Abstract
Purpose of Review
We will highlight the biological processes across a women’s lifespan from young adulthood through menopause and beyond that impact blood pressure and summarize women’s representation in hypertension clinical trials.
Recent Findings
Throughout their lifetime, women potentially undergo several unique sex-specific changes that may impact their risk of developing hypertension. Blood pressure diagnostic criteria for pregnant women remains 140/90 mmHg and has not been updated for concordance with the 2017 ACC/AHA guideline due to a lack of data. Although on a population level, women develop hypertension at later ages than men, new data shows women’s BP starts to increase as early as the third decade Understanding how age and sex both contribute to hypertension in elderly women is crucial to identify optimal blood pressure and treatment targets.
Summary
Effective screening, monitoring, and treatment of hypertension throughout a women’s lifespan is necessary to reduce CVD risk. We highlight several gaps in the literature pertaining to understanding sex-specific hypertension mechanisms.
Keywords: hypertension, women, sex differences, clinical trials
Introduction
Hypertension is a leading modifiable risk factor for cardiovascular disease and is highly prevalent worldwide.(1) The prevalence of hypertension is known to vary by sex and age, and the impact of increased blood pressure is different for men and women. (2) For a comparable 10mmHg increase in systolic blood pressure, women experience a 25% increase in CVD risk while men risk is only 15% higher.(3) Sex-specific differences in blood pressure (BP) have been noted since the early 1900’s when women were first observed to have lower BP compared to men of a similar age.(4) BP, and consequently hypertension prevalence, is lower in women from adolescence until menopause or the fifth decade of life,(5–7) following which the prevalence of hypertension increases steeply in women.(8) Despite the higher prevalence of hypertension in men, a recent study of 32,833 individuals (17,733 women [54%]) followed for over four decades, demonstrated that women actually have a steeper increase in BP as early as the third decade that continues throughout the life course.(7) These differences persisted even after adjustment for multiple cardiovascular risk factors. Taken together, these sex differences in BP across the life course may have important implications for the diagnosis and treatment of hypertension in men and women, though currently there are no sex-specific guidelines for the diagnosis or treatment of hypertension.
In this review, we highlight a variety of sex-specific biological processes across a women’s lifespan from young adulthood through menopause and older age that impact BP and hypertension (Figure 1). We will also emphasize areas where future studies are needed to further understand these differences in hypertension mechanisms and treatment, highlighting the importance of prespecified analyses by sex in clinical trials. Although beyond the scope of this review in which we focus on biological sex and hypertension, gender - a social construct – also influences blood pressure and hypertension. Gender inequities and different socialization patterns across genders may affect cardiovascular risk.(9)
Figure 1.
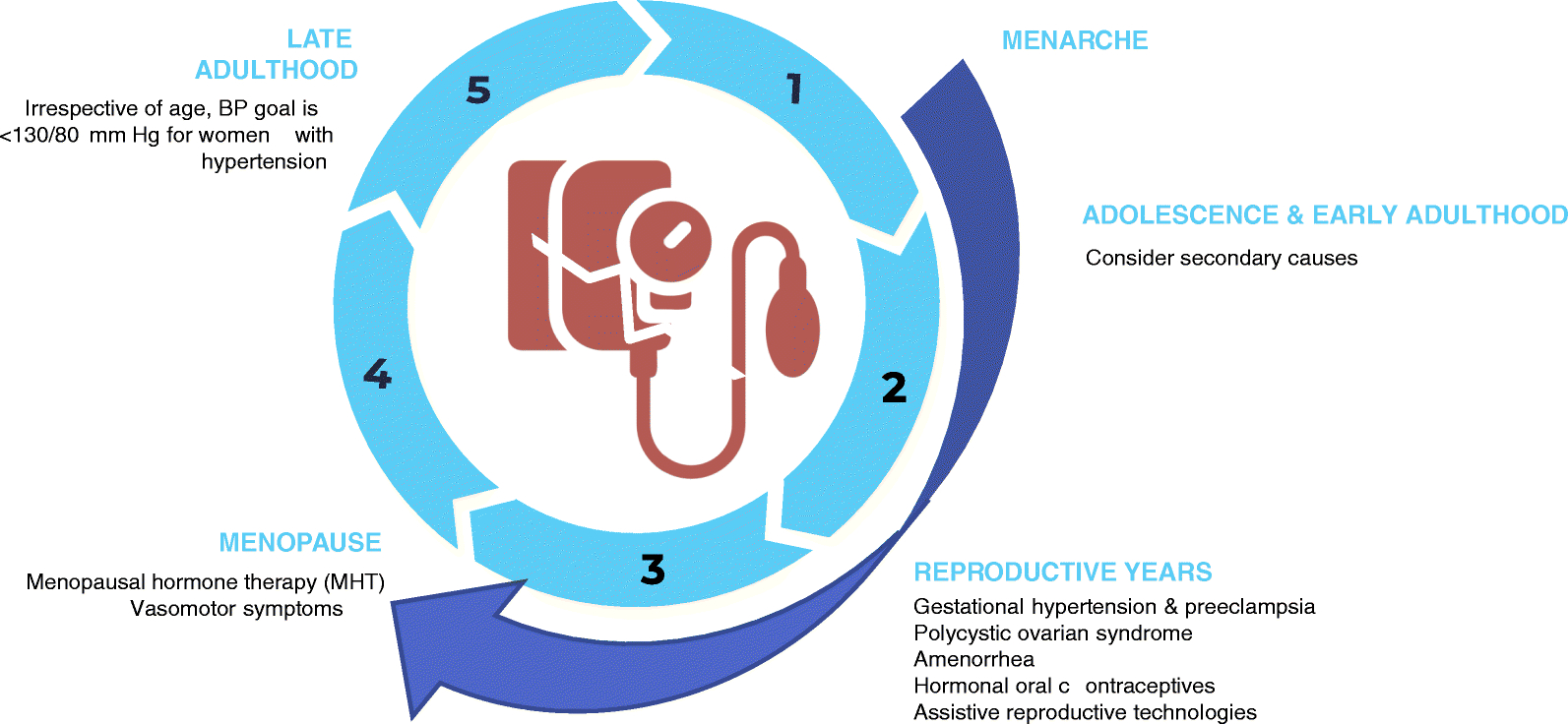
Issues unique to hypertension and blood pressure in women across the lifespan.
