Abstract
Introduction
Thoracic CT is a useful tool in the early diagnosis of patients with COVID-19. Typical appearances include patchy ground glass shadowing. Thoracic radiotherapy uses daily cone beam CT imaging (CBCT) to check for changes in patient positioning and anatomy prior to treatment through a qualitative assessment of lung appearance by radiographers. Observation of changes related to COVID-19 infection during this process may facilitate earlier testing improving patient management and staff protection.
Methods
A tool was developed to create overview reports for all CBCTs for each patient throughout their treatment. Reports contain coronal maximum intensity projection (MIP's) of all CBCTs and plots of lung density over time. A single therapeutic radiographer undertook a blinded off-line audit that reviewed 150 patient datasets for tool optimisation in which medical notes were compared to image findings. This cohort included 75 patients treated during the pandemic and 75 patients treated between 2014 and 2017. The process was repeated retrospectively on a subset of the 285 thoracic radiotherapy patients treated between January–June 2020 to assess the efficiency of the tool and process.
Results
Three patients in the n = 150 optimisation cohort had confirmed COVID-19 infections during their radiotherapy. Two of these were detected by the reported image assessment process. The third case was not detected on CBCT due to minimal density changes in the visible part of the lungs.
Within the retrospective cohort four patients had confirmed COVID-19 based on RT-PCR tests, three of which were retrospectively detected by the reported process.
Conclusion
The preliminary results indicate that the presence of COVID-19 can be detected on CBCT by therapeutic radiographers.
Implications for practice
This process has now been extended to clinical service with daily assessments of all thoracic CBCTs. Changes noted are referred for oncologist review.
Keywords: Radiotherapy, CBCT, Cone-beam CT, COVID-19 corona virus
Introduction
In December 2019, initial cases of COVID-19 were reported in Wuhan China, and infections rapidly spread internationally.1 On the 30th January 2020 the World Health Organisation (WHO) declared the outbreak as a public health emergency of international concern. Advanced age and co-morbidities have both been linked with poor outcomes of COVID-19.2
Clinical presentation of the infection varies greatly, with early predication indicating up to 80% patients could be asymptomatic, a figure since revised to 17–20%3 , 4 Symptomatic patients experience a range of symptoms including fever, dry cough, and dyspnoea while more severe effects include respiratory failure, multi-organ failure and death.5 Patients who are elderly or have underlying co-morbidities are disproportionally affected by COVID-19 and at increased risk of adverse outcomes.6 , 7 Cancer patients are particularly vulnerable with data indicating a higher risk of intensive care unit (ICU) admissions, ventilator requirements and death compared with the general population.2 , 8, 9, 10 Fatality rates ranging from 4 to 11% have been reported for patients of an advanced age or with comorbidities. These factors are associated with higher cancer incidence and could explain the link between COVID-19 and cancer.10 , 12 , 14
Chest computed tomography (CT) can be a useful tool for early diagnosis of COVID-19 with the majority of patients displaying similar CT findings.11 The most common observations on CT are ground-glass opacification (GGO's), air bronchograms, crazy-paving patterns, and thickening of the adjacent pleura.12 The disease distribution in the initial chest CT is typically confined to the middle and lower lobes of the lung and is pre-dominantly peripheral. As disease advances, follow up CT shows consolidation and coalescing infiltrates as the central and upper lungs become affected.13
Cancer patients receiving radiotherapy need to attend daily for treatment without any delays, interruptions or premature termination to avoid suboptimal oncologic outcomes.15, 16, 17 Daily hospital visits can increase the risk COVID-19 exposure, especially due to the risk of spread from asymptomatic patients.14
Cone beam computed tomography (CBCT) is used routinely for image guided radiotherapy (IGRT) to assure positioning and visualisation of any anatomical changes.18 CBCT is a medical imaging technique which forms a 3D representation of the patient.19 Local IGRT protocols specify daily CBCT for all lung and oesophageal patients, facilitating assessment of healthy lung tissue.20 Research demonstrates despite that inferior image quality in CBCT (compared to diagnostic CT) retrospective identification of COVID-19 characteristics is feasible.21 Fifty percent of patients can have radiographic abnormalities before the onset of symptoms; CBCT may therefore allow early clinical detection and treatment of COVID-19. Early diagnosis could improve patient outcomes with timely detection of COVID-19 correlating with improved prognosis11 in addition to improving departmental safety through permitting appropriate management of COVID-19 positive patients. This manuscript reports on the use of radiotherapy CBCTs and a semi-automated process to detect COVID-19 changes in lung anatomy by therapeutic radiographers as a feasibility study for the multicentre CATCH (COVID associated temporal changes) study.
