Abstract
Recent developments in immune checkpoint inhibitors (ICIs) have provided new treatment strategies for advanced cancer. However, ICIs lead to an imbalance between T cell–mediated inflammatory responses and immune tolerance in the myocardium. Here we report the first case that implicates the contribution of ICI-induced vasculitis to myocardial injury. (Level of Difficulty: Intermediate.)
Key Words: myocardial injury, pembrolizumab, vasculitis
Abbreviations and Acronyms: CK, creatinine kinase; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; PD-1, programmed cell death protein–1; PD-L1, programmed cell death protein–1 ligand 1
Graphical abstract
History of Presentation
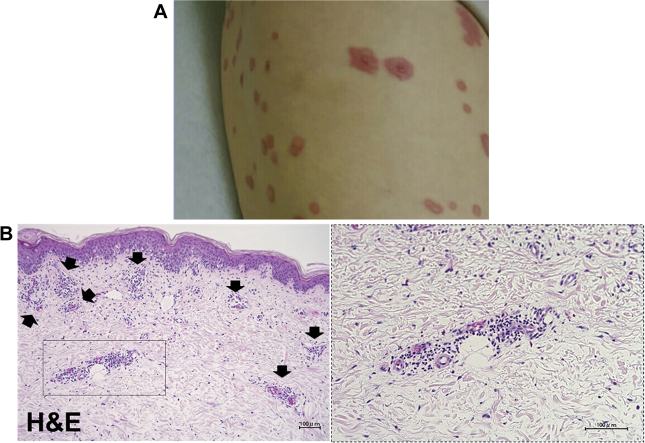
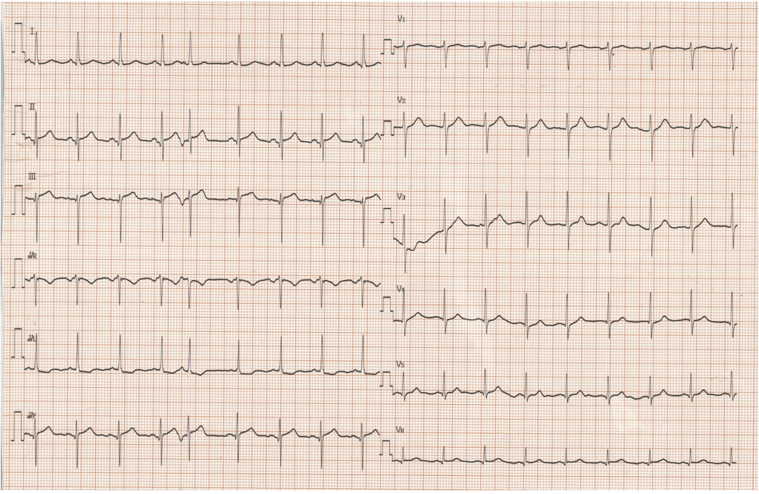
A 79-year-old female patient underwent surgery for malignant melanoma of the right jaw. Upon identification of cervical lymph node metastases, immunotherapy with a programmed cell death protein–1 (PD-1) inhibitor, pembrolizumab, was initiated at a dose of 200 mg every 3 weeks. After 10 cycles of pembrolizumab, the treatment was discontinued because of exanthema on her limbs and trunk (Figure 1A). A femoral skin biopsy was performed, and the patient was treated with oral prednisolone 30 mg/day. Histopathologically, the biopsy specimen showed infiltration of lymphocytes, neutrophils, and eosinophils surrounding the small blood vessels (Figure 1B). After 1 month on prednisolone, the patient’s skin condition deteriorated, and she was hospitalized for treatment with intravenous immunoglobulin therapy at 17.5 g/day (0.4 g/kg/day). As no improvement was observed after 5 days of intravenous immunoglobulin treatment, 3-day steroid pulse therapy (methylprednisolone 1,000 mg/day) was added to her drug regimen, followed by oral administration of prednisolone 45 mg/day. Her condition improved, and the dose of prednisolone was tapered to 5 mg/day for 2 weeks. At this time, the patient experienced acute onset of aphasia and right homonymous hemianopsia. Computed tomography revealed a cerebral hemorrhage in the left parietal and occipital lobe, followed by sudden onset of chest pain 3 days later. Electrocardiography showed no specific ST-segment changes, and transthoracic echocardiography revealed a left ventricular ejection fraction of 80% without reduced local wall motion. C-reactive protein was 2.02 mg/dl, and serum levels of creatinine kinase (CK) and CK-MB were within their normal ranges at 40 IU/l and 9 IU/l, respectively, although serum troponin T was measured at 0.077 ng/ml, which was somewhat higher than the normal value (<0.014 ng/ml). Electrocardiography performed on the following day revealed slight ST-segment elevation in leads II, III, and aVF (Figure 2) and increases in serum CK, CK-MB, and troponin T levels to 391 IU/l, 54 IU/l, and 1.340 ng/ml, respectively.
