4 patients demonstrated HL without hyperglycemia and that only patients with an HL-hyperglycemia syndrome had a significantly lower value of DO2 seems to confirm that, in our specific clinical environment, HL and hyperglycemia are linked by the causative factor of a poor DO2, leading on 1 hand to lactate production through the anaerobic pathway and on the other hand to a vicious cycle of lactate production due to poor ability to use glucose through the aerobic pathway.2,5,10 Reduced oxygen content in cases of acute anemia is usually compensated by reduced blood viscosity with increased blood flow in the microcirculation and by a compensatory increase in cardiac output.12 This last mechanism may be impaired during CPB, where pump flow is usually adjusted on the basis of the patient's body surface area and temperature, not the Hb value. On the basis of our data, the main rationale for explaining HL during CPB is a DO2 inadequate to guarantee the needed oxygen consumption of the patient.
In the present study, we investigated the role of potentially modifiable factors related to CPB surgery in determining postoperative HL and hyperglycemia.11 Our results demonstrate, in a relatively large series of patients treated at different sites, that a DO2i <270 mL/min/m2 with O2ERi >35% and low CI (<2.4 L/min/m2) with SvO2 <65% during CPB are associated with HL and hyperglycemia and DO2i >290 mL/min/m2 with O2ERi <25% and CI >2.4 L/min/m2 with SvO2 >75% during CPB are associated with a low incidence of HL and hyperglycemia. Various preoperative factors or comorbidities may create the right environment for HL during CPB. Age, female sex, congestive heart failure, low left ventricular ejection fraction, hypertension, atherosclerosis, diabetes, preoperative Hb value, redo or complex surgery, and emergency procedures were found to be risk factors for HL by Demers and colleagues,1 who reported an HL incidence of 18%. Some of these factors were confirmed in our study, and other new factors were identified; however, our study population had a significantly shorter CPB duration and a lower degree of hemodilution during CPB. Given that both these factors seem to favor the onset of HL, the lower HL rate in our population is reasonably explained. The role of CPB duration in the determination of HL during CPB has been highlighted by other authors.1
Some study limitations should be acknowledged. First, the design of this analysis compares 2 different extracorporeal circulation management methods. In relation to the available literature, the values taken of 75% for SvO2 and 25% for O2ERi are not directly comparable because the roller pump used in group B does not correlate the calculated heart rate with the measured heart rate. Second, several patients had peripheral cannulation for CPB, which does not allow us to make a comparison between peripheral versus central cannulation. Moreover, during conventional management; we believed it appropriate not to use hypothermia because the calculated data that we were monitoring corresponded to the set objectives of 2.4 L/min flow; this nonmodification of management is intrinsically part of the retrospective nature of the study. Finally, the study focused on CPB with the use of a roller pump and does not consider the centrifuge, but it is also necessary to consider that, with its limitations, the roller pump is predominant in the daily use of cardiac surgery centers.18 The pump flow is delivered with a roller pump, often the flow management is calculated and not measured with an ultrasonic flowmeter,19 this often involves an overestimation (eg, due to occlusion of the rotor, technique with which the occlusion is made, vacuum-assisted venous drainage use, hypothermia, viscosity, positioning of the cannula, or material of the pump). Our center used a roller pump with a half silicone tube, and an occlusion of 1 cm2/min on a three-eighths meter high column. For reasons described above, a 0.3 ± 0.2 index discrepancy occurred with ultrasound monitoring that allowed us to evaluate lower cardiac indexes that we could not have evaluated without this gap.
Conclusions
This retrospective observational study showed that management of DO2i in relation to O2ERi was 16% more specific in terms of negative predictive value for HL during CPB compared with the use of CI in relation to SvO2. Group A patients showed a significant reduction in the incidence of intraoperative lactate peak, correlated with postoperative reduction of serum creatinine value, mechanical ventilation time, and ICU stay, compared with group B patients.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Ignazio Condello, PhD, summarizes the results of the study and explains the relevance of the research for the readers of the Journal. Video available at: https://www.jtcvs.org/article/S2666-2507(20)30189-9/fulltext.
