Abstract
Several neurological presentations have been reported following coronavirus 2019 (COVID-19) infection. This case report describes three myasthenia gravis (MG) patients presented following COVID-19 infection. We report three adult patients with myasthenic Gravis and COVID-19 infection. The patients are between 38 and 61 years old. Case 1 is a 61-year-old woman with progressive dysphagia, nasal speech, ocular ptosis, diplopia, and proximal muscle weakness for 10 days. She had a COVID-19 infection 6 weeks ago. Case 2 is a 57-year-old man with clinical symptoms of muscular fatigability, diplopia, ptosis, and dysphagia for a week and a positive COVID-19 infection 10 days ago. Case 3 is a 38-year-old woman with fatigability, ptosis, dysphagia, and a diagnosis of COVID-19 infection 4 weeks ago. All patients had a positive RT-PCR for COVID-19 infection by nasopharyngeal swab test and a high-level acetylcholine receptor antibody in the serum. All patients were treated with pyridostigmine and prednisolone with a favorable outcome. MG may appear following COVID-19 infection, and the role of molecular mimicry and latent MG activation should be considered the cause of the disease onset.
Keywords: Coronavirus 2019, COVID-19, Myasthenia gravis, Case report, Post-infection
Graphical Abstract
1. Introduction
Myasthenia Gravis (MG) is an autoimmune disorder caused by the destruction of the post-synaptic neuromuscular junction due to the production of antibodies against nicotinic acetylcholine receptors (AChR) at the neuromuscular junction [1]. The most common clinical manifestations of this disorder are fatigability and weakness of skeletal muscle with varying degrees, which may be generalized or focal, including ptosis, diplopia, nasal speech, dysphagia, muscular weakness of the limbs, and respiratory distress [2]. Previous studies have demonstrated a possible etiological role of different viral infections in MG [3]. The novel coronavirus outbreak of SARS-CoV-2 (COVID-19) first commenced in Wuhan, China, in December 2019 [3]. On 19 February 2020, Iran described the first infected cases with COVID-19 in Qom city. The virus infects various systems, most commonly involving the respiratory and gastrointestinal tracts [3]. While fever, pneumonia of varying severity, and diarrhea are significant features of COVID-19; nevertheless, the association of various neurological disorders with the COVID-19 has been well established, such as stroke, acute disseminated encephalitis seizure, and Guillan Barre syndrome (GBS) [4], [5], [6]. Likewise, some studies reported the association of MG disease with COVID-19 infection [7], [8], [9]. Restivo et al. described three myasthenic patients in Italy following infection with COVID-19 [9]. Sriwastava et al. reported the association of new-onset ocular myasthenia gravis in a patient with COVID‑19 [7]. Herein, we describe the clinical manifestation of three cases of myasthenia gravis following COVID-19 infection.
2. Case presentation
2.1. Patient 1
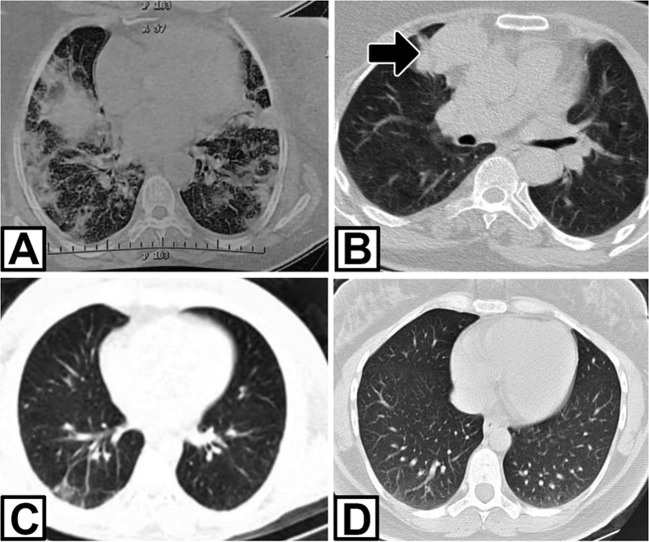
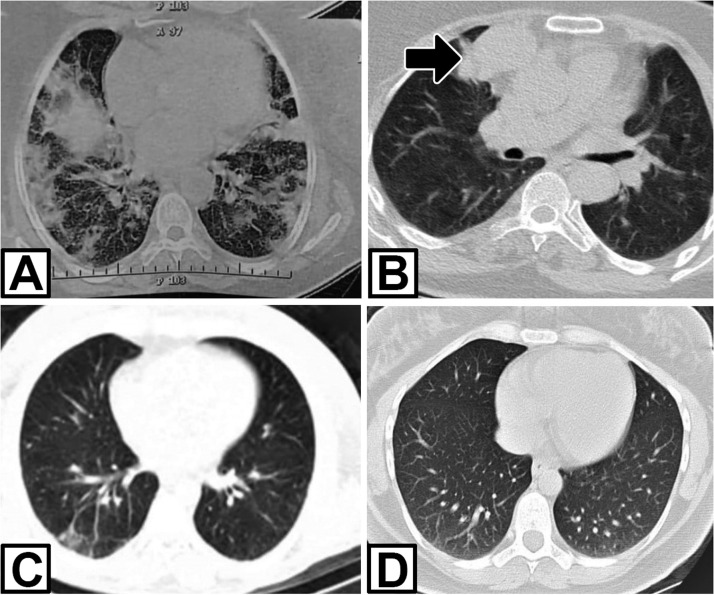
A 61-year-old woman was admitted in mid-November 2020 with a history of COVID-19 infection six weeks ago. The patient complained of dysphagia, nasal speech, ocular ptosis, diplopia, proximal muscle weakness, and dyspnea, which had been progressively worsened for ten days. COVID-19 infection had been confirmed with positive reverse transcription-polymerase chain reaction (RT-PCR) assay on the nasal-pharyngeal swab and bilateral ground-glass opacities in the lung computed tomography (CT) 6 weeks ago ( Fig. 1). The patient had been treated with Azithromycin and Remdesivir. After five days, she had been afebrile with mild signs of respiratory problems. At the time of admission to our hospital, she had bilateral ptosis, impaired counting, and nasal speech. Muscle strength was decreased in the proximal upper and lower limbs, with a medical research council (MRC) score of grade 3. The severity of MG was class IIIb according to myasthenia gravis foundation American (MGFA). The chest CT demonstrated thymoma. 