Abstract
A 40-year-old woman with history of atopy and peripheral eosinophilia presented with clinical signs of heart failure. Echocardiography revealed a restrictive cardiomyopathy with biventricular thrombi. Hypereosinophilic syndrome resulting in eosinophilic myocarditis (Loeffler’s syndrome) was diagnosed. This case highlights the workup, diagnosis, and management of hypereosinophilic syndrome with eosinophilic myocarditis. (Level of Difficulty: Advanced.)
Key Words: cardiac MRI, cardiomyopathies, eosinophilic myocarditis, Loeffler’s endocarditis
Abbreviations and Acronyms: CMR, cardiac magnetic resonance imaging; EGPA, eosinophilic granulomatosis with polyangiitis; FISH, fluorescence in situ hybridization; HES, hypereosinophilic syndrome; IL, interleukin; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; RV, right ventricular
Graphical abstract
A 40-year-old woman presented to her internist with 2 weeks of dyspnea on exertion, lower extremity edema, headaches, and peripheral neuropathy. N-terminal pro-brain natriuretic peptide was 3,126 pg/ml. Furosemide was initiated, and she was referred for outpatient cardiology evaluation. Due to progressive dyspnea, she instead presented to the emergency department. On examination, she was afebrile, with blood pressure 122/88 mm Hg, pulse 96 beats/min, respirations 16 breaths/min, pulse oximetry 96% on room air. Her examination showed no jugular venous distension, clear lungs bilaterally, and a soft systolic murmur was heard at the right upper sternal border along with a split S2. No peripheral edema was noted. Dermatologic and neurologic examinations were unremarkable.
Learning Objectives
-
•
To develop a broad differential diagnosis with specific consideration of eosinophilic myocarditis in patients with new-onset heart failure and history of atopy.
-
•
To understand clinical, laboratory, and imaging findings associated with eosinophilic myocarditis.
-
•
To learn treatment strategies for eosinophilic myocarditis to include high-dose steroids, anticoagulation, guideline-directed medical therapy, and anti-IL-5 therapy with routine follow-up imaging to assess response.
Past Medical History
The patient's medical history included: ulcerative colitis on sulfasalazine; intermittent urticaria and angioedema; allergic rhinitis on cetirizine, ranitidine, and montelukast; and migraines on topiramate.
Differential Diagnosis
The differential diagnosis included eosinophilic myocarditis (Loeffler’s syndrome), hypereosinophilic syndrome (HES), eosinophilic granulomatosis with polyangiitis (EGPA), drug-induced eosinophilia, and leukemia/myeloproliferative disorder (Table 1).
Table 1.
Hypereosinophilic Syndromes and Clinical Manifestations
| Syndrome | Symptoms | Diagnostic Criteria | Organ Involvement |
|---|---|---|---|
| Eosinophilic myocarditis∗ | Chest pain, dyspnea, fatigue, palpitations, syncope | Peripheral eosinophilia, ESR, CRP, troponin, CK, ECG, echocardiogram, cardiac MRI, endomyocardial biopsy | Cardiovascular, blood, lungs |
| Hypereosinophilic syndrome (HES) | Dyspnea, cough, urticaria, abdominal pain, vomiting, fever, arthritis, neuropathy | Peripheral eosinophils >1,500/μl without other identifiable causes AND presence of organ damage | Skin, lungs, gastrointestinal, nervous, blood, cardiovascular |
| Eosinophilic granulomatosis with polyangiitis | Asthma, sinusitis, proteinuria, abdominal pain, neuropathy, rash | ANCA, urinalysis, CT chest and sinus, PFTs, echocardiogram, ESR, CRP, tissue biopsy | Skin, lungs, nervous, kidneys |
| Drug-induced eosinophilia | Rash, oliguria, cough, abdominal pain, neuropathy | Peripheral eosinophilia, Diagnosis of exclusion, tissue biopsy | Skin, blood, lungs, kidneys, nervous, gastrointestinal |
| Leukemia/Myeloproliferative disorders | Weight loss, fevers, night sweats, fatigue | Peripheral eosinophilia, JAK2 V617F, FIP1L1/PDGFRa analysis, bone marrow biopsy, peripheral smear | Bone, blood |
ANCA = anti-neutrophil cytoplasmic antibody; CK = creatine kinase; CRP = C-reactive protein; CT = computed tomography; ECG = electrocardiogram; ESR = erythrocyte sedimentation rate; FIP1L1/PDGFRa = FIP1-like 1/platelet-derived growth factor receptor alpha; JAK2 V617F = Janus kinase; MRI, magnetic resonance imaging; PFT = pulmonary function test.
Eosinophilic myocarditis (Loeffler’s syndrome) is a potential manifestation of the disease entities, not exclusive from them.
