Abstract
Pulmonary sarcomatoid carcinoma (PSC) is a rare subtype of non‐small cell lung cancer (NSCLC) with an extremely poor prognosis making it a therapeutic challenge. However, the development of genetic variation molecular diagnosis and targeted agents has brought the treatment of such malignancies to the precision era. Co‐existing mutations of EGFR and MET have been reported in NSCLC, but rarely found in PSC. We herein present a rare case of a 74‐year‐old female patient diagnosed with PSC, carrying an activating mutation in exon 21 L858R of EGFR and a concurrent MET amplification prior to treatment. Combined application of gefitinib and crizotinib, inhibitors targeting EGFR and MET, respectively, was prescribed. The patient experienced a partial response and was stable for 9.7 months off therapy. The observation stresses the importance of genetic testing and paves the way for combined targeted strategies in PSC.
Keywords: crizotinib/gefitinib, EGFR mutation, MET amplification, non‐small cell lung cancer, Pulmonary sarcomatoid carcinoma
PSC patients harboring both EGFR mutation and MET amplification may benefit from combined EGFR and MET inhibitors. The combination of these two drugs in PSC is rarely reported some more large‐scale clinical trials are required to confirm this result.
1. INTRODUCTION
Pulmonary sarcomatoid carcinoma (PSC), a rare subtype of non‐small cell lung cancer (NSCLC) characterized by poor differentiated, highly invasive, and early systemic metastases, accounts for only 0.4% of all pulmonary malignancies. The overall prognosis is worse than other subtypes of NSCLC. 1 The rapid development of molecular detection and deep understanding of tumor (U.S. vs. U.K spelling) biology highlight the important role of targeted drugs in NSCLC. Some patients with NSCLC harboring specific driver mutations could benefit from targeted therapies. 2 , 3 Until now, due to its rarity, high aggressiveness, and difficulty in diagnosis, no large‐scale prospective studies of PSC are available. Thus, the efficacy of some targeted options in the treatment of PSC is not clear. We report here a novel PSC case, with mutation of EGFR exon 21 L858R and MET amplification, that benefited from combined target therapies with gefitinib and crizotinib.
2. CASE PRESENTATION
A 74‐year‐old female patient was admitted to hospital in June 4, 2017, because of severe dyspnea who required high‐flow oxygen to maintain normal blood oxygen saturation. In addition, the patient presented with cough, sputum, an evident swollen face, and decreased respirations bilaterally. Laboratory tests revealed high leucocyte counts up to 18.23 × 109/L and hypoalbuminemia. A PET/CT (Figure 1) scan showed a mass in the right upper lung lobe measuring 3.0 × 1.6 cm and several uncertain small pulmonary nodules. The mediastinal window displayed bilateral pleural effusions, a pericardial effusion, and bilateral enlargement mediastinal lymph nodes. No abnormal hypermetabolic activity was found in her abdomen, bones, or brain. Her clinical stage was T1N3M1. A thoracentesis was performed under ultrasound guidance in order to alleviate her dyspnea. Subsequently, a core needle biopsy was carried out on her left supraclavicular lymph node. Immunohistochemistry (IHC) analysis showed diffuse positivity for cytokeratin and vimentin consistent with a diagnosis of sarcomatoid cancer of lung origin (Figure 2). Genetic testing using DNA‐based next‐generation sequencing (NGS) was also performed and indicated the co‐existence of an EGFR (exon 21 L858R) mutation and MET amplification. Given her advanced age, multiple metastases in mediastinal lymph nodes, and poor performance status (ECOG > 2), she was not a suitable candidate for chemotherapy. The genetic testing results, concurrent EGFR and MET mutations, led us to use a combined targeted strategy with gefitinib 250 mg orally once daily and crizotinib administered 250 mg twice daily, inhibitors targeting EGFR and MET, respectively. After two months of treatment, most enlarged mediastinal lymph nodes disappeared or reduced significantly, the pericardial effusion vanished, and pleural effusion remained stable. A follow‐up CT (Figure 3) scan eight months later showed the right upper lung lobe mass was shrunken and pleural effusion observably reduced consistent with a good partial response. The patient has maintained a lasting and ongoing partial response for 9.7 months off therapy without evident clinical pulmonary symptoms.
