Abstract
Background
Dialysis and kidney transplant patients are vulnerable populations for COVID-19 related disease and mortality.
Methods
We conducted a prospective study exploring the eight week time course of specific cellular (interferon-γ release assay and flow cytometry) or/and humoral immune responses (ELISA) to SARS-CoV-2 boost vaccination in more than 3100 participants including medical personnel, dialysis patients and kidney transplant recipients using mRNA vaccines BNT162b2 or mRNA-1273.
Results
SARS-CoV-2-vaccination induced seroconversion efficacy in dialysis patients was similar to medical personnel (> 95%), but markedly impaired in kidney transplant recipients (42%). T-cellular immunity largely mimicked humoral results. Major risk factors of seroconversion failure were immunosuppressive drug number and type (belatacept, MMF-MPA, calcineurin-inhibitors) as well as vaccine type (BNT162b2 mRNA). Seroconversion rates induced by mRNA-1273 compared to BNT162b2 vaccine were 97% to 88% (p < 0.001) in dialysis and 49% to 26% in transplant patients, respectively. Specific IgG directed against the new binding domain of the spike protein (RDB) were significantly higher in dialysis patients vaccinated by mRNA-1273 (95%) compared to BNT162b2 (85%, p < 0.001). Vaccination appeared safe and highly effective demonstrating an almost complete lack of symptomatic COVID-19 disease after boost vaccination as well as ceased disease incidences during third pandemic wave in dialysis patients.
Conclusion
Dialysis patients exhibit a remarkably high seroconversion rate of 95% after boost vaccination, while humoral response is impaired in the majority of transplant recipients. Immunosuppressive drug number and type as well as vaccine type (BNT162b2) are major determinants of seroconversion failure in both dialysis and transplant patients suggesting immune monitoring and adaption of vaccination protocols.
Key words: epidemiology, COVID-19, SARS-CoV-2 vaccination, mRNA-1273, BNT162b2, tozinameran, dialysis patients, kidney transplant recipients, medical personnel, humoral and cellular immune response, clinical decision-making, guidelines
Research in context.
Evidence before this study
Dialysis patients and kidney transplant recipients experience a high percentage of complicated COVID-19 disease courses with markedly increased mortality compared to normal population. To date, SARS-CoV-2 vaccines such as BNT162b2 mRNA (Pfizer/BioNTech) or mRNA-1273 (Moderna) have undergone clinical testing in the general population only, with COVID-related protection rates up to 95% after two vaccinations. While few and mostly incomplete SARS-CoV-2 specific vaccination data exist for dialysis patients or kidney transplant recipients so far, we and others demonstrated reduced vaccination success rates for other vaccines in either vulnerable group compared to the general population. However, there are no systematic data on evolution of humoral and cellular response either in dialysis or in kidney transplant cohorts. To assess the availability of the data, results from database source National Library of Medicine (https://pubmed.ncbi.nlm.nih.gov) have been searched. For our search, following terms have been used: COVID-19, vaccination, SARS-CoV-2, BNT162b2, mRNA-1273, Biontech, Moderna, immunosuppression, antibody, humoral, T cells.
Added value of this study
This analysis of more than 3100 Saxonian study participants directly compares for the first time SARS-CoV-2 vaccination induced humoral and cellular immune response rates and qualities in immunocompetent medical personal, immunocompromised dialysis patients, and immunosuppressed kidney transplant recipients at four to five weeks after the second vaccine dose. Hereby, dialysis patients exhibited a remarkably high seroconversion rate after vaccination of 95% (similar to the tested medical personnel) but required a booster vaccination for this excellent result. In contrast, the majority of kidney transplant recipients did not show seroconversion even five weeks after booster vaccination. Independently from the analyzed patient group, the failed humoral response was associated with significantly lower frequencies of SARS-CoV-2 reactive CD4+ T helper cells. The number and type of immunosuppressive drugs in both dialysis patients and transplant recipients are most critical risk factors for vaccination failure. The type of mRNA vaccine does not make any difference in seroconversion for medical personnel but apparently for immunocompromised dialysis patients and especially for transplant recipients under immunosuppressive therapy. Hereby, mRNA-1273 was remarkably more effective than BNT162b2 mRNA. SARS-CoV-2 infection occurring in dialysis patients before booster vaccination caused severe COVID-19 with a high mortality rate. In contrast, SARS-CoV-2 infection after booster vaccination caused either mildly symptomatic disease (< 10%) or predominantly asymptomatic (> 90%) disease. Our Saxonian dialysis network data with > 5000 dialysis patients demonstrate that during third wave pandemia COVID-19 disease incidences in dialysis patients ceased despite steep incidence increases in normal population additionally indicating vaccination effectivity.
Implications of all the available evidence
Dialysis patients exhibit a remarkably high seroconversion rate of 95% after boost vaccination, while humoral response is impaired in the majority of transplant recipients. Immunosuppressive drug number and type as well as vaccine type (BNT162b2) are major determinants of seroconversion failure in both dialysis patients and transplant recipients. Patients under immunosuppressive therapy should be monitored for vaccination-related seroconversion and potentially need additional vaccinations or modified vaccination protocols or even modifications of their immunosuppressive therapy. Our data suggest that certain mRNA vaccines (mRNA-1273) may induce a more frequent seroconversion rate in immunocompromised dialysis patients and kidney transplant recipients but not in normal population (medical personnel). SARS-CoV-2 vaccination seems to be safe and highly protective after boost vaccination in dialysis patients.
Alt-text: Unlabelled box
1. Introduction
The vulnerable populations of dialysis patients (DP) and kidney transplant recipients (KTR) experience a high percentage of complicated COVID-19 disease courses. They show a markedly increased mortality compared to normal population [1]. In Saxony, a federal state with about four million inhabitants, a very high COVID-19 prevalence was shown in the second pandemic wave starting in October 2020. To better understand the disease progression of COVID-19 in the described population, we established a corresponding network in the first pandemic wave, in March 2020. This network includes almost all nephrology centers in Saxony with about 5000 DP, 1000 KTR and 800 medical personnel (MP). In weekly intervals, we thus exchanged COVID-19 disease cases and results in the groups of DP, KTR and MP. In the dialysis centers, patients were tested for SARS-CoV-2 infection by RT-PCR if they presented one of the classic symptoms (fever, cough, shortness of breath, myalgias, diarrhea, or other symptoms consistent with such an infection) or if they were in contact with a person with RT-PCR-confirmed disease. Routine PCR screening without a cause was not part of good medical practice of the dialysis centers.
We identified 50 KTR, more than 700 DP, and 150 MP with symptomatic COVID-19 disease since October 2020 through this network, allowing us to monitor disease progression. While none of the MP died from COVID-19, approximately 10% of the affected KTR and 20% of the DP died in this short period despite extensive precautions (hygiene rules, history and fever screening, individual transports, FFP-2 masks, etc.) in the centers. In Saxony, dialysis patients represent about 0.1% of the general population. Nevertheless, until vaccination became available, they accounted for close to 5% of all COVID-19-related deaths in Saxony [2].
To date, SARS-CoV-2 vaccines such as BNT162b2 mRNA (Pfizer/BioNTech) or mRNA-1273 (Moderna) have undergone clinical testing in the general population only, with COVID-related protection rates up to 95% after two vaccinations. While very few and mostly incomplete SARS-CoV-2 specific vaccination data exist for DP or KTR so far, we and others demonstrated reduced vaccination success rates for other vaccines in either DP or KTR compared to the general population [3], [4], [5].
The presentation of our network data with high COVID-19 prevalence and mortality rates led to a coordinated vaccination campaign by the State Ministry of Social Affairs within a few weeks. A rapid vaccination schedule thus became possible in all nephrology centers in Saxony. This was the starting point for an investigator-driven, prospective observational study. This study investigates the SARS-CoV-2-specific humoral as well as cellular immune response in patients and MP at defined intervals after appropriate vaccination (DIA-Vacc study).
Within more than 3100 participants, we studied the evolution of SARS-CoV-2 specific humoral and T-cellular immune responses in “immunosuppressed” KTR, “immunocompromised” DP as well as “immunocompetent” MP after SARS-CoV-2 infection and/or vaccination using either BNT162b2 mRNA or mRNA-1273 vaccines. The detailed characterization of vaccine-specific immunity was performed by ELISA and surrogate neutralization assay for antibody detection as well as interferon gamma release assays, and flow cytometry measurements for T-cell response.