Hypertension in Young Adulthood
BP trajectory tracks from childhood through adulthood, and elevations in childhood BP are a risk factor for later life hypertension and cardiovascular events.(10–12) This association does not differ between sexes,(13) but the cardiovascular phenotype of essential hypertension has been shown to differ in young men compared to women. In an analysis of 3,145 healthy participants of the UK Enigma study, a long term follow up study of 18–49 year old individuals, men with hypertension had a predominately “cardiac” phenotype of hypertension with low or normal peripheral vascular resistance, while women had predominately a “vascular” phenotype of hypertension with elevated peripheral vascular resistance, augmentation index and aortic pulse wave velocity. (14)
As previously mentioned, young men have a higher prevalence of hypertension than women of a similar age, and the beneficial effects of female-predominant sex hormones such as estrogen may partially explain lower BP in women. This phenomenon has been extensively explored in previous literature.(15–18) Briefly, the first mechanism by which estrogen can be protective in women is through its modulation of the renin-angiotensin-aldosterone-system (RAAS). Estrogen increases angiotensinogen synthesis and decreases renin and angiotensin converting enzyme (ACE) activity and consequently decreases angiotensin 2 levels, thus shifting balance to the depressor pathways of the RAAS. Estrogen also decreases the prohypertensive effects of endothelin 1 by decreasing its production as well as the expression of endothelin receptor types A and B. Similarly, the estrogenic compound 17β-estradiol increases nitric oxide synthase and decreases angiotensin 2 production leading to vasodilation. Estrogen therefore can lead to a decrease in BP through several mechanisms. On the other hand, testosterone, which is lower in premenopausal women than men, shifts the balance of the RAAS system to the pressor pathway, i.e. it increases vasoconstriction and sodium retention along with BP. Therefore, in premenopausal women the effect of a relatively higher level of estrogen coupled with a lower level of testosterone results in lower BP compared to men of a similar age. Of note, BP in women is lowest during the luteal phase of the menstrual cycle when estrogen levels are at their peak.(19–21)
Derangements in the hormonal milieu of reproductive aged women can negate the expected sex-specific lower BP in women. The most common example of this is polycystic ovarian syndrome (PCOS) which is characterized by elevated testosterone levels, irregular menstrual cycles, excessive body hair, reduced fertility, increased risk of diabetes and obesity, and hypothalamic hypoestrogenaemia – an abnormal pituitary regulation of estrogen leading to low plasma estrogen.(22) Similarly, obese premenopausal women, who often have altered hormonal levels, have a higher risk of hypertension compared to lean women.(23, 24) The effect of increase body mass index on BP is greater in women than men, however the mechanism behind this has not been explored.(25) Other behavioral risk factors for hypertension such as smoking and low levels of physical activity can also contribute to high BP in young women.(26) However, whether a sex-specific impact of these modifiable risk factors on BP exists is not well elucidated.
It is important to note that unlike in adults, hypertension in adolescents (12–18 years) and young adults (19–39 years) is mostly due to secondary etiologies and an age-based approach to determining the underlying and potentially correctible cause is recommended.(27) As outlined in Table 1, fibromuscular dysplasia is more common in young women.(27–29) Medications are also an important potentially reversible cause of hypertension. Despite the beneficial effects of endogenous estrogen on BP, the chronic use of oral combined hormonal contraceptives (CHC), those containing both an estrogen and progestin, is associated with BP elevation and overt hypertension in ~5% of high dose users (at least 50 microgram of estrogen and 104mg of progestin).(30–33) In a prospective cohort of 68,000 women, those taking low dose oral CHCs had a 1.5 times greater risk of developing hypertension.(34) Although not dose-dependent, the development of hypertension in women taking oral CHCs appears to be related to the cumulative duration of use. For every 5-year increment of using oral contraceptives, risk of hypertension increased by 13% (95%CI: 1.03–1.25. Cessation reverses the effect on BP with a return to pretreatment levels within 3 months of discontinuation.(34) It has been shown that among hypertensive women on oral CHCs (n=72) discontinuation of CHC had the same BP lowering effect as the addition of an antihypertensive medication.(35) Of note, the use of progestin only pills does not appear to have a significant effect on BP.(36) Alternatives to oral CHCs including progesterone-only depot injections, subcutaneous estrogen implants or levonorgestrel-releasing intrauterine devices are safe and widely available.(37–39)
Table 1.
| Causes | Definition/Description | Age | Sex-specific |
|---|---|---|---|
| Coarctation of the Aorta | Narrowing of the descending aorta | 12–18 | No However, should be suspected in young women with Turner syndrome, Takayasu arteritis, lupus erythematosus, and with other rheumatologic diseases |
| Renal Parenchymal Disease | Includes various disorders of the kidney | 12–18 and 19–39 | No |
| Thyroid Dysfunction | Thyroid hormone affects the systemic vasculature and cardiac output (Hypothyroidism or hyperthyroidism) | 19–39 | Yes, more common in men |
| Fibromuscular Dysplasia | Vascular disorder of unknown etiology that causes narrowing mainly of renal arteries and leads to decreased renal perfusion | 19–39 | Yes, affects mostly women |
| Polycystic Ovarian Syndrome (PCOS) | Heterogeneous syndrome primarily characterized by ovulatory dysfunction and hyperandrogenism | 12–18 and 19–39 | Only women |
| Endocrine disorders | Includes but not limited to:
|
12–18 and 19–39 | No |
| Psychological | Includes but not limited to:
|
12–18 and 19–39 | No |
| Other Renal Diseases | Includes renovascular disease and primary kidney disease | 12–18 and 19–39 | No |
| Pharmacological causes | Include but not limited to:
|
12–18 and 19–39 | No (except oral contraceptives in women only) |
The mechanism behind the effect of oral contraceptives on BP is not clearly understood. The estrogen component (ethinyl estradiol) in oral CHCs increases the hepatic production of angiotensinogen and activates the RAAS, which may increase vascular resistance. Ethinyl estradiol also has prothrombotic and proinflammatory effects.(40) Oral CHCs have also been shown to increase circulating uric acid, impair the endothelin dependent nitric oxide pathway, elevate proinflammatory biomarkers and lead to endothelial dysfunction.(41, 42)
The American College of Obstetricians and Gynecology 2019 Practice Bulletin endorses the US Medical Eligibility Criteria recommendations for the initiation of CHCs.(43) Women with high BP (systolic 140–159 mmHg or diastolic 90–99 mmHg), and those with hypertension who are age >35 years should avoid CHCs if possible. CHC use is contraindicated for women with systolic BP ≥160 mmHg or diastolic BP ≥ 100mmHg or vascular disease. For women ≤ 35 years with controlled hypertension who are otherwise healthy, evidence suggests that they can continue combined oral contraceptives if they show no evidence of end-organ damage and do not smoke. For all other women on antihypertensive medications, use of oral contraceptives is left up to clinical judgement of the physician and patient acceptability of non-hormonal methods or progestin-only pills. After initiating oral contraceptives, BP should be checked within 2 to 4 weeks and at follow up visits every 6 months to monitor for the development of hypertension. CHCs should be discontinued and another form of contraception should be considered if BP increases markedly in the absence of other causes. BP measurement before or during the initiation of progestin-only pills, subdermal implants, depot-medroxyprogesterone acetate, or levonorgestrel-releasing intrauterine is not indicated.(44)
Hypertension in Pregnancy
Overview of hypertensive disorders of pregnancy
It is important to note that special consideration should be given to hypertension during pregnancy. The definitions of the four hypertension disorders of pregnancy (chronic hypertension, gestational hypertension, preeclampsia/eclampsia, and preeclampsia superimposed on chronic hypertension) are found in Table 2 and these disorders complicate up to 10% of pregnancies.(45–47) Classification depends on the timing of development of hypertension or persistence of hypertension postpartum along with the presence or absence of target organ damage. The American College of Obstetricians and Gynecologists hypertension guidelines do not incorporate the 2017 ACC/AHA BP guidelines recommendation to reclassify prehypertension as elevated BP (systolic BP 120–129 mmHg) and stage 1 hypertension (systolic BP 130–139 mmHg/diastolic BP 80–89 mmHg), and continue to use the cut-off of 140/90 mmHg for the diagnosis of hypertension.(48) Future research is needed to clarify the impact of implementing a consistent definition of hypertension in pregnant and non-pregnant adult women.