References
- 1.Demers P., Elkouri S., Martineau R., Couturier A., Cartier R. Outcome with high blood lactate levels during cardiopulmonary bypass in adult cardiac surgery. Ann Thorac Surg. 2000;70:2082–2086. doi: 10.1016/s0003-4975(00)02160-3. [DOI] [PubMed] [Google Scholar]
- 2.Ranucci M., Isgrò G., Romitti F., Mele S., Biagioli B., Giomarelli P. Anaerobic metabolism during cardiopulmonary bypass: the predictive value of carbon dioxide derived parameters. Ann Thorac Surg. 2006;81:2189–2195. doi: 10.1016/j.athoracsur.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 3.Maillet J.M., Le Besnerais P., Cantoni M., Nataf P., Ruffenach A., Lessana A., et al. Frequency, risk factors, and outcome of hyperlactatemia after cardiac surgery. Chest. 2003;123:1361–1366. doi: 10.1378/chest.123.5.1361. [DOI] [PubMed] [Google Scholar]
- 4.Landow L. Splanchnic lactate production in cardiac surgery patients. Crit Care Med. 1993;21(2 suppl):S84–S91. doi: 10.1097/00003246-199302001-00015. [DOI] [PubMed] [Google Scholar]
- 5.Boldt J., Piper S., Murray P., Lehmann A. Case 2-1999. Severe lactic acidosis after cardiac surgery: sign of perfusion deficits. J Cardiothorac Vasc Anesth. 1999;13:220–224. doi: 10.1016/s1053-0770(99)90093-9. [DOI] [PubMed] [Google Scholar]
- 6.Totaro R., Raper R.F. Epinephrine induced lactic acidosis following cardiopulmonary bypass. Crit Care Med. 1997;25:1693–1699. doi: 10.1097/00003246-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Weil M.H., Afifi A.A. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock) Circulation. 1970;41:989–1001. doi: 10.1161/01.cir.41.6.989. [DOI] [PubMed] [Google Scholar]
- 8.Rashkin M.C., Bosken C., Baughman R.P. Oxygen delivery in critically ill patients. Relationship to blood lactate and survival. Chest. 1985;87:580–584. doi: 10.1378/chest.87.5.580. [DOI] [PubMed] [Google Scholar]
- 9.Takala J., Uusaro A., Parviainen I., Ruokonen E. Lactate metabolism and regional lactate exchange after cardiac surgery. New Horiz. 1996;4:483–492. [PubMed] [Google Scholar]
- 10.Ranucci M., De Toffol B., Isgrò G., Romitti F., Conti D., Vicentini M. Hyperlactatemia during cardiopulmonary bypass: determinants and impact on postoperative outcome. Crit Care. 2006;10:R167. doi: 10.1186/cc5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raper R.F., Cameron G., Walker D., Bovey C.J. Type B lactic acidosis following cardiopulmonary bypass. Crit Care Med. 1997;25:46–51. doi: 10.1097/00003246-199701000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Habib R.H., Zacharias A., Schwann T.A., Riordan C.J., Durham S.J., Shah A. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–1450. doi: 10.1016/s0022-5223(02)73291-1. [DOI] [PubMed] [Google Scholar]
- 13.Mehta R.L., Kellum J.A., Shah S.V., Molitoris B.A., Ronco C., Warnock D.G., et al. Acute Kidney Injury Network: a report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta R.H., Grab J.D., O'Brien S.M., Bridges C.R., Gammie J.S., Haan C.K., et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- 15.de Somer F., Mulholland J.W., Bryan M.R., Aloisio T., Van Nooten G.J., Ranucci M. O2 delivery and CO2 production during cardiopulmonary bypass as determinants of acute kidney injury: time for a goal-directed perfusion management? Crit Care. 2011;15:R19. doi: 10.1186/cc10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revelly J.P., Tappy L., Martinez A., Bollmann M., Cayeux M.C., Berger M.M., et al. Lactate and glucose metabolism in severe sepsis and cardiogenic shock. Crit Care Med. 2005;33:2235–2240. doi: 10.1097/01.ccm.0000181525.99295.8f. [DOI] [PubMed] [Google Scholar]
- 17.Ensinger H., Geisser W., Brinkmann A., Wachter U., Vogt J., Radermacher P., et al. Metabolic effects of norepinephrine and dobutamine in healthy volunteers. Shock. 2002;18:495–500. doi: 10.1097/00024382-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Baker R.A., Willcox T.W. Australian and New Zealand perfusion survey: equipment and monitoring. J Extra Corpor Technol. 2006;38:220–229. [PMC free article] [PubMed] [Google Scholar]
- 19.Puis L., Milojevic M., Boer C., De Somer F.M.J.J., Gudbjartsson T., van den Goor J., et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Interact Cardiovasc Thorac Surg. 2020;30:161–202. doi: 10.1093/icvts/ivz251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ignazio Condello, PhD, summarizes the results of the study and explains the relevance of the research for the readers of the Journal. Video available at: https://www.jtcvs.org/article/S2666-2507(20)30189-9/fulltext.