Methods
The work reported was approved through local quality improvement and clinical audit committee (local QICA project 2749) and our local umbrella ethics approval (Ethics REC ref. 17/NW/0060).
Patient data
The project was undertaken in two phases with two patient cohorts. The first, the “optimisation cohort”, retrospectively included a arbitrary convenient sample of 150 patients treated with curative intent radiotherapy and daily online CBCT imaging. Seventy-five patients were treated during the pandemic (i.e., after March 2020). This cohort was used to optimise the semi-automatic reporting tool. The optimisation cohort also included 75 patients treated prior to the pandemic (i.e. between 2014 and 2017) as controls, to test it false positives could be reported. The second cohort, the “evaluation cohort”, retrospectively included all radical lung and oesophagus patients receiving radiotherapy at our institution between January and June 2020 including the 75 from the optimisation cohort.
Thoracic treatment at the authors institute involves daily CBCT's, with treatment fractionation ranging from 3 to 33 fractions.
Semi-automatic reporting tool development
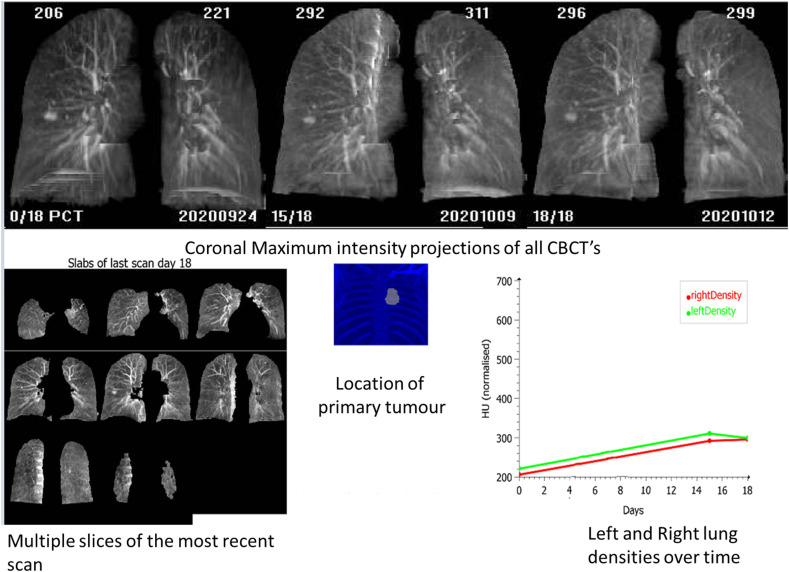
CBCT scans were acquired with Elekta XVI (Elekta Oncology systems, Copenhagen, Sweden), version 5.01 to 5.03. Each daily CBCT was converted into a coronal maximum intensity projection (MIP). MIP rendering is a standard CT image analysis technique that produces a single 2D image that is a projection of the highest attenuating voxels of the image encountered by X-ray beam and approximates a planar radiograph.22 , 17 , 9 We create the MIP images using the standard software libraries from the Elekta XVI system (the MIP operation is available in most common image processing libraries). The tool reads the planning CT (pCT) and all available CBCTs for a patient and sequentially plots the MIP images in date order and generates a visual timeline of lung appearance. For that purpose CBCTs were registered to the pCT using a rectangular region of interest (ROI) drawn to fit tightly around both lungs. The pCT lung contours with a small negative margin were then used to mask the lungs for density analysis and MIP generation in the pCT and all CBCTs (i.e., MIP images were restricted to the lung tissues alone). Pixel values outside the lungs were used for normalisation of CBCT image intensities and all images were presented with a fixed level and window. Density values were also plotted. Fig. 1 shows an example of the tool's output.