Investigations
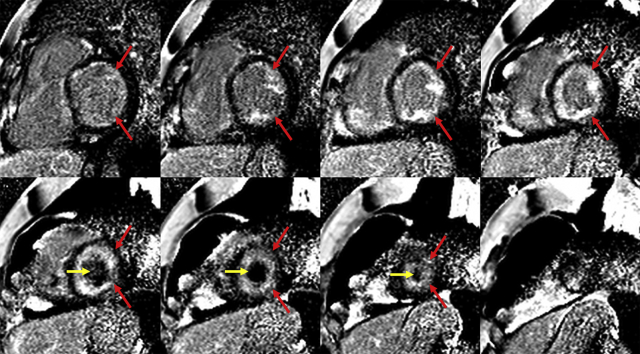
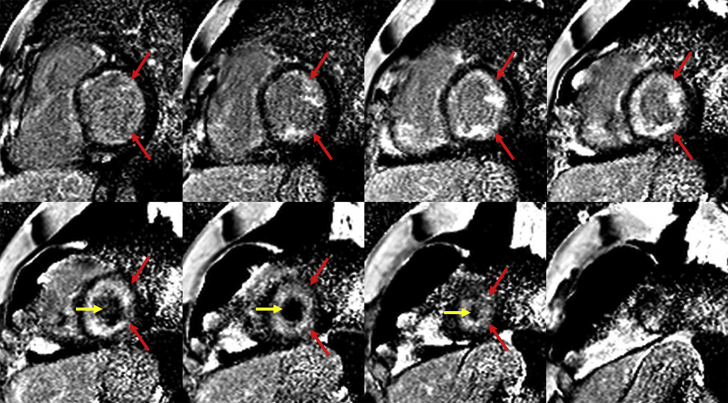
Laboratory testing showed N-terminal pro-brain natriuretic peptide 478 pg/ml, troponin 0.10 ng/ml, D-dimer 1,600 ng/ml, complete blood count showed 37% eosinophils: 2,700 absolute, serum creatinine 1.43 mg/dl. Urinalysis was unrevealing. Serum immunoglobulin E 309 kU/l. Chest computed tomography scan showed no pulmonary embolism. Transthoracic echocardiogram displayed left ventricular ejection fraction (LVEF) of 55% to 60%, a large echogenic mass in the left ventricular (LV) apex and smaller echogenic mass in the right ventricle (RV), pulmonary artery systolic pressure of 48 mm Hg, and right atrial dilation (Video 1). Cardiac magnetic resonance imaging (CMR) demonstrated a large layering LV apical thrombus and smaller RV apical thrombus, reduced LV longitudinal shortening, and diffuse subendocardial late gadolinium enhancement (LGE) consistent with endomyocardial fibrosis (Video 2, Figures 1 and 2).
Figure 1.
Cardiac Magnetic Resonance Late Gadolinium Enhancement Images: Eosinophilic Myocarditis
Short-axis late gadolinium enhancement imaging demonstrated diffuse subendocardial enhancement (red arrows) consistent with endomyocardial fibrosis and a diagnosis of eosinophilic myocarditis. The left ventricular thrombus (yellow arrows) is also noted as a region of hypointensity within the apical left ventricular cavity.
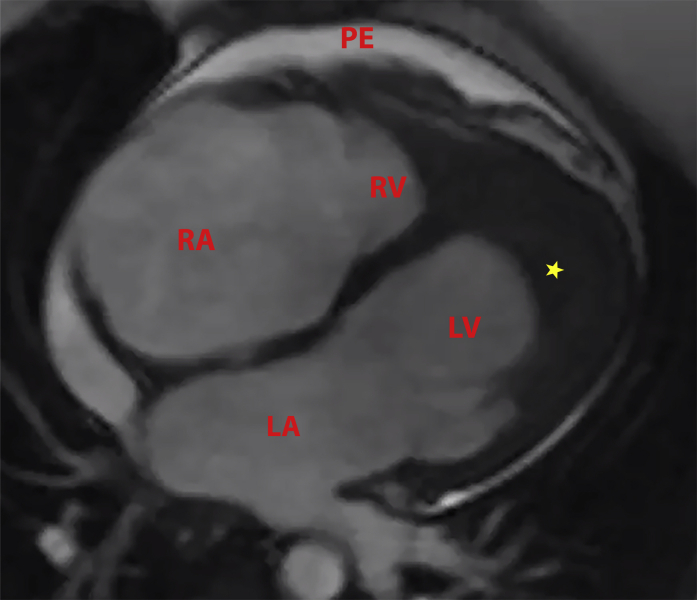
Figure 2.