3-hertz repetitive nerve stimulation (RNS) of the facial, median, and accessory nerves showed 15–27% decrement, suggesting a post-synaptic neuromuscular junction disorder. Acetylcholine receptor binding antibody (AchR-Ab) in the serum was elevated (10 pmol/L; normal value, < 0.4 pmol/L). Brain MRI was normal. The patient was treated with plasma exchange, pyridostigmine bromide 60 mg four times a day, and prednisone 1 mg/kg with significant improvement. After 15 days, the patient was discharged with mild weakness, without dysphagia and dyspnea, and an O2 saturation level of 98% at the room air. She was referred to a thoracic surgeon for thymoma surgery.
Fig. 1.
Lung CT scan of three patients. A: grand glass opacity at bilateral lung in patient 1; B: the patient 1 after treatment COVID-19. Thymoma revealed at right mediastinum (arrow); C and D: mild bilateral opacity in lower lobe in patients 2 and 3, respectively.
2.2. Patient 2
A 57-year-old man was referred to us in October 2020 with muscle fatigue, diplopia, ptosis, and dysphagia from a week ago. He reported a history of chronic heart failure (CHF) and implantable cardioverter-defibrillators (ICD) since ten years ago. He described a dry cough, fatigue, and fever as high as 38.5 °C for six days, before developing fatigue and ptosis. Chest CT scan indicated signs of COVID-19 pneumonia in the lower lobe of the lung, and RT-PCR assay on nasal-pharyngeal swab sample was positive for COVID-19. A few days later, proximal weakness of the upper limbs, diplopia, and dysphagia to solid foods became apparent (MGFA class IIb). He had mild left ptosis, mild bilateral facial weakness, and mild proximal upper limb weakness on examination. The slow RNS (3-hertz) of the facial, median, ulnar, and radial nerves showed a 10–40% decremental response. Serum AChR antibody level was raised (8 pmol/L; normal value < 0.4 pmol/L). Thymus was normal on chest CT scan. We prescribed pyridostigmine 60 mg three times a day and prednisone 25 mg daily. Dysphagia and muscle weakness improved after seven days.
2.3. Patient 3
A 38-year-old woman was referred to our clinic for fatigability, ptosis, and dysphagia in June 2020. She described myalgia, fatigue, coughing, and fever four weeks ago. A diagnosis of COVID-19 infection was made based on a chest CT scan and positive RT-PCR on the nasal-pharyngeal swab sample. Supportive treatment had been started at home. After four weeks, the patient experienced diplopia, ptosis, generalized fatigue, and dysphagia. On the physical examination, the patient had bilateral ptosis and mild limited eye movements. She had a proximal upper and lower weakness (MRC grade 4). Deep tendon reflexes were normal. AchR-Ab was positive with a titer higher than 8 nmol/L (normal < 0.4 nmol/l), and anti-MuSK Ab was negative. Repetitive nerve stimulation was performed, and it was compatible with a post-synaptic neuromuscular transmission disorder with a decremented response of 30–40% at facial and radial nerves. Chest CT scan disclosed mild bilateral lower lobe opacities in the lung without thymoma (Fig. 1). The brain MRI was normal. According to the combination of these findings, the patient was diagnosed with seropositive generalized myasthenia gravis (class IIb MGFA). Pyridostigmine 240 mg and prednisolone 25 mg daily were started, with a significant improvement in her symptoms.
