Abstract
Background:
Patients with chronic hypertension are at increased risk for superimposed preeclampsia. The 2016 American College of Obstetricians and Gynecologists (ACOG) guideline recommended initiating 81 mg daily aspirin for all pregnant women with chronic hypertension to prevent superimposed preeclampsia.
Objective:
1) To evaluate the rates of implementation of the 2016 ACOG guideline over time; 2) To evaluate the effectiveness of aspirin for the prevention of superimposed preeclampsia and other adverse maternal and neonatal outcomes in women with chronic hypertension before and after this guideline.
Study design:
This is a retrospective study of women with chronic hypertension who delivered at Thomas Jefferson University Hospital from 1/2014 through 6/2018. This cohort of women with chronic hypertension was divided into 2 groups, before and after the ACOG recommendation published in 7/2016. Daily 81 mg aspirin was initiated between 12 to 16 weeks. We excluded multiple gestations and incomplete records. The primary outcome was incidence of superimposed preeclampsia, and secondary outcomes were incidence of superimposed preeclampsia with or without severe features, small for gestational age, and preterm birth <37 weeks. Subgroup analysis based on risk stratification was evaluated in women with chronic hypertension requiring antihypertensive medication, history of preeclampsia and pre-gestational diabetes.
Results:
We identified 457 pregnant women with chronic hypertension, 203 in the post-ACOG group and 254 in the pre-ACOG group. Aspirin 81 mg was offered to 142 (70%) in the post-ACOG group and 18 (7.0%) in the pre-ACOG group. Maternal demographics were not significantly different. The overall incidence of superimposed preeclampsia was not significantly different: 87 (34.3%) vs. 72 (35.5%), p=0.79 in the pre and post ACOG guideline groups, respectively. Superimposed preeclampsia with severe features significantly increased: 32 (12.6%) vs 9 (4.4%), p<0.01 while superimposed preeclampsia without severe features significantly decreased: 55 (21.7%) vs 63 (31.0%) p=0.03. There were no significant differences in small for gestational age neonates or preterm birth <37 weeks incidences between groups. There were no significant differences in the subgroup analysis based on the severity of chronic hypertension requiring antihypertensive medication, history of preeclampsia, or pregestational diabetes.
Conclusion:
After the adoption of the ACOG guidelines in 70% of the cohort, superimposed preeclampsia, small for gestational age, and preterm birth were not significantly decreased after implementation of aspirin 81mg initiated between 12 to 16 weeks of gestation.
Keywords: Low dose aspirin, aspirin, superimposed preeclampsia, preeclampsia, chronic hypertension, ACOG, small for gestational age, preterm birth
Condensation:
Low dose aspirin does not prevent superimposed preeclampsia in women with chronic hypertension.
INTRODUCTION
Preeclampsia is a hypertensive disorder that affects millions of pregnant women on a global scale. It increases the risk of preterm birth (PTB), end-organ damage, maternal mortality, and future cardiovascular disease such as coronary artery disease and stroke.1 Hypertensive disorder in pregnancy is the second most common direct cause of maternal mortality worldwide (14%).2 Superimposed preeclampsia occurs when women with chronic hypertension (CHTN) develop preeclampsia. It is characterized by worsening or uncontrollable hypertension, new onset of proteinuria, or significantly increased preexistent proteinuria.3
The pathophysiology underlying preeclampsia is not fully defined.4, 5 It has been theorized that there are two stages of preeclampsia: 1) inadequate placental implantation6, 7 and 2) a maternal hypertensive state resulting from placental hypoxia, oxidative stress, and inflammation.8, 9 In normal pregnancy, trophoblasts invade the myometrium and induce remodeling of the uterine spiral arteries to low resistance vessels, allowing for enhanced uteroplacental blood flow.4, 10 In preeclampsia, shallow invasion of the myometrium causes poor remodeling of the spiral arteries, leading to reduced uteroplacental perfusion.7, 10 The metabolic stress results in the release of secondary inflammatory mediators such as thromboxane A2 into the maternal bloodstream, which causes endothelial dysfunction and reduces endothelial-derived vasodilator properties, leading to vasoconstriction and increased maternal blood pressure (BP).11 Release of other mediators such as soluble receptor (sFLT) for vascular endothelial growth factors (VEGF), placental growth factor (PlGF), type 1 angiotensin II receptor antibodies, hypoxia inducible factors (HIF) have all been implicated in multiple pathophysiological mechanisms to produce the clinical syndrome preeclampsia.10, 12 Pre-existing conditions such as hypertension, diabetes, and inflammatory states such as autoimmune disease are believed to contribute to poor placentation, decreased uteroplacental perfusion, and overall level of inflammation4–8, 11. Low dose aspirin has been proposed to decrease the risk of preeclampsia, through inhibition of cyclooxygenase-1 in the arachidonic acid pathway, decreasing thromboxane A2 production.13
In September 2014, the United States Preventative Services Task Force (USPSTF)14 conducted a systematic review to identify the risk factors for which physicians should recommend low dose aspirin to prevent the incidence of preeclampsia: history of preeclampsia, multifetal gestation, chronic hypertension, type 1 or type 2 diabetes, renal disease, or autoimmune disease. In July 2016, the American College of Obstetricians and Gynecologists (ACOG) endorsed the USPSTF recommendation in offering 81 mg low dose aspirin for patients with these high risk factors between 12 and 28 weeks of gestation.15 Guidelines in multiple countries now recommend that low-dose aspirin prophylaxis should be considered for women with more than one of several moderate risk factors for preeclampsia.1, 15–18
The objective of this study was to evaluate 1) the implementation of 2016 ACOG guideline recommending low dose aspirin in high-risk patients over time and 2) the effectiveness of aspirin in preventing superimposed preeclampsia and adverse maternal and neonatal outcomes in women with CHTN.
METHODS
Study and Population
This is a retrospective cohort study of all patients with singleton gestation and diagnosis of CHTN who had prenatal care since the first trimester and delivered at Thomas Jefferson University Hospital from January 2014 to June 2018. The diagnosis of CHTN was made by patient history, current prescription of antihypertensive medication, or elevated BP defined as ≥140 mmHg systolic or ≥90 mmHg diastolic identified on two occasions before 20 weeks of gestation. Pregnancy dating was confirmed by ultrasound before 20 weeks of gestation. We divided this cohort of women with CHTN into two groups: pre-ACOG guideline and post-ACOG guideline. The post-ACOG group consisted of women who were less than 16 weeks after the ACOG guideline was issued in July 2016. Our practice recommended daily low dose 81 mg aspirin to start between 12 to 16 weeks of gestation to patients with CHTN, and it was continued until delivery. Patients with multiple gestations, major fetal malformations, known genetic conditions, incomplete medical records or incomplete delivery data were excluded from the study. The Institutional Review Board at Thomas Jefferson University Hospital approved this study; IRB # 14D.96 was first approved on 03/15/2014, reviewed annually, and last approved on 5/23/2019.
Variables
All data was obtained from electronic medical records, including demographic data such as age, race, body mass index (BMI, kg/m2), obesity (BMI > 30 kg/m2), nulliparity, and smoking during pregnancy. Diagnosis of pre-gestational diabetes, history of preeclampsia, and other chronic medical conditions were also recorded. Data regarding prescription of aspirin including date and gestational age at starting date were collected. Patient adherence to aspirin was collected through prenatal visit documentation from patient self-reporting. Tablet count was not feasible.
Diagnostic criteria for superimposed preeclampsia with and without severe features were based on the 2013 ACOG Hypertension in Pregnancy Task Force.3 Superimposed preeclampsia was diagnosed by a sudden increase in previously well-controlled BP or escalation of antihypertensive medications to control BP, new onset of proteinuria or a sudden increase in known proteinuria prior to or early in pregnancy. Sudden increases in BP or proteinuria were not well defined by the 2013 ACOG Hypertension in Pregnancy guideline. In our practice, we defined sudden increase in BP as previously well-controlled BP: normal (<140 mmHg systolic or <90 mmHg diastolic) or mild range BP (140–159 mmHg systolic or 90–104 mmHg diastolic) in clinic and by self-monitoring at home that subsequently worsened to severe range BP (≥160 mmHg systolic or ≥105 mmHg diastolic) as confirmed by the clinic or hospital, requiring initiation or escalation of antihypertensive medications to achieve a goal of mild range BP’s. Escalation of medication was defined as the initiation of antihypertensive medication, increase in prior antihypertensive medication or addition of a second antihypertensive agent in order to meet the goal of mild range BP’s. The treating provider determined the choice of antihypertensive. Sudden increase in proteinuria was defined as a 50% increase in proteinuria compared to baseline pre-pregnancy or first trimester proteinuria. In the absence of a 24-hour urine protein collection, often due to non-compliance, a urine protein/creatinine ratio (UPC) was obtained. A sudden increase in proteinuria was also defined as a UPC ≥ 0.5 with a baseline UPC ≥ 0.3.