Table 2.
Hypertensive disorders of pregnancy
In pregnancy, hypertension is defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg confirmed on 2 or more occasions and severe HTN is systolic blood pressure ≥160 mmHg and/or diastolic blood pressure ≥110 mmHg confirmed on 2 or more measurements at least 15 min apart
| Timing of Diagnosis | Additional Information | |
|---|---|---|
| Chronic hypertension | Predating pregnancy or occurring at <20 weeks of gestation or persists ≥ 12 weeks post delivery | Prevalent in 0.9–1.5% of pregnant women 20–50% with chronic hypertension may develop preeclampsia |
| Gestational Hypertension | ≥ 20 weeks after gestation | Prevalent in 6–7% 10–25% can progress to having preeclempsia |
| Preeclampsia* | ≥ 20 weeks after gestation | Prevalent in 5–7% Definition: Hypertension AND Proteinuria (≥300 mg per 24-hour urine collection or protein:creatinine ratio ≥0.3 or urine dipstick reading ≥2+) OR Any new onset feature of severe preeclampsia*
|
| Chronic hypertension with superimposed preeclampsia |
Definition:
Chronic hypertension AND a sudden increase in BP previously well controlled or an increase in antihypertensive therapy to control BP OR New onset proteinuria or increase in proteinuria |
BP: blood pressure
- Thrombocytopenia (platelet count <100*109 L)
- Impaired liver function not accounted for by other causes: abnormally elevated liver transaminase levels (at least twice the normal concentration) or severe persistent right upper quadrant or epigastric pain unresponsive to medication
- Progressive renal insufficiency (serum creatinine concentration >1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease.
- Pulmonary edema
- New onset headache unresponsive to medication and not accounted for by other causes
- Visual disturbances
Overview of BP goal and antihypertensives in pregnancy
Another notable difference between hypertension in the pregnant and non-pregnant adult woman is the difference in recommended BP targets during pregnancy. There are many differences among international hypertensive disorders of pregnancy guidelines regarding the threshold for antihypertensive medication initiation and treatment targets and a full discussion of the topic is outside the scope of this review.(49) During pregnancy, women should be transitioned to the preferred antihypertensive medications nifedipine, labetalol, and/or methyldopa, which is often done in consultation with an obstetrician/gynecologist or maternal fetal medicine specialist.(46) Women who are hypertensive and planning to become pregnant or are pregnant should not be treated with angiotensin converting enzyme inhibitors/angiotensin receptor blockers or direct renin inhibitors due to potential for teratogenesis (intrauterine growth restriction, neonatal renal failure, oligohydramnios and death).(46) Atenolol, a beta blocker, should also be avoided as it may cause intrauterine growth restriction and low birth weight.(46) Thiazide diuretics can be continued during pregnancy if they were used before and should be stopped if pregnant women develop preeclampsia to avoid worsening intravascular volume depletion.(50, 51)
BP measurement during pregnancy
The United States Preventive Task Force (USPSTF) recommends BP measurement to screen for preeclampsia at each prenatal visit for women without a known diagnosis of a hypertensive disorder to enable early diagnosis and monitoring and minimize the risk of adverse maternal/fetal events.(52) Similar to recommendations from the 2017 ACC/AHA for non-pregnant adults, prior to diagnosing hypertension in a pregnant women, BP measurements should be repeated and confirmed to be elevated on two separate occasions. The exception in pregnancy occurs when there is concern for severe hypertension, a medical emergency defined as systolic BP ≥160 and/or diastolic BP ≥110 mmHg. In this situation, the measurement should be repeated within a short interval (15 min) to confirm or refute the diagnosis. Another difference in the diagnostic criteria used for pregnant women versus other groups of adults is that out of office definitions of hypertension are not incorporated into current guidelines. Several ongoing and recently published studies examining correlations between office and out of office BP in pregnant women have yet to be incorporated into ACOG recommendations.(53–56) Using a validated device to measure BP is of utmost importance given the consequences of hypertension in pregnancy. A systematic review identified BP devices validated for accuracy in pregnancy which are listed in Table 3 along with subsequent devices that have been approved.(57–61)
Table 3.