FIGURE 1.
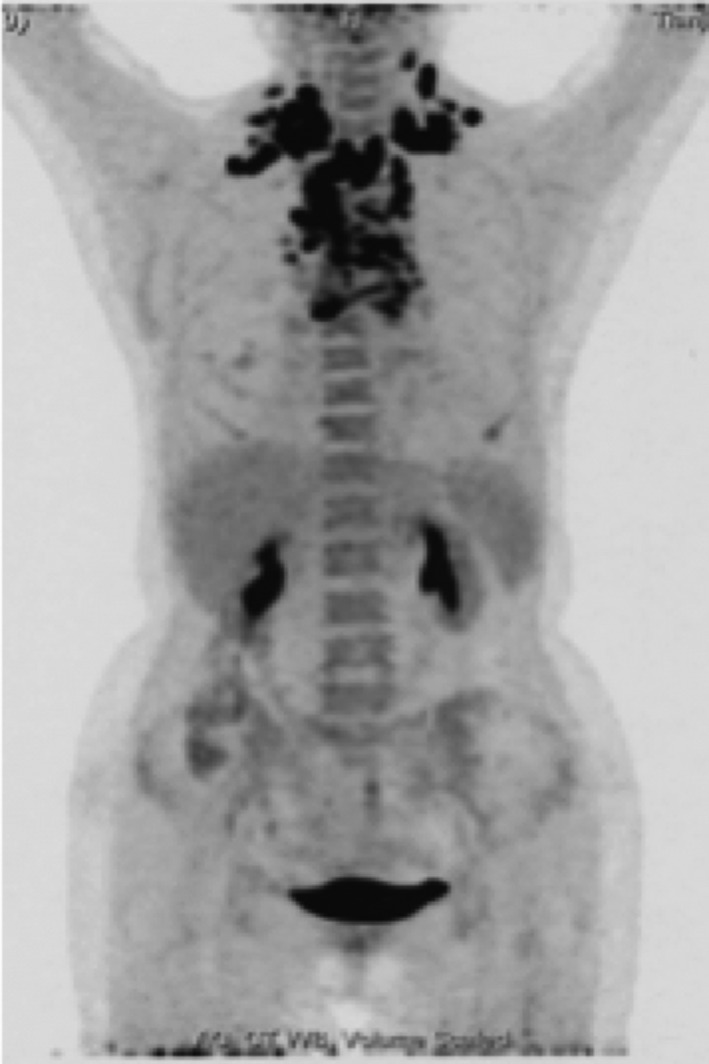
PET/CT scan (2017.06) of a 74‐year‐old female patient showed high metabolic nodules in her right upper lobe. Multiple lymph nodes are seen in bilaterally in the neck, supraclavicular region, mediastinum, and right upper lung
FIGURE 2.
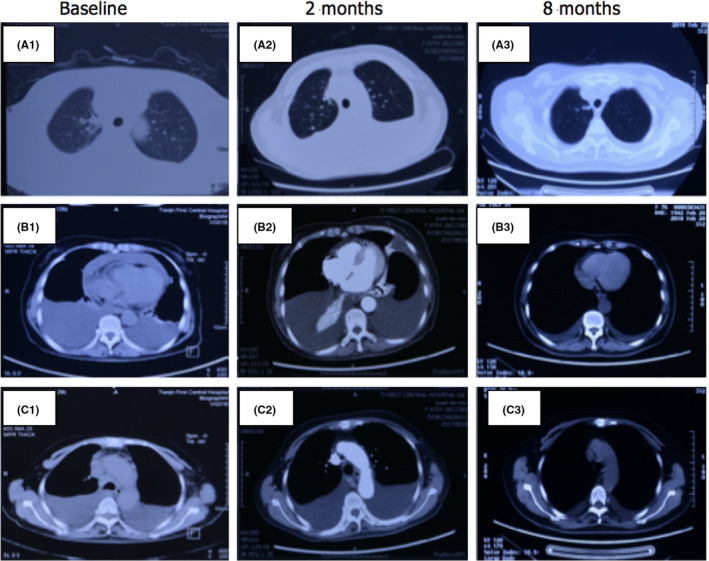
CT scan comparison between prior treatment (A1, B1, C1) and response to gefitinib and crizotinib combined therapy 2 months later(A2, B2, C2) and 8 months later(A3, B3, C3). The mass in the right upper lung lobe shrunk, most enlarged mediastinal lymph nodes disappeared or reduced significantly, the pericardial effusion vanished, and the pleural effusion reduced observably
FIGURE 3.