Superimposed preeclampsia with severe features was diagnosed when any of the following were present: severe range of BP ≥160 mmHg systolic or ≥105 mmHg diastolic despite escalation of antihypertensive therapy (uncontrolled severe BP), platelet count <100.000/microliter, elevated liver transaminases (two times the upper limit of normal concentration according to institutional laboratory), new onset or worsening renal insufficiency (creatinine ≥ 1.1 mg/dL or twice the baseline value), pulmonary edema or severe persistent right upper quadrant/epigastric pain or cerebral/visual disturbances unresponsive to medication.3
Indications for delivery, delivery information, maternal complications and neonatal outcomes such as birthweight in grams, small for gestational age (SGA) defined as less than 10th percentile according to Fenton growth chart,19 Apgar at 5 minutes, neonatal intensive care unit (NICU) admission rates, and incidence of mechanical ventilation, respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage or sepsis were also collected.
Our institutional practice regarding management of patients with CHTN included increasing the frequency of prenatal visits to every 2–4 weeks and prescribing automated home blood pressure devices for self-monitoring and reporting at prenatal visits. Blood pressure devices were not standardized as the brand was determined by insurance coverage or out of pocket payment. Patients brought them to the clinic for teaching as needed. We emphasized the importance of appropriate cuff size given the high incidence of obesity in our population. Patients notified physicians of elevated blood pressures by phone or electronic message which were used to triage for further clinical evaluation. All decisions regarding diagnoses, interventions, and medications were based on blood pressure readings confirmed by standardized instruments in the clinic, emergency room, or labor and delivery unit. Chronic antihypertensive medication was continued if patients were taking them prior to the first prenatal visit. Medications with potential fetal adverse effects were substituted. Medication was initiated if BP was ≥ 160mmHg systolic or ≥105 mmHg diastolic as recommended by ACOG. Baseline labs were requested at the first prenatal visit to evaluate for end-organ damage; these included complete blood count, electrolytes, liver function tests, creatinine, 24-hour urine protein, EKG, HbA1c or early 1-hour glucose test if not already diagnosed with diabetes mellitus, and an ophthalmology consultation. Reports of dating and anatomy ultrasounds were reviewed for gestational age, major fetal malformations, and intrauterine growth restriction (IUGR). Serial fetal growth evaluations were done every 4 weeks for patients with CHTN per TJUH protocol. IUGR was defined by estimated fetal weight less than 10th percentile on the Hadlock et al growth curve.20 Patients diagnosed with IUGR were followed by non-stress tests (NST) twice a week and weekly umbilical artery Doppler and amniotic fluid index. Women diagnosed with superimposed preeclampsia without severe features continued outpatient observation with weekly visits, laboratories and twice a week NSTs, while those with severe features were admitted to the hospital for daily surveillance to determine the appropriate time for delivery. Indications for delivery were ≥ 37 weeks for those with superimposed preeclampsia without severe features and ≥ 34 weeks for those with superimposed preeclampsia with severe features according to the 2013 ACOG Hypertension in Pregnancy Task Force.3 The primary outcome was the overall incidence of superimposed preeclampsia compared in the pre and post ACOG groups and the implementation of the low dose aspirin guideline. Secondary outcomes were incidence of superimposed preeclampsia with and without severe features, gestational age at diagnosis of preeclampsia, gestational age at delivery, and incidence of preterm delivery before 37, 34, and 28 weeks of gestation.
Analysis of incidence of superimposed preeclampsia with and without severe features pre and post ACOG guidelines was conducted in the following subgroups: 1) Only CHTN without other risk factors, 2) Severe CHTN requiring antihypertensive medications, 3) CHTN and history of preeclampsia, and 4) CHTN and pre-gestational diabetes.
Statistics
Statistical analysis was conducted using Statistical Package for Social Sciences (SPSS) version. 22 (IBM Inc., Armonk, NY, USA). Data are shown as means ± standard deviation (SD) or number (percentage). Differences between pre-ACOG group and post-ACOG groups were analyzed using Chi-square test or Fisher’s exact test for categorical variables. Results of primary and secondary outcomes were presented as odds ratio (OR) or as mean difference with 95% of confidence interval (CI). In the presence of any significant demographic confounders, adjusted ORs (aOR) were calculated after adjusting for the confounders. Sub-group analysis by risk factors and nonparametric data were compared using Wilcoxon and Mann-Whitney tests. P value < 0.05 was considered statistically significant.
RESULTS
Eight thousand one hundred and eighty women with singleton pregnancy delivered at TJUH between January 2014 and June 2018. Among them, 652 (8.03%) had a diagnosis of CHTN at discharge, and 457 (70%) of them had complete records for analysis. The pre-ACOG group had 254 (55.5%) patients and the post-ACOG group had 203 (44.5%) patients.
Maternal Demographics
There were no statistically significant differences in the demographic characteristics between the pre- and post-ACOG groups. (Table 1). As a result, we did not need to adjust for any confounders in the main outcomes. Dating ultrasound by crump rump length before 14 weeks was done in 388 (85%) of women, and all women had anatomy ultrasound and serial fetal growth evaluations.
Table 1.
Maternal Demographics
| Characteristic | Pre ACOG N=254 |
Post ACOG N=203 |
P - value |
|---|---|---|---|
| Maternal age | 32.7±5.4 | 31.9±6.2 | 0.12 |
| Race | |||
| African American | 160 (63.0) | 128 (63.1) | 1.0 |
| White | 62 (24.4) | 46 (22.7) | 0.74 |
| Hispanic | 12 (4.7) | 13 (6.4) | 0.53 |
| Asian | 12 (4.7) | 7 (3.4) | 0.64 |
| Other | 8 (3.15) | 9 (4.4) | 0.62 |
| Gravida | 3.9±2.6 | 3.99±2.7 | 0.98 |
| Nulliparous | 77 (30.3) | 53 (26.1) | 0.34 |
| History of Preterm birth | 66 (26.0) | 60 (29.5) | 0.4 |
| BMI | 35.0±9.3 | 34.89±8.3 | 0.84 |
| Obesity BMI >30 | 169 (66.5) | 141 (69.45) | 0.54 |
| History of Preeclampsia | 55 (21.7) | 61 (30.1) | 0.051 |
| Pregestational diabetes | 35 (13.8) | 29 (14.3) | 0.23 |
| Gestational diabetes | 37 (14.6) | 26 (12.8) | 0.64 |
| Thyroid disorders | 10 (4.92) | 10 (3.93) | 0.29 |
| Antiphospholipid syndrome | 1 (0.4) | 0 (0) | 0.69 |
| Autoimmune disorder | 11 (4.3) | 5 (2.4) | 0.31 |
| Vascular disease | 4 (1.57) | 1 (0.4) | 0.38 |
| Sickle cell disease | 4 (1.57) | 1 (0.5) | 0.38 |
| Thromboembolism | 11 (4.33) | 2 (0.98) | 0.10 |
| Renal disease | 16 (6.3) | 5 (2.4) | 0.16 |
| Smoking | 34 (16.74) | 52 (20.47) | 0.85 |
| Substance abuse | 20 (7.87) | 15 (7.39) | 0.59 |
| Antihypertensive medication | 117 (46.06) | 61 (30.04) | 0.11 |
| Prescribed aspirin | 18 (7.0) | 142 (70.0) | <0.01 |
| Verbally reported taking aspirin | 15 (5.9) | 135 (66.5) | <0.01 |
| Gestational age at aspirin prescription | 14.94 ±6.3 | 14.97 ±4.3 | 0.98 |
Values reported as n (%) or mean ± standard.