Learning Objectives
-
•
To review ICI-induced vasculitis.
-
•
To propose a new adverse event, ICI-induced myocardial vasculitis.
Figure 1.
Macroscopic and Pathological Findings of Skin Lesion
(A) Femoral exanthema. (B) Staining of femoral exanthema. The exanthema on the patient’s femoral and skin biopsy specimen revealed infiltration of lymphocytes, neutrophils, and eosinophils around the small blood vessels. H&E = hematoxylin and eosin.
Figure 2.
Initial Electrocardiogram
An electrocardiogram obtained 1 day after the onset of chest pain revealed slight ST-segment elevation in leads II, III, and aVF.
Medical History
The patient had a history of malignant melanoma and hypertension.
Differential Diagnosis
In this patient, acute coronary syndrome was most suspected, followed by myocarditis, pericarditis, and stress cardiomyopathy.
Investigations
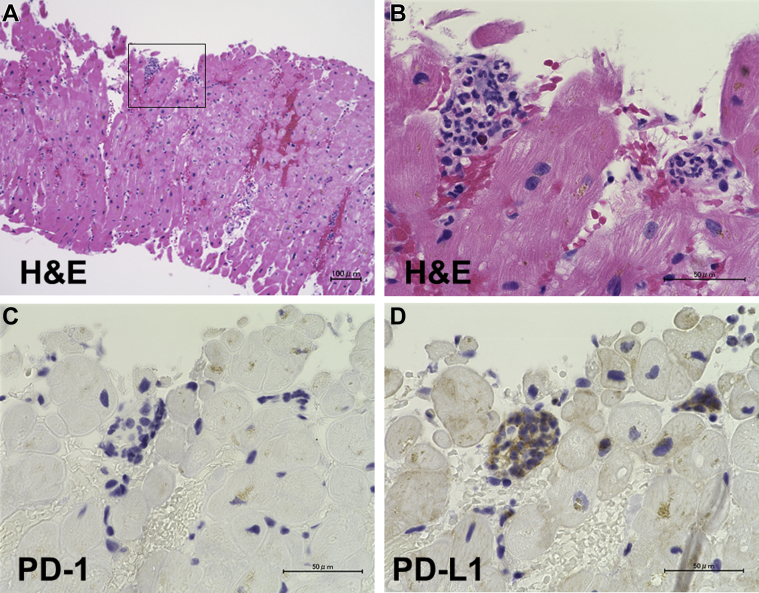
To exclude acute myocardial infarction, emergency left heart catheterization was performed. Coronary angiography showed normal coronary arteries, and left ventriculography detected focal reduced motion of the inferior wall (Video 1). On subsequent pathological evaluation, endomyocardial biopsy was performed at the right ventricular septum. We collected more than 2 myocardial samples to minimize sampling errors. There were no inflammatory infiltrates within the myocardium in any sample (Figure 3A), and many neutrophils and a few eosinophils and lymphocytes negative for PD-1 were detected in the regions surrounding the small blood vessels, in association with obstruction of the vascular lumina (Figures 3B and 3C). Furthermore, we also detected prominent expression of immunoreactive PD-1 ligand 1 (PD-L1) in association with the infiltrates at the small vessels (Figure 3D), a finding we attributed to pembrolizumab-mediated oversuppression of PD-1. These results strongly suggested that the myocardial injury in this case was not related to myocarditis but due to myocardial vasculitis associated with pembrolizumab treatment.
Figure 3.
Staining of Myocardial Tissue
Hematoxylin and eosin (H&E) staining of myocardial tissue revealed no inflammatory cell infiltrates within the myocardium (A). Neutrophils and eosinophils were detected around the small blood vessels in association with obstruction at the vascular lumina (B). Immunostaining identified programmed cell death protein–1 (PD-1)–negative (C) and PD-1 ligand 1 (PD-L1)–positive (D) cells.
Management
Although repeat steroid pulse therapy was considered, we decided to continue the existing steroid treatment because on the day after endomyocardial biopsy, the patient’s chest pain disappeared, and CK, CK-MB, and troponin T levels improved to 105 U/L, 8 IU/L, and 0.700 ng/ml, respectively. CRP reached its peak value of 19.14 mg/dl at 5 days after the onset of chest pain, then tended to improve, decreasing to 1.07 mg/dl 25 days later.