Figure 1.
Sample reports generated by the CBCT COVID-19 imaging tool. The tool provides a single report containing sequentially positioned lung MIP images, multiple slices of the last CBCT to show the most up-to-date anatomy in more detail (typically of use when the tool is used for online daily assessment), an image indicating the location of the tumour, and a longitudinal plot of the right and left lung mean density over time. All images are labelled with the day since the planning CT scan. Lung density values (mean in ‘HU’) are also shown in the MIP images. Because of scatter, the apparent mean HU in the CBCTs always is higher than in the planning CT.
Rigid image registration was chosen over non-rigid, because the latter tended to be affected by COVID-19 lesions, driving these higher density regions towards the chest walls and reducing their visibility. The margin used to mask the lungs during the analysis was shrunk by 3 mm as a compromise between sensitivity for small changes occurring in the peripheral lungs and resilience to lung contour changes that could lead to tissues outside the lungs (e.g. the ribs) being mistaken for changes in lung density. Prior to MIP generation, images were blurred in the A-P direction (σ = 1 cm) with a 2 cm triangular kernel to reduce contrast from normal lung structures while maintaining infection contrast. Typically, computation time was less than 1 min per report.
Using the optimisation cohort, a therapeutic radiographer assessed all images and MIP summary reports blindly for potential COVID related changes. This involved reviewing density change graphs for any significant change followed by a review of each MIP for any significant density changes throughout the treatment, with particular focus on changes suspicious for COVID-19. Algorithm settings were optimised during this phase and fixed thereafter.
The observer categorised suspicious density changes following indications identified by previous research, including GGO's distributed primarily in the lower and middle lobes of the lung that progresses superiorly.13 Medical and radiotherapy records were reviewed for COVID diagnosis, known symptoms or documented COVID typical changes and compared to image findings. Records were stored locally in MOSAIQ®, the integrated information system used to collect patient imaging and radiotherapy treatment information.23 Within this initial cohort RT-PCR (Reverse Transcriptase Polymerase Chain Reaction) test was not routinely carried out, therefore asymptomatic patients were not identified and not all symptomatic patients were tested meaning many patients could have been COVID-19 positive without detection.
Semi-automatic reporting tool evaluation
The second phase repeated this process retrospectively on the evaluation cohort to assess the efficiency of the tool and process. Two radiographers independently assessed the reports generated by the tools with the radiotherapy imaging record (Rad1), and evaluated medical notes (Rad2). Rad1 was blinded to patients’ medical notes and COVID status. The results of potential COVID changes on CBCT or reported in the radiotherapy notes were later correlated to the medical notes.
Results
Optimisation cohort
Three of 150 patients had confirmed COVID-19 based on RT-PCR test. Two were diagnosed retrospectively using the reported CBCT assessment tool and process. The other was not detected on CBCT, as there were minimal lung parenchymal changes observed. Fig. 2 shows the report of two of those COVID-19 positive patients.