ACOG: American College of Obstetricians and Gynecologist
BMI: Body mass index
ACOG Guideline Implementation
Aspirin was offered to 142/203 (70%) women post-ACOG and 18/254 (7%) pre-ACOG. Implementation of the guideline increased over time from 24.2% in 2016 to 82.6% in 2018, (Figure 1). After the ACOG guideline was released, patients with CHTN and history of preeclampsia were more likely to be prescribed aspirin than patients with only CHTN 48/55 (87.3%) vs 72/114 (63.3%), p<0.01) and had better documentation regarding adherence to the prescribed aspirin than patients with only CHTN 45/55 (81.8%) vs 65/114 (57.0%), p<0.01). (Figure 2)
Figure 1.
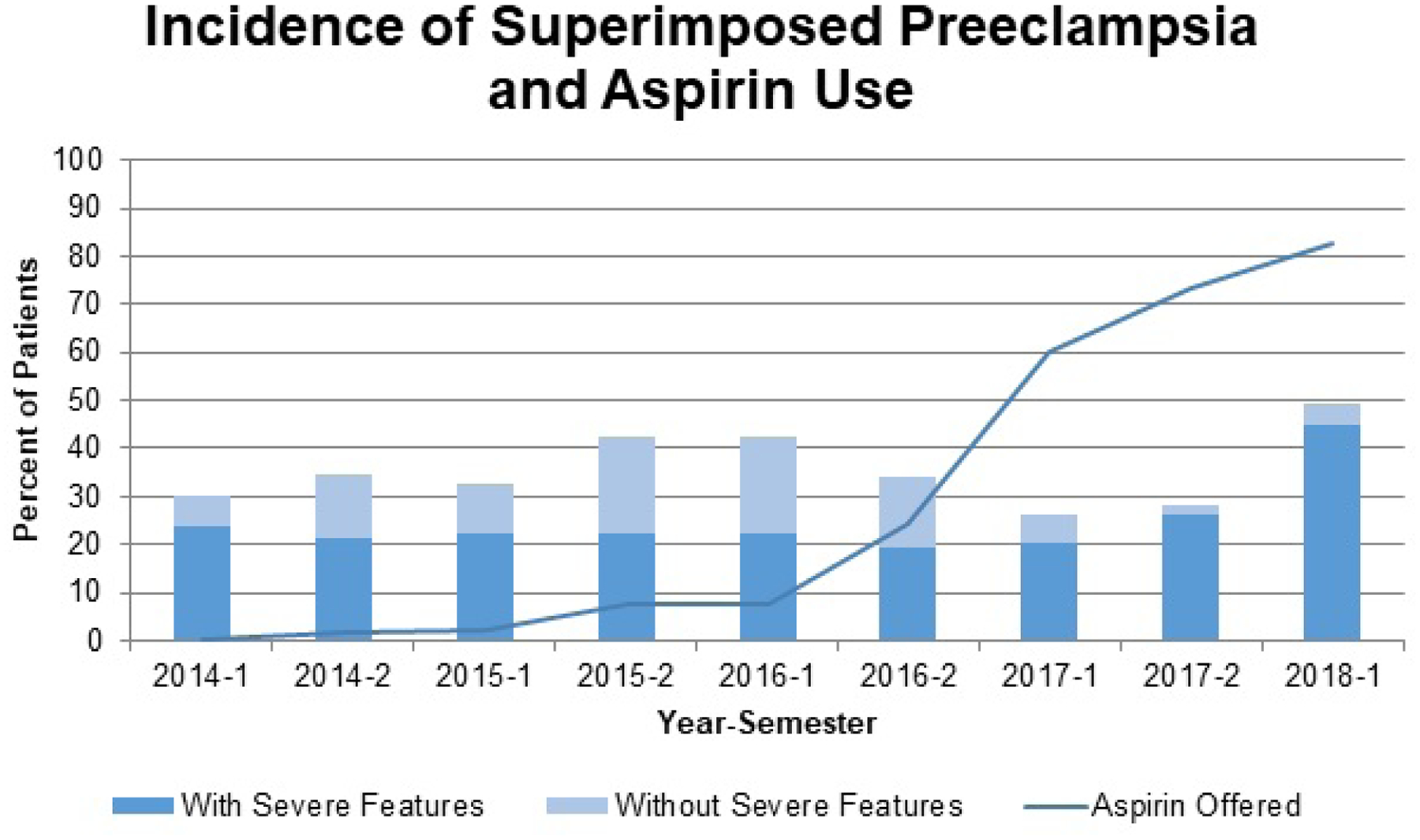
Incidence of Superimposed Preeclampsia and Aspirin Use
Percent of patients who developed superimposed preeclampsia with and without severe features and percent of patients who were offered aspirin over time. Time presented as year-semester.
Figure 2.
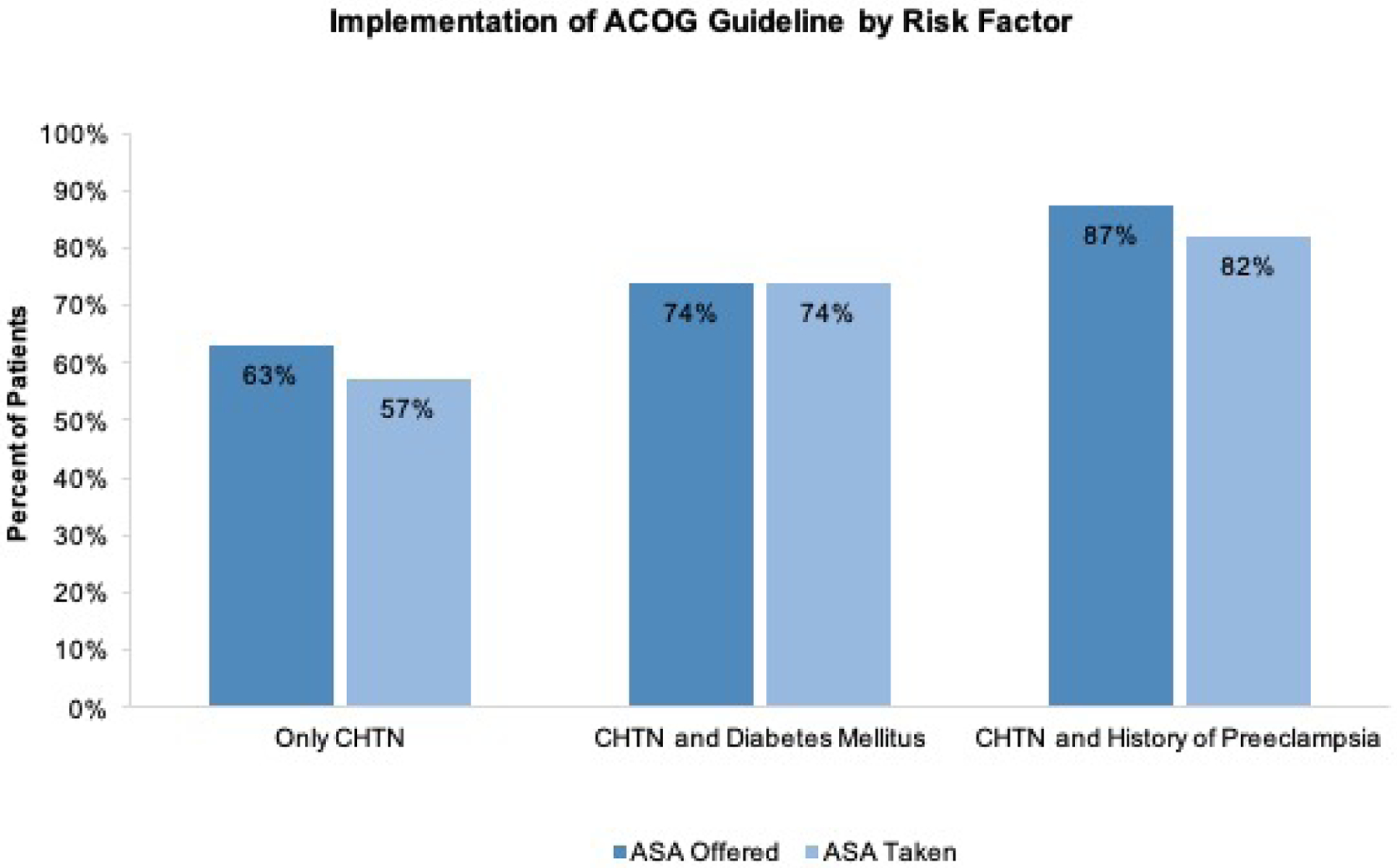
Implementation of ACOG Guideline
Percent of patients who were offered aspirin and taking aspirin by subgroup.