3. Discussion
At the start of the spread of SARS-CoV-2 throughout the world, it was believed that this infection is principally targeting the pulmonary system; however, other systemic involvement, especially central and peripheral nervous system disorders, were rapidly revealed.
The present study described three patients with MG who showed their first presentations after infection with COVID-19. COVID-19 was confirmed by chest CT scan and RT-PCR. All patients had positive RNS and high serum AChR antibody levels in favor of MG. None of the patients had a history of autoimmune disease or other neurological disorders. In our study, the time interval between the onset of myasthenic symptoms and COVID-19 was 10–30 days. In three patients reported by Restivo et al., this time interval was 5–7 days [9], while in the reports by Huber et al. [8] and Sriwastava et al. [7], this was two weeks. The first of our patients had thymoma in the chest CT.
In the first patient with thymoma, we hypothesize that SARS-CoV-2 infection or Azithromycin administration may have unmasked or triggered a latent myasthenia gravis in this patient. Previously, latent MG unmaking has been described by antibiotic medications such as Aminoglycosides or neuromuscular blocking agents [10], [11] or infections such as varicella or West Nile virus [12], [13].
In general, there are two potential explanations for our patients. First, the SARS-CoV-2, like other viruses, can play a role in activating latent autoimmune diseases such as MG. Second, the antigenic similarity and interaction between the SARS-CoV-2 epitope and the nicotinic acetylcholine receptor at the neuromuscular junction termed molecular mimicry could be responsible for MG induction; however, such associations are far to be demonstrated, and requires further complementary evidence [14].
4. Conclusion
We described three patients with MG who began to manifest shortly after the COVID-19 infection. It is not easy to find definite cause-and-effect relationships between MG manifestation and COVID-19 infection; however, latent MG activation by the virus and molecular mimicry may play a role in the initiation of MG.
Acknowledgment
The authors request to thank all the patients for their consent to publish the case report.
Disclosure statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Gilhus N.E., Owe J.F., Hoff J.M., Romi F., Skeie G.O., Aarli J.A. Myasthenia Gravis: a review of available treatment approaches. Autoimmune Dis. 2011;2011 doi: 10.4061/2011/847393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melzer N., Ruck T., Fuhr P., Gold R., Hohlfeld R., Marx A., Melms A., Tackenberg B., Schalke B., Schneider-Gold C., Zimprich F., Meuth S.G., Wiendl H. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J. Neurol. 2016;263:1473–1494. doi: 10.1007/s00415-016-8045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of Coronavirus Disease 2019 (COVID-19) J. Gen. Intern. Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okhovat A.A., Ansari B., Hemasian H., Haghi-Ashtiani B., Advani S., Ziaadini B., Abdi S., Sikaroudi H., Nafissi S., Fatehi F. Guillain-Barre syndrome in patients with coronavirus disease-2019: report of six cases and review of literature. Curr. J. Neurol. 2020;19:122–130. doi: 10.18502/cjn.v19i3.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdi S., Ghorbani A., Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J. Neurol. Sci. 2020;416 doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sriwastava S., Tandon M., Kataria S., Daimee M., Sultan S. New onset of ocular myasthenia gravis in a patient with COVID-19: a novel case report and literature review. J. Neurol. 2021;268:2690–2696. doi: 10.1007/s00415-020-10263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber M., Rogozinski S., Puppe W., Framme C., Höglinger G., Hufendiek K., Wegner F. Postinfectious onset of Myasthenia Gravis in a COVID-19 patient. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.576153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Restivo D.A., Centonze D., Alesina A., Marchese-Ragona R. Myasthenia Gravis associated with SARS-CoV-2 infection. Ann. Intern. Med. 2020;173:1027–1028. doi: 10.7326/L20-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunsire M.F., Clarke S.G., Stedmon J.J. Undiagnosed myasthenia gravis unmasked by neuromuscular blockade. Br. J. Anaesth. 2001;86:727–730. doi: 10.1093/bja/86.5.727. [DOI] [PubMed] [Google Scholar]
- 11.Hussain N., Hussain F., Haque D., Chittivelu S. A diagnosis of late-onset Myasthenia gravis unmasked by topical antibiotics. J. Community Hosp. Intern. Med. Perspect. 2018;8:230–232. doi: 10.1080/20009666.2018.1487245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha A., Batra P., Vilhekar K.Y., Chaturvedi P. Post-varicella myasthenia gravis. Singap. Med. J. 2007;48:e177–e180. [PubMed] [Google Scholar]
- 13.Leis A.A., Szatmary G., Ross M.A., Stokic D.S. West nile virus infection and myasthenia gravis. Muscle Nerve. 2014;49:26–29. doi: 10.1002/mus.23869. [DOI] [PubMed] [Google Scholar]
- 14.Fujinami R.S., von Herrath M.G., Christen U., Whitton J.L. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin. Microbiol. Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]