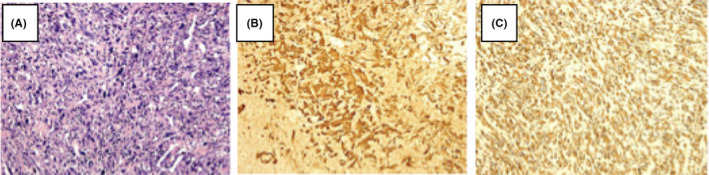
Histopathological observation (HE, 200× magnification) of a biopsy from the left supraclavicular lymph node of a 74‐year‐old female patient showing diffuse distribution of large and highly pleomorphic tumor cells with marked atypia, and many neutrophils infiltrating in interstitial tissue (A); Immunohistochemical stains(IHC, 200× magnification) of the same tissue showing diffuse positivity of cytokeratin (B) and vimentin (C)
3. DISCUSSION
We describe a case of a patient with sarcomatoid carcinoma of pulmonary origin, which harbored concomitant mutations in EGFR and MET. The diagnosis of PSC mainly depends on the morphology and immunohistochemical staining under electron microscopy. Immunohistochemistry (IHC) analysis must show positivity of cytokeratin and is frequently positive in CK18, CK7, AE1/3, and CAM 5.2. Moreover, vimentin and CEA are also positive. 4 IHC analysis on the patient's metastatic lymph node showed diffuse positivity of cytokeratin and vimentin, consistent with the diagnosis of PSC. PSC is known to have an extremely poor prognosis. Vieira et al. showed that PSC is typically resistant to conventional first‐line chemotherapy, with a median progression‐free survival (PFS) only 2.0 months. 5 Thus, an active search for new therapeutic options to improve the prognosis of such tumors is urgently needed. Patients with NSCLC and specific driver mutations, such as EGFR‐ or ALK‐mutated may get remarkable benefit from targeted therapies. 2 , 3 Updated guidelines suggest tissue detection of PSC patients with potential genetic abnormalities to guide individualized precision therapy with targeted drugs. 6
Because of the limited number of studies, the frequency of EGFR aberrations in PSC remains unknown. Italiano et al. 7 found no EGFR mutations in 22 PSC patients, while Leone et al. 8 found 2 EGFR exon 19 deletions f 22 patients with PSC(When I looked at Leone's paper they actually reported on 23 patients—their initial patient and 22 additional patients. Look at Table 1). What's more, the effectiveness of EGFR‐targeted therapies on PSC is unclear. Atsuhito Ushiki et al. 9 described a case of PSC with deletion of EGFR exon 19 treated with gefitinib with a poor response, partly attributed to primary or acquired resistance. On the contrary, Zou Fangwen et al. 10 reported a patient with PSC and an EGFR exon 21 mutation who benefited from erlotinib treatment. The potential role of EGFR‐TKIs in the treatment of PSC thus still requires much larger sample studies.
TABLE 1.