Oscillometric blood pressure devices validated for accuracy during pregnancy
| Setting | Validated Devices |
|---|---|
| Ambulatory | BP Lab, Spacelabs 90207, Welch Allyn QuietTrak, PHYSIO-PORTUP |
| Office | A&D UM-101, Dinamap ProCare 400, Microlife 3AS1-2, Nissei DS-400, Omron HEM-907, Omron MIT, Omron MIT Elite, Terumo ES-H51, Welch Allyn Vital Signs |
| Home | Microlife 3BTO-A, Microlife WatchBP Home, Omron HEM 705 CP, Omron M7, Omron MIT, Andon iHealth Track, Omron HEM-9210T, Omron BP760N, Omron BP765 |
| Office or Home | Omron MIT Elite, Omron T9P |
Hypertension in the postpartum period
Women who develop hypertension during pregnancy have an increased risk of prehypertension/hypertension in the year after delivery and an increase risk of developing other cardiometabolic disorders such as diabetes mellitus and hypercholesterolemia.(62, 63) For every 13 mmHg increase in systolic BP in early and late pregnancy, risk of cardiovascular mortality increased by 20% and 14% respectively.(64) Preeclampsia has also been shown to be associated with a 4-foldincrease in incident heart failure and 2-fold increase in incident coronary heart disease, stroke, and cardiovascular death.(65)
In the majority of women, BP falls during the first and second trimesters and approaches its pre-pregnancy levels during the third trimester. In this setting, gestational hypertension and preeclampsia usually begin to resolve within 2 weeks postpartum and should resolve fully within 12 weeks. If hypertension persists, a diagnosis of chronic hypertension should be considered with appropriate secondary work-up for underlying etiologies as clinically indicated.(66–68) In other women, postpartum hypertension can present as either persistent or new onset hypertension (3–6 days postpartum) with prevalence rates varying widely 0.3–27.5% depending on the group studied.(67) New onset postpartum hypertension may be due to a large volume of intravenous fluid administered during delivery, loss of pregnancy associated vasodilation, use of nonsteroidal anti-inflammatory drugs postdelivery, as a side-effect of medications used for the treatment of postpartum hemorrhage, or as extravascular fluid mobilizes following delivery and often resolves in a similar timeline of gestational hypertension.(62, 69) Of note, preeclampsia can also arise de novo postpartum.(70)
Assistive reproductive technologies and hypertension
The use of assisted reproductive technologies (ART) to help treat infertility is rising worldwide.(71, 72) A recent systematic review found that there is a significant increased risk of preeclampsia in women who use ART (all types) for conception vs. natural conception (relative risk [RR]= 1.71, 95% CI: 1.11–2.62).(73) Invasive ARTs, including intrauterine insemination, in vitro fertilization, intracytoplasmic sperm injection, oocyte donation, single or double embryo transfer, and transfer after fresh and frozen cycles, are associated with increased risk of gestational hypertension (RR= 1.79 [1.24, 2.57]), preeclampsia (RR= 1.75 [1.50, 2.03]) and all pregnancy related hypertensive disorders (RR= 1.54 [1.39–1.70]) regardless of gestation order.(74) Similarly non-invasive ART (ovulation induction) was associated with an increase in preeclampsia (RR= 1.56 [1.27, 1.91]) and all hypertensive disorders of pregnancy (RR= 1.24 [1.05, 1.45]).(74) A population based cohort study from national health registries in Sweden, Denmark and Norway found that hypertensive disorders of pregnancies were found in 6% of ART singleton pregnancies and 13% of ART twin pregnancies.(75) Risk of hypertensive disorders was higher in ART singleton pregnancies compared to spontaneously conceived singleton pregnancies with all ART modalities (fresh cycle, frozen cycles, invitro fertilization, intracytoplasmic sperm injection). The highest risk in singleton pregnancies was observed after frozen–thawed cycles [odds ratio (OR) = 1.41 (1.27, 1.56)]. For twin pregnancies higher risk of hypertensive disorders was only observed after frozen-thawed cycles compared to spontaneous twin pregnancies [OR= 1.57 (1.34, 1.85)]. The mechanism by which ART increases risk of preeclampsia is unclear especially given the multitude of potential confounders associated with infertility itself that may affect the association between ART and preeclampsia. However a few hypothesis exist, first recurrent spontaneous miscarriages which are common with ART are associated with an increased risk of preeclampsia.(76) Second, placental insufficiency, a hallmark of preeclampsia, may result upon transfer of the conceptus into the uterine cavity of the woman and the altered hormonal environment in the endometrium.(77, 78) Third, ART may contribute to oxidative stress which increases the risk of preeclampsia.(73) Additionally, absence of the corpus luteum in programmed cycles - common with in vitro fertilization specifically with frozen thawed embryo transfer - may increase the risk of abnormal maternal cardiovascular adaptation to pregnancy leading to an increased risk of preeclampsia.(79) Lastly, while women with chronic hypertension that predates pregnancy are known to be at heightened risk for the development of superimposed preeclampsia during pregnancy, to our knowledge the potential interaction between chronic hypertension and ART has not been rigorously examined.(77)
There have been conflicting results regarding the potential long-term effect of ART on hypertension risk.(80, 81) In one study of nonobese women aged 43 years and older who conceived by ART mean systolic and diastolic BP was higher for up to three years after delivery and age at menopause age was significantly earlier compared to women who conceived without ART.(82)
Hypertension and Menopause
The prevalence of hypertension in postmenopausal women is higher than that in men of a comparable age and 75% of postmenopausal women (age >60 years) in the U.S. have hypertension.(83) During menopause, estradiol levels decrease thus activating the RAAS and leading to vasoconstriction. There is also an increase in salt sensitivity(17). Additionally, androgen production continues in postmenopausal women and may increase arterial stiffness and vascular inflammation leading to endothelial dysfunction and increased BP. However, findings are inconsistent in the literature as far as the role of estrogen/androgen on hypertension in postmenopausal women. (25, 83–85) Severity of menopausal symptoms should also be asked about. Studies have shown women who experience vasomotor symptoms such as hot flashes have higher awake and asleep blood pressure when compared to women without hot flashes.(86) It is important to note that it is difficult to separate the exact role of hormonal changes from aging on BP. Another mechanism contributing to hypertension in postmenopausal women is an increase in sympathetic activation that could be due to increased body weight and redistribution of body fat as well as increased leptin levels.(25) Postmenopausal women are also more likely to have a non-dipping BP pattern which is associated with poorer cardiovascular outcomes and more target organ damage in women compared to men.(87, 88)