2. Methods
2.1. Study design
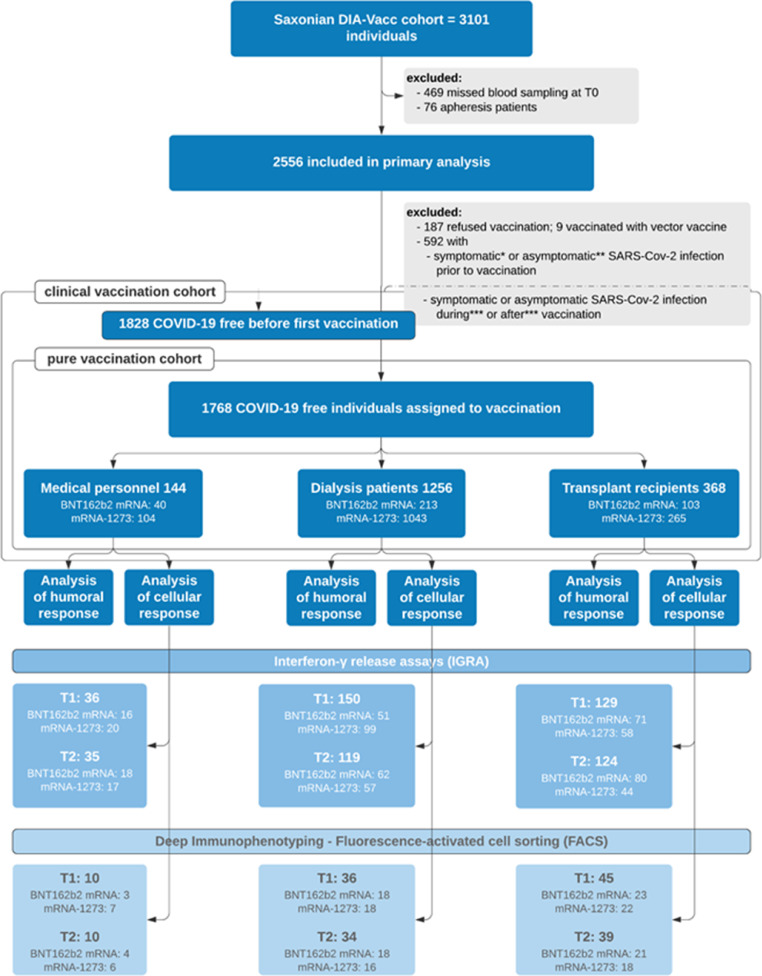
The investigator-driven, non-interventional, prospective, observational trial was started in 26 out of 36 Saxonian nephrology centers from January 15th to February 24th, exploring the time course of a specific cellular or/and humoral immune response to disease and/or SARS-CoV-2 vaccination in MP, DP, and KTR. Of all 36 dialysis centers in Saxony, the first 26 committing dialysis centers were recruited for the DIA-Vacc study and later requests could not be considered due to funding restrictions. For the study results reported here, a “pure vaccination cohort” was created excluding retrospectively all symptomatically and asymptomatically COVID-19 infected or deceased participants before, during and after vaccination (up to T2) to assess a purely vaccination-related immune response as indicated by the study flow chart (Fig. 1). The clinical vaccination cohort consists of the pure vaccination cohort but includes all participants, who experienced symptomatic or asymptomatic COVID-19 disease (or death) strictly during or after vaccination to assess clinical outcome of vaccination. Study start (T0) was immediately before first vaccination. Further monitoring time points were three (BNT162b2 mRNA) to four (mRNA-1273) weeks later before second vaccination (T1) and about eight weeks after study start (T2; five to four weeks after the booster vaccination respectively). By vaccine availability during January (BNT162b2 mRNA) and February (mRNA-1273) 2021 only the first four dialysis centers being assigned to the vaccination campaign, received BNT162b2 mRNA, while all other following dialysis centers received mRNA-1273 vaccine for both vaccinations. Neither any dialysis center nor any patient or MP nor the study center (Dresden) had a choice or influence regarding the type of vaccine, which was assigned in the order of contacting the central vaccination institute in Saxony. Information to all dialysis centers about the start of the vaccination campaign was distributed by the central vaccination institute via email at the same time.
Fig. 1.
Study cohort
* Symptomatic participants = clinical infection, PCR proven ** Asymptomatic participants before 1st vaccination = IgG against Spike S1 ≥ 35•2 BAU/ml or IgG against NCP ≥ 1•1 ratio *** Participants with symptomatic [clinical infection, PCR proven] or asymptomatic [IgG NCP ≥ 1.1 ratio] SARS-CoV-2 infection during or after vaccination period were analyzed separately for the assessment of safety and serological efficacy and are added to the pure vaccination cohort in the corresponding analysis (clinical vaccination cohort). T0 = before first vaccination; T1 = 3–4 weeks after first vaccination; T2 = 4–5 weeks after first vaccination; Analysis of humoral response included measurement of IgA and IgG antibodies against the SARS-CoV-2 S1 protein at time point T0 and additionally IgG antibodies against the novel binding domain S1/RDB of the ACE2 receptor at time point T2. At both time points, additional IgG antibodies against the SARS-CoV-2 nucleocapsid (NCP) were measured. Analysis of the cellular immune response included deep immunophenotyping by FACS analysis and interferon-gamma release assays. Patients in the FACS analysis were all included in the group of IGRA measurements (reciprocal not true). All consented participants from 4 centers adjacent to the study coordination center were selected for IGRA measurement (logistic reasons). The selection took into account the respective number of patients per group (MP/DP/KR) and vaccine type and center. One of the 4 centers with T-cell isolation did not participate in PBMC isolation and thus deep immunophenotyping. For the latter, participants were selected from all 3 groups in which a sufficient number of cells (at least 107/time point and participant) was ensured for the planned analyses at least at the time points T0 and T2 before cryopreservation and after PBMC isolation (technical reasons). BNT162b2 and mRNA-1273 account for the distinct vaccine types. Different indications of numbers stand for the corresponding case numbers in the corresponding subgroups.
In all study participants (eligibility if > 18 years old and signed informed consent) at T0 and T2, SARS-CoV-2 specific IgG- or IgA-antibody reactions (Euroimmun [6], [7], [8], [9]) against the Spike protein subunit S1 and IgG-antibodies against the nucleocapsid protein subunit (NCP) were analyzed. In addition, the receptor binding domain (RBD) antibody formation suggesting neutralizing activity against the SARS-CoV-2 virus was also examined at T2 [10]. The antibody response measurements at T1 were restricted to representative subgroups, while T0 and T2 measurements were done in all study participants. For all antibody measurements Euroimmun ELISAs on Euroimmun analyzers were used. A positive serologic response was defined as de novo antibody development (seroconversion) at T1 or T2.
To provide a detailed characterization of the cellular SARS-CoV-2 immune response, two independent assays were performed at all time points in representative subgroups (Fig. 1). Hereby, a SARS-CoV-2 specific interferon-γ release assay (IGRA [11]) as well as in-depth immunophenotyping using flow cytometry (FACS [12]) were applied, as previously described. The exact procedure and analysis is further described in the Supplementary Appendix.
2.2. End points
The primary end point was the positive humoral immune response eight weeks after vaccination as defined by de novo positivity of either IgG- or IgA- anti-SpikeS1 antibodies without development of virus-specific NCP antibodies. Secondary end points were development of vaccination-induced de novo T-cellular immunity as well as clinical safety and serological as well as cellular immune response parameters.
2.3. Statistical analysis
Statistical analysis was performed using the R Environment for statistical Computing [13], version 4.0.4. Categorical variables are summarized as frequencies and percentages; continuous variables are reported as median and interquartile range. All applied statistical tests are two-sided, and the significance level of 5% is used for hypothesis testing. No adjustments for multiple comparison were performed. For the analysis of the cellular immune response, differences between groups for categorical variables were assessed using the chi-squared test. For continuous variables, a t-test or the Mann-Whitney U test was employed, as appropriate. The dynamics of quantitative variables were analyzed employing the paired t-test, assuming a normal distribution for the differences between the initial and follow-up visit. Correlation size and significance were calculated using Spearman's correlation coefficient. The analysis of association between the serological vaccination response and risk factors of interest was carried out using a multiple logistic regression model and generalized estimating equations (with clinical center as a blocking factor). Immunosuppressive therapy was considered to be a major risk factor. Some studies [14] observed a substantial difference in seroconversion response after administering different vaccines, and therefore, in our analysis, we included the vaccine type as a risk factor. We also adjusted for the number of comorbidities, gender, age, body mass index (BMI) [15], as potential confounders. In addition, we investigated whether the seroconversion and Hepatitis B vaccination responses are associated. In the patients receiving immunosuppressive therapy, the possible influence of individual therapies and their combinations was assessed using logistic regression, and the explanatory power of each immunosuppressive drug was also explored by means of a penalized regression model (elastic net approach [16]).
3. Results
3.1. Basic study cohort characteristics
Of more than 3100 study participants (Table S1), a total of 1768 participants fulfilled all “pure vaccination cohort” requirements, of whom 144 MP, 1256 DP, and 368 KTR were monitored at and up to eight weeks after vaccination (Table 1a). The MP cohort is characterized by younger age, female predominance, and few drug treated comorbidities. The dialysis/transplant cohort is about 20/10 years older, has a male predominance, of whom almost all have multiple drug requiring comorbidities and long times on dialysis/transplantation, respectively. 95% of all dialysis patients were treated with hemodialysis and 5% with peritoneal dialysis. Within the dialysis group 63 patients (5%) were exposed to current immunosuppressive therapy, while with the exception of an identical twin transplantation 100% of KTR received immunosuppressants.
