Summary
Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum of different conditions which are characterized by hepatic steatosis in the absence of secondary causes. It is currently the most common chronic liver disease worldwide, and its estimated prevalence is about 1.5-6.5%. The only histological finding of steatosis (“simple” steatosis) represents the uncomplicated form of NAFLD, while non-alcoholic steatohepatitis (NASH) is its inflammatory subtype associated with disease progression to cirrhosis and hepatocellular carcinoma (HCC), and represents the major indication for liver transplantation. NASH is still a diagnostic and therapeutic challenge for clinicians and liver biopsy is currently the only accepted method to reliably distinguish NASH from “simple” steatosis. From the histological perspectives, NAFLD and NASH continue to be an area of active interest for pathologists, with a specific focus on better methods of evaluation, morphologic clues to pathogenesis, and predictors of fibrosis progression. This review focuses on histopathology of NAFLD in adults, with the aim to provide a practical diagnostic approach useful in the clinical routine.
Key words: fatty liver, steatohepatitis, non-alcoholic, metabolic syndrome
Introduction
“Fatty liver” is a very common condition defined by the presence of intra-hepatocytes lipid droplets, commonly referred to as steatosis. It encompasses a wide spectrum of hepatic alterations with a wide range of etiological and clinical-pathological features 1.
Non-alcoholic fatty liver disease (NAFLD) is an umbrella term used to comprise a wide spectrum of conditions, which are characterized by hepatic steatosis in absence of secondary causes, including significant alcohol consumption, chronic use of medications and hereditary disorders. An international expert membership on liver pathology has recently suggested an update of nomenclature, and the definition of “Metabolic Dysfunction Associated Fatty Liver Disease” (MAFLD) has been proposed as a more appropriate term to describe liver diseases associated with known metabolic dysfunctions 2. Waiting for a worldwide acceptance of this new term and a refinement of diagnostic criteria to define MAFLD, the term NAFLD is used in this review.
NAFLD is the most common chronic liver disease worldwide. Its estimated prevalence is around 1.5-6.5% on global population 3, although its absolute prevalence worldwide is unknown and is tightly connected to genetic and environmental factors 4. Its prevalence appears to be increasing, with an estimated 3.6 million of new cases annually. The estimated annual medical costs directly attributable to NAFLD exceed 35 billion euros in 4 large European countries (United Kingdom, France, Germany, and Italy).
Simple steatosis represents the uncomplicated form of NAFLD, while non-alcoholic steatohepatitis (NASH) is its inflammatory subtype which is associated with disease progression, development of cirrhosis and, eventually, hepatocellular carcinoma (HCC), and a possible need for liver transplantation should be considered. Indeed, due to the decline in hepatitis C virus patients, NASH-correlated cirrhosis is already the major indication for liver transplantation.
NASH can be a diagnostic and therapeutic challenge for clinicians 5. Although several non-invasive tests have been developed, liver biopsy is currently the only accepted method to reliably distinguish NASH from “simple” steatosis. Moreover, from a histopathological perspective, NAFLD and NASH continues to be an area of increasing interest for liver pathologists, with specific focus on better methods of assessment, morphologic features for pathogenesis, and predictive markers of fibrosis progression 6.
This review focuses on histopathology of NAFLD in adults, with the aim to provide a practical diagnostic approach useful in the clinical routine.
Overview on clinical aspects of NAFLD
NAFLD is considered the hepatic manifestation of metabolic syndrome (MeS), characterized by obesity, insulin resistance or type 2 diabetes, dyslipidemia and hypertension 7. MeS is the third death cause due to cardiovascular disease and extra-hepatic neoplasms 8,9. The criteria for MeS diagnosis are reported in Table I 10,11. NAFLD may develop in lean individuals 12. Lean-NAFLD is defined as hepatic steatosis in patients with a BMI < 25 kg/m2 (or < 23 kg/m2 in Asian individuals) in absence of “significant” alcohol intake 13. Initially described in Asian populations, it is reported that, even among European individuals, approximately 20% of patients are considered as lean NASH.
Table I.