Cardiac Magnetic Resonance 4-Chamber View
Four-chamber cardiac magnetic resonance imaging shows left atrial (LA) and right atrial (RA) enlargement, left ventricular (LV) chamber size decreased by presence of large layering LV thrombus (∗), small right ventricular size (RV) with small RV thrombus, and a small pericardial effusion (PE).
Evaluation for hematologic causes of peripheral eosinophilia, including Epstein-Barr virus, human immunodeficiency virus, hepatitis C virus, serum protein electrophoresis, serum free light chains, tryptase, genetic abnormalities (BCR-ABL, JAK2 V617F, flow cytometry), was negative, fluorescence in situ hybridization analysis was without rearrangements. p-ANCA was negative, c-ANCA demonstrated weak positivity (1:160). Testing for antiphospholipid syndrome was negative. Computed tomography of the abdomen and pelvis showed mild congestive hepatopathy but no evidence of malignancy.
Management
After consultation with hematology, rheumatology, and allergy/immunology services, the patient was started on high-dose methylprednisolone, then transitioned to oral prednisone at 1 mg/kg/day and tapered by 10 mg/day each week. She demonstrated good clinical response to high-dose steroids, and her eosinophil count remained undetectable with steroid taper. As an outpatient, treatment was initiated with anti-interleukin (IL)-5 therapy (mepolizumab). She was anticoagulated with heparin and transitioned to warfarin. She was diuresed with bumetanide and spironolactone given inadequate response to furosemide.
Discussion
Eosinophilic myocarditis, or Loeffler’s syndrome, is a hypereosinophilic syndrome defined by organ damage with 20% of cases having cardiac involvement and persistent eosinophilia (1). Although endomyocardial biopsy is the gold standard for diagnosis of eosinophilic myocarditis, the sensitivity is only 54% (2).
The most likely etiology of this patient’s peripheral eosinophilia is primary HES, but the differential diagnosis includes EGPA, malignancy/lymphoproliferative disorder, and drug-induced eosinophilia (Table 1). A diagnosis of HES requires the presence of 1,500 peripheral eosinophils/microliter, no alternative etiologies, and organ damage due to hypereosinophilia (3). On presentation, our patient had an elevated serum creatinine of 1.3 to 1.5 mg/dl without proteinuria. Her renal function improved with diuresis, arguing against renal involvement. Cardiac and neurologic involvement hinted at EGPA, but without asthma or sinusitis (despite a positive c-ANCA), this was not diagnostic of vasculitis. EGPA is a small vessel vasculitis and is not associated with development of thrombi. Because she remained symptomatic despite discontinuation of sulfasalazine, drug-induced eosinophilia is unlikely.
Eosinophilic myocarditis is a major cause of morbidity and mortality among patients with HES and should be considered even in patients who lack peripheral eosinophilia (4, 5, 6, 7). Eosinophil-mediated heart damage evolves through 3 stages: an acute necrotic stage, an intermediate stage characterized by thrombus formation along the damaged endocardium, and a fibrotic stage characterized by altered cardiac function due to restrictive cardiomyopathy and/or entrapment of the chordae tendineae leading to mitral and tricuspid regurgitation (7). Interestingly, it appears these are not discrete stages but rather an overlapping syndrome. CMR in eosinophilic myocarditis most often demonstrates subendocardial LGE (57.1%) (8), and in the acute setting, as in this case, can present with diffuse subendocardial LGE (Figure 1) (9). Our patient also developed biventricular apical thrombi (Figure 2).
In HES, eosinophil-based granules contain cytotoxic proteins that act as platelet agonists and increase vascular permeability while activating factor XII, a procoagulant that predisposes to thrombosis (8). There are no guidelines for treatment of eosinophilic myocarditis and the mortality rate approaches >20% if eosinophil count does not normalize (8). In a 2017 review, corticosteroids were frequently used (77.7%) in systemic conditions with primary indications such as EGPA (87.0%) or HES (86.7%). Thus, treatment revolves around reducing cardiac involvement by reducing peripheral eosinophilia. In some cases, a second immunosuppressive agent (8), such as mepolizumab, an anti-IL-5 receptor monoclonal antibody, can be used to block IL-5–mediated eosinophil differentiation and survival. By blocking IL-5, mepolizumab reduces the number of circulating and tissue eosinophils. Mepolizumab is approved by the Food and Drug Administration for the treatment of EPGA and has a well-tolerated side-effect profile. Previous case reports have shown that in eosinophilic myocarditis, anti-IL-5 therapy resulted in improved LVEF and stabilized cardiac function (10). There are no current guidelines for type or duration of therapy, but because of the high risk of progression and worsening fibrosis should tissue eosinophilia recur, we continued mepolizumab indefinitely.