Table 1a.
Baseline characteristics of SARS-CoV-2 unexposed persons / patients of the DIA-Vacc pure vaccination cohort at study start (T0).
| Variable | Category | MP | DP | KTR |
|---|---|---|---|---|
| Number | evaluable | 144 | 1256 | 368 |
| Age (years) | mean ± SD | 48 ± 11.9 | 67.6 ± 14 | 57.3 ± 13.7 |
| Male Sex | n / % | 34 / 23.6 | 818 / 65.1 | 241 / 65.5 |
| BMI (kg/m2) | Mean ± SD | 25.7 ± 4.9 | 27.5 ± 5.7 | 26.4 ± 4.8 |
| Cause of end stage renal disease | n / % | n.a. | 1014 / 80.7 | 222 / 60.2 |
| Diabetes-Hypertension-Vascular disease | n / % | n.a. | 605 / 48.2 | 62 / 16.8 |
| Glomerulonephritis-Interstitial nephritis | n / % | n.a. | 258 / 20.5 | 94 / 25.5 |
| Vasculitis | n / % | n.a. | 40 / 3.2 | 11 / 3 |
| Polycystic kidney disease | n / % | n.a. | 111 / 8.8 | 55 / 14.9 |
| Unknown | n / % | n.a. | 242 / 19.3 | 146 / 39.7 |
| Drug treated comorbidities | n / % | 32 / 22.2 | 1203 / 95.8 | 330 / 89.7 |
| Diabetes mellitus | n / % | 4 / 2.8 | 430 / 34.2 | 72 / 19.6 |
| Cardiovascular disease | n / % | 25 / 17.4 | 1155 / 92 | 316 / 85.9 |
| Lung disease | n / % | 6 / 4.2 | 79 / 6.3 | 23 / 6.2 |
| Liver cirrhosis | n / % | 0 / 0 | 18 / 1.4 | 4 / 1.1 |
| Cancer | n / % | 0 / 0 | 58 / 4.6 | 10 / 2.7 |
| None | n / % | 112 / 77.8 | 53 / 4.2 | 38 / 10.3 |
| Type of dialysis | n.a. | 1256 / 100 | n.a. | |
| Hemodialysis | n / % | n.a. | 1198 / 95.4 | n.a. |
| Peritonealdialysis | n / % | n.a. | 58 / 4.6 | n.a. |
| Time on dialysis (years) | mean ± SD | n.a. | 5.7 ± 5.6 | 6.6 ± 6.6 |
| On transplant waiting list | n / % | n.a. | 164 / 13.1 | n.a. |
| Time on transplantation (years) | mean ± SD | n.a. | n.a. | 9.9 ± 6.8 |
| Previous transplantation | n / % | n.a. | 93 / 7.4 | 56 / 15.2 |
| Hepatitis B vaccination failure | n / % | 2 / 1.4 | 263 / 20.9 | 32 / 8.7 |
| Flu vaccination winter 2020/2021 | n / % | 82 / 56.9 | 907 / 72.2 | 210 / 57.1 |
| On immunosuppressive therapy | n / % | 1 / 0.7 | 63 / 5 | 367 / 99.7 |
| Corticosteroids | n / % | 0 / 0 | 42 / 3.3 | 178 / 48.4 |
| Calcineurin-Inhibitor | n / % | 0 / 0 | 20 / 1.6 | 322 / 87.5 |
| MMF/MPA | n / % | 0 / 0 | 15 / 1.2 | 280 / 76.1 |
| mTOR-Inhibitor | n / % | 0 / 0 | 2 / 0.2 | 59 / 16 |
| Belatacept | n / % | 0 / 0 | 2 / 0.2 | 17 / 4.6 |
| T-cell depleting ab | n / % | 0 / 0 | 0 / 0 | 0 / 0 |
| B-cell depleting ab | n / % | 0 / 0 | 4 / 0.3 | 0 / 0 |
| Other | n / % | 1 / 0.7 | 3 / 0.2 | 5 / 1.4 |
| Type of vaccine | ||||
| BNT162b2 mRNA | n / % | 40 / 27.8 | 213 / 17 | 103 / 28 |
| mRNA-1273 | n / % | 104 / 72.2 | 1043 / 83 | 265 / 72 |
T0 = before first vaccination; T1 = 3–4 weeks after first vaccination; T2 = 8 weeks after first vaccination.
For this evaluation all patients with asymptomatic* or documented symptomatic** COVID-19 disease before and during vaccination up to T2 were excluded. Hepatitis B vaccination failure definition - patients with unsuccessful vaccination after at least four attempts; MP = Medical Personnel; DP = Dialysis Patients; KTR = Kidney Transplant Recipient; MMF-MPA = mycophenolate mofetil or mycophenolic acid.
*Asymptomatic COVID-19 disease definition - neither knowledge nor symptoms of COVID-19 disease, but IgG-antibody reaction to nucleocapsid (T0, T1 or T2) or to the Spike protein subunit S1 (only T0) of the SARS-CoV-2 virus is positive.
**Symptomatic COVID-19 disease definition - SARS-CoV-2 PCR positive patients with clinical symptoms.
3.2. Study end points
3.2.1. Immune response rates to vaccination at T1 and T2 in the pure vaccination cohort
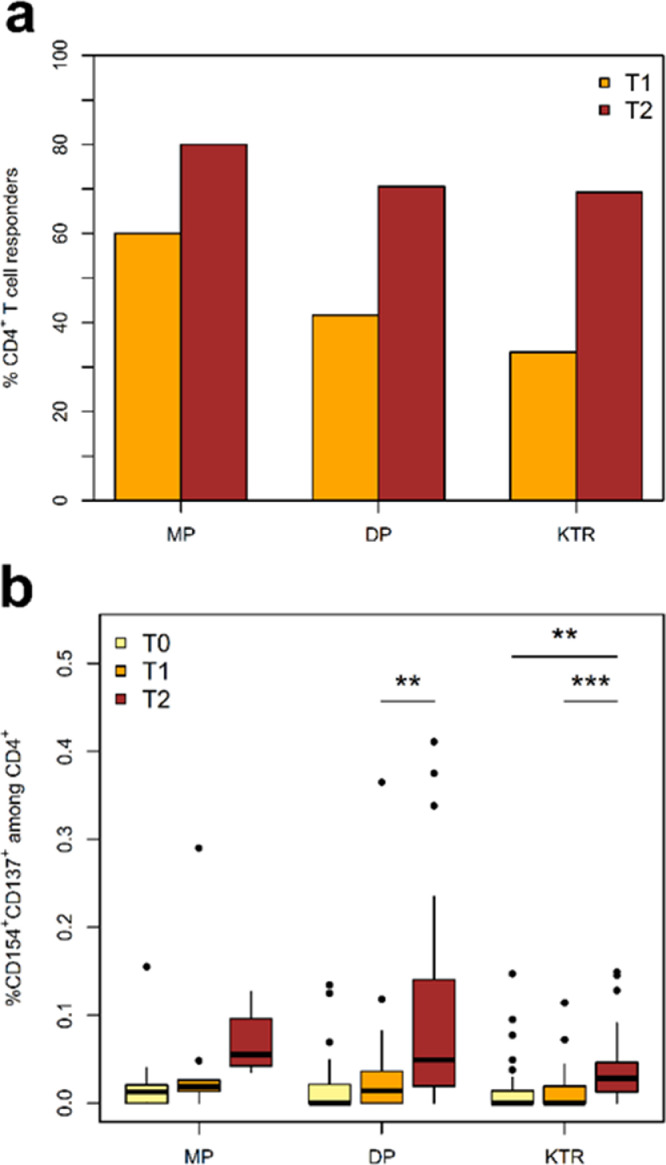
Seroconversion rates in the MP cohort were excellent reaching 96% at T1 and 99% at T2 (Table 1b). In general, IgA-anti SpikeS1 antibody results were comparable to IgG-anti SpikeS1 antibody results and sensitivity could be slightly increased using both measurements. All MP with seroconversion developed SARS-CoV-2 RBD antibodies at T2. The cellular vaccination response rate as indicated by a positive IGRA test was at 81% at T1 and 86% at T2 (Table 1b). Data obtained by FACS revealed comparable incidences. Due to the pre-existing SARS-CoV-2-cross-reactive T-cells known to be detectable by FACS in unexposed patients [17], vaccine-directed T-cell response by FACS was defined as more than twofold-increase of S-reactive T-cells compared to the pre-vaccination (T0) T-cell response. Positive cellular response was observed in 60% of MP at T1 and 80% at T2, respectively (Fig. 2a).
Table 1b.