Menopausal Hormone Therapy
Menopausal Hormone Therapy (MHT), previously known as hormone replacement therapy includes both combined estrogen and progestin and estrogen alone for women who have undergone hysterectomy. MHTs have been shown to have little to no effect on BP. In the Women’s Health Initiative (WHI), a randomized placebo-controlled trial of 16,000 women examining the effect of MHT on cardiovascular outcomes participants randomized to MHT had a 1.5 mmHg increase in systolic BP compared to those on placebo after 5.2 years.(89) The Kronos Early Estrogen Prevention Study examined the effect of two different formulations of MHT [daily oral conjugated equine estrogen (0.45 mg), or weekly transdermal 17β-estradiol (50μg) each with intermittent progesterone (200 mg daily 12 days of the month)] on recently postmenopausal women with no hypertension pressure at baseline.(90) Neither MHT formulation produced any significant change to BP. In the Postmenopausal Estrogen/Progestin Interventions trial, among 875 healthy postmenopausal women, the use of estrogen with or without progestin did not affect BP after 3 years. Some studies have found that estrogen replacement therapy reduces ambulatory BP and result in greater dipping in nighttime BP.(91, 92) However, MHT should not be used only for primary prevention of cardiovascular disease given the increased risk of coronary heart disease, stroke, and venous thromboembolic risk in women on MHT as shown in the WHI study especially for women who are >10 years postmenopausal.(93–97)
Hypertension in Elderly Women
Hypertension prevalence increases with age and a steep rise in rates occur in women starting as early as the third decade of life.(7) Hypertension rates are higher in women than men aged 65 years and older.(98) The mechanism behind increasing BP in elderly women are linked to a decline in nitric oxide synthesis after menopause and a decline in endothelial function thus leading to impaired endothelium mediated vasodilation.(99, 100) Other non-specific sex mechanism related to aging contribute to a rise in BP with age such as inflammation, oxidative stress, comorbidities, and arterial stiffness.(101, 102) NHANES data (2011–2014) showed that among individuals ≥75 years, 81.2% of women have hypertension vs. 73.4% of men (Figure 1).(98) Compared to young and middle aged women, elderly women also have more severe hypertension and lower rates of BP control.(98)
Irrespective of sex and age, the current recommended BP goal is <130/80 mmHg for all adults, except for pregnant women as previously discussed.(48) This is in line with the findings from the Systolic BP Intervention Trial (SPRINT) that showed that intensive (systolic BP <120 mmHg) vs. standard (<140 mmHg) BP lowering resulted in significantly lower rates of fatal and nonfatal cardiovascular events and all-cause mortality even in the prespecified subgroups of women and adults ≥75 years.(103, 104) There was no difference in number of participants who experienced injurious falls or prevalence of orthostatic hypotension in the SPRINT in the intensive vs. standard group.(104) The Hypertension in the Very Elderly Trial (HYVET) which enrolled participants over the age of 80 (60% women) showed that hypertensive treatment vs. no treatment significantly reduced cardiovascular and all-cause mortality, fatal and non-fatal stroke and heart failure even after adjustment for sex.(105) Thus, it is important to treat hypertension in elderly women and men. Not only that, but controlling BP as a way to optimize cardiovascular health and may reduce cognitive decline and prevent dementia in the elderly irrespective of sex.(106–109) However, the benefit of antihypertensive treatment on frailty is still not well elucidated as frail adults are usually excluded form clinical trials such as SPRINT and HYVET. Currently, there are no specific BP treatment guidelines for the frail or age specific guidelines for adults, and future studies are needed to fill this important gap in evidence.
Representation of Women in Hypertension Clinical Trials
A systematic review of 740 cardiovascular clinical trials between 2010 and 2017 using data from ClinicalTrials.gov showed that despite making up 51% percent of the population, women represent only 38.2% of trial participants.(110, 111) Women’s representation in trials varied by disease state and was highest in trials of pulmonary hypertension and hypertension (46.2%) and lowest in trials on heart failure, acute coronary syndrome, arrythmia, and coronary heart disease. (110) The National Heart, Lung, and Blood Institute (NHLBI) reinstated the importance of including women in cardiovascular clinical trials consistent with the epidemiology of the disease and the need to include and need to report valid analysis of women in a recent perspective piece.(112) Future trials of hypertension lowering must include women across the lifespan to address the various issues we have presented herein. Previous trials have included predominantly menopausal and postmenopausal women, which may limit their generalizability.
Conclusion
Hypertension affects women throughout their lifetime, and exacerbating factors vary by age. Use of oral contraceptives, pregnancy, assisted reproductive technology and menopause are unique hypertension risk factors in women that should be taken into account when screening and managing hypertension. Despite scientific advances, sex-specific gaps in the evidence base remain. These include: 1) understanding whether there should be sex-specific thresholds for the diagnosis and treatment of hypertension across a women’s lifespan specifically during pregnancy and in the elderly, 2) sex specific impact of modifiable risk factors on blood pressure, such as obesity, 3) understanding the long term impact of ART on hypertension risk. We advocate for inclusion of women of all ages in all cardiovascular related trials given the physiological changes women go through across their lifetime.
Funding
Dr. Bello has research funding from NIH/NHLBI (K23 HL136853 R01 HL153382)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent. This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Dr. Bello has nothing to disclose.
Dr. Ghazi has nothing to disclose.