Maternal Outcomes
The overall incidence of superimposed preeclampsia was unchanged 87/254 (34.3%) vs 72/203 (35.5%), p=0.79 when the pre-ACOG and post-ACOG groups were compared. The incidence of superimposed preeclampsia without severe features was significantly decreased 32/254 (12.6%) vs 9/203 (4.4%), p<0.01 while the incidence of superimposed preeclampsia with severe features was significantly increased 55/254 (21.7%) vs 63/203 (31.0%), p=0.02 in the pre and post ACOG groups, respectively. There were no differences regarding gestational age at diagnosis of superimposed preeclampsia, indications of delivery, mode of delivery, maternal or fetal complications. There were seven cases of stillbirths in both groups and no maternal or neonatal deaths (Table 2). Even when evaluating only women who were offered aspirin in the post-ACOG group 142 (70%) vs the pre-ACOG group, the incidence of superimposed preeclampsia: 57/142 (40.1%) vs. 87/254 (34.3%); odds ratio (OR) 1.3, 95% confidence interval (CI): 0.84 to 1.9), p=0.27, remained not significantly different while superimposed preeclampsia with severe features was again significantly increased in the post-ACOG group 52/142 (36.6%) vs 55/254 (21.6%) OR 2.1 (1.3 – 3.3), p=0.002.
Table 2.
Maternal Outcomes
| Maternal Outcomes | Pre ACOG N=254 |
Post ACOG N =203 |
P - value |
|---|---|---|---|
| Superimposed preeclampsia | 87 (34.3) | 72 (35.5) | 0.79 |
| Without severe features | 32 (12.6) | 9 (4.4) | <0.01 |
| With severe features | 55 (21.7) | 63 (31.0) | 0.02 |
| GA at diagnosis of superimposed preeclampsia | 34.1±4.0 | 33.8±4.1 | 0.65 |
| Mode of delivery | |||
| Caesarean | 102 (40.16) | 94 (46.3) | 0.19 |
| Indication for delivery | |||
| Completed 37 weeks | 159 (62.6) | 131 (64.53) | 0.69 |
| Fetal indication | 30 (11.81) | 30 (14.78) | 0.4 |
| Lab abnormalities | 7 (2.76) | 13 (6.4) | 0.06 |
| Uncontrolled severe BPa | 28 (11.02) | 36 (17.73) | 0.04 |
| Persistent maternal symptoms | 17 (6.69) | 14 (6.9) | 1.0 |
| Spontaneous onset of labor | 46 (18.11) | 31 (15.27) | 0.45 |
| Placental abruption | 5 (1.97) | 4 (1.97) | 1.0 |
| Eclampsia | 0 (0) | 0 (0) | - |
| HELLP Syndrome | 0 (0) | 3 (1.48) | 0.25 |
| Pulmonary edema | 2 (0.7) | 1 (0.4) | 1.0 |
| Magnesium at delivery | 53 (20.87) | 48 (23.65) | 0.5 |
| Maternal complications | |||
| Postpartum hemorrhage | 27 (10.63) | 32 (15.8) | 0.11 |
| Maternal ICU admission | 4 (1.57) | 2 (1.0) | 0.59 |
| Maternal brain imaging | 1 (0.39) | 4 (2.0) | 0.19 |
| Maternal mortality | 0 (0) | 0 (0) | - |
| Fetal complications | |||
| Antenatal steroids | 41 (16.14) | 39 (19.21) | 0.45 |
| Intrauterine growth restriction | 21 (8.3) | 17 (8.4) | 0.92 |
| Abnormal umbilical artery Doppler | 10 (3.9) | 6 (3.0) | 0.57 |
| Stillbirth | 2 (0.99) | 7 (2.7) | 0.19 |
Values reported as n (%) or mean ± standard
ACOG: American College of Obstetricians and Gynecologist; BP: Blood pressure; HELLP: hemolysis, elevated liver enzymes, low platelet count; ICU Intensive care unit.
Uncontrolled severe blood pressure (BP) is defined as BP not controlled after several intravenous doses of antihypertensive medications.
Women who were prescribed aspirin in the post-ACOG group (70%) vs women who were not prescribed aspirin in the post-ACOG group (30%) were more likely to be older and have a history of preeclampsia. After adjusting for these confounding factors (age and history of preeclampsia), there was no difference in overall incidence of superimposed preeclampsia 15/61 (24.6%) vs 57/142 (40.1%), aOR 1.81 (0.90 – 3.64), but there was a significant increase in superimposed preeclampsia with severe features in patients who were prescribed aspirin 11/61 (18.0%) vs 52/142 (36.6%), aOR 2.36 (1.10–5.06). (Supplementary Material Table 2)
Women who developed superimposed preeclampsia with severe features were evaluated separately to compare patients pre-ACOG and post-ACOG. While there were no differences in inherent maternal demographics, post-ACOG patients were less likely to be on antihypertensive medication 35/55 (63.6%) vs 19/63 (30.2%). After adjusting for this, there were no differences in gestational age at delivery or adverse perinatal outcomes when the pre-ACOG and post-ACOG groups were compared. (Supplementary Material Table 3 and 4)
There were no differences in the overall incidence of superimposed preeclampsia or presence of severe features when the subgroups were analyzed: history of preeclampsia, pre-gestational diabetes, or severity of hypertension based on the need for antihypertensive medication (Table 3).
Table 3.
Subgroup analysis. Incidence of Superimposed Preeclampsia without and with Severe Features
| Pre ACOG N=254 |
Post ACOG N=203 |
P-value | |
|---|---|---|---|
| Incidence of Superimposed Preeclampsia without Severe Features | |||
| ONLY CHTNa | 53/170 (31.2) | 34/119 (28.6) | 0.70 |
| CHTN with Anti-hypertensive Meds | 46/117 (39.3) | 22/61 (36.1) | 0.75 |
| CHTN and History of Preeclampsia | 25/55 (45.4) | 28/61 (45.9) | 1.0 |
| CHTN and Pre-gestational Diabetes | 13/35 (37.1) | 12/29 (41.4) | 0.8 |
| Incidence of Superimposed Preeclampsia with Severe Features | |||
| Only CHTNa | 34/170 (20.0) | 31/119 (26.0) | 0.25 |
| CHTN with Antihypertensive Meds | 35/117 (29.9) | 19/61 (31.1) | 0.87 |
| CHTN and History of Preeclampsia | 15/55 (27.3) | 25/61 (40.9) | 0.17 |
| CHTN and Pre-gestational Diabetes | 8/35 (22.8) | 9/29 (31.0) | 0.57 |
Values reported as n (%).
ACOG: American College of Obstetricians and Gynecologists
CHTN: Chronic hypertension
Only CHTN group (women with history of preeclampsia, or medical conditions as pregestational diabetes, renal disease, vascular disease, antiphospholipid syndrome, autoimmune disorder, sickle cell disease were excluded)
When analyzing both pre and post-ACOG cohorts combined, the incidence of overall superimposed preeclampsia women with both CHTN and history of preeclampsia was significantly increased when compared with women with only CHTN: 53/116 (45.7%) vs. 87/289 (30.1%) OR 1.9; (95%CI: 1.25–3.0). The incidence of superimposed preeclampsia with severe features was also increased in patients with both CHTN and history of preeclampsia: 40/116 (34.4%) vs. 65/289 (22.5%) OR 1.8; (95%CI: 1.12–2.9) respectively.
Preterm Birth and Neonatal Outcomes
There were no significant differences in the overall gestational age at delivery, incidence of PTB at <37, <34 or <28 weeks when comparing the post-ACOG and pre-ACOG groups. There were no significant differences in neonatal outcomes before and after the ACOG guideline (Table 4). The findings in gestational age and preterm birth remained non-significant even when patients were subdivided by the presence of superimposed preeclampsia with severe features, (Supplementary Material Table 4).