Criteria for Metabolic Syndrome (MeS) diagnosis defined by The National Cholesterol Educational Program Adult Treatment Panel III (NCEP-ATP) (from Grundy et al., 2005, mod.) 10.
| At least 3 of the 5 following criteria should be present: |
|
The diagnosis of NAFLD requires evidence of hepatic steatosis in absence of other causes of liver fat accumulation. From the clinical point of view, no specific physical signs can confirm the diagnosis of NAFLD. In the practice, it is suspected when an increase in serum aminotransferase levels are found in a patient with features of MeS. Indeed, elevation of transaminase is the most common laboratory modification in NAFLD patients, usually mild (less than twice the upper limit of normal) irrespectively of the severity of disease. Of note, almost 80% of patients with NAFLD do not show any biochemical abnormality and the diagnosis can be established incidentally, even in advanced stage of disease. A number of other non-specific laboratory findings can be detected (such as increased γ-glutamyl transferase and alkaline phosphatase). Elevated serum ferritin levels with normal transferrin saturation can be detected in an acute phase and it appears to be related with the progression towards a fibrotic stage 3,14. A high prevalence of low-title antinuclear antibody (ANA; ≤ 1:160) and anti-smooth muscle antibody (ASMA; ≤ 1:40) has been reported in NAFLD patients, usually with a normal range of IgG 15.
Pathology of NAFLD
From a histological point of view, NAFLD patients may show “simple” steatosis (NAFL) or NASH. Although the natural history and prognostic features of NAFLD remain controversial, NAFL is largely considered a benign condition with minimal risk of progression or clinical impact, while NASH represents the progressive and prognostically relevant form of this disease. Therefore, the differential diagnosis between NAFL and NASH play an important consideration in routine practice, and the efficacy of new drugs to induce resolution of NASH is considered a key endpoint in clinical trials. Distinguishing NASH from NAFL represents the major indication to perform a liver biopsy. Once a diagnosis of NASH has been established, the information about severity of disease, particularly about fibrosis status, provides prognostic clues 16.
Due to the epidemic spread of obesity, diabetes and associated fatty liver disease, performing liver biopsy in all these patients is unaffordable. Current guidelines recommend biopsy for patients with NAFLD who are at increased risk of steatohepatitis and/or advanced fibrosis stage and for patients in whom any coexisting liver diseases cannot be ruled out. Much effort has been done to develop non-invasive tests to identify NASH 17,18 and algorithms have been proposed to help clinicians to decide when a liver biopsy should be performed17. A detailed description on non-invasive tests and clinical diagnostic algorithms is beyond the aims of this review.
“Simple” steatosis
In NAFLD, steatosis is typically macrovescicular, and is normally located in perivenular areas (acinar zone 3) and easily recognized by a light microscope on Hematoxylin-Eosin (H&E) stain (Fig. 1). It appears as an empty and optically clear vacuole, because lipids are removed during tissue processing. Hepatocytes with a foamy appearance with numerous tiny vacuoles can be seen in microvescicular steatosis, but it is never a prominent feature (Fig. 2). Microvescicular steatosis tends to be present in more severe cases of steatohepatitis, it is due to a mitochondrial injury and can be life-threatening. As steatosis increases, its zonal distinction disappears and steatotic hepatocytes are equally distributed in all the acinar zones.
Figure 1.
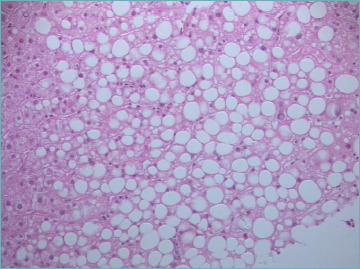
Macrovescicular steatosis. (Hematoxylin and Eosin, H&E; original magnification 20x).
Figure 2.
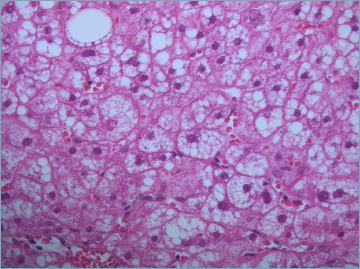
Microvescicular steatosis is characterized by tiny lipid droplets filling the hepatocyte cytoplasm (H&E; original magnification 60x).
Steatosis in more than 5% of hepatocytes is generally accepted as a working definition of a fatty liver. This is an arbitrary threshold based on the assumption that minimal changes have no clinical relevance 19. It has been demonstrated that ALT and AST show significant changes when steatosis increases, thus, it supports the clinical guidelines of 5% cut-off for abnormal steatosis 20. Quantification of steatosis severity as mild (5-33%), moderate (34-66%), and severe (> 66%) should be specified in final pathological report. Grading of steatosis should be performed at low to medium magnification.
Steatosis alone is unspecific and can be seen in various condition. Alcohol abuse, drug, toxins and ischemic damage also share the preferential zone 3 location 21. Correlation with clinical information is essential to understand whether steatosis is related to NAFLD or to other causes.