References
- 1.Collaborators GRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134(6):441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei YC, George NI, Chang CW, Hicks KA. Assessing Sex Differences in the Risk of Cardiovascular Disease and Mortality per Increment in Systolic Blood Pressure: A Systematic Review and Meta-Analysis of Follow-Up Studies in the United States. PLoS One. 2017;12(1):e0170218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ROBINSON SC, BRUCER M. RANGE OF NORMAL BLOOD PRESSURE: A STATISTICAL AND CLINICAL STUDY OF 11,383 PERSONS. Arch Intern Med (Chic). 1939;64(3):409–444. doi: 10.1001/archinte.1939.00190030002001 [DOI] [Google Scholar]
- 5.Syme C, Abrahamowicz M, Leonard GT, Perron M, Richer L, Veillette S, et al. Sex differences in blood pressure and its relationship to body composition and metabolism in adolescence. Arch Pediatr Adolesc Med. 2009;163(9):818–25. [DOI] [PubMed] [Google Scholar]
- 6.Joyner MJ, Wallin BG, Charkoudian N. Sex differences and blood pressure regulation in humans. Exp Physiol. 2016;101(3):349–55. [DOI] [PubMed] [Google Scholar]
- 7.Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, et al. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020;5(3):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● Women have a steeper increase in blood pressure compared to men beginning as early as the third decade
- 8.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021:CIR0000000000000950. [DOI] [PubMed] [Google Scholar]
- 9.O’Neil A, Scovelle AJ, Milner AJ, Kavanagh A. Gender/Sex as a Social Determinant of Cardiovascular Risk. Circulation. 2018;137(8):854–64. [DOI] [PubMed] [Google Scholar]
- 10.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84(4):633–41. [PubMed] [Google Scholar]
- 11.Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119(2):237–46. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117(25):3171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo D, Cheng Y, Zhang H, Ba M, Chen P, Li H, et al. Association between high blood pressure and long term cardiovascular events in young adults: systematic review and meta-analysis. BMJ. 2020;370:m3222. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● There is a progressive associaction between blood pressure and cardiovascular eve
- 14.Nardin C, Maki-Petaja KM, Miles KL, Yasmin, McDonnell BJ, Cockcroft JR, et al. Cardiovascular Phenotype of Elevated Blood Pressure Differs Markedly Between Young Males and Females: The Enigma Study. Hypertension. 2018;72(6):1277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53(3):688–708. [DOI] [PubMed] [Google Scholar]
- 16.Khalil RA. Sex hormones as potential modulators of vascular function in hypertension. Hypertension. 2005;46(2):249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pechère-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens. 2004;17(10):994–1001. [DOI] [PubMed] [Google Scholar]
- 18.Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14(3):185–201. [DOI] [PubMed] [Google Scholar]
- 19.Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, et al. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol. 1997;273(5):F777–82. [DOI] [PubMed] [Google Scholar]
- 20.Dunne FP, Barry DG, Ferriss JB, Grealy G, Murphy D. Changes in blood pressure during the normal menstrual cycle. Clin Sci (Lond). 1991;81(4):515–8. [DOI] [PubMed] [Google Scholar]
- 21.Karpanou EA, Vyssoulis GP, Georgoudi DG, Toutouza MG, Toutouzas PK. Ambulatory blood pressure changes in the menstrual cycle of hypertensive women. Significance of plasma renin activity values. Am J Hypertens. 1993;6(8):654–9. [DOI] [PubMed] [Google Scholar]
- 22.Bentley-Lewis R, Seely E, Dunaif A. Ovarian hypertension: polycystic ovary syndrome. Endocrinol Metab Clin North Am. 2011;40(2):433–49, ix-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–72. [DOI] [PubMed] [Google Scholar]
- 24.Sharabi Y, Grotto I, Huerta M, Grossman E. Susceptibility of the influence of weight on blood pressure in men versus women: lessons from a large-scale study of young adults. Am J Hypertens. 2004;17(5 Pt 1):404–8. [DOI] [PubMed] [Google Scholar]
- 25.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. 2012;14(3):254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everett B, Zajacova A. Gender differences in hypertension and hypertension awareness among young adults. Biodemography Soc Biol. 2015;61(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010;82(12):1471–8. [PubMed] [Google Scholar]
- 28.Wenger NK, Arnold A, Bairey Merz CN, Cooper-DeHoff RM, Ferdinand KC, Fleg JL, et al. Hypertension Across a Woman’s Life Cycle. J Am Coll Cardiol. 2018;71(16):1797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappadis SL, Somers MJ. Hypertension in adolescents: a review of diagnosis and management. Curr Opin Pediatr. 2003;15(4):370–8. [DOI] [PubMed] [Google Scholar]
- 30.Weir RJ, Briggs E, Mack A, Naismith L, Taylor L, Wilson E. Blood pressure in women taking oral contraceptives. Br Med J. 1974;1(5907):533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisch IR, Frank J. Oral contraceptives and blood pressure. JAMA. 1977;237(23):2499–503. [PubMed] [Google Scholar]
- 32.Meade TW, Haines AP, North WR, Chakrabarti R, Howarth DJ, Stirling Y. Haemostatic, lipid, and blood-pressure profiles of women on oral contraceptives containing 50 microgram or 30 microgram oestrogen. Lancet. 1977;2(8045):948–51. [DOI] [PubMed] [Google Scholar]
- 33.Wilson ES, Cruickshank J, McMaster M, Weir RJ. A prospective controlled study of the effect on blood pressure of contraceptive preparations containing different types and dosages of progestogen. Br J Obstet Gynaecol. 1984;91(12):1254–60. [DOI] [PubMed] [Google Scholar]
- 34.Chasan-Taber L, Willett WC, Manson JE, Spiegelman D, Hunter DJ, Curhan G, et al. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation. 1996;94(3):483–9. [DOI] [PubMed] [Google Scholar]
- 35.Lubianca JN, Moreira LB, Gus M, Fuchs FD. Stopping oral contraceptives: an effective blood pressure-lowering intervention in women with hypertension. J Hum Hypertens. 2005;19(6):451–5. [DOI] [PubMed] [Google Scholar]
- 36.Hussain SF. Progestogen-only pills and high blood pressure: is there an association? A literature review. Contraception. 2004;69(2):89–97. [DOI] [PubMed] [Google Scholar]
- 37.Aisien AO, Enosolease ME. Safety, efficacy and acceptability of implanon a single rod implantable contraceptive (etonogestrel) in University of Benin Teaching Hospital. Niger J Clin Pract. 2010;13(3):331–5. [PubMed] [Google Scholar]
- 38.Dorflinger LJ. Metabolic effects of implantable steroid contraceptives for women. Contraception. 2002;65(1):47–62. [DOI] [PubMed] [Google Scholar]
- 39.Morin-Papunen L, Martikainen H, McCarthy MI, Franks S, Sovio U, Hartikainen AL, et al. Comparison of metabolic and inflammatory outcomes in women who used oral contraceptives and the levonorgestrel-releasing intrauterine device in a general population. Am J Obstet Gynecol. 2008;199(5):529.e1–.e10. [DOI] [PubMed] [Google Scholar]
- 40.Shufelt CL, Bairey Merz CN. Contraceptive hormone use and cardiovascular disease. J Am Coll Cardiol. 2009;53(3):221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olatunji LA, Seok YM, Igunnu A, Kang SH, Kim IK. Combined oral contraceptive-induced hypertension is accompanied by endothelial dysfunction and upregulated intrarenal angiotensin II type 1 receptor gene expression. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(11):1147–57. [DOI] [PubMed] [Google Scholar]
- 42.Machado RB, Fabrini P, Cruz AM, Maia E, da Cunha Bastos A. Clinical and metabolic aspects of the continuous use of a contraceptive association of ethinyl estradiol (30 microg) and gestodene (75 microg). Contraception. 2004;70(5):365–70. [DOI] [PubMed] [Google Scholar]
- 43.ACOG Practice Bulletin No. 206: Use of Hormonal Contraception in Women With Coexisting Medical Conditions. Obstet Gynecol. 2019;133(2):e128–e50. [DOI] [PubMed] [Google Scholar]
- 44.Curtis KM JT, Tepper NK, et al. U.S. Selected Practice, Recommendations for Contraceptive Use MRR, 65(No., 10.15585/mmwr.rr6504a1R-D. [DOI]
- 45.Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237–e60. [DOI] [PubMed] [Google Scholar]
- 46.ACOG Practice Bulletin No. 203: Chronic Hypertension in Pregnancy. Obstet Gynecol. 2019;133(1):e26–e50. [DOI] [PubMed] [Google Scholar]
- 47.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. [DOI] [PubMed] [Google Scholar]
- 48.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e426–e83. [DOI] [PubMed] [Google Scholar]
- 49.Sinkey RG, Battarbee AN, Bello NA, Ives CW, Oparil S, Tita ATN. Prevention, Diagnosis, and Management of Hypertensive Disorders of Pregnancy: a Comparison of International Guidelines. Curr Hypertens Rep. 2020;22(9):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Churchill D, Beevers GD, Meher S, Rhodes C. Diuretics for preventing pre-eclampsia. Cochrane Database Syst Rev. 2007(1):CD004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Committee Opinion No. 623: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2015;125(2):521–5. [DOI] [PubMed] [Google Scholar]
- 52.United States Preventive Task Force. Preeclempsia Screening. https://www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/preeclampsia-screening. Accessed December 28, 2020.
