Abstract
The FilmArray Pneumonia Panel has proven to be an effective tool for rapid detection of main respiratory pathogens. However, its rational use needs appropriate knowledge and formation regarding its indication and interpretation. Herein, we provide some advices to help with success of its daily routine use, particularly in critically ill ventilated COVID-19 patients.
Clinical Trial registration number: NCT04453540.
Keywords: Covid-19, Bacterial coinfection, Antimicrobial stewardship, Pneumonia, Critically ill, Molecular diagnosis
1. Introduction
Since the start of the pandemic Covid-19 outbreak, molecular respiratory panel such as FilmArray Pneumonia Panel (FAPP; bioMérieux, France) has been widely used in critically ill patients for bacterial coinfections management. Regarding its performance for pathogens and antimicrobial resistance detection (Suppl. Table 1 ), all authors highlighted FAPP interest for antimicrobial stewardship, especially antibiotic sparing (Caméléna et al., 2020; Ginocchio et al., 2021; Furukawa et al., 2021; Maataoui et al., 2021; Mitton et al., 2021; Zacharioudakis et al., 2021). However, FAPP interpretation could be challenging (Maataoui et al., 2021; Mitton et al., 2021). Indeed, as evoked by Maataoui et al., one of the reasons of a non-optimal use of FAPP was the “lack of knowledge and confidence in the test” (Maataoui et al., 2021). The present study reports the lessons from the implementation of FAPP during the first COVID-19 outbreak, when a training on “how to use FAPP” could not be performed due to the work overload.
Table 1.
Clinical characteristics of patients
| Variables | Patients (n = 90) |
| Male Sex | 72 (80.0) |
| Age (years) | 65 [58.3-70.0] |
| Obesity (BMI >40 kg/m2) | 6 (6.6) |
|
Comorbidities: Hypertension Diabetes Immune deficiency Chronic respiratory failure Chronic hemodialysis Cirrhosis |
50 (55.5) 27 (30.0) 18 (20) 10 (11.0) 1 (1.0) 1 (1.0) |
|
ICU data: SAPS 2 score ICU LOS (days) IMV duration (days) ECMO |
44 [36-61] 23 [14-37] 17 [11-27] 10 (11) |
| ICU mortality | 25 (28) |
Data are presented as: n (%) – median [IQR].
Immune deficiency = diabetes, neoplasia, transplant, neutropenia; aplasia, immunosuppressive therapy; SAPS 2 score = Simplified Acute Physiology Score II; LOS = Length of stay; IMV = invasive mechanical ventilation.
This is a multicenter retrospective analysis (clinicalTrial.gov NCT04453540) of all critically ill patients who were admitted to the Nancy and Reims University Hospitals (six ICUs) from March to May 2020, with COVID-19 and respiratory failure requiring invasive mechanical ventilation (IMV). The local institutional ethics committee approved this study (Comité d’éthique du CHRU de Nancy, N°CO-20). Informed consent was obtained from all participants and/or their legal guardians. Presence of SARS-CoV-2 was diagnosed using RT-PCR. All patients with suspicion of bacterial pneumonia were eligible. The decision to prescribe FAPP was at the discretion of the clinician. Only patients with concomitant FAPP, conventional culture (CC) and Gram stain were included. Samples were endotracheal aspirates (ETA) and bronchoalveolar lavages (BAL). Results of the FAPP and Gram stain were available for the intensivists within four hours. A first result of the CC was available after one day with a definitive result within five days. For quantitative culture, only the bacteria above the following threshold were considered: 104 CFU/mL for BAL and 105 CFU/mL for ETA. Phenotypic drug susceptibility testing was performed according to the recommendations of the Antibiogram Committee of the French Society for Microbiology (CA-SFM)/European Committee for Antibiotic Susceptibility Testing (EUCAST). A multidisciplinary expert committee (MEC) composed of intensivists, infectious disease specialists and microbiologists from both centers analyzed retrospectively the contribution of FAPP compared to CC in the treatment decision of pneumonia according to criteria from Weiss et al. (Weiss et al., 2015). Antibiotics used to treat any concomitant infection were not considered by the MEC. Early bacterial coinfections, represented by community-acquired pneumonia (CAP), were defined as infections occurring during the first 48h of ICU admission. The ventilator-associated pneumonia (VAP) were defined as infections occurring after 48h of IMV. Multiple tests from the same patient were considered independent when performed during distinct infectious episodes. Categorical data were analyzed using chi-square test or Fisher's exact test. Statistical analyses were performed by an independent statistician using SAS 9.4 software (SAS Institute, Inc, Cary, N.C.).