Table 4.
Neonatal Outcomes
| Pre ACOG N=254 |
Post ACOG N=203 |
P-value | |
|---|---|---|---|
| Gestational age at delivery (weeks) | 36.8±9.1 | 36.9±3.2 | 0.29 |
| PTD <37 weeks | 60 (23.62) | 53 (26.11) | 0.54 |
| PTD <34 weeks | 28 (11.02) | 25 (12.32) | 0.67 |
| PTD <28 weeks | 9 (3.54) | 6 (2.96) | 0.73 |
| Birthweight (g) | 2880±808 | 3040±2188 | 0.32 |
| Small for gestational age | 32 (12.6) | 19 (9.36) | 0.29 |
| Apgar at 5 minutes <7 | 14 (5.5) | 13 (6.4) | 0.69 |
| NICU admission | 84 (33.07) | 69 (33.99) | 0.84 |
| RDS | 26 (10.24) | 25 (12.32) | 0.55 |
| Ventilator required | 15 (5.91) | 20 (9.85) | 0.16 |
| IVH Grade 3,4 | 2 (0.8) | 1 (0.5) | 1.0 |
| NEC | 4 (1.57) | 1 (0.5) | 0.38 |
| Sepsis | 3 (1.18) | 3 (1.48) | 0.1 |
Values reported as n (%) or mean ± standard.
ACOG: American College of Obstetricians and Gynecologists; PTD: Incidence of preterm delivery, NICU: Neonatal intensive care unit. RDS: Respiratory distress syndrome; IVH: Intraventricular hemorrhage; NEC: Necrotizing enterocolitis.
In both pre and post-ACOG groups combined, the incidence of preterm birth < 37 weeks in the entire cohort was 113/457 (24.7%). Women with CHTN and superimposed preeclampsia had an increased incidence of preterm birth <37 weeks 74/159 (46.5%) vs 39/298 (13.1%) p<0.0001; OR 5.7; (95% CI, 3.6–9.1) when compared with women with CHTN without superimposed preeclampsia. There was even higher risk if diagnosed with superimposed preeclampsia with severe features: 68/118 (57.6%) vs 39/298 (13.1%) p<0.0001; OR 9.0; (95% CI, 5.5–14.8).
COMMENT
Principal Findings
The overall implementation rate of the 2016 ACOG guideline for preeclampsia prevention with low dose aspirin 81mg before 16 weeks to women with singleton pregnancy and CHTN was 70%. During 2018, it increased to 82%. The overall incidence of superimposed preeclampsia was unchanged before and after the ACOG guideline; moreover, the incidence of superimposed preeclampsia with severe features was significantly increased. There were no differences in the gestational age at diagnosis of superimposed preeclampsia, PTB by gestational age or SGA.
Results
Our cohort seems to have a high incidence of overall superimposed preeclampsia (35%), recurrent superimposed preeclampsia (45.7%), superimposed preeclampsia with severe features (25%) and perinatal complications, but despite the differences in population and diagnostic criteria over time, our results are consistent with other publications. In an individual participant data (IPD) meta-analysis to calculate the recurrence risk of hypertensive disorders of pregnancy (HDP), 236/581 (41%) women with CHTN developed recurrence of HDP.21 In a prospective cohort from United Kingdom, 180/822 (22%) of women with CHTN developed superimposed preeclampsia; half of them had early-onset of superimposed preeclampsia (<34 weeks) with 48% incidence of IUGR and 51% of preterm delivery among them.22 In a retrospective cohort of women with CHTN from Thailand, 130/300 (43.3%) developed superimposed preeclampsia with significantly increased incidence of SGA, low birth weight, asphyxia, and neonatal intensive care unit admission.23 In a retrospective cohort of 447 women with CHTN from Dallas, Texas; women with baseline proteinuria >300 mg/24 hours were statistically significantly more likely to develop superimposed preeclampsia 44/56 (79%) when compared with women with CHTN and negative proteinuria 193/391 (49%) aOR, 4.22; (95%CI, 2.8–8.5 p<0.001) with increased risk of PTB and SGA.24 These studies indicate that patients with CHTN have a significant risk for developing superimposed preeclampsia and associated complications, requiring much attention and intervention.
We also noticed an increased incidence of superimposed preeclampsia with severe features in the post-ACOG group. After further analysis, we believe this is secondary to a learning curve after the publication of the 2013 ACOG guidelines with new diagnostic criteria and management for superimposed preeclampsia and superimposed preeclampsia with severe features. We hypothesize possible underdiagnosis in the subsequent few years (pre-ACOG group). Some diagnostic parameters such as the definition of sudden increase in blood pressure, indications for initiation or escalation of antihypertensive medications, and the definition of significant increase of proteinuria were unclear, requiring the creation of internal guidelines.
The novelty of this retrospective study is that it evaluated rates of superimposed preeclampsia in a singular high-risk cohort with CHTN and analyzed sub-groups with additional risk factors. The USPSTF performed a systematic review of randomized control trials (RCT) that had subjects at high risk for developing preeclampsia.14 However, “high risk” was defined differently in each trial. The publications span from 1993 to 2012 prior to the ACOG Task Force publication that redefined superimposed preeclampsia diagnostic criteria. The USPSTF included 23 studies in its analysis; 13 of them were used to calculate the risk of preeclampsia, 7 studies included women with history of CHTN,25–31 and only one reported subgroup analysis for women with CHTN.25 (Table 5). According to the USPSTF, a pooled analysis of preeclampsia from trials of women at risk, sorted by sample size, showed a decrease in the incidence of preeclampsia by 24% RR, 0.76; (95% CI, 0.62–0.95). One of the largest trials in the USPSTF was the Maternal Fetal Medicine Unit (MFMU) study,25 an RCT from 1998 that prescribed 60 mg of aspirin between 13–26 weeks of gestation. It found that in women with CHTN (n=763), aspirin did not decrease the incidence of superimposed preeclampsia: 26% vs placebo 25%, RR, 1.1; (95% CI, 0.8–1.4). While the diagnostic criteria for CHTN was similar to our cohort study, the diagnostic criteria for superimposed preeclampsia at that time was more similar to the current definition of superimposed preeclampsia with severe features. Other RCTs have evaluated low dose of aspirin (60–150mg) for preeclampsia prevention in high-risk population including women with CHTN.32–37 The number of participating women with CHTN varied from 7% to 53%, but only one trial37 presented stratified data for CHTN. (Table 5) The ASPRE trial prescribed 150 mg of daily aspirin starting at 11–14 weeks of gestation to 1776 women at high risk for preterm preeclampsia.37 Preterm preeclampsia occurred in 13 (1.6%) in the aspirin group, versus 35 (4.3%) in the placebo group OR, 0.38; (95% CI, 0.20 to 0.74; p=0.004). A secondary analysis of this trial found that in women with CHTN, there was no significant difference in preeclampsia diagnosed before 37 weeks of gestation: 10.2% (5/49) in the aspirin group and 8.2% (5/61) in the placebo group aOR, 1.29; (95% CI, 0.33–5.12), even with >90% compliance. The low sample size of these subgroups of women may have affected this outcome, as the subgroup of CHTN was 110/1620 (6.7%).38 Table 5 summarizes major RCT studies using low dose aspirin for preeclampsia prevention that included women with CHTN, stratified by inclusion into the USPSTF analysis. There were 13773 women, including 3051/12881 (23%) with CHTN, but only two studies presented sub-analysis results. These results indicate the need for more studies evaluating specifically women with CHTN.
Table 5.