Vaccination response at T1 (3–4 weeks) and at T2 time point (8 weeks after first vaccination) in SARS-CoV-2 unexposed persons/patients of the DIA-Vacc pure vaccination cohort.
| Variable | Category | MP T1 | DP T1 | KTR T1 | MP T2 | DP T2 | KTR T2 |
|---|---|---|---|---|---|---|---|
| Number | evaluable | 55 | 278 | 144 | 134 | 1136 | 333 |
| Humoral response | |||||||
| IgG-Ab or IgA-Ab Spike S1 positive | n /% | 53 / 96.4 | 172 / 61.9 | 11 / 7.6 | 132 / 98.5 | 1083 / 95.3 | 140 / 42 |
| IgG-Ab Spike S1 positive | n /% | 53 / 96.4 | 140 / 50.4 | 7 / 4.9 | 132 / 98.5 | 1074 / 94.5 | 112 / 33.6 |
| Number_IgA measurements | 50 | 245 | 134 | 125 | 1026 | 312 | |
| IgA-Ab Spike S1 positive | n /% | 44 / 88 | 127 / 51.8 | 9 / 6.7 | 123 / 98.4 | 873 / 85.1 | 112 / 35.9 |
| IgG- and IgA-Ab Spike S1 positive patients | n /% | 44 / 80 | 95 / 34.2 | 5 / 3.5 | 123 / 91.8 | 864 / 76.1 | 84 / 25.2 |
| number_rbd measurements | n.a. | n.a. | n.a. | 122 | 991 | 120 | |
| neutralizing-ab (rbd) positive | n /% | n.a. | n.a. | n.a. | 122 / 100 | 938 / 94.7 | 79 / 65.8 |
| IgG-Ab Nucleocapsid positive | n /% | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| T-cell response: IGRA subgroup | Category | MPT1 | DPT1 | KTRT1 | MPT2 | DPT2 | KTRT2 |
| Number | n | 36 | 150 | 129 | 35 | 119 | 124 |
| Interferon-γ release assay (IGRA) positive | n /% | 29 / 80.6 | 66 / 44 | 10 / 7.8 | 30 / 85.7 | 93 / 78.2 | 37 / 29.8 |
| IGRA-test positive or any positive humoral response | n /% | 34 / 94.4 | 104 / 69.3 | 17 / 13.2 | 34 / 97.1 | 116 / 97.5 | 58 / 46.8 |
| IGRA-test positive and any positive humoral response | n /% | 27 / 75 | 50 / 33.3 | 2 / 1.6 | 30 / 85.7 | 86 / 72.3 | 18 / 14.5 |
MP = Medical Personnel; DP = Dialysis Patients; KTR = Kidney Transplant Recipient; Interferon-γ release assay = IGRA; ELISA = enzyme-linked immunosorbent assay; FACS = fluorescence-activated cell sorting; T0 = before first vaccination; T1 = 3–4 weeks after first vaccination; T2 = 8 weeks after first vaccination;.
For this evaluation, all participants with asymptomatic* or documented symptomatic** COVID-19 disease before and during vaccination up to T2 were excluded. Humoral vaccination responses were assessed as positive, when de novo production of the antibody to the Spike S1 (IgA or IgG) protein or RBD (IgG) subunit was measured. Patients with COVID-19 disease and/or asymptomatic COVID-19 disease by de novo reaction to the nucleocapsid protein during vaccination (up to T2) were excluded. A positive T-cellular response to vaccination as assessed by interferon-γ release assay (IGRA) turned from a negative result on T0 to positive on T1 or T2, respectively (≥ 100 mIU/ml.
A positive IGRA response required de novo positivity above a threshold value of 100 mIU/ml, as being recommended by the manufactures.
*Asymptomatic COVID-19 disease definition - neither knowledge nor symptoms of COVID-19 disease, but IgG-antibody reaction to nucleocapsid (T0, T1 or T2) or to the Spike protein subunit S1 (only T0) of the SARS-CoV-2 virus is positive.
**Symptomatic COVID-19 disease definition - SARS-CoV-2 PCR positive patients with clinical symptoms.
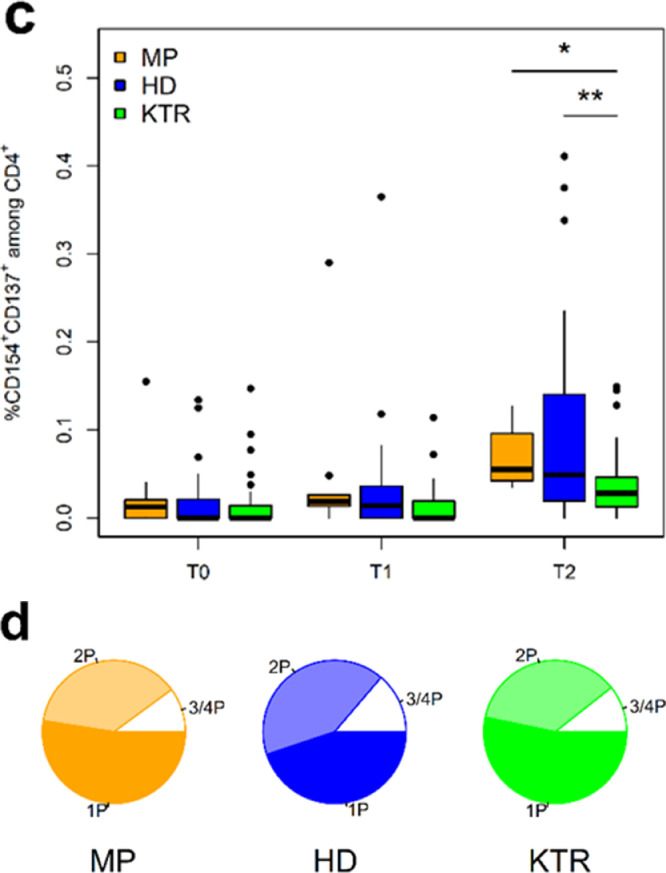
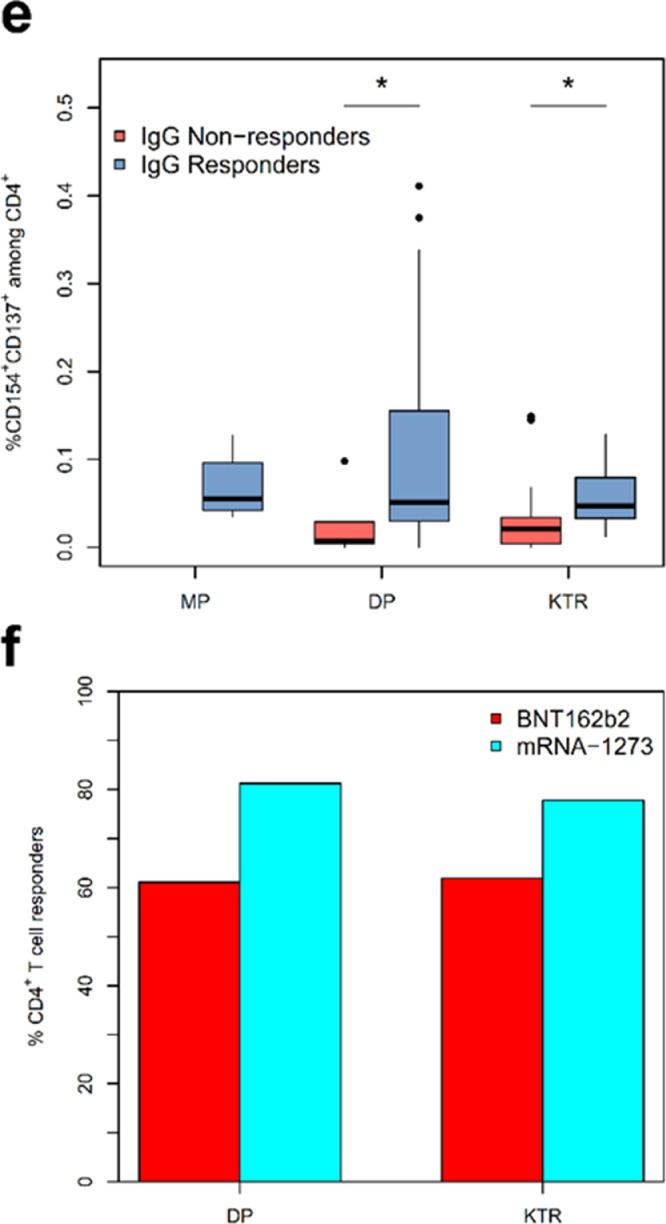
Fig. 2.