- 53.Tucker KL, Bankhead C, Hodgkinson J, Roberts N, Stevens R, Heneghan C, et al. How Do Home and Clinic Blood Pressure Readings Compare in Pregnancy? Hypertension. 2018;72(3):686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mikami Y, Takai Y, Era S, Ono Y, Saitoh M, Baba K, et al. Provisional criteria for the diagnosis of hypertension in pregnancy using home blood pressure measurements. Hypertens Res. 2017;40(7):679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dougall G, Franssen M, Tucker KL, Yu LM, Hinton L, Rivero-Arias O, et al. Blood pressure monitoring in high-risk pregnancy to improve the detection and monitoring of hypertension (the BUMP 1 and 2 trials): protocol for two linked randomised controlled trials. BMJ Open. 2020;10(1):e034593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pealing LM, Tucker KL, Mackillop LH, Crawford C, Wilson H, Nickless A, et al. A randomised controlled trial of blood pressure self-monitoring in the management of hypertensive pregnancy. OPTIMUM-BP: A feasibility trial. Pregnancy Hypertens. 2019;18:141–9. [DOI] [PubMed] [Google Scholar]
- 57.Bello NA, Woolley JJ, Cleary KL, Falzon L, Alpert BS, Oparil S, et al. Accuracy of Blood Pressure Measurement Devices in Pregnancy: A Systematic Review of Validation Studies. Hypertension. 2018;71(2):326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Den Heuvel JFM, Lely AT, Franx A, Bekker MN. Validation of the iHealth Track and Omron HEM-9210T automated blood pressure devices for use in pregnancy. Pregnancy Hypertens. 2019;15:37–41. [DOI] [PubMed] [Google Scholar]
- 59.Topouchian J, Hakobyan Z, Asmar J, Gurgenian S, Zelveian P, Asmar R. Clinical accuracy of the Omron M3 Comfort. Vasc Health Risk Manag. 2018;14:189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi H, Yoshika M, Yokoi T. Validation of two automatic devices for the self-measurement of blood pressure according to the ANSI/AAMI/ISO81060–2:2009 guidelines: the Omron BP765 (HEM-7311-ZSA) and the Omron BP760N (HEM-7320-Z). Vasc Health Risk Manag. 2015;11:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abou-Dakn M, Wenzel S. Validation of the PHYSIO-PORT UP ambulatory blood pressure monitor in pregnant women according to the European Society of Hypertension International Protocol revision 2010. J Hum Hypertens. 2018;32(11):770–4. [DOI] [PubMed] [Google Scholar]
- 62.Black MH, Zhou H, Sacks DA, Dublin S, Lawrence JM, Harrison TN, et al. Hypertensive disorders first identified in pregnancy increase risk for incident prehypertension and hypertension in the year after delivery. J Hypertens. 2016;34(4):728–35. [DOI] [PubMed] [Google Scholar]
- 63.Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, et al. Hypertensive Disorders of Pregnancy and Maternal Cardiovascular Disease Risk Factor Development: An Observational Cohort Study. Ann Intern Med. 2018;169(4):224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luoto R, Kharazmi E, Whitley E, Raitanen J, Gissler M, Hemminki E. Systolic hypertension in pregnancy and cardiovascular mortality: a 44-year follow-up study. Hypertens Pregnancy. 2008;27(1):87–94. [DOI] [PubMed] [Google Scholar]
- 65.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2). [DOI] [PubMed] [Google Scholar]
- 66.Mikami Y, Matsumoto T, Kano K, Toriumi T, Somei M, Honda MJ, et al. Current status of drug therapies for osteoporosis and the search for stem cells adapted for bone regenerative medicine. Anat Sci Int. 2014;89(1):1–10. [DOI] [PubMed] [Google Scholar]
- 67.Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206(6):470–5. [DOI] [PubMed] [Google Scholar]
- 68.Podymow T, August P. Postpartum course of gestational hypertension and preeclampsia. Hypertens Pregnancy. 2010;29(3):294–300. [DOI] [PubMed] [Google Scholar]
- 69.Goel A, Maski MR, Bajracharya S, Wenger JB, Zhang D, Salahuddin S, et al. Epidemiology and Mechanisms of De Novo and Persistent Hypertension in the Postpartum Period. Circulation. 2015;132(18):1726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol. 2004;190(5):1464–6. [DOI] [PubMed] [Google Scholar]
- 71.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406. [DOI] [PubMed] [Google Scholar]
- 72.Davies MJ, Rumbold AR, Moore VM. Assisted reproductive technologies: a hierarchy of risks for conception, pregnancy outcomes and treatment decisions. J Dev Orig Health Dis. 2017;8(4):443–7. [DOI] [PubMed] [Google Scholar]
- 73.Almasi-Hashiani A, Omani-Samani R, Mohammadi M, Amini P, Navid B, Alizadeh A, et al. Assisted reproductive technology and the risk of preeclampsia: an updated systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomopoulos C, Salamalekis G, Kintis K, Andrianopoulou I, Michalopoulou H, Skalis G, et al. Risk of hypertensive disorders in pregnancy following assisted reproductive technology: overview and meta-analysis. J Clin Hypertens (Greenwich). 2017;19(2):173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad PR, et al. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum Reprod. 2015;30(7):1724–31. [DOI] [PubMed] [Google Scholar]
- 76.Tandberg A, Klungsøyr K, Romundstad LB, Skjærven R. Pre-eclampsia and assisted reproductive technologies: consequences of advanced maternal age, interbirth intervals, new partner and smoking habits. BJOG. 2015;122(7):915–22. [DOI] [PubMed] [Google Scholar]
- 77.Thomopoulos C, Tsioufis C, Michalopoulou H, Makris T, Papademetriou V, Stefanadis C. Assisted reproductive technology and pregnancy-related hypertensive complications: a systematic review. J Hum Hypertens. 2013;27(3):148–57. [DOI] [PubMed] [Google Scholar]