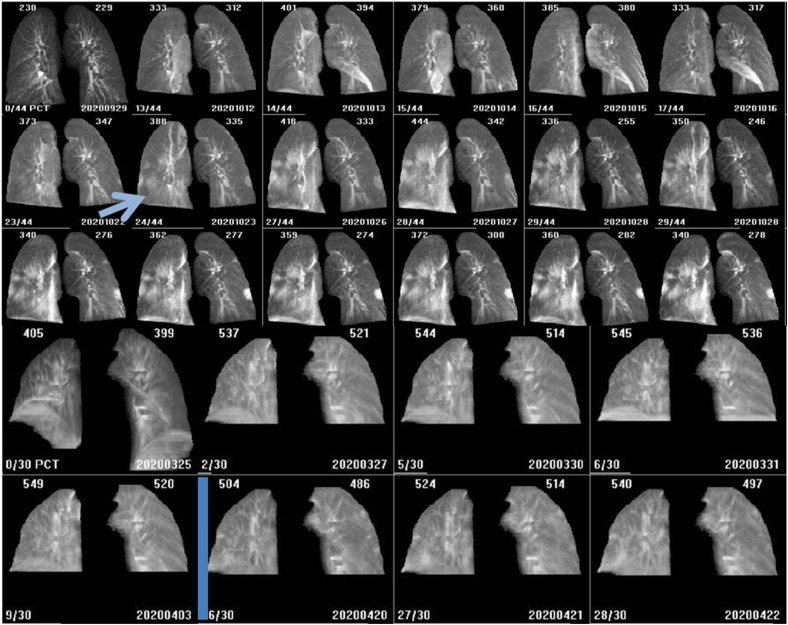
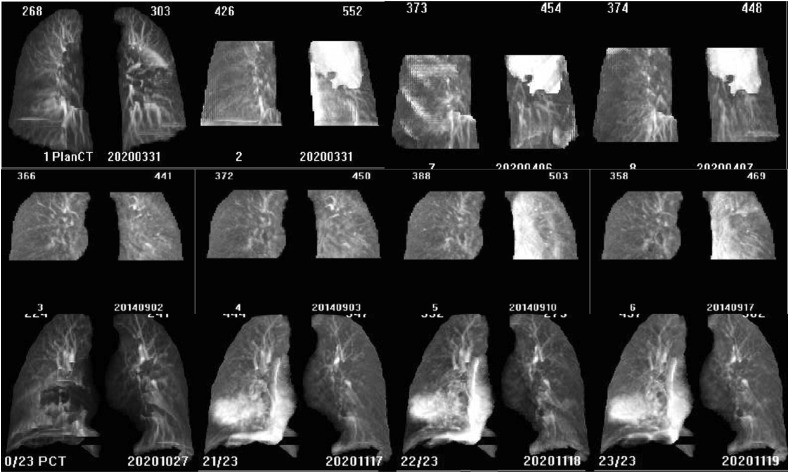
Figure 2.
Examples of COVID-19 positive patients (optimisation cohort). Top – these images show lung density changes identified by the observer at fraction 9 of 25 (arrow). This patient became symptomatic on fraction 11 of 25 with a cough and received a RT-PCR test. The results were established on fraction 12 of 25 as positive. Bottom - CBCT images were acquired in this patient for whom COVID-19 was not detected through CBCT analysis. The changes in lung density over time are minimal. Treatment has paused for this patient following COVID-19 diagnosis until longer symptomatic (for 17 days) in line with Trust COVID response protocols at the time. Density changes may have occurred during the pause in treatment where no CBCTs were made. This is an unavoidable limitation of retrospective evaluation.
The observer identified suspicious lung density changes over the course of radiotherapy on CBCT images from a further 32 patients. Fifteen were treated since the start of the COVID-19 pandemic and 17 were treated prior to 2020. This research confirmed lung density changes during the course of radiotherapy treatment are not specific to COVID-19, occurring in 23 % of patients. Table 1 summarises these findings.
Table 1.
Summary of optimisation cohort.
| Optimisation Cohort | |
|---|---|
| Number of Patients (1–33 images per patient) reviewed | 150 |
| Number of scans determined to need further review | 34 |
| Number of Patients with Confirmed/Suspected COVID | 3 |
| Number of patients with COVID detected by CBCT alone | 2 |
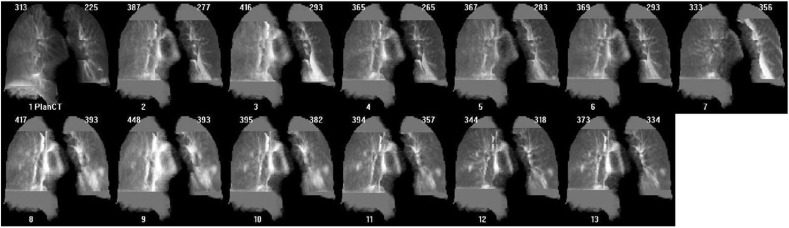
For images reviewed retrospectively by a radiographer, changes were not assessed by a clinician nor were the patients tested for COVID-19 based on these results. One patient's MIPs demonstrated changes to lung density similar to those reported in patients with COVID-19 (Fig. 3 ) but no clinical symptoms were reported within imaging and clinical notes. If seen prospectively this patient would have been referred for further review and RT-PCR COVID-19 test.