Analysis of SARS-CoV-2-reactive CD4+ T-cell helper response by multi-parameter flow cytometry. a) Incidence of SARS-CoV-2-reactive CD4+ T cell responders. A T-cell response is defined as a twofold increase or more in the frequency of activated T cells between T0 and T1 or T2. b) Kinetics of activated Spike-reactive CD4+ T helper cells at and following vaccination. Shown frequencies are after correction for background activation. c) Magnitude of SARS-CoV-2 Spike-reactive CD4+ T helper cell response in MP, DP, and KTR cohorts at different time points. Shown frequencies are after correction for background activation. d) Spike-reactive cytokine-producing CD4+ T cells detected at T2 in study cohorts. Depicted is the total frequency of cytokine IFNg, TNF, IL2 or Granzyme B producing CD4+ T cells, which produce only one cytokine (1P), or simultaneously produce two (2P), three or four (3P or 4P) cytokines. e) Magnitude of SARS-CoV-2 Spike-reactive CD4+ T helper cell response in humoral responders and non-responders as defined by IgG serology. f) Incidence of SARS-CoV2-reactive CD4+ T helper cell responders in DP and KTR patients vaccinated by BNTb162b or mRNA-1273 vaccine. Depicted are the responses at T2.
Asterisks indicate the level of significance for the paired t-test (b) and Mann-Whitney U test (c and e): * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
In the DP cohort, seroconversion rate was 62% at T1 and 95% at T2 (Table 1b). RBD antibodies were detected in 95% of seroconversion positive DP at T2. Cellular immune response was detected in 44% at T1 and 78% at T2 by IGRA. In line with the data on humoral immunity, FACS data demonstrated a delay in the increase of the frequencies of SARS-Cov-2-reactive CD4+ T helper cells detected following vaccination. Thus, a significant increase in the magnitude of vaccine-reactive CD4+ T-cell response could be observed in DP at T2 as compared to pre-vaccination T0 and T1. No significant increase could be observed between T0 and T1 (Fig. 2b).
The seroconversion rate in the KTR cohort was low with 8% at T1 and 42% at T2 (Table 1b). RBD antibodies were detected in 65% of all antibody positive KTR at T2. IGRA turned positive in 8% at T1 and 30% at T2 and IGRA values were overall lower than in MP and DP. Analyzing the kinetics of S-reactive CD4+ T helper cells by FACS in KTR patients following vaccination demonstrated a significant delay in T cell response similar to DP patients with significant increase only occurring after the boost vaccination (Fig. 2b). Compared to MP and DP, KTR showed significantly lower frequencies of SARS-CoV-2-reactive CD4+ T helper cells (Fig. 2c).
Furthermore, we evaluated the functionality of the detected SARS-CoV-2-reactive CD4+ T cells as defined by singular or simultaneous production of different effector cytokines. Similar kinetic patterns could be found for CD4+ T cells producing IFN-γ, TNF-α, and IL-2 in DP and KTR cohorts with a strong delay in the T cell response from T0 to T2 (Fig. S3a–c). KTR demonstrated detectable but significantly lower frequencies of cytokine producing CD4+ T helper cells as compared to MP and DP (Fig. S3d–f). Despite their lower frequencies, KTR showed bi- and tri-functional CD4 T cells (being attributed to antiviral protection [18], in equivalent proportions compared to MD, DP (Fig. 2d).
Trying to understand the differences in seroconversion ability, we considered the generation of IgG in context of cellular immunity. Since CD4+ T helper cells play an essential role in the antibody generation by B cells, we compared the frequencies of activated CD4+ T helper cells in patients with and without seroconversion at T2. Interestingly, patients with IgG showed a significantly higher frequency of SARS-CoV-2-reactive CD4+ T helper cells including those with effector memory phenotype (Figs. 2e, S3g).
3.3. Risk factors for seroconversion failure in DP and KTR cohorts
The results of the analysis of risk factors for seroconversion failure are summarized in Tables 2a (DP) and 2b (KTR). Immunosuppressive drug therapy and type of vaccine were identified as major independent risk factors for a negative seroconversion after SARS-CoV-2 vaccination (Table 2a). In addition, BMI, time on dialysis for DP and patient age, time on transplantation for KTR were independently linked to seroconversion after vaccination. These results were in agreement with those obtained using the generalized estimating equations (GEE) approach (Tables S3 and S4). In addition, to investigate whether the effect of immunosuppressive therapy differs between these patient groups, we fitted a multiple logistic regression model for the combined data set, where the interaction between the number of IS drugs and group was included as a predictor. This interaction turned out to be statistically significant (Likelihood-ratio test, p-value = 0.019). According to the fit results, shown in Table S5, the chance of non-response for KTR patients is, on average, four times higher than for dialysis patients. Table 2d depicts different success rates of vaccination-induced seroconversion dependent on the number of immunosuppressive drugs and some typical combinations in DP or KTR.
Table 2b.
Multiple logistic regression analysis for a negative humoral immune response to SARS-CoV-2 vaccination compared to a positive immune response in unexposed kidney transplant recipients of the DIA-Vacc pure vaccination cohort between T0 and T2.
| Risk factor | OR | Wald CI | p-value | |
|---|---|---|---|---|
| Sex | Male | Ref. | ||
| Female | 0.866 | [0.526; 1.425] | 0.571 | |
| Age | per year | 1.027 | [1.008; 1.047] | 0.006 |
| BMI | Per unit | 0.989 | [0.942; 1.039] | 0.669 |
| Time on transplantation | per year | 0.946 | [0.911; 0.982] | 0.004 |
| Number of comorbidities | per one | 0.952 | [0.653; 1.387] | 0.797 |
| Hepatitis B vaccination failure | No | Ref. | ||
| Yes | 0.449 | [0.189; 1.065] | 0.069 | |
| Number of IS drugs | Per one | 2.055 | [1.338; 3.157] | 0.001 |
| Vaccine type | BNT162b2 mRNA | Ref. | ||
| mRNA-1273 | 0.356 | [0.205; 0.616] | < 0.001 | |
Ref. = reference category; IS means immunosuppression; T0 = before first vaccination; T2 = 8 weeks after first vaccination.
Comparator is the kidney transplant recipient cohort, which shows a positive immune reaction to vaccination as defined by de novo development of any IgG-Ab or IgA-Ab against the Spike S1 subunit on T2. A negative vaccination response was defined by negative results of both above tests at T2.
Table 2a.
Multiple logistic regression analysis for a negative humoral immune response to SARS-CoV-2 vaccination compared to a positive response in unexposed dialysis patients of the DIA-Vacc pure vaccination cohort between T0 and T2.
| Risk factor | OR | Wald CI | p-value | |
|---|---|---|---|---|
| Sex | Male | Ref. | ||
| Female | 1.337 | [0.683; 2.617] | 0.397 | |
| Age | per year | 1.002 | [0.980; 1.025] | 0.858 |
| BMI | per unit | 0.937 | [0.878; 1.000] | 0.051 |
| Time on dialysis | per year | 0.933 | [0.874; 0.996] | 0.037 |
| Number of comorbidities | per one | 0.932 | [0.562; 1.546] | 0.987 |
| Hepatitis B vaccination failure | No | Ref. | ||
| Yes | 1.024 | [0.491; 2.134] | 0.621 | |
| IS drugs | None | Ref. | ||
| At least one | 10.034 | [4.668; 21.568] | < 0.001 | |
| Vaccine type | BNT162b2 mRNA | Ref. | ||
| mRNA-1273 | 0.224 | [0.119; 0.421] | < 0.001 | |
Ref. = reference category; T0 = before first vaccination; T2 = 8 weeks after first vaccination.
IS means immunosuppression. Comparator is the dialysis patient cohort, which shows a positive immune reaction to vaccination as defined by de novo development of any IgG-Ab or IgA-Ab against the Spike S1 subunit on T2. A negative vaccination response was defined by negative results of both above tests at T2.
Table 2d.
Success/positive rates of humoral vaccination-related de novo immune response dependent on immunosuppression number, type or vaccine type at T2.
| Factors | category | DP | KTR | MP |
|---|---|---|---|---|
| Total Numbers | n | 1136 | 368 | 144 |
| Immunosuppressive drugs | n /% | 63 / 6 | 367 / 99•8 | 1 / 0•7 |
| No IS drug | % of group number | 96% of 1048 | 100% of 1 | n.a. |
| One IS drug | % of group number | 78% of 37 | 84% of 19 | n.a. |
| Two IS drugs | % of group number | 62% of 13 | 43% of 180 | n.a. |
| Three IS drugs | % of group number | n.a. | 35% of 128 | n.a. |
| CS | % of group number | 82% of 28 | n.a. | n.a. |
| CS/CNI | % of group number | n.a. | 56% of 25 | n.a. |
| CNI/MMF-MPA | % of group number | n.a. | 39% of 123 | n.a. |
| CNI/mTOR-I | % of group number | n.a. | 38% of 13 | n.a. |
| CS/CNI/MMF-MPA | % of group number | n.a. | 34% of 102 | n.a. |
| CS/CNI/mTOR-I | % of group number | n.a. | 50% of 12 | n.a. |
| Belatacept alone or in a combination | % of group number | n.a. | 19% of 16 | n.a. |
| Vaccine Type | % of group number | 95% of 1136 | 42% of 333 | 99% of 134 |
| BNT162b2 mRNA | % of group number | 88% of 200* | 26% of 99* | 97% of 39 |
| mRNA-1273 | % of group number | 97% of 936 | 49% of 234 | 99% of 95 |
MP = Medical Personnel; DP = Dialysis Patients; KTR = Kidney Transplant Recipient; IS means immunosuppression. CNI = Calcineurin-Inhibitor; MMF-MPA = mycophenolate mofetil or mycophenolic acid; mTOR-I = mTOR-inhibitors; CS = glucocorticosteroids; T2 = 8 weeks after first vaccination.