Randomized control trials using aspirin for preeclampsia prevention that included women with chronic hypertension.
| Included in USPSTF Review | Author | ASA Dosage (mg daily) | Preeclampsi a in ASA group | Preeclampsi a in Placebo group | OR | P value | % of patients with CHTN | Incidenc e of Preeclam psia in CHTN patients | GA at ASA initiation (weeks) |
|---|---|---|---|---|---|---|---|---|---|
| Yes | Viinikka 199330 | 50 | 9/97 | 11/100 | 0.83 (0.33 – 2.10) | 0.82 | 83% | No data | 15–16 |
| Yes | CLASP 199426 | 60 | 257/3449 | 290/3437 | 0.87 (0.73 – 1.04) | 0.14 | 20% | No data | 12 – 32 |
| Yes | MFMU 199825 | 60 | 229/1273 | 253/1266 | 0.88 (0.72 – 1.07) | 0.21 | 30% | 26% ASA vs 25% placebo (p = 0.66) | 13–26 |
| Yes | Ayala 201327 | 100 | 11/176 | 22/174 | 0.49 (0.25–0.99) | N/A | No data | 12–16 | |
| Yes | Grab 200028 | 100 | 3/22 | 2/21 | 1.50 (0.23 – 10.02) | 1.00 | 44% | No data | 18 |
| Yes | Hermida 199729 | 100 | 3/50 | 7/50 | 0.43 (0.12–1.56) | N/A | No data | 12–16 | |
| Yes | Villa 201231 | 100 | 8/61 | 11/60 | 0.67 (0.25 – 1.81) | 0.46 | 17% | No data | 12–13 |
| No | ECPPA 199632 | 60 | 32/496 | 30/494 | 1.07 (0.64 – 1.78) | 0.90 | 47% | No data | 12–32 |
| No | Byaruhanga 199833 | 75 | 17/113 | 23/117 | 0.72 (0.36 – 1.44) | 0.39 | 16% | No data | 20–28 |
| No | Vainio 200234 | 86 | 2/43 | 10/43 | 0.2 (0.05–0.86) | 34% | No data | 12–14 | |
| No | Ebrashy 200535 | 75 | 26/74 | 40/65 | - | - | 35% | No data | 14–16 |
| No | Zhao, 201264 | 75 | 22/120 | 64/122 | - | - | N/A | No data | 13–16 |
| No | Odibo 201536 | 81 | 3/16 | 3/14 | 0.85 (0.14 – 5.07) | 1.00 | 53% | No data | 18–32 |
| No | Stanescu 201565 | 150 | 0/100 | 2/50 | 0.96 (0.91 – 1.02) | 0.11 | N/A | No data | 11–14 |
| No | ASPRE 201737 | 150 | 66/798 | 94/822 | 0.70 (0.50 – 0.97) | 0.04 | 7% | 5/49 ASA vs 5/61 placebo 1.22 (0.33 – 4.49) P = 1.0 | 11–14 |
| Total | 688/6888 | 862/6835 | |||||||
USPSTF: United States Preventative Services Task Force; ECPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant women. ECPPA (Estudo Colaborativo para Prevencao da Pre-eclampsia com Aspirina) Collaborative Group; CHTN: chronic hypertension; MFMU: Maternal Fetal Medicine Units network; ASPRE: Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia; N/A: not available; ASA: aspirin; GA: gestational age; OR: odds ratio.
Multiple meta-analyses have been conducted;39–43 some suggest that low dose aspirin for preeclampsia prevention is more effective if initiated prior to 16 weeks.39 In addition to decreasing overall preeclampsia by 43% and severe preeclampsia by 53%, aspirin also demonstrated to decrease the incidence of fetal growth restriction by 44%40, perinatal death, and early onset of preeclampsia prior to 34 weeks.41 New analysis showed that the beneficial effect of aspirin is also dose-dependent, with a greater reduction of all outcomes with a daily dose of aspirin of ≥100 mg if initiated prior to 16 weeks RR, 0.33; 95% CI, 0.19–0.57 p<0.0001).40 A Cochrane review (46 RCTs with 32,891 women) identified a more modest reduction of 17% in the risk of pre-eclampsia associated with the use of antiplatelet agents RR, 0.83; (95% CI, 0.77 to 0.89).42 An individual participant data meta-analysis including 32,217 women and 32,819 infants recruited from 31 RCTs showed no significant difference in the effects of antiplatelet therapy for women with CHTN: 293/1678 (17.5%) in the aspirin group developed superimposed preeclampsia versus 295/1625 (18.15%) in the control group RR, 0·97; (95% CI, 0·84–1·12 p=0·28).44 No significant difference was found when women were randomized to initiating aspirin before 16–20 weeks’ gestation compared with those randomized at or after 16–20 weeks. They reported a 10% reduction in the incidence of preeclampsia.43, 44
Our findings are consistent with RCTs and IPD meta-analysis reporting that low dose aspirin may not be sufficient for preeclampsia prevention in patients with chronic hypertension.25, 38
The mechanism for the lack of efficacy of low dose aspirin in preeclampsia prevention for certain subgroups of women has been proposed. Broadly, aspirin is required at higher concentrations in pregnancy. Evaluation of different doses of aspirin (100mg vs 150mg) showed that there was a reduction in the total drug metabolite concentration in pregnant vs. non-pregnant women, likely due to altered pharmacokinetics and increased clearance.45 Obesity has been implicated as a factor in poor response to low dose aspirin. Obesity limits the absorption of aspirin, and platelet regeneration occurs at a higher rate. This allows for increased renewal of cyclooxygenase-1, decreasing the time-dependent effect of aspirin.46 In a secondary analysis of the MFMU RCT,25, 47 maternal serum thromboxane B2 (TXB2) (an indirect measure of thromboxane A2) levels were drawn at three different times of pregnancy and stratified by BMI. Obese women, especially those with BMI > 40 kg/m2, had higher median TXB2 levels in both the second and third trimesters, and lower rates of complete TXB2 inhibition by aspirin when compared with non-obese women. Chronic diseases as CHTN, diabetes, lupus, renal disease, antiphospholipid syndrome have an increased risk of preeclampsia, likely due to inflammatory factors that may affect the endometrium and uterine and ovarian vasculature prior to pregnancy, altering the implantation process and placentation in the first trimester.6 The trophoblastic invasion occurs in two waves; the first wave is decidual invasion of spiral arteries at 8 to 10 weeks and the second wave invasion into myometrial segments at 16 to 18 weeks.48 Changes in the timing of aspirin initiation could be an option. Studies in women that underwent in vitro fertilization have shown that preconception low dose aspirin is associated with improved implantation rates, and increased blood flow velocity in the uterine and ovarian arteries with lower pulsatility index values.49 RCTs have shown that preconception initiation of 75–100 mg aspirin is safe in pregnancy,50–52 A systematic review evaluating low dose aspirin initiated prior to conception or before 11 weeks53 in women with infertility, or recurrent pregnancy loss showed no difference in the incidence of preeclampsia 11/404 (2.7%) vs. 25/415 (6%) RR 0.52 (CI: 0.23–1.17, p=0.12), but showed a significant decrease in PTB < 37 weeks: 35/657 (5.3%) vs. 65/659 (10%) RR 0.52 (CI: 0.27–0.97, p=0.04). These studies have a low incidence of preeclampsia, as this question was not in their original design.. A recent multicenter double blinded placebo control trial in low risk nulliparous women, prescribed 81 mg aspirin between 6 0/7 and 13 6/7 weeks of pregnancy. It not only showed a significantly decreased in overall PTB <37 weeks but showed also significantly decreased PTB < 34 weeks with hypertensive disorders of pregnancy: 8/5780 (0.1%) vs. 21/5764 (0.4%) RR: 0.38 (0.17–0.85) p-0.015.54 Timing in aspirin initiation needs to be factored in when evaluating low dose aspirin efficacy in preeclampsia prevention.
The Fetal Medicine Foundation (FMF) has developed a prediction model for preeclampsia based on maternal risk factors, uterine artery pulsatility index, mean arterial pressure (MAP), serum pregnancy-associated plasma protein-A, and placental growth factor multiple of the median values.55, 56 After screening 35,948 singleton pregnancies, 1058 (2.9%) experienced preeclampsia. This algorithm predicted 75% (95% CI, 70–80%) of preterm-preeclampsia and 47% (95% CI, 44–51%) of term-preeclampsia, at a false positive rate of 10%. This prediction model, validated in Asia,57 Australia,58 and the United States,59 was compared with the United Kingdom NICE guidelines16 and ACOG recommendations,15 demonstrating that the FMF screening performance to predict preterm preeclampsia was far superior than the traditional approach with the use of maternal factors.60 This prediction model may assist with better candidate selection for preeclampsia prevention medications in women with CHTN.