NASH
Diagnosis of NASH requires i) steatosis (more than 5%), and ii) both lobular inflammation and ballooning degeneration of hepatocytes with a mainly zone 3 distribution. Other pathological features (such as portal inflammation, Mallory-Denk bodies, glycogenated nuclei, apoptosis, megamitochondria, iron deposition) may be seen, but they are neither necessary nor enough to establish a diagnosis of NASH.
Hepatocyte Ballooning is defined as enlarged hepatocytes (more than 1.5-2 times the normal size, corresponding approximately to a cellular diameter more than 30 μm) with round (instead of polygonal) shape and rarefied cytoplasm (Fig. 3). Bedossa et al., have recently considered two grade of ballooning: i) grade 1 (or small) ballooning characterized by a round hepatocyte with typical pale reticulated cytoplasm with almost no variation in size compared to normal hepatocytes, and ii) grade 2 ballooning characterized by more conventional ballooning 22. It has been questioned that this distinction can lead to an over-diagnosis of ballooning and, subsequently, of NASH, due to a strong similarity of grade 1 ballooning with cytoplasmic glycogenosis, which is a condition often observed in NAFLD. A useful clue to distinguish grade 1 ballooning from cytoplasmic glycogenosis is the cell shape, which is rounded in case of ballooning while remains polygonal in case of glycogenosis (Fig. 4). True ballooning degeneration can be difficult to properly identify, and the inter-observer agreement is far from being perfect. The consistency in ballooning detection is higher if the enlargement of cells is considered a prerequisite for diagnosis. Keratin stains can help in ballooning detection; indeed, it has been demonstrated that ballooned hepatocytes lose CK8-18. Moreover, they are usually surrounded by collagen fibers that can be easily highlighted by collagen stains 23. The feathery degeneration associated with injury due to cholestasis (“cholate stasis”) may mimic hepatocyte ballooning. The location (periportal in cholate stasis and pericentral in NASH) and the associated changes (bile ductular reaction/ bile duct injury versus steatosis) make the distinction usually easy.
Figure 3.

Mallory-Denk bodies appear as cytoplasmic hyaline inclusions of ballooned hepatocytes (arrow). (H&E; original magnification 60x).
Figure 4.

Hepatocyte glycogenosis. The hepatocyte cytoplasm appears homogeneously clear. It may mimic ballooning degeneration but note that the cell contour remains polygonal. Several glycogenated nuclei are also evident (arrow). (H&E; original magnification 20x).
Lobular inflammation is usually mild and characterized by small foci of inflammatory cells, mainly lymphocytes (usually a mixture of CD4+/CD8+ T lymphocytes) and macrophages, sometimes associated with hepatocyte dropout. Few plasma cells may be seen and small aggregates of neutrophils are rare, unless Mallory-Denk bodies are present. In case of a severe lobular inflammation, other or concomitant causes should be considered, mainly alcohol or drugs. Lipogranulomas (steatotic hepatocyte or fat droplet surrounded by lymphocytes, macrophages and rare eosinophils) are frequently detected in NASH (Fig. 5). They are not indicative of active inflammation and, therefore, they are not considered for assessment of lobular inflammation 23.
Figure 5.

Fat droplet surrounded by lymphocytes and macrophages represents a lipogranulomas (arrow). Lipogranulomas are frequently found in fatty liver; they are not considered in the assessment of lobular inflammation. (H&E; original magnification 40x).
Subjects with NASH may develop fibrosis and about 20% show a progression to cirrhosis. Typical NASH fibrosis is characterized by a sinusoidal collagen deposition which usually begins in zone 3, giving rise to a delicate framework around single hepatocytes (Fig. 6). Periportal fibrosis develops subsequently in most cases, followed by bridging (mainly central-to-portal) fibrosis. NAFLD cirrhosis may be indistinguishable from cirrhosis due to other etiologies. Of note, steatosis may completely disappear at cirrhotic stage. In the past, this led to a misdiagnosis of cryptogenic cirrhosis. Specific stains for collagen such as Masson trichrome, van Gieson, reticulin and Sirius red are recommended, particularly at earlier stages 24.
Figure 6.

NASH early stage: delicate perisinusoidal collagen deposition in zone 3. High quality connective tissue stains are required for a correct assessment. (Van Gieson stain; original magnification 20x).
Most studies have shown that the stage of fibrosis is an independent predictor of overall- and liver-related mortality, regardless of the presence or severity of other histological features 