Vaccination response rates in drug combinations of less than 10 patients were reported as n.a.;.
statistical significance using p < 0•001 compared to other vaccine type;.
Since immunosuppressive drug therapy and drug number were independent risk factors for seroconversion failure in both the DP and KTR cohort, we further examined the weight of each individual immunosuppressive drug type in all patients with immunosuppressive drug therapy using both conventional and penalized regression models. Belatacept, MMF/MPA and calcineurin-inhibitors were associated with higher seroconversion failure rates compared to mTOR-I and glucocorticoids.
The influence of IS on humoral response was explored using a penalized logistic regression model estimated using the elastic net approach, which is illustrated in Fig. S4. The fit results were in an overall agreement with the outcome of the conventional logistic regression (see Table 2c). Belatacept, MMF/MPA, CNI have a significant influence on the humoral response, while the influence of glucocorticosteroids and mTOR-inhibitors is much weaker.
Table 2c.
Risk factor assessment of individual immunosuppressive drugs regarding humoral vaccination failure at T2 based on logistic regression.
| IS class | OR | Wald CI | p-value | |
|---|---|---|---|---|
| Steroids | No | Ref | ||
| Yes | 1.374 | [0.885; 2.132] | 0.157 | |
| CNI | No | Ref | ||
| Yes | 3.604 | [1.798; 7.222] | <0.001 | |
| MMF/MPA | No | Ref | ||
| Yes | 3.7938 | [2.243; 6.430] | <0.001 | |
| mTORi | No | Ref | ||
| Yes | 1.935 | [0.968; 3.869] | 0.062 | |
| Belatacept | No | Ref. | ||
| Yes | 7.085 | [1.973; 25.446] | 0.003 | |
IS means immunosuppression; Steroids = glucocorticosteroids; CNI = calcineurin-inhibitor; MMF-MPA = mycophenolate mofetil or mycophenolic acid; mTOR-I = mTOR-inhibitors; T2 = 8 weeks after first vaccination.
Vaccine type is surprisingly also a predominant risk factor for seroconversion failure in DP and KTR, but not in MP cohort (Table 2d). In contrast to MP, in the DP cohort the seroconversion success rate in BNT162b2 mRNA was about 10% lower compared to mRNA-1273 vaccine (87•5 vs. 97%; p = 0•001). Within DP with seroconversion, antibodies against the receptor binding domain (RDB; suggestive for neutralization) were detected in 85% after vaccination with BNT162b2 but in 95% with mRNA-1273 (P < 0•001). In the KTR cohort, seroconversion rate was almost twice as high with the mRNA-1273 (49%) compared to BNT162b2 mRNA (26%) vaccine (p < 0•001). In line with the seroconversion rate, a higher number of patients demonstrating cellular immune response as defined by > twofold increase of SARS-CoV-2-reactive CD4+ T cells after vaccination with mRNA1273 as compared to BNT162b2. This difference was observed in all cohorts albeit non-significant, possibly due to the low number of analyzed patients (Fig. 2f).
3.3.1. Clinical outcome after SARS-CoV-2 vaccination
The percentage and outcome of de novo COVID-19 disease or side effects during the first three to four and eight weeks after vaccination were monitored (Table 3). Between T0 and T1, none of MP or KTR experienced any COVID-19 disease. Nevertheless, 17 patients (1.3%) of the DP cohort experienced PCR-positive COVID-19 disease, of whom five out of 17 (29%) died. In contrast, between T1 and T2, only eight patients in all groups experienced symptomatic COVID-19 disease with a mild time course without hospitalization and three of MP, 35 of DP, and four of KTR experienced asymptomatic COVID-19 disease. The mode of transmission was unknown for at least half of the SARS-CoV-2 cases. In the remaining cases transmission in the private domain dominated.
Table 3.
Clinical events during vaccination at T1 (3–4 weeks) and T2 (8 weeks after fist vaccination) in SARS-CoV-2 unexposed persons/patients of the DIA-Vacc pure vaccination cohort.
| Variable | Category | MP T1 | DP T1 | KTR T1 | MP T2 | DP T2 | KTR T2 |
|---|---|---|---|---|---|---|---|
| Number | evaluable | 148 | 1304 | 376 | 148 | 1304 | 376 |
| Vaccination side effects | n /% | 37 / 25 | 126 / 9.7 | 112 / 29.8 | 59 / 39.9 | 315 / 24.2 | 119 / 31.6 |
| Arm pain | n /% | 26 / 17.6 | 88 / 6.7 | 94 / 25 | 33 / 22.3 | 183 / 14 | 78 / 20.7 |
| Joint pain | n /% | 4 / 2.7 | 6 / 0.5 | 7 / 1.9 | 14 / 9.5 | 43 / 3.3 | 10 / 2.7 |
| Fever | n /% | 3 / 2 | 9 / 0.7 | 5 / 1.3 | 18 / 12.2 | 50 / 3.8 | 14 / 3.7 |
| Shivering | n /% | 3 / 2 | 5 / 0.4 | 7 / 1.9 | 15 / 10.1 | 81 / 6.2 | 6 / 1.6 |
| Severe allergic reaction | n /% | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| others | n /% | 13 / 8.8 | 38 / 2.9 | 29 / 7.7 | 30 / 20.3 | 147 / 11.3 | 73 / 19.4 |
| hospitalization due to vaccination | n /% | 0 / 0 | 5 / 0.4 | 3 / 0.8 | 0 / 0 | 5 / 0.4 | 6 / 1.6 |
| Asymptomatic COVID-19 disease | n /% | 0 / 0 | 0 / 0 | 0 / 0 | 3 / 2 | 35 / 2.7 | 4 / 1.1 |
| Symptomatic COVID-19 disease with | n /% | 0 / 0 | 17 / 1.3 | 0 / 0 | 1 / 0.7 | 3 / 0.2 | 4 / 1.1 |
| Cough | n /% | 0 / 0 | 10 / 0.8 | 0 / 0 | 1 / 0.7 | 0 / 0 | 2 / 0.5 |
| headache | n /% | 0 / 0 | 5 / 0.4 | 0 / 0 | 1 / 0.7 | 1 / 0.1 | 2 / 0.5 |
| Fever | n /% | 0 / 0 | 8 / 0.6 | 0 / 0 | 0 / 0 | 0 / 0 | 3 / 0.8 |
| Dyspnoe | n /% | 0 / 0 | 4 / 0.3 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| Dysgeusia/smelling disorder | n /% | 0 / 0 | 1 / 0.1 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| Rash | n /% | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| Diarrhoe | n /% | 0 / 0 | 0 / 0 | 0 / 0 | 1 / 0.7 | 0 / 0 | 1 / 0.3 |
| Hospitalization necessary | n /% | 0 / 0 | 10 / 0.8 | 0 / 0 | 0 / 0 | 0 / 0 | 1 / 0.3 |
| Intensive care necessary | n /% | 0 / 0 | 3 / 0.2 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| Mechanical ventilation necessary | n /% | 0 / 0 | 1 / 0.1 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| Death due to/with COVID-19 | n /% | 0 / 0 | 5 / 0.4 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| SARS-Cov-2 transmission likely due to | n /% | 0 / 0 | 16 / 1.2 | 0 / 0 | 1 / 0.7 | 3 / 0.2 | 4 / 1.1 |
| Medical personnel | n /% | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| Patients | n /% | 0 / 0 | 3 / 0.2 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| carpool | n /% | 0 / 0 | 2 / 0.2 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
| Private domain | n /% | 0 / 0 | 3 / 0.2 | 0 / 0 | 1 / 0.7 | 3 / 0.2 | 4 / 1.1 |
| unknown | n /% | 0 / 0 | 8 / 0.6 | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
T0 = before first vaccination; T1 = 3–4 weeks after first vaccination; T2 = 8 weeks after first vaccination.
For this evaluation, all participants with previous asymptomatic* or documented symptomatic** COVID-19 disease before vaccination (T0) were excluded and de novo COVID-19 cases during vaccination up to T2 were assessed. Symptomatic COVID-19 disease was documented for SARS-CoV-2 PCR positive patients with clinical symptoms between T1 and T0 as well as between T2 and T1. Asymptomatic COVID-19 disease was assessed by participants without knowledge or symptoms of COVID-19 disease, but de novo IgG-antibody reaction to the nucleocapsid subunit of the SARS-CoV-2 virus independent on other measures.