Strengths and Limitations
Our study has a number of strengths. We evaluated the implementation of the ACOG guideline recommending low dose of aspirin for preeclampsia prevention in one of the populations at highest risk for preeclampsia, those with CHTN, and evaluated the effect of additional risk factors, such as severity of disease based on use of antihypertensive medication, history of preeclampsia and pre-gestational diabetes on rates of superimposed preeclampsia with and without severe features. The single institution design of the study allows for standardized diagnostic criteria and practice management in accordance with the ACOG Task Force on Hypertension in Pregnancy.3 Another strength of the study is the selection of high-risk patients based only on past medical history rather than biomarkers or uterine artery Doppler and can be replicated in other communities where those biomarkers are not available..61
Our study does have some limitations. It is a retrospective cohort, and therefore we are unable to make conclusions regarding causality. The sample size is small and maybe underpowered to detect a small clinical difference in the two groups. Due to lack of clarity of diagnostic criteria for superimposed preeclampsia from the ACOG recommendations, we developed institutional guidelines to define sudden increase of hypertension and proteinuria, which may differ slightly from other institutional interpretations. We did not collect MAP information at the first prenatal visit. The home BP monitoring was not standardized and was self-reported. Very few women brought their devices for calibration. We did not have the funding for standardized BP devices or monitoring systems. However, self-reported measurements were used to screen women for further evaluation, and clinical decisions were only made after confirming BP using a standardized BP device in a clinical setting. Adherence to aspirin could not be reported with complete accuracy, as it was based on patient report and chart documentation, and we were not able to monitor pill intake. Our patient population includes patients with multiple risk factors for preeclampsia including African American race, multiple comorbidities, and obesity, and thus, our results may not be universally applicable. We did not include other well-known risk factors of preeclampsia as family history of preeclampsia or conception by in vitro fertilization.
Research Implications
Further studies on the dosing, timing, and evaluation of the efficacy of aspirin in preeclampsia prevention are needed on women with chronic hypertension, as they have a significant risk of developing superimposed preeclampsia and adverse outcomes. Using a higher dose of aspirin, 150mg, or 162mg (2 tablets), as suggested by the International Federation of Gynecology and Obstetrics (FIGO) recommendation, may be an option.62 The efficacy of preconception initiation of aspirin at different doses needs to be studied in high-risk population. The impact of maternal BMI on the appropriate dose of aspirin requires pharmacokinetic studies. Lastly, evaluation of lower goals for BP control in women with CHTN (<140 or <90 mmHg) vs. (<150 or <100 mmHg) and their effect on the incidence of superimposed preeclampsia and severe features.63 Evaluation of other high-risk subgroups including women with history of preeclampsia, diabetes mellitus, multiple pregnancies, renal disease or autoimmune disease needs to be studied independently. If an RCT studying women with CHTN and low dose aspirin for superimposed preeclampsia prevention is planned, enrollment of 3400 women will be needed to decrease the risk from 30% to 25.5% (by 15%) with an 80% power and 95% confidence interval.
Conclusions
Our findings showed an overall 70% institutional adherence to the 2016 ACOG recommendation of 81mg aspirin in patients with CHTN for superimposed preeclampsia prevention. However, the daily 81 mg of aspirin initiated between 12 to 16 weeks of pregnancy did not decrease the incidence of superimposed preeclampsia, severe features, SGA or PTB in patients with CHTN.
Supplementary Material
American Journal of Obstetrics and Gynecology at a Glance:
- Why was the study conducted?
- To evaluate the ACOG recommendation of low dose aspirin 81mg for pregnant women with chronic hypertension
- What are the key findings?
- Superimposed preeclampsia, small for gestational age, and preterm birth were not significantly decreased after implementation of daily 81 mg aspirin initiated between 12 to 16 weeks of gestation.
- What does this study add to what is already known?
- Low dose aspirin for women with chronic hypertension as recommended by ACOG or USPSTF does not prevent superimposed preeclampsia or other adverse maternal and neonatal outcomes.
- Further studies on the dosing, timing, and efficacy of aspirin in preeclampsia prevention are needed for women with specific risk factors.
Acknowledgment
We appreciate the statistical assistance of Alex Knee, MS. Assistant Professor, Dept. of Medicine, UMMS-Baystate. Program Manager, Epidemiology/Biostatistics Research Core Baystate Medical Center Office of Research
Financial Support:
No financial support was received for this study. Rupsa C. Boelig is supported by NIH grant T32 GM008562.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors report no conflicts of interest.
Data from this manuscript was presented as a poster presentation at the 2018 American College Obstetrics and Gynecologist Annual Meeting in Austin, Texas, April 27–30, 2018.
REFERENCES
- 1.WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. Geneva; 2011. [PubMed] [Google Scholar]
- 2.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global health 2014; 2(6): e323–33. [DOI] [PubMed] [Google Scholar]
- 3.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013; 122(5): 1122–31. [DOI] [PubMed] [Google Scholar]
- 4.Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. Bmj 2019; 366: l2381. [DOI] [PubMed] [Google Scholar]
- 5.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol 1998; 179(5): 1359–75. [DOI] [PubMed] [Google Scholar]
- 6.Staff AC. The two-stage placental model of preeclampsia: An update. J Reprod Immunol 2019; 134–135: 1–10. [DOI] [PubMed] [Google Scholar]
- 7.Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol 2015; 213(4 Suppl): S115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine 1999; 222(3): 222–35. [DOI] [PubMed] [Google Scholar]
- 9.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 2009; 30 Suppl A: S43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nature reviews Nephrology 2014; 10(8): 466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Possomato-Vieira JS, Khalil RA. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Advances in pharmacology 2016; 77: 361–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nature reviews Nephrology 2014; 10(9): 531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benigni A, Gregorini G, Frusca T, et al. Effect of low-dose aspirin on fetal and maternal generation of thromboxane by platelets in women at risk for pregnancy-induced hypertension. N Engl J Med 1989; 321(6): 357–62. [DOI] [PubMed] [Google Scholar]
- 14.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Annals of internal medicine 2014; 160(10): 695–703. [DOI] [PubMed] [Google Scholar]
- 15.ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet Gynecol 2018; 132(1): e44–e52. [DOI] [PubMed] [Google Scholar]
- 16.Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London; 2010. [PubMed] [Google Scholar]
- 17.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, Canadian Hypertensive Disorders of Pregnancy Working G. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy hypertension 2014; 4(2): 105–45. [DOI] [PubMed] [Google Scholar]
- 18.Lowe SA, Bowyer L, Lust K, et al. The SOMANZ Guidelines for the Management of Hypertensive Disorders of Pregnancy 2014. The Australian & New Zealand journal of obstetrics & gynaecology 2015; 55(1): 11–6. [DOI] [PubMed] [Google Scholar]
- 19.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC pediatrics 2013; 13(1): 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology 1991; 181(1): 129–33. [DOI] [PubMed] [Google Scholar]
- 21.van Oostwaard MF, Langenveld J, Schuit E, et al. Recurrence of hypertensive disorders of pregnancy: an individual patient data metaanalysis. Am J Obstet Gynecol 2015; 212(5): 624 e1–17. [DOI] [PubMed] [Google Scholar]
- 22.Chappell LC, Enye S, Seed P, Briley AL, Poston L, Shennan AH. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: a prospective study. Hypertension 2008; 51(4): 1002–9. [DOI] [PubMed] [Google Scholar]