- 78.Daniel Y, Schreiber L, Geva E, Amit A, Pausner D, Kupferminc MJ, et al. Do placentae of term singleton pregnancies obtained by assisted reproductive technologies differ from those of spontaneously conceived pregnancies? Hum Reprod. 1999;14(4):1107–10. [DOI] [PubMed] [Google Scholar]
- 79.Conrad KP, Baker VL. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol. 2013;304(2):R69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Udell JA, Lu H, Redelmeier DA. Long-term cardiovascular risk in women prescribed fertility therapy. J Am Coll Cardiol. 2013;62(18):1704–12. [DOI] [PubMed] [Google Scholar]
- 81.Westerlund E, Brandt L, Hovatta O, Wallén H, Ekbom A, Henriksson P. Incidence of hypertension, stroke, coronary heart disease, and diabetes in women who have delivered after in vitro fertilization: a population-based cohort study from Sweden. Fertil Steril. 2014;102(4):1096–102. [DOI] [PubMed] [Google Scholar]
- 82.Rosato E, Perrone G, Capri O, Galoppi P, Candelieri M, Marcoccia E, et al. Hypertension and early menopause after the use of assisted reproductive technologies in women aged 43 years or older: Long-term follow-up study. J Obstet Gynaecol Res. 2016;42(12):1782–8. [DOI] [PubMed] [Google Scholar]
- 83.Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54(1):11–8. [DOI] [PubMed] [Google Scholar]
- 84.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens. 2011;24(7):740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reckelhoff JF, Fortepiani LA. Novel mechanisms responsible for postmenopausal hypertension. Hypertension. 2004;43(5):918–23. [DOI] [PubMed] [Google Scholar]
- 86.Gerber LM, Sievert LL, Warren K, Pickering TG, Schwartz JE. Hot flashes are associated with increased ambulatory systolic blood pressure. Menopause. 2007;14(2):308–15. [DOI] [PubMed] [Google Scholar]
- 87.Routledge FS, McFetridge-Durdle JA, Dean CR. Stress, menopausal status and nocturnal blood pressure dipping patterns among hypertensive women. Can J Cardiol. 2009;25(6):e157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McSweeney JC, Rosenfeld AG, Abel WM, Braun LT, Burke LE, Daugherty SL, et al. Preventing and Experiencing Ischemic Heart Disease as a Woman: State of the Science: A Scientific Statement From the American Heart Association. Circulation. 2016;133(13):1302–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. [DOI] [PubMed] [Google Scholar]
- 90.Kling JM, Lahr BA, Bailey KR, Harman SM, Miller VM, Mulvagh SL. Endothelial function in women of the Kronos Early Estrogen Prevention Study. Climacteric. 2015;18(2):187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Butkevich A, Abraham C, Phillips RA. Hormone replacement therapy and 24-hour blood pressure profile of postmenopausal women. Am J Hypertens. 2000;13(9):1039–41. [DOI] [PubMed] [Google Scholar]
- 92.Cagnacci A, Rovati L, Zanni A, Malmusi S, Facchinetti F, Volpe A. Physiological doses of estradiol decrease nocturnal blood pressure in normotensive postmenopausal women. Am J Physiol. 1999;276(4):H1355–60. [DOI] [PubMed] [Google Scholar]
- 93.Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke: a meta-analysis. BMJ. 2005;330(7487):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–77. [DOI] [PubMed] [Google Scholar]
- 95.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166(3):357–65. [DOI] [PubMed] [Google Scholar]
- 96.Salpeter SR, Walsh JM, Greyber E, Salpeter EE. Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med. 2006;21(4):363–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dubey RK, Imthurn B, Zacharia LC, Jackson EK. Hormone replacement therapy and cardiovascular disease: what went wrong and where do we go from here? Hypertension. 2004;44(6):789–95. [DOI] [PubMed] [Google Scholar]
- 98.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 99.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–6. [DOI] [PubMed] [Google Scholar]
- 100.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aging Sun Z., arterial stiffness, and hypertension. Hypertension. 2015;65(2):252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buford TW. Hypertension and aging. Ageing Res Rev. 2016;26:96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA. 2016;315(24):2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–98. [DOI] [PubMed] [Google Scholar]
- 106.Benetos A, Petrovic M, Strandberg T. Hypertension Management in Older and Frail Older Patients. Circ Res. 2019;124(7):1045–60. [DOI] [PubMed] [Google Scholar]
- 107.de Bruijn RF, Bos MJ, Portegies ML, Hofman A, Franco OH, Koudstaal PJ, et al. The potential for prevention of dementia across two decades: the prospective, population-based Rotterdam Study. BMC Med. 2015;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–99. [DOI] [PubMed] [Google Scholar]
- 109.Chen KH, Henderson VW, Stolwyk RJ, Dennerstein L, Szoeke C. Prehypertension in midlife is associated with worse cognition a decade later in middle-aged and older women. Age Ageing. 2015;44(3):439–45. [DOI] [PubMed] [Google Scholar]
- 110.Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL. Women’s Participation in Cardiovascular Clinical Trials From 2010 to 2017. Circulation. 2020;141(7):540–8. [DOI] [PubMed] [Google Scholar]; ●● Women are still disproportionately represented in cardiovascular trials
- 111.U.S. Census Bureau QuickFacts. (2019). https://www.census.gov/quickfacts/fact/table/US/PST045219 (Accessed on January 28, 2021).
- 112.Pearson GD, Mensah GA, Rosenberg Y, Stoney CM, Kavounis K, Goff DC. National Heart, Lung, and Blood Institute cardiovascular clinical trial perspective. Am Heart J. 2020;224:25–34. [DOI] [PubMed] [Google Scholar]