Follow-Up
Our patient continued a steroid taper and initiated mepolizumab 300 mg subcutaneously every 4 weeks. Her absolute eosinophil count remained zero despite tapering prednisone to 5 mg daily. She remains on bumetanide and spironolactone with normalization of her renal function and improvement in her lower extremity edema. Dyspnea improved, and her diuretic requirement decreased. Because of ease of use, the patient elected to switch her anticoagulation to apixaban 5 mg twice daily following a comprehensive discussion of the off-label use of direct oral anticoagulants for management of ventricular thrombus, with the understanding of the inherent equipoise in the literature. Transthoracic echocardiogram at 6 and 14 weeks showed persistent but smaller LV apical thrombus, and preserved LVEF. CMR performed 3 months after treatment initiation showed LVEF 52%, persistent LV thrombus with resolution of RV thrombus, and improving but persistent myocardial edema indicative of resolving inflammation. Following resolution of RV thrombus, RV biopsy was performed and showed moderate subendocardial fibrosis on trichrome stain (Figure 3) without a significant number of inflammatory cells, concerning for progression to the fibrotic stage of eosinophilic myocarditis. The patient has mild peripheral neuropathy without further systemic symptoms.
Figure 3.
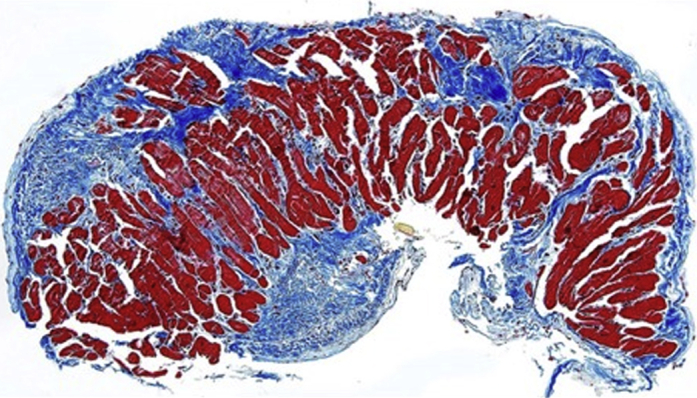
Right Ventricular Biopsy
Trichrome stain showing moderate subendocardial fibrosis (blue staining).
Conclusions
In patients with a history of atopy and hypereosinophilia presenting with heart failure, the diagnosis of eosinophilic myocarditis should be considered, particularly in the presence of cardiac dysfunction or ventricular thrombi. A thorough clinical investigation for the etiology of eosinophilia should be undertaken. In the case of eosinophilic myocarditis and/or HES, initiation of immunosuppression with high-dose steroids in conjunction with anticoagulation and guideline-directed medical therapies for heart failure is the treatment strategy of choice. A steroid-sparing regimen including anti-IL-5 therapy can be considered. We recommend co-management by a multidisciplinary team including cardiology and allergy/immunology specialists.
Author Relationship With Industry
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
References
- 1.Kuchynka P., Palecek T., Masek M. Current diagnostic and therapeutic aspects of eosinophilic myocarditis. BioMed Res Int. 2016;2016:2829583. doi: 10.1155/2016/2829583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizkallah J., Desautels A., Malik A. Eosinophilic myocarditis: two case reports and review of the literature. BMC Res Notes. 2013;6:538. doi: 10.1186/1756-0500-6-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn J.E., Groh M., Lefevre G. (A critical appraisal of) classification of hypereosinophilic disorders. Front Med (Lausanne) 2017;4:216. doi: 10.3389/fmed.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chusid M.J., Dale D.C., West B.C., Wolff S.M. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore) 1975;54:1–27. [PubMed] [Google Scholar]
- 5.Ogbogu P.U., Rosing D.R., Horne M.K., 3rd Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:457–475. doi: 10.1016/j.iac.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Take M., Sekiguchi M., Hiroe M. Clinical spectrum and endomyocardial biopsy findings in eosinophilic heart disease. Heart Vessels Suppl. 1985;1:243–249. doi: 10.1007/BF02072403. [DOI] [PubMed] [Google Scholar]
- 7.Weller P.F., Bubley G.J. The idiopathic hypereosinophilic syndrome. Blood. 1994;83:2759–2779. [PubMed] [Google Scholar]
- 8.Brambatti M., Matassini M.V., Adler E.D., Klingel K., Camici P.G., Ammirati E. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol. 2017;70:2363–2375. doi: 10.1016/j.jacc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Mavrogeni S.I., Sfikakis P.P., Koutsogeorgopoulou L. Cardiac tissue characterization and imaging in autoimmune rheumatic diseases. J Am Coll Cardiol Img. 2017;10:1387–1396. doi: 10.1016/j.jcmg.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Song T., Jones D.M., Homsi Y. Therapeutic effect of anti-IL-5 on eosinophilic myocarditis with large pericardial effusion. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2016-218992. bcr-2016–218992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.