Histopathologic and molecular data
| Case no. | Age/Gender | Location | Size (cm) | Histotype | EGFR mutation | EFGR FISH | K‐RAS |
|---|---|---|---|---|---|---|---|
| 1 | 70/M | LLL | 3.1 | Scc | wt | Amp | wt |
| 2 | 54/M | LUL | 8 | SCC | wt | Pol | wt |
| ADC component | wt | Pol | wt | ||||
| 3 | 78/M | LLL | 4.5 | SCC | wt | Pol | wt |
| 4 | 56/M | L Lung | 8 | SCC | wt | Pol | Cod 12 TGT |
| ADC component | wt | Pol | wt | ||||
| 5 | 44/F | LUL | 5 | SCC | wt | Neg | wt |
| 6 | 59/M | LLL | 3 | SCC | wt | Neg | Cod 12 GAT |
| ADC component | wt | Neg | Cod 12 GAT | ||||
| 7 | 64/M | Carena | 2 | SCC | wt | Neg | wt |
| 8 | 62/M | R Lung | 2.5 | SCC | wt | Neg | wt |
| 9 | 81/F | R Lung | 7 | SCC | wt | Neg | wt |
| 10 | 81/F | RUL | 4.5 | SCC | Exon 19 del | Pol | wt |
| ADC component | Exon 19 del | Pol | wt | ||||
| 11 | 72/M | RLL | 3.5 | SCC | wt | Pol | wt |
| ADC component | wt | Pol | wt | ||||
| 12 | 57/F | R Lung | 2.7 + 5.5 | SCC | wt | Neg | wt |
| 13 | 69/F | RUL | 8 | SCC | wt | Neg | wt |
| 14 | 52/M | RUL | 3 | SCC | wt | Neg | wt |
| 15 | 45/F | ML | Bx | SCC | wt | Neg | wt |
| 16 | 53/F | RUL | 3.9 | SCC | wt | Pol | wt |
| 17 | 72/M | LLL | 4.2 | SCC | Exon 19 del | Neg | wt |
| ADC component | Exon 19 del | Neg | wt | ||||
| 18 | 76/M | RUL | 6 | SCC | wt | Neg | wt |
| 19 | 79/M | RUL | 4.5 | SCC | wt | Amp | wt |
| 20 | 77/M | LLL | 8.5 | SCC | wt | Neg | Cod 12 TGT |
| 21 | 80/M | LUL | 8 | SCC | wt | Pol | wt |
| 22 | 70/M | RUL | 4.5 | SCC | wt | Pol | wt |
| 23 | 84/F | R Lung | Bx | SCC | wt | Neg | wt |
Abbreviations: ADC, adenocarcinoma; AMP, amplified; and Bx, bronchial biopsy; LL, left lung; LLL, left lower lobe; LUL, left upper lobe; ML, middle lobe; Neg, negative; Pol, polysomy; RL, right lung; RLL, right lower lobe; RUL, right upper lobe; SCC, sarcomatoid carcinoma; wt, wild‐type.
MET mutation is a relatively common phenomenon in PSC. Numerous studies have found a higher incidence (approximately 20%–30%) of MET exon 14 skipping mutation than in lung adenocarcinomas. 11 , 12 Crizotinib, a potent MET inhibitor, has shown efficacy in lung adenocarcinomas with MET exon 14 splicing alterations. 13 This was further confirmed in PSC. 12 In contrast, studies on MET amplification in PSC are limited. Preliminary reports of MET‐amplified adenocarcinomas treated with crizotinib showed partial responses in 4 of 12 patients. Moreover, the high copy number category (MET/CEP7 ratio ≥ 5) by fluorescence in situ hybridization (FISH) could produce a better response. 14
Molecular analysis indicates that the MET pathway intersects with EGFR signaling pathways. One study found a high level MET copy number was a negative prognostic factor for NSCLC patients. 15 Furthermore, MET amplification is a confirmed mechanism of acquired resistance to first‐generation EGFR‐TKIs. 16 In EGFR‐TKIs‐resistant NSCLC, about 5%–25% patients were found to have MET amplification. 17 Professor Wu Yilong 18 proposed that, if EGFR‐TKIs‐resistance is due to MET amplification, the EGFR pathway should still remain sensitive, and in order to overcome resistance, the MET inhibitor should be combined with the original EGFR‐TKIs instead exchanging the agents. Theoretically, concurrent application of EGFR‐TKIs and MET inhibitors could overcome the resistance. In preclinical HCC827ER cell line model, 19 MET amplification was discovered after continued exposure to erlotinib. The combination use of erlotinib and E7050 (small molecule MET inhibitor) markedly inhibited ErbB3 phosphorylation and suppressed downstream signaling pathway compared with erlotinib or E7050 alone. These in vitro data indicate that concurrent EGFR/MET inhibitors may enhance erlotinib sensitivity and increase synergistic anti‐tumor activity. Scagliotti GV et al. 20 found Erlotinib plus tivantinib (a MET receptor inhibitor) produced a dramatic response compared to erlotinib monotherapy in EGFR‐mutated NSCLC.