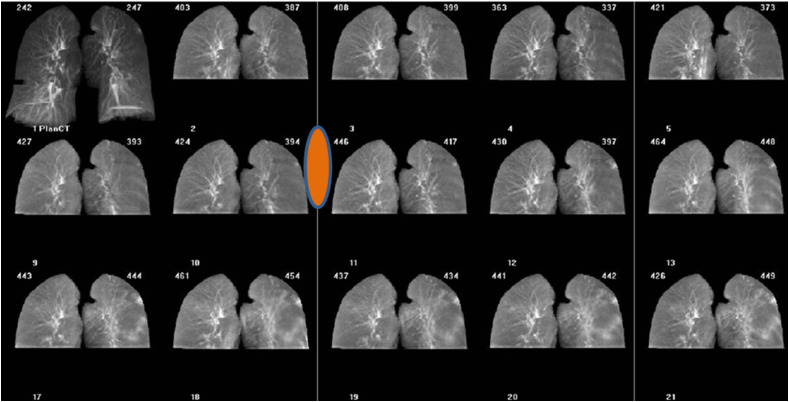
Figure 3.
Example of COVID-19 positive patient report (retrospective cohort). Patient MIP's reviewed retrospectively demonstrating COVID-19 like lung density changes, start of change shown by orange symbol. Patient was asymptomatic and therefore not RT-PCT tested. Part of the observed HU variation demonstrated in the graph is due to differences in imaging systems used, as the patient was moved between treatment units. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Retrospective cohort
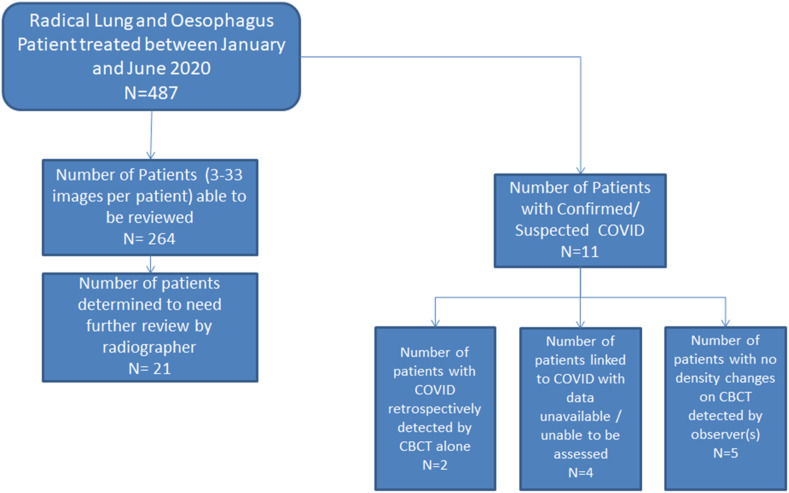
Four of 258 patients reviewed were confirmed to have COVID-19 by RT-PCR test. Three of these patients were retrospectively (and blinded) identified independently using CBCT reports, all prior to the date of clinical detection. The fourth patient had no visible lung density changes on CBCT. There were 7 patients with symptoms suspicious of COVID-19 not confirmed by RT-PCR test, none of whom had visible lung density changes. If reviewed prospectively, 3 of these 11 confirmed/suspected patients would have been escalated for further review based on lung density changes.
Observers identified 23 patients that if seen prospectively would have been highlighted for further evaluation. Unfortunately, none of these patients were tested for COVID-19 given the limited testing capacity at the time. Details are summarised in Fig. 4 .
Figure 4.
Summary of retrospective cohort.
Mean time taken for observers to review all MIPs together per patient was 12 s (range 9–16 s, SD 1.8 s). Beyond the detection of COVID-19, the MIP reports facilitated the observation of other lung density changes including atelectasis and pleural effusion as well as non COVID-19 pneumonia (Fig. 5 ). Fig. 6 shows a patient treated in 2014 with lung CBCT appearance typical of COVID-19 infection, demonstrating that the usefulness of the tool could be extended to aggressive infections outside of COVID-19.