Discussion
Immune checkpoint inhibitors (ICIs) have provided a paradigm shift in cancer treatments, extending survival particularly in patients with cancer at advanced stages who could not overcome their incurable conditions (1). One critical disadvantage of ICI treatment is the possibility of immune-related adverse events (irAEs) (2). Untoward activation of the immune system may contribute to adverse events associated with ICI therapy (3). In recent years, cardiovascular complications of ICI therapy, especially ICI-associated myocarditis, with an incidence of fatality of 30% to 50%, have attracted attention (4,5). In addition to ICI-associated myocarditis, ICI-associated vasculitis is also an important irAE (4). An observational, retrospective pharmacovigilance study identified 82 cases of vasculitis among 31,321 adverse events (0.26%) reported in patients receiving ICIs, with a mortality rate of 6.1% (6). Although vasculitis associated with ICI therapy could affect any size blood vessel, a systematic review of case reports (7) indicated that large-vessel vasculitis (giant-cell arteritis and aortitis) and vasculitis of the nervous system (primary angiitis of the central nervous system and isolated vasculitis of the peripheral nervous system) were among the most common types associated with ICI therapy. Here, we report a case of myocardial injury due to myocardial vasculitis in a patient undergoing treatment for malignant melanoma with pembrolizumab. To the best of our knowledge, this is the first report of myocardial vasculitis associated with ICI therapy. Although the mechanisms underlying the development of vasculitis in this setting are not clearly understood, PD-1 inhibition enhances the activation of CD4+ T cells and can result in the release of proinflammatory cytokines and cytotoxic mediators within the arterial tissue. A significant point to be emphasized is that these factors can also promote remodeling processes that ultimately lead to intimal proliferation and vascular occlusion (8). Grabie et al. (9) reported that although the initiation of cardiac injury is dependent on T cells, secondary inflammatory events involving polymorphonuclear leukocytes play an essential role in myocardial damage in a mouse model. Furthermore, PD-L1 is up-regulated in the myocardium during drug-induced cardiac injury. This expression of PD-L1 was dependent on interferon gamma produced by infiltrating T cells and performing an important role in protecting the myocardium from immune-mediated excessive inflammation (9).
Our findings, which include PD-1-negative neutrophil infiltration together with PD-L1 up-regulation in the periarterial region, may reflect cytokine-induced inflammatory processes. Likewise, myocardial vasculitis with neutrophils dominant, rather than lymphocyte infiltration into the myocardium, may explain the limited efficacy of prednisolone in this case. As such, during prednisolone tapering, one should consider the possibility that additional irAEs such as vasculitis may develop.
Follow-Up
One month later, transthoracic echocardiography showed a left ventricular ejection fraction of 62% without reduced local wall motion, and CK, CK-MB, and troponin T levels were further improved to 16 IU/l, 3 IU/l, and 0.050 ng/ml, respectively. No signs of worsening myocardial vasculitis were detected.
Conclusions
ICIs including pembrolizumab may cause myocardial injury due not only to myocarditis but also to myocardial vasculitis.
Author Relationship With Industry
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Left Ventriculography at Emergency Left Heart Catheterization
Left ventriculography revealed focal reduced motion of the inferior wall.
References
- 1.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdel-Wahab N., Shah M., Suarez-Almazor M.E. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 4.Ball S., Ghosh R.K., Wongsaengsak S. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol. 2019;74:1714–1727. doi: 10.1016/j.jacc.2019.07.079. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L., Awadalla M., Mahmood S.S. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41:1733–1743. doi: 10.1093/eurheartj/ehaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salem J.E., Manouchehri A., Moey M. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daxini A., Cronin K., Sreih A.G. Vasculitis associated with immune checkpoint inhibitors—a systematic review. Clin Rheumatol. 2018;37:2579–2584. doi: 10.1007/s10067-018-4177-0. [DOI] [PubMed] [Google Scholar]
- 8.Cadena R.H., Abdulahad W.H., Hospers G.A.P. Checks and balances in autoimmune vasculitis. Front Immunol. 2018;9:1–11. doi: 10.3389/fimmu.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabie N., Gotsman I., DaCosta R. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell-mediated injury in the heart. Circulation. 2007;116:2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left Ventriculography at Emergency Left Heart Catheterization
Left ventriculography revealed focal reduced motion of the inferior wall.