Overall, 344 patients with a positive SARS-CoV-2 RT-PCR were admitted in the participating ICUs of whom 90 fulfilled eligibility criteria. Samples were 74 ETA and 45 BAL. Characteristics and ICU data are presented in Table 1. Bacteriological results were presented in Tables 2 and 3 . The rate of clinically confirmed CAP and VAP were 5.0% and 40.3%, respectively. Bacterial pathogens were detected by FAPP (45.4%) and/or by CC (38.7%) in 41 and 34 ETA and in 13 and 12 BAL, respectively. The adequacy between FAPP and CC in pathogen detection was better (P = 0.017) for BAL (95.6%) than for ETA (79.7%). Staphylococcus aureus and Pseudomonas aeruginosa were the most prevalent pathogens. Two cases of negative FAPP (no detection of Morganella morganii) have led to inappropriate discontinuation of empirical antibiotic therapy. Two Extended-Spectrum β-lactamase ESBL (not-CTX-M)-producing Enterobacter cloacae and one 3GC-resistant P. aeruginosa were isolated by CC without detection of resistance gene by FAPP. Regarding the six samples with methicillin-resistance of S. aureus (MRSA) detected in FAPP, only three have a S. aureus-positive culture and all where methicillin-susceptible (MSSA). According to MEC analysis, FAPP-based therapeutic decision was concordant with CC-based therapeutic decision in 91% for BAL compared with 69% for ETA (P = 0.009). The most contribution of FAPP regarding antibiotic prescription was antibiotic spare (Table 2). However, we observed that intensivists considered FAPP for treatment only in 42.0% (50/119) of cases.
Table 2.
Bacteriological results according to the type of pneumonia and contribution of the panel FAPP on antibiotic prescription
|
Samples (n = 119) |
||
| CAP | VAP | |
| Type of suspected pneumonia | 27 (22.7) | 92 (77.3) |
|
Confirmed diagnostic of pneumonia (% among suspected / % among total) |
6 (22.2 / 5.0) |
48 (52.2 / 40.3) |
|
Type of samples: ETA BAL |
15 12 |
59 33 |
| Antibiotics 48h prior to samples (n = 55) | 15 (55.6) | 40 (43.5) |
|
Bacterial copathogens: Staphylococcus aureus Pseudomonas aeruginosa Haemophilus influenzae Escherichia coli Klebsiella pneumoniae Enterobacter cloacae Klebsiella aerogenes Proteus spp. Serratia marcescens Streptococcus agalactiae Moraxella catarrhalis Mycoplasma pneumoniae Morganella morganii Hafnia alvei Providencia stuartii |
FAPP / CC 0 / 0 0 / 0 4 / 0 0 / 0 1 / 1 0 / 0 0 / 0 0 / 0 0 / 0 0 / 0 1 / 0 1 / NA NA / 0 NA / 0 NA / 0 |
FAPP / CC 17 / 12 11 / 11 6 / 3 9 / 5 6 / 6 5 / 5 4 / 2 4 / 3 3 / 3 3 / 1 1 / 1 0 / NA NA / 2 NA / 1a NA / 1a |
|
Resistance detection: MRSA 3GC-R Gram-negative bacilli |
FAPP / AST 0 / 0 0 / 0 |
FAPP / AST 6 / 0 5 / 6b |
| Type of pneumonia | CAP | VAP |
|
Contribution of FAPP at first intensivist decisionc No modification of empirical antibiotics Speeded-up adequate antibiotic Antibiotic spared Inappropriate antibiotic treatment Inappropriate stopped antibiotic |
0 2 (20.0) 8 (80.0) 0 0 |
3 (7.5) 9 (22.5) 20 (50.0) 7 (17.5) 1 (2.5) |
|
Contribution of FAPP based on MEC analysise No modification of empirical antibiotics Speeded-up adequate antibiotic Antibiotic spared Inappropriate antibiotic treatment Inappropriate stopped antibiotic |
1 (3.7) 4 (14.8) 22 (81.5) 0 0 |
11 (12.0) 13 (14.1) 56 (60.9) 10 (10.9) 2 (2.2) |
Data are presented as: n (%).