Figure 5.
Example of non-COVID-19 related changes demonstrated on the reports. Top - Atelectasis; Middle - Pleural effusion; Bottom - Pneumonia.
Figure 6.
Example of patient (treated in 2014) with lung density changes comparable to COVID-19 changes.
Discussion
Our tool was designed to facilitate rapid review of thoracic CBCT by therapeutic radiographers to detect COVID-19 infections. This tool allows radiographers to asses each CBCT without having to review each image slice, and typically less than 1 min is spent on reviewing a patient.
This work reports the first systematic screening of CBCT scans acquired during RT for COVID-19. The ability to assess COVID-19 and non COVID-19 pneumonia on CT by radiologists has been investigated with promising results for accuracy with 83%, 80% and 60% reported.24 , 25 In a study comparing repeat RT-PCR testing with CT-based diagnosis, 75% of patients with positive CT but negative RT-PCR findings later tested positive on RT-PCR.14 Similar results were found within He et al. (2020)26 study who also identified 5 patients who had characteristic chest CT findings with negative initial PCR which later positive RT-PCR tests. 40% of patients had improvement in CT findings before serial RT-PCR results converting from positive to negative, suggesting RT-PCR results lag behind radiographic findings.27 It does need to be considered that all trials were conducted on a small sample size and in single institutions so generalisation is restricted. Combining the RT-PCR and the use of imaging in the form of CBCT or diagnostic CT may provide more accurate results. Research has shown that a combined approach has improved sensitivity from 79 to 94%.14
This work demonstrates similar assessment is feasible using daily CBCT routinely acquired as part of thoracic radiotherapy standard of care. Within our retrospective cohort of 285 patients, 4 had confirmed COVID-19 identified through RT-PCR testing. Three of these were also identified independently using CBCT imaging data only. The remaining patient may not have been identified due to a two week gap in treatment where no imaging occurred. Another reason could be linked to COVID-19 not always affecting the lungs but other organ systems, meaning no changes within the lungs occurred.30 Seven patients within this cohort with suspected COVID-19 were not tested to confirm this as there was no routine patient testing at the time, however may have been referred onward for confirmation based on CBCT imaging if seen prospectively.
This work was limited by the use of patient screening measures, resulting in a low prevalence of COVID-19 in patients treated during the recruitment period, reducing the statistical confidence in our findings. These results should therefore be considered as proof of principle. We have now implemented the assessment as part of the local daily workflow, enabling continuous, on-going evaluation. A multi-centre study would allow faster accumulation of COVID-19 positive events; the CATCH study is a multicentre study currently being established for this purpose. Another limitation was the retrospective nature of this work, during a period with limited COVID-19 RT-PCR testing capacity. As such it is unknown if asymptomatic patients were missed, and conversely, if the 23 patients identified who would have been highlighted for further investigation were infected or not.
The radiographer assessment team found the use of MIPs in the daily reports, with the images aligned to a common frame of reference useful. The main advantage of using this technique is that it simplifies visualisation regions of highest density change28 , 14 and reducing the volume of data to be reviewed, reducing the review time required per patient. Systematic review of the time required for assessment was beyond the scope of this work.
One disadvantage of MIPs is the loss in spatial resolution and amount of data available,29 which may lead to missing information indicative of COVID-19. However, Jabeen et al. (2019) demonstrated a MIP technique has a high sensitivity and specificity in the thorax, detecting even small pulmonary nodules missed on conventional axial images.29
The observers recognised from the comparison of the overview reports and corresponding image review notes that not all changes observed on the real-time CBCT were observed on the reconstructed MIPs. One example included a patient whose image notes indicated lung density changes in the left upper lobe that was not apparent on the MIP's or the density graphs. This could be due to loss in data as images are projected or the margins and image processing used in the analysis.
The use of a more automated method was proposed to reduce resources, with automation being well established in reducing the rates of medical errors through removing the human involvement within checks and procedures.31 The initial plan for a clinical prospective review was to use the density graphs produced to indicate any density variation. Only the MIP's of patients with density changes demonstrated would be reviewed by radiographers. However, the observers determined that a combined auto-manual approach would be required as graphs were not accurate enough as a single decision point. In practice, treatment radiographers would have to assess all MIP images and use the graphs as more of an aid. Within a prospective clinical assessment, radiographers could also review the CBCT data should any changes appear suspicious and the patient's clinical notes provided a complete picture of the patient's situation.