After failure of previous EGFR‐TKIs, osimertinib is given to control the disease while MET amplification can occur with or without loss of the T790M mutation. MET gene amplification is the main cause of bypass pathway activation as resistance mechanism to EGFR‐TKIs. Several studies have demonstrated that the use of crizotinib with osimertinib has the potential to overcome resistance in osimertinib‐resistant EGFR‐mutant NSCLC cell lines with MET gene amplification. As a result, the combination of crizotinib and osimertinib could be an effective therapeutic strategy in MET amplification at the time of acquired resistance to osimertinib.
The histopathologic profile of our case is in accordance with that of PSC. Molecular testing proved deletion in exon 21 of EGFR and a concurrent MET amplification, which has not been reported in PSC before. The patient's pathological type also was consistent with sarcomatoid carcinoma, rather than adenocarcinoma. While the role of MET amplification in primary resistance of EGFR‐TKIs remains unclear, and the efficacy of EGFR‐TKIs in PSC is not yet certain, 9 the patient was in poor condition so given that multiple clinical trials confirming the feasibility and safety of this combination therapy, 19 , 20 , 21 concurrent treatment with gefitinib and crizotinib was given. The results were marked improvements in imaging findings, performance status, and a prolonged partial response for 9.7 months. The use of combination therapy of two targeted drugs to overcome drug resistance is a very new idea. Our case highlights the importance of comprehensive genetic testing for a better understanding of drug resistance and selection of appropriate targeted options.
Moreover, there are side effects of the treatment, because both of the drugs will cause different type of adverse effects. For crizotinib, the most common adverse reactions are vision disorder, nausea, diarrhea, vomiting, edema, and constipation. Vision disorders including visual impairment, photopsia, vision blurred, and vitreous floaters were reported in clinical trials. For gefitinib, drug‐related adverse events with an incidence of diarrhea, rash, acne, dry skin, nausea, and vomiting, the higher dose showed a higher rate for most of these adverse events.
4. CONCLUSION
PSC has an extremely poor prognosis and limited therapies. With the development of the individualized treatment concept, the identification of existing mutations by genetic testing has paved the way for multiple targeted combined therapies. According to our report, PSC patients harboring both EGFR mutation and MET amplification may benefit from combined EGFR and MET inhibitors. The combination of these two drugs in PSC is rarely reported some more large‐scale clinical trials are required to confirm this result.
AUTHOR CONTRIBUTIONS
Xiaomeng Wang collected the data and wrote the initial draft. Jie Cao and Weijiao Du visualized or presented the data. Weihong Zhang involved in data curation. Shui Cao involved in critical review, commentary or revision—including pre‐ or post‐publication stages.
CONSENT STATEMENT
We declared that there was no interest conflict on our manuscript. The patient has provided informed consent for publication of the case. Data are available on request from the authors.