ETA = endotracheal aspirate; BAL = bronchoalveolar lavage; CAP = community-acquired pneumonia (defined as infections occurring during the first 48h of ICU admission); VAP = ventilator-associated pneumonia; FAPP = FilmArray® Pneumonia Panel; CC = conventional culture; AST = antimicrobial susceptibility testing; MRSA = methicillin-resistant S. aureus; 3GC-R = third generation cephalosporins resistance; NA = not applicable (species not detected either by the FAPP or by the CC); MEC = multidisciplinary expert committee.
a The isolation of H. alvei and P. stuartii in CC had no impact on antibiotic therapy as they were covered by the antibiotics administered following the detection of other pathogens detected by FAPP.
b Among 3GC-resistant Gram-negative bacilli, 3 CTX-M were detected by both FAPP and CC, 2 CTX-M were detected only by FAPP, 2 ESBL not belonging to CTX-M as well as one 3GC-resistant P. aeruginosa were detected only by CC.
c A contribution of FAPP at first intensivist decision was noted in 50 samples (42.0%).
d Decrease unnecessary antibiotic use (interruption or de-escalation).
e Theoretical contribution of FAPP after MEC analysis of the 119 samples (100.0%).
Table 3.
Bacteriological results according to the type of respiratory samples
|
Samples (n = 119) |
||
| ETA (n = 74) | BAL (n = 45) | |
|
Type of pneumonia CAP: suspected/confirmed VAP: suspected/confirmed |
15 / 5 59 / 34 |
12 / 1 33 / 14 |
| Antibiotics 48h prior to samples (n = 55) | 32 (58) | 23 (42) |
|
Positive direct examination Presence of Gram + Presence of Gram – Polymicrobial |
39 (53) 6 (15) 9 (23) 24 (62) |
14 (33) 3 (21) 1 (7) 10 (72) |
|
Infection polymicrobial FAPP (n = 18) Mean number of bacteria detected CC (n = 8) Mean number of bacteria detected |
14 (77) 2.2 5 (62) 2.2 |
4 (23) 2.25 3 (38) 2 |
|
Bacterial copathogens: Staphylococcus aureus Pseudomonas aeruginosa Haemophilus influenzae Escherichia coli Klebsiella pneumoniae Enterobacter cloacae Klebsiella aerogenes Proteus spp. Serratia marcescens Streptococcus agalactiae Moraxella catarrhalis Mycoplasma pneumoniae Morganella morganii Hafnia alvei Providencia stuartii |
FAPP / CC 12 / 6 10/ 10 7 / 2 7 / 4 6 / 6 3 / 3 3 / 1 4 / 3 2 / 2 2 / 1 2 / 1 1 / NA NA / 1 NA / 1a NA / 1a |
FAPP / CC 5 / 6 1 / 1 3 / 1 2 / 1 1 / 1 2 / 2 1 / 1 0 / 0 1 / 1 1 / 0 0 / 0 0 / NA NA / 1 NA / 0 NA / 0 |
|
Resistance detection: MRSA 3GC-R Gram-negative bacillib |
FAPP / AST 5 / 0 4 / 3 |
FAPP / AST 1 / 0 1 / 3 |
Data are presented as: n (%).
ETA = endotracheal aspirate; BAL = bronchoalveolar lavage; CAP = community-acquired pneumonia (defined as infections occurring during the first 48h of ICU admission); VAP = ventilator-associated pneumonia; FAPP = FilmArray Pneumonia Panel; CC = conventional culture; AST = antimicrobial susceptibility testing; MRSA = methicillin-resistant S. aureus; 3GC-R = third generation cephalosporins resistance; NA = not applicable (species not detected either by the FAPP or by the CC).
a The isolation of H. alvei and P. stuartii in CC had no impact on antibiotic therapy as they were covered by the antibiotics administered following the detection of other pathogens detected by FAPP.
b Among 3GC-resistant Gram-negative bacilli, 3 CTX-M were detected by both FAPP and CC, 2 CTX-M were detected only by FAPP, 2 ESBL not belonging to CTX-M as well as one 3GC-resistant P. aeruginosa were detected only by CC.
These results confirmed the usefulness of FAPP to rapidly diagnose bacterial coinfection. However, there is a room for improvement of its use and interpretation. Herein, we suggest four tips for a tailored use of FAPP in critically ill ventilated patients:
2.1. Training for mastering FAPP by the intensivists is required for successful utilization in the daily routine practice.
We believe that an appropriate knowledge about FAPP performance and results interpretation should led to a better antibiotic use. Therefore, a collaboration between microbiologists and intensivists is mandatory.