Fast daily reviews of the evolution of changes in patients’ lung densities, aided by tools similar to those we report, could permit the identification of asymptomatic COVID-19 and other infections, enabling appropriate management steps to be taken. Research has shown that up to 46% of lung cancer patients experience an infection during their radiotherapy,32 with early diagnosis meaning earlier medication, reducing probability of gaps in treatment.
Following this initial retrospective work, prospective reviews have begun by two therapeutic radiographers who access on-treatment lung and oesophagus patients daily. Changes noted by radiographers that are deemed as suspicious are referred for oncologist review.
Conclusion
Lung density changes on CBCT suggestive of COVID-19 can be identified by Therapeutic Radiographers. With the current wave of COVID-19, it is likely that many more cases will be detected shortly. This work is particularly pertinent in clinical set-up where frontline RT-PCR testing for COVID-19 is not available routinely, but may also find applications in the monitoring of lung tissue changes beyond the current pandemic.
Conflict of interest statement
None.
Acknowledgements
The authors acknowledge support from the NIHR Manchester BRC, BRC-1215-20007 and Manchester CRUK RADNET(C147/A18083) and (C147/A25254)
References
- 1.Rothan H.A., Byrareddy S.N. The epidemeology and pathogensis of coronavirus (Covid-19) outbreak. J Autoimmun. 2020;109(January):1–4. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee L., Cazier J., Starkey T., Briggs S., Arnold R., Bisht V., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:309–316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heneghan C., Brassey J., Jefferson T. COVID-19: what proportion are asymptomatic? CEBM. 2020. https://www.cebm.net/covid-19/covid-19-what-proportion-are-asymptomatic/ Published.
- 4.Byambasuren O., Cardona M., Bell K., Clark J., McLaws M.L., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. J Association of Medical Microbiology and Infectious Disease Canada. 2020;5(4):223–234. doi: 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhikari S.P., Meng S., Wu Y., Mao Y., Ye R., Wang Q., et al. A scoping review of 2019 Novel Coronavirus during the early outbreak period: Epidemiology, causes, clinical manifestation and diagnosis, prevention and control. Infect Dis Poverty. 2020:1–12. doi: 10.21203/rs.2.24474/v1. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhal T. Review on COVID19 disease so far. Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:1–2. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., Zhu F., Wang C., Wang J., Chen R., Jia P., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan , China. European Society for Medical Oncology. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. 2020;(January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Y., Jin R., Zhao J., Li W., Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21(4) doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabarriti R., Brodin P., Maron M., Tome W., Halmos B., Guhan C., et al. Extent of prior lung irradiation and mortality in COVID-19 Patients with a cancer history. advances in radiation oncology. 2020;5:707–710. doi: 10.1016/j.adro.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang G., Gong T., Wang G., Wang J., Guo X., Cai E., et al. Timely diagnosis and treatment shortens the time to resolution of coronavirus disease (COVID-19) pneumonia and lowers the highest and last CT scores from sequential chest CT. Cardiopulm Imaging. 2020;215:367–373. doi: 10.2214/AJR.20.23078. [DOI] [PubMed] [Google Scholar]
- 12.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. Am J Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 14.Youssef I., Donahue B., Flyer M., Thompson S., Huang A., Gallant F. Covert COVID-19: cone beam computed tomography lung changes in an asymptomatic patient receiving radiation therapy. Advances in Radiation Oncology. 2020;5:715–721. doi: 10.1016/j.adro.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bese N.S., Sut P.A., Ahmet O. The effect of treatment interruptions in the postoperative irradiation of breast cancer. Oncology. 2005;69(3):214–223. doi: 10.1159/000087909. https://pubmed.ncbi.nlm.nih.gov/16127290/ [DOI] [PubMed] [Google Scholar]