Wang X, Cao J, Du W, Zhang W, Cao S. Response to gefitinib/crizotinib combination in a pulmonary sarcomatoid carcinoma patient harboring concurrent EGFR mutation and MET amplification. Clin Case Rep. 2021;9:e04487. 10.1002/ccr3.4487
First author: Xiaomeng Wang
Co‐first author: Jie Cao
REFERENCES
- 1. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. World J Surg Oncol. 2013;11:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scagliotti G, Stahel RA, Rosell R, Thatcher N, Soria JC. ALK translocation and crizotinib in non‐small cell lung cancer: an evolving paradigm in oncology drug development. Eur J Cancer. 2012;48(7):961‐973. [DOI] [PubMed] [Google Scholar]
- 3. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121‐128. [DOI] [PubMed] [Google Scholar]
- 4. Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134(1):49‐54. [DOI] [PubMed] [Google Scholar]
- 5. Vieira T, Girard N, Ung M, et al. Efficacy of first‐line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol. 2013;8(12):1574‐1577. [DOI] [PubMed] [Google Scholar]
- 6. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243‐1260. [DOI] [PubMed] [Google Scholar]
- 7. Italiano A, Cortot AB, Ilie M, et al. EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: implications for anti‐EGFR treatment of a rare lung malignancy. Int J Cancer. 2009;125(10):2479‐2482. [DOI] [PubMed] [Google Scholar]
- 8. Leone A, Graziano P, Gasbarra R, et al. Identification of EGFR mutations in lung sarcomatoid carcinoma. Int J Cancer. 2011;128(3):732‐735. Author reply 736. [DOI] [PubMed] [Google Scholar]
- 9. Ushiki A, Koizumi T, Kobayashi N, et al. Genetic heterogeneity of EGFR mutation in pleomorphic carcinoma of the lung: response to gefitinib and clinical outcome. Jpn J Clin Oncol. 2009;39(4):267‐270. [DOI] [PubMed] [Google Scholar]
- 10. Zou F, Xie G, Ma JA, Zhou DA, Jiang YI, Zheng JY. Epidermal growth factor receptor mutation heterogeneity analysis of pulmonary sarcomatoid carcinoma successfully treated with erlotinib: a case report. Oncol Lett. 2015;9(5):2239‐2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tong JH, Yeung SF, Chan AW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non‐small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048‐3056. [DOI] [PubMed] [Google Scholar]
- 12. Liu X, Jia Y, Stoopler MB, et al. Next‐generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol. 2016;34(8):794‐802. [DOI] [PubMed] [Google Scholar]
- 13. Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015;5(8):842‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Camidge DR, Ou SH, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c‐MET‐amplified non‐small cell lung cancer (NSCLC). J Clin Oncol. 2014;32(suppl):9070. [Google Scholar]
- 15. Park S, Choi YL, Sung CO, et al. High MET copy number and MET overexpression: poor outcome in non‐small cell lung cancer patients. Histol Histopathol. 2012;27(2):197‐207. [DOI] [PubMed] [Google Scholar]
- 16. Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039‐1043. [DOI] [PubMed] [Google Scholar]
- 17. Gelsomino F, Facchinetti F, Haspinger ER, et al. Targeting the MET gene for the treatment of non‐small‐cell lung cancer. Crit Rev Oncol Hematol. 2014;89(2):284‐299. [DOI] [PubMed] [Google Scholar]
- 18. Wu YL, Soo RA, Locatelli G, Stammberger U, Scagliotti G, Park K. Does c‐MET remain a rational target for therapy in patients with EGFR TKI‐resistant non‐small cell lung cancer. Cancer Treat Rev. 2017;61:70‐81. [DOI] [PubMed] [Google Scholar]
- 19. Nakagawa T, Takeuchi S, Yamada T, et al. Combined therapy with mutant‐selective EGFR inhibitor and MET kinase inhibitor for overcoming erlotinib resistance in EGFR‐mutant lung cancer. Mol Cancer Ther. 2012;11(10):2149‐2157. [DOI] [PubMed] [Google Scholar]
- 20. Scagliotti GV, Shuster D, Orlov S, et al. Brief Report: tivantinib in combination with erlotinib versus erlotinib alone for EGFR mutant NSCLC: an exploratory analysis of the phase 3 MARQUEE study. J Thorac Oncol. 2018;13(6):849‐854. [DOI] [PubMed] [Google Scholar]
- 21. Ou S‐H, Govindan R, Eaton KD, et al. Phase I results from a study of crizotinib in combination with erlotinib in patients with advanced nonsquamous non‐small cell lung cancer. J Thorac Oncol. 2017;12(1):145‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]