3.2. FAPP should be performed on BAL to avoid over-diagnosis of bacterial coinfection.
Lower relevance of FAPP results from ETA compared to BAL for treatment could be explained by detection of not significant bacteria from the tracheobronchial colonization. However, if BAL could not be performed, ETA could be used with cautious interpretation of FAPP results.
4.3. Conventional culture should be systematically performed in parallel.
To detect bacteria not included in the FAPP (Mitton et al., 2021) and to confirm resistance gene detection. For Gram-negative bacilli, FAPP detects only CTX-M ESBL. Moreover, as previously described (Webber et al., 2020), FAPP led to over-detection of MRSA that could lead to an overuse of anti-MRSA antibiotics, especially in case of local ecology with low prevalence of MRSA. Indeed, among the 6 samples with MRSA detected in FAPP (5 ETA and 1 BAL), only three (2 ETA and 1 BAL) had a S. aureus-positive culture and all where MSSA. Such discordance could be explained either by the co-occurrence of a S. aureus with an empty SCCmec cassette and methicillin-resistant negative coagulase staphylococci, or by a mixed specimen of MSSA and MRSA, respectively above and below the threshold of culture detection.
5.4. Therapeutic decision must be re-evaluated with the result of 2-days conventional culture.
The delay of 2 days for definitive CC interpretation should cover slowing growing bacteria (low bacterial load or prior antimicrobial treatment) as well as drug susceptibility testing results. Moreover, of 65 negative-FAPP, 62 (95.4%) showed 5-days negative culture and 3 (4.6%) were positive (for outside-panel bacteria) but within 2 days of culture. Consequently, in absence of i) severity criteria, namely septic shock or severe ARDS (according to Berlin criteria), and of ii) Gram-negative bacilli at Gram stain, empirical antibiotic therapy could be stopped based on a negative-FAPP result.
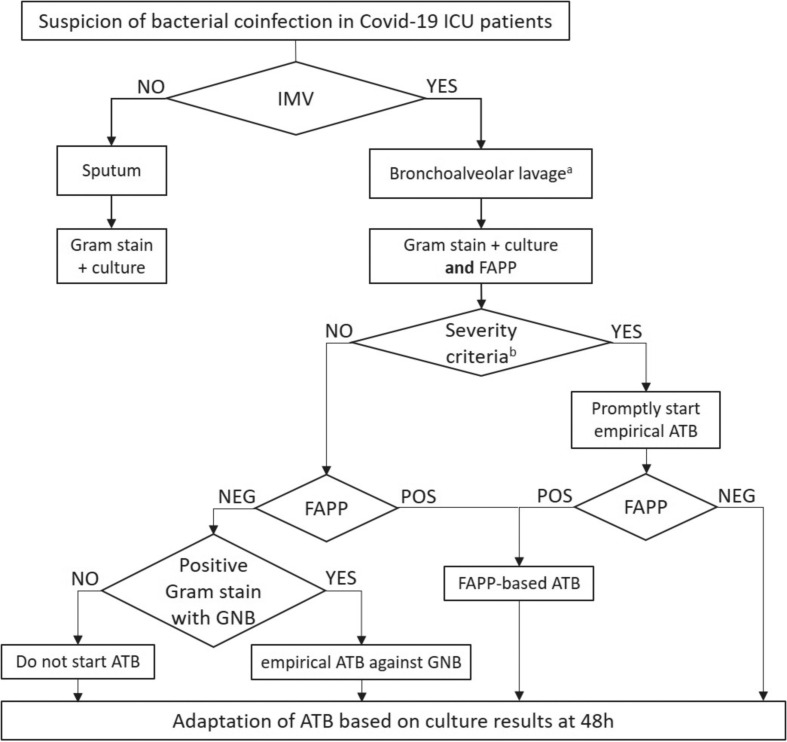
In the present study, a FAPP use based on these tips would allow 65.6% of antibiotic spare in bacterial coinfection and a better adequacy of empirical antibiotic treatment. Regarding VAP, FAPP should consider local ecology for optimal interpretation, especially for resistance detection (i.e. P. aeruginosa with non-enzymatic resistance). Based on our results, we propose an algorithm to improve the use of FAPP for antibiotic stewardship at the bedside (Fig. 1 ). Further studies are now warranted to demonstrate that rational use of FAPP will also improve patient outcome.
Fig. 1.