*Asymptomatic COVID-19 disease definition - neither knowledge nor symptoms of COVID-19 disease, but IgG-antibody reaction to nucleocapsid (T0, T1 or T2) or to the Spike protein subunit S1 (only T0) of the SARS-CoV-2 virus is positive.
**Symptomatic COVID-19 disease definition - SARS-CoV-2 PCR positive patients with clinical symptoms.
Vaccination side effects were overall mild (Table 3). In DP, mild to moderate symptoms appeared to be less frequent compared to the MP and KTR cohorts. A hospitalization event after vaccination was not documented in the MP group and rarely seen (up to one percent) in DP or KTR.
Finally, surrogate vaccination efficacy in the Saxonian dialysis center network is also indicated by a reduced COVID-19 incidence in DP compared to normal population during third pandemic wave (Fig. S2).
4. Discussion
In Saxonian nephrology centers high COVID-19 incidences and high mortality rates are reported. 10% of kidney transplant recipients and up to 20% of dialysis patients die with COVID-19. A lockdown in the federal state and closing of border crossings could not change this, nor could hygiene measures or organizational precautionary measures. SARS-CoV-2 vaccination appears to be a possible and perhaps best life-saving option. To date, however, data on the vaccination response to SARS-CoV-2 immunization for immunocompromised dialysis patients and immunosuppressed kidney transplant recipients have hardly been available. Both patient groups were not adequately considered in early vaccination studies.
Similar excellent seroconversion rate as in MP was observed in DP (> 95%), but only in DP boost vaccinations were required to achieve such high vaccination serological immunogenicity. In contrast, KTR demonstrated markedly impaired seroconversion rates of 8% after the first and 42% after the boost vaccination. Similarly weak vaccination response was reported in previous studies on transplant recipients by Benotmane et al. [19,20]. Of note, the humoral response alteration in KTR included not only the quantity but also the functionality as reflected by the markedly lower frequencies of RBD-antibodies, being suggestive for virus neutralization, in seroconverted KTR as compared to MP or DP.
A comprehensive understanding of the vaccination-induced alterations in SARS-CoV-2 specific immunity can enable alternative vaccination design strategies or development of novel vaccine compositions. Accordingly, an in-depth characterization of SARS-CoV-2-reactive T cell immunity has accompanied the monitoring of antibody generation in all three groups in our study. Of interest, the data on cellular immunity was in line with the data on humoral immunity demonstrating a comparable magnitude of SARS-CoV-2-reactive CD4+ T helper cells producing Th1 cytokines between MP and DP and significantly decreased frequencies in KTR. The most obvious explanation is immunosuppressive therapy applied in KTR. In fact, a logistic regression analysis demonstrated type and number of immunosuppressive medications as risk factors for the seroconversion failure in DP and KTR cohorts. Thus, an inverse link could be observed between the seroconversion rate and the number of the used immunosuppressive drugs in both cohorts. In addition, the type of drugs determined the vaccination response with the most negative impact provided by costimulation-blocker belatacept, the antimetabolite MMF/MPA, or calcineurin-inhibitors as compared to mTOR-inhibitors or glucocorticosteroids. The data on mTOR-inhibitors is in line with the previously described beneficial effect for vaccination efficacy in Influenza study [21] and might be of direct clinical relevance considering its known anti-rejection properties comparable to MMF/MPA.
However, immunosuppression cannot alone be responsible for the low vaccination serological immunogenicity in KTR, since vaccination success up to 100% was reported in context of other vaccination settings [22]. In KTR, SARS-CoV-2-reactive humoral response comparable to immune competent population has been reported in context of COVID-19 by other and our studies [12,23]. The difference in the immune response as compared to natural infection may be explained by the broad repertoire of antigenic stimuli provided by the whole SARS-CoV-2- in comparison to Spike-protein of vaccine. In fact, we and others demonstrated antigenic properties of two other proteins Membrane and Nucleocapsid. In addition, immunogenic dominance of SARS-CoV-2 proteins differs within the population with a proportion of patients demonstrating very few or even no Spike-reactive T cells but high frequencies of M- or N-reactive T cells [12].
In line with these observations, we found in our study that the type and amount of antigenic vaccine stimulation played an important role for immunocompromised DP or immunosuppressed KTR. In contrast to equivalent vaccine immunogenicity in the MP cohort, the type of vaccine was also an independent risk factor for the failed humoral immunity in the DP and KTR cohorts. Seroconversion rates in DP and KTR was significantly higher in mRNA-1273 vaccinated patients with the difference being especially evident in KTR as demonstrated by seroconversion rates of 26% versus 49% for BNT162b2 and mRNA-1273 vaccines, respectively. Similar differences in vaccine responses were reported by Boyarski [14,24] for KTR after the first dose, but less pronounced after boost vaccination. Differences to our study results may relate to the lack of exclusion of asymptomatically COVID-19 diseased transplant recipients before and after vaccination in their study (no measurement of NCP antibodies, no examination before vaccination). Seroconversion rates separately reported for mRNA-1273 [19] and BNT162b2 [25] in kidney transplant recipients are also in line with our observation. The simplest explanation for the higher mRNA-1273 vaccine immunogenicity in DP and KTR could be the three times higher dose, a better thermostability and handling. Being neglectable for immunocompetent individuals, these factors might be important in patients with impaired immunity, where strong stimuli are required. Further factors influencing vaccine immunogenicity include antigenic motifs, mRNA modifications and lipid formulation [26,27], but their comparison is outside the scope of the study. In addition, the response to inactivated vector-based vaccines, at least in primary non-responders, would be of great interest. Also, mucosal delivery which induces a stronger immunological memory would be highly interesting to investigate [28].
Another important finding is an unexpectedly high seroconversion rate in DP. In fact, a high percentage of vaccination failure has been reported in different previous studies on vaccinations against other pathogens such as HBV or influenza [29]. Uremia, inadequate dialysis, use of low biocompatibility dialysis material, hyperparathyroidism, anemia, iron overload and malnutrition have been considered as factors leading to ineffective vaccination in DP against other infectious diseases like i.e. Hepatitis B vaccination [30]. Nevertheless, the Spike-directed vaccination serological response was with 95% extremely high as compared to 50–60% in HBV vaccination [29]. Except the principle differences in antigenic motifs, the main differences between the previous vaccines and vaccines applied in our study is their type and mode of delivery into the host cell. Besides the typical antigenic presentation, immunogenicity can be increased by mRNA through direct activation of endosomal pattern recognition receptors and by lipid formulation via induction of type I interferon expression in dendritic cells [26]. It seems that this type of antigenic presentation or stimulation may overcome uremia-induced immune alterations in DP but probably is not strong enough to generate Spike-specific immune response in immunosuppressed patients. Consequently, additional boosting and or a stronger (higher dose) stimulus should be applied in non-responders. mRNA vaccines as used in this study cohort appeared safe in all groups similar to the phase 3 clinical trials for BNT162b2 [31] and mRNA-1273 [32]. Vaccination side effects were frequent but usually mild and appeared less frequent in the DP cohort. Side effects leading to hospitalization did not occur in the MP cohort and rarely (up to 1%) in DP or KTR most likely relating to the marked differences in comorbidities between these cohorts.
While no causal link between vaccination and disease incidences or mortality can be proven in an observational diagnostic study, COVID-19 disease monitoring (RT-PCR testing when presenting with symptoms or in case of contact with a confirmed case, as already described above) demonstrated a severity switch with 29% mortality in 17 DP up to T1 to asymptomatic disease in 92% of 38 cases and no mortality or hospitalization between T1 and T2. Additionally, disease incidences in the Saxonian dialysis centers compared to the normal population overproportionally decreased or rather ceased after vaccination despite start of third pandemic wave (Fig. S2). Hereby, it needs to be considered that about 80% of all cohorts received both vaccinations up to the end of march 2021, when vaccination rates of overall Saxonian population was still below 10%.
Limitations of our study include the observational, non-randomized study character, the selection bias towards personnel and patients interested in SARS-CoV-2 vaccination and lack of demographic matching between different cohorts. Although the former cannot be controlled for, the latter was accounted for in the multivariate analyses by including demographic factors as covariates. In addition, the results of non-parametric tests comparing the subpopulations receiving BNTb162b versus mRNA-1273 among the DP or KTR patients with respect to the risk factors of interest did not reveal any statistically significant differences, given the significance level of 5% (after a Bonferroni correction, 0•7%), see Tables S6 and S7 in the Supplementary Appendix). Therefore, no statistical evidence of confounding between the vaccine type and these risk factors was found in the data. Due to our large study size and purely vaccination related measurements of both humoral and cellular immune responses, we believe that these principle results should be applicable in general for dialysis and kidney transplant patients with similar characteristics and should be further confirmed in prospective randomized trials. Nevertheless, the influences of BMI, time on dialysis for DP and patient age, time on transplantation for KTR may be responsible for further variation regarding vaccination-related seroconversion rates dependent on specific cohort composition.