- 16.Chen A.M., Yoshizaki T., Hus S., Mikaeilian A., Cao M. Image-guided adaptive radiotherapy improves acute toxicity during intensity-modulated radiation therapy for head and neck cancer. J Radiat Oncol. 2018;7(2):139–145. doi: 10.1007/s13566-017-0336-1. [DOI] [Google Scholar]
- 17.González Ferreira J.A., Jaén Olasolo J., Azinovic I., Jeremic B. Effect of radiotherapy delay in overall treatment time on local control and survival in head and neck cancer: review of the literature. Rep Practical Oncol Radiother. 2015;20(5):328–339. doi: 10.1016/j.rpor.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan H., Zhen X., Cerviño L., Jiang S.B., Jia X. Progressive cone beam CT dose control in image-guided radiation therapy. Med Phys. 2013;40(6):1–7. doi: 10.1118/1.4804215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scarfe W., Farman A. What is cone-beam CT and how does it work? Dent Clin. 2008;52(4):707–730. doi: 10.1016/j.cden.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Duffton A., Harrow S., Lamb C., McJury M. An assessment of cone beam CT in the adaptive radiotherapy planning process for non-small-cell lung cancer patients. Br J Radiol. 2016;89(1062) doi: 10.1259/bjr.20150492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suppli M.H., Riisgaard de Blanck S., Elgaard T., Josipovic M., Pøhl M. Early appearance of coronavirus disease 2019 associated pulmonary infiltrates during daily radiotherapy imaging for lung cancer. J Thorac Oncol. 2020;15(6):1081–1084. doi: 10.1016/j.jtho.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawel N., Seifert B., Luetolf M., Boehm T. Effect of slab thickness on the CT detection of pulmonary nodules: use of sliding thin-slab maximum intensity projection and volume rendering. Am J Roentgenol. 2009;192(5):1325–1329. doi: 10.2214/AJR.08.1689. [DOI] [PubMed] [Google Scholar]
- 23.Elekta MOSAIQ: radioation onology system. 2018. https://www.elekta.com/dam/jcr:d79cff58-91ce-4d4c-b27d-472dc38da371/MOSAIQ-RO-brochure.pdf Available at:
- 24.Inui S., Fujikawa A., Jitsue M., Kunishima N., Watanabe S., Suzuki Y., et al. Chest CT findings in cases from the cruise ship “diamond princess” with coronavirus disease 2019 (COVID-19) Radiology: Cariothoracic Imaging. 2020;2(2):1–7. doi: 10.1148/ryct.2020204002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai Y., Yao L., Wei T., Tian F., Jin D., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. J Am Med Assoc. 2020;323(13):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J., Luo L., Luo Z., Lyu J., Ng M., Shen X. Diagnostic performance between CT andinitial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir Med. 2020;168:105980. doi: 10.1016/j.rmed.2020.105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):32–40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilburn-Toppin F., Arthurs O., Tasker A., Set P. Detection of pulmonary nodules at paediatric CT: maximum intensity projections and axial source images are complementary. Pediatr Radiol. 2013;43:820–826. doi: 10.1007/s00247-012-2597-6. [DOI] [PubMed] [Google Scholar]
- 29.Jabeen N., Qureshi R., Sattar A., Baloch M. Diagnostic accuracy of maximum intensity projection in diagnosis of malignant pulmonary nodules. Cureus. 2019;11(11) doi: 10.7759/cureus.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg S., Garg M., Pranhakar N., Malhotra P., Agarwal R. Unraveling the mystery of Covid-19 cytokine storm: from skin to organ systems. Dermatol Ther. 2020;33(6) doi: 10.1111/dth.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aron R., Dutta S., Janakiraman R., Pathak P. The impact of automation of systems on medical errors: evidence from field research. Inf Syst Res. 2011;22(3):429–446. [Google Scholar]
- 32.Sarihan S., Ercan I., Saran A., Cetintas S.K., Akalin H., Engin K. Evaluation of infections in non-small cell lung cancer patients treated with radiotherapy. Canc Detect Prev. 2005;29(2):181–188. doi: 10.1016/j.cdp.2004.11.001. [DOI] [PubMed] [Google Scholar]