Clinical algorithm for initiating antibiotics using FAPP in bacterial coinfection of critically ill COVID-19 patients. IMV, invasive mechanical ventilation; BAL, bronchoalveolar lavage; FAPP, FilmArray® Pneumonia Panel; ATB, antibiotics; GNB, Gram-negative bacilli. a Endotracheal aspirate samples could be used but need cautious interpretation regarding the risk of over-diagnosis due to tracheobronchial colonization; b Septic shock (according to SEPSIS-3) or severe ARDS (according to Berlin criteria).
Ethics consideration
The institutional ethics committee approved this study (Comité d’éthique du CHRU de Nancy, N°CO-20). All experiments were performed in accordance with guidelines and regulations. Informed consent was obtained from all participants and/or their legal guardians.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The sponsor was CHRU de Nancy (Direction de la Recherche et de l'Innovation).
Author contribution
Emmanuel Novy (EN) and Corentine Alauzet (CA) participated equally in this work. EN, CA and Thomas Guillard (TG) contributed substantially to the study design and the writing of the manuscript. EN, CA, TG, Carine Thivilier (CT), Antoine Goury (AG) contributed to the acquisition, analysis and interpretation of data. CT and AG made critical revision of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest pertaining to this study.
Acknowledgment
Provided and cared for study patients and general supervision: Bruno Mourvillier, Bruno Levy, Gérard Audibert, Marie-Reine Losser, Philippe Guerci, Sébastien Gibot, Alain Lozniewski and Christophe de Champs. Collected data: Anne-Elisabeth Manteaux, Tom Alix, Nejla Aissa, Gautier Julien and Michel Bonnivard. Statistical analysis: Eliane Albuisson.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.diagmicrobio.2021.115507.
Appendix. Supplementary materials
References
- Caméléna F, Moy A-C, Dudoignon E, Poncin T, Deniau B, Guillemet L, et al. Performance of a multiplex polymerase chain reaction panel for identifying bacterial pathogens causing pneumonia in critically ill patients with COVID-19. Diagn Microbiol Infect Dis. 2020;99 doi: 10.1016/j.diagmicrobio.2020.115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginocchio CC, Garcia-Mondragon C, Mauerhofer B, Rindlisbacher C, EME Evaluation Program Collaborative Multinational evaluation of the BioFire FilmArray Pneumonia plus Panel as compared to standard of care testing. Eur J Clin Microbiol Infect Dis. 2021;40:1609–1622. doi: 10.1007/s10096-021-04195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa D, Kim B, Jeng A. Real-life utilization of BioFire Filmarray pneumonia panel as an antibiotic stewardship tool. Infect Dis (Lond) 2021;53:308–313. doi: 10.1080/23744235.2020.1866774. [DOI] [PubMed] [Google Scholar]
- Maataoui N, Chemali L, Patrier J, Tran Dinh A, Le Fèvre L, Lortat-Jacob B, et al. Impact of rapid multiplex PCR on management of antibiotic therapy in COVID-19-positive patients hospitalized in intensive care unit. Eur J Clin Microbiol Infect Dis. 2021 doi: 10.1007/s10096-021-04213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitton B, Rule R, Said M. Laboratory evaluation of the BioFire FilmArray Pneumonia plus panel compared to conventional methods for the identification of bacteria in lower respiratory tract specimens: a prospective cross-sectional study from South Africa. Diagn Microbiol Infect Dis. 2021;99 doi: 10.1016/j.diagmicrobio.2020.115236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharioudakis IM, Zervou FN, Dubrovskaya Y, Inglima K, See B, Aguero-Rosenfeld M. Evaluation of a Multiplex PCR Panel for the Microbiological Diagnosis of Pneumonia in Hospitalized Patients: Experience from an Academic Medical Center. Int J Infect Dis. 2021;104:354–360. doi: 10.1016/j.ijid.2021.01.004. [DOI] [PubMed] [Google Scholar]
- Weiss E, Zahar J-R, Lesprit P, Ruppe E, Leone M, Chastre J, et al. Elaboration of a consensual definition of de-escalation allowing a ranking of β-lactams. Clin Microbiol Infect. 2015;21 doi: 10.1016/j.cmi.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Webber DM, Wallace MA, Burnham CA, Anderson NW. Evaluation of the BioFire FilmArray Pneumonia Panel for Detection of Viral and Bacterial Pathogens in Lower Respiratory Tract Specimens in the Setting of a Tertiary Care Academic Medical Center. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00343-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.