In conclusion, based on a large prospective clinical and immunological data set obtained within the Saxonian dialysis network under homogenous and centralized conditions, we demonstrated several clinically relevant observations:
-
(a)
Dialysis patients show high percentage of serological vaccination response, however, two vaccinations are required to obtain antiviral protection as demonstrated by immunological data and the clinical data of vaccination breakthrough up to four weeks after the boost vaccination;
-
(b)
Kidney transplant recipients demonstrate an impaired humoral and cellular immunity, which correlated with the type and number of immunosuppressive agents;
-
(c)
Not only immunosuppression but also the type of vaccine influenced the vaccination response in dialysis patients and especially in kidney transplant recipients.
Our study results indicate that SARS-CoV-2 vaccination seems to be safe but adaption of vaccination protocols including additional boost vaccinations or modified vaccination protocols or types should be considered in these vulnerable patients according to our short term observational data. Long term studies need to follow up on these findings.
Funding
This study was funded by the Else Kröner Fresenius Stiftung, Bad Homburg v. d. H., grant number Fördervertrag EKFS 2021_EKSE.27.
EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany provided antibody ELISAs and interferon-gamma release assays for this study.
CRediT authorship contribution statement
Julian Stumpf: Data curation, Visualization, Writing – original draft. Torsten Siepmann: Data curation. Tom Lindner: Data curation. Claudia Karger: Data curation. Jörg Schwöbel: Data curation, Visualization, Writing – original draft. Leona Anders: Data curation. Robert Faulhaber-Walter: Data curation. Jens Schewe: Data curation. Heike Martin: Data curation. Holger Schirutschke: Data curation. Kerstin Barnett: Data curation. Jan Hüther: Data curation. Petra Müller: Data curation. Torsten Langer: Data curation. Thilo Pluntke: Data curation. Kirsten Anding-Rost: Data curation. Frank Meistring: Data curation. Thomas Stehr: Data curation. Annegret Pietzonka: Data curation. Katja Escher: Data curation. Simon Cerny: Data curation. Hansjörg Rothe: Data curation. Frank Pistrosch: Data curation. Harald Seidel: Data curation. Alexander Paliege: Data curation. Joachim Beige: Data curation. Ingolf Bast: Data curation. Anne Steglich: Data curation, Conceptualization. Florian Gembardt: Data curation, Conceptualization. Friederike Kessel: Data curation, Conceptualization. Hannah Kröger: Data curation, Conceptualization. Patrick Arndt: Data curation, Conceptualization. Jan Sradnick: Data curation, Conceptualization. Kerstin Frank: Data curation, Conceptualization. Anna Klimova: Formal analysis, Project administration. René Mauer: Formal analysis, Project administration. Xina Grählert: Formal analysis, Project administration. Moritz Anft: Data curation, Conceptualization. Arturo Blazquez-Navarro: Formal analysis, Project administration. Timm H Westhoff: Data curation. Ulrik Stervbo: Data curation, Conceptualization. Torsten Tonn: Data curation, Conceptualization. Nina Babel: Writing – original draft, Data curation. Christian Hugo: Data curation, Visualization, Writing – original draft.
Declaration of Competing Interest
PA, NB, KB, IB, AB-N, SC, KE, RF-W, KF, FG, XG, CH, JH, CK, FK, AK, HK, TL, TL, HM, RM, FM, PM, AP, AP, FP, TP, HR, JS, HS, JS, HS, TS, JS, AS, TS, US, JS, TW, TT, LA, KA-R, MA have no conflict of interests.
JB has a relationship with the German Ministry of Health via Hannover Medical School and receives study coordination and per-patient fees for Crit-CoV-U study (proteomic prediction of COVID-19 severity). The study has been supported by a grant from the Else-Kröner-Fresenius-Stiftung.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.lanepe.2021.100178.
Appendix. Supplementary materials
References
- 1.Jager K.J., Kramer A., Chesnaye N.C. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Infektionsfälle in Sachsen - sachsen.de. 04.05.2021, 12:30 2021. https://www.coronavirus.sachsen.de/infektionsfaelle-in-sachsen-4151.html.
- 3.Friedrich P., Sattler A., Muller K., Nienen M., Reinke P., Babel N. Comparing humoral and cellular immune response against HBV vaccine in kidney transplant patients. Am J Transpl. 2015;15(12):3157–3165. doi: 10.1111/ajt.13380. [DOI] [PubMed] [Google Scholar]
- 4.Roch T., Giesecke-Thiel C., Blazquez-Navarro A. Generation of HBsAg-reactive T- and B-cells following HBV vaccination in serological non-responders under hemodialysis treatment. Eur J Immunol. 2021 doi: 10.1002/eji.202048756. [DOI] [PubMed] [Google Scholar]
- 5.Hirzel C., Kumar D. Influenza vaccine strategies for solid organ transplant recipients. Curr Opin Infect Dis. 2018;31(4):309–315. doi: 10.1097/QCO.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 6.Padoan A., Sciacovelli L., Basso D. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clin Chim Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio-Acero R., Castelletti N., Fingerle V. In search for the SARS-CoV-2 protection correlate: a head-to-head comparison of two quantitative S1 assays in a group of pre-characterized oligo-/asymptomatic patients. Infect Dis Ther. 2021;(Jun 16) doi: 10.1007/s40121-021-00475-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Müller L., Andrée M., Moskorz W. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. medRxiv. 2021 2021.03.03.21251066. [Google Scholar]
- 9.Meyer B., Torriani G., Yerly S. Validation of a commercially available SARS-CoV-2 serological immunoassay. Clin Microbiol Infect. 2020;26(10):1386–1394. doi: 10.1016/j.cmi.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EUROIMMUN Medizinische Labordiagnostika AG aPc. SARS-CoV-2 NeutraLISA. 31.03.2021 2021. https://www.coronavirus-diagnostics.com/documents/Indications/Infections/Coronavirus/EI_2606_D_UK_F.pdf.
- 11.Aiello A., Najafi Fard S., Petruccioli E. Spike is the most recognized antigen in the whole-blood platform in both acute and convalescent COVID-19 patients. Int. J. Infect. Dis. 2021;106:338–347. doi: 10.1016/j.ijid.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thieme C.J., Anft M., Paniskaki K. Robust T Cell Response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients. Cell Rep Med. 2020;1(6) doi: 10.1016/j.xcrm.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Team RDC . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: a language and environment for statistical computing, r foundation for statistical computing. [Google Scholar]
- 14.Boyarsky B.J., Werbel W.A., Avery R.K. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grupper A., Rabinowich L., Schwartz D. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;(Apr 18) doi: 10.1111/ajt.16615. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 17.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betts M.R., Ambrozak D.R., Douek D.C. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75(24):11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benotmane I., Gautier-Vargas G., Cognard N. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99(6):1487–1489. doi: 10.1016/j.kint.2021.03.014. Epub 2021 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benotmane I., Gautier-Vargas G., Cognard N. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99(6):1498–1500. doi: 10.1016/j.kint.2021.04.005. Published online 2021 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannick J.B., Del Giudice G., Lattanzi M. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268):268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 22.Eckerle I., Rosenberger K.D., Zwahlen M., Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS ONE. 2013;8(2):e56974. doi: 10.1371/journal.pone.0056974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Candon S., Guerrot D., Drouot L. T cell and antibody responses to SARS-CoV-2: experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am J Transplant. 2021;21(2):854–863. doi: 10.1111/ajt.16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyarsky B.J., Werbel W.A., Avery R.K. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korth J., Jahn M., Dorsch O. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech) Viruses. 2021;13(5):756. doi: 10.3390/v13050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batty C.J., Heise M.T., Bachelder E.M., Ainslie K.M. Vaccine formulations in clinical development for the prevention of severe acute respiratory syndrome coronavirus 2 infection. Adv Drug Deliv Rev. 2021;169:168–189. doi: 10.1016/j.addr.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nance K.D., Meier J.L. Modifications in an Emergency: the Role of N1-Methylpseudouridine in COVID-19 Vaccines. ACS Cent Sci. 2021;7(5):748–756. doi: 10.1021/acscentsci.1c00197. Published online 2021 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Windpessl M., Bruchfeld A., Anders H.J. COVID-19 vaccines and kidney disease. Nat Rev Nephrol. 2021;17(5):291–293. doi: 10.1038/s41581-021-00406-6. Epub 2021 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen S.K., Brown R.S., Jr Hepatitis B treatment: lessons for the nephrologist. Kidney Int. 2006;70(11):1897–1904. doi: 10.1038/sj.ki.5001908. [DOI] [PubMed] [Google Scholar]
- 30.Vlassopoulos D. Recombinant hepatitis B vaccination in renal failure patients. Curr Pharm Biotechnol. 2003;4(2):141–151. doi: 10.2174/1389201033489900. [DOI] [PubMed] [Google Scholar]
- 31.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.