- 23.Boriboonhirunsarn D, Pradyachaipimol A, Viriyapak B. Incidence of superimposed preeclampsia among pregnant Asian women with chronic hypertension. Hypertension in pregnancy 2017; 36(2): 226–31. [DOI] [PubMed] [Google Scholar]
- 24.Morgan JL, Nelson DB, Roberts SW, Wells CE, McIntire DD, Cunningham FG. Association of Baseline Proteinuria and Adverse Outcomes in Pregnant Women With Treated Chronic Hypertension. Obstet Gynecol 2016; 128(2): 270–6. [DOI] [PubMed] [Google Scholar]
- 25.Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 1998; 338(11): 701–5. [DOI] [PubMed] [Google Scholar]
- 26.CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. Lancet 1994; 343(8898): 619–29. [PubMed] [Google Scholar]
- 27.Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiology international 2013; 30(1–2): 260–79. [DOI] [PubMed] [Google Scholar]
- 28.Grab D, Paulus WE, Erdmann M, et al. Effects of low-dose aspirin on uterine and fetal blood flow during pregnancy: results of a randomized, placebo-controlled, double-blind trial. Ultrasound Obstet Gynecol 2000; 15(1): 19–27. [DOI] [PubMed] [Google Scholar]
- 29.Hermida RC, Ayala DE, Iglesias M, et al. Time-dependent effects of low-dose aspirin administration on blood pressure in pregnant women. Hypertension 1997; 30(3 Pt 2): 589–95. [DOI] [PubMed] [Google Scholar]
- 30.Viinikka L, Hartikainen-Sorri AL, Lumme R, Hiilesmaa V, Ylikorkala O. Low dose aspirin in hypertensive pregnant women: effect on pregnancy outcome and prostacyclin-thromboxane balance in mother and newborn. British journal of obstetrics and gynaecology 1993; 100(9): 809–15. [DOI] [PubMed] [Google Scholar]
- 31.Villa PM, Kajantie E, Raikkonen K, et al. Aspirin in the prevention of pre-eclampsia in high-risk women: a randomised placebo-controlled PREDO Trial and a meta-analysis of randomised trials. BJOG 2013; 120(1): 64–74. [DOI] [PubMed] [Google Scholar]
- 32.ECPPA: randomised trial of low dose aspirin for the prevention of maternal and fetal complications in high risk pregnant women. ECPPA (Estudo Colaborativo para Prevencao da Pre-eclampsia com Aspirina) Collaborative Group. British journal of obstetrics and gynaecology 1996; 103(1): 39–47. [DOI] [PubMed] [Google Scholar]
- 33.Byaruhanga RN, Chipato T, Rusakaniko S. A randomized controlled trial of low-dose aspirin in women at risk from preeclampsia. Int J Gynaecol Obstet 1998; 60(2): 129–35. [DOI] [PubMed] [Google Scholar]
- 34.Vainio M, Kujansuu E, Iso-Mustajarvi M, Maenpaa J. Low dose acetylsalicylic acid in prevention of pregnancy-induced hypertension and intrauterine growth retardation in women with bilateral uterine artery notches. BJOG 2002; 109(2): 161–7. [DOI] [PubMed] [Google Scholar]
- 35.Ebrashy A, Ibrahim M, Marzook A, Yousef D. Usefulness of aspirin therapy in high-risk pregnant women with abnormal uterine artery Doppler ultrasound at 14–16 weeks pregnancy: randomized controlled clinical trial. Croatian medical journal 2005; 46(5): 826–31. [PubMed] [Google Scholar]
- 36.Odibo AO, Goetzinger KR, Odibo L, Tuuli MG. Early prediction and aspirin for prevention of preeclampsia (EPAPP) study: a randomized controlled trial. Ultrasound Obstet Gynecol 2015; 46(4): 414–8. [DOI] [PubMed] [Google Scholar]
- 37.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med 2017; 377(7): 613–22. [DOI] [PubMed] [Google Scholar]
- 38.Poon LC, Wright D, Rolnik DL, et al. Aspirin for Evidence-Based Preeclampsia Prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol 2017; 217(5): 585 e1–e5. [DOI] [PubMed] [Google Scholar]
- 39.Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 2010; 116(2 Pt 1): 402–14. [DOI] [PubMed] [Google Scholar]
- 40.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol 2017; 216(2): 110–20 e6. [DOI] [PubMed] [Google Scholar]
- 41.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol 2018; 218(3): 287–93 e1. [DOI] [PubMed] [Google Scholar]
- 42.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev 2007; (2): CD004659. [DOI] [PubMed] [Google Scholar]
- 43.Meher S, Duley L, Hunter K, Askie L. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: an individual participant data meta-analysis. Am J Obstet Gynecol 2017; 216(2): 121–8 e2. [DOI] [PubMed] [Google Scholar]
- 44.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA, Group PC. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007; 369(9575): 1791–8. [DOI] [PubMed] [Google Scholar]
- 45.Shanmugalingam R, Wang X, G MU, et al. A pharmacokinetic assessment of optimal dosing, preparation and chronotherapy of aspirin in pregnancy. Am J Obstet Gynecol 2019. [DOI] [PubMed] [Google Scholar]
- 46.Norgard NB. Obesity and Altered Aspirin Pharmacology. Clinical pharmacokinetics 2018; 57(6): 663–72. [DOI] [PubMed] [Google Scholar]
- 47.Finneran MM, Gonzalez-Brown VM, Smith DD, Landon MB, Rood KM. Obesity and laboratory aspirin resistance in high-risk pregnant women treated with low-dose aspirin. Am J Obstet Gynecol 2019; 220(4): 385 e1–e6. [DOI] [PubMed] [Google Scholar]
- 48.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983; 4(4): 397–413. [DOI] [PubMed] [Google Scholar]
- 49.Rubinstein M, Marazzi A, Polak de Fried E. Low-dose aspirin treatment improves ovarian responsiveness, uterine and ovarian blood flow velocity, implantation, and pregnancy rates in patients undergoing in vitro fertilization: a prospective, randomized, double-blind placebo-controlled assay. Fertility and sterility 1999; 71(5): 825–9. [DOI] [PubMed] [Google Scholar]
- 50.Ahrens KA, Silver RM, Mumford SL, et al. Complications and Safety of Preconception Low-Dose Aspirin Among Women With Prior Pregnancy Losses. Obstet Gynecol 2016; 127(4): 689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schisterman EF, Silver RM, Lesher LL, et al. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014; 384(9937): 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sjaarda LA, Radin RG, Silver RM, et al. Preconception Low-Dose Aspirin Restores Diminished Pregnancy and Live Birth Rates in Women With Low-Grade Inflammation: A Secondary Analysis of a Randomized Trial. J Clin Endocrinol Metab 2017; 102(5): 1495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaemsaithong P, Cuenca-Gomez D, Plana MN, Gil MM, Poon LC. Does low-dose aspirin initiated before 11 weeks’ gestation reduce the rate of preeclampsia? Am J Obstet Gynecol 2019. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman MK, Goudar SS, Kodkany BS, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet 2020; 395(10220): 285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Gorman N, Wright D, Syngelaki A, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am J Obstet Gynecol 2016; 214(1): 103 e1–e12. [DOI] [PubMed] [Google Scholar]
- 56.O’Gorman N, Wright D, Poon LC, et al. Accuracy of competing-risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet Gynecol 2017; 49(6): 751–5. [DOI] [PubMed] [Google Scholar]
- 57.Chaemsaithong P, Pooh RK, Zheng M, et al. Prospective evaluation of screening performance of first-trimester prediction models for preterm preeclampsia in an Asian population. Am J Obstet Gynecol 2019; 221(6): 650 e1–e16. [DOI] [PubMed] [Google Scholar]
- 58.Park F, Russo K, Williams P, et al. Prediction and prevention of early-onset pre-eclampsia: impact of aspirin after first-trimester screening. Ultrasound Obstet Gynecol 2015; 46(4): 419–23. [DOI] [PubMed] [Google Scholar]
- 59.Sonek J, Krantz D, Carmichael J, et al. First-trimester screening for early and late preeclampsia using maternal characteristics, biomarkers, and estimated placental volume. Am J Obstet Gynecol 2018; 218(1): 126 e1–e13. [DOI] [PubMed] [Google Scholar]
- 60.O’Gorman N, Wright D, Poon LC, et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol 2017; 49(6): 756–60. [DOI] [PubMed] [Google Scholar]
- 61.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal diagnosis and therapy 2013; 33(1): 8–15. [DOI] [PubMed] [Google Scholar]
- 62.Poon LC, Shennan A, Hyett JA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet 2019; 145 Suppl 1: 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 2014; 2: CD002252. [DOI] [PubMed] [Google Scholar]
- 64.Zhao YM, Cheung MS, Lin Z, Sun J. Enantioselective synthesis of beta, gamma-unsaturated alpha-fluoroesters catalyzed by N-heterocyclic carbenes. Angewandte Chemie 2012; 51(41): 10359–63. [DOI] [PubMed] [Google Scholar]
- 65.Stanescu AD, Banica R, Sima RM, Ples L. Low dose aspirin for preventing fetal growth restriction: a randomised trial. J Perinat Med 2018; 46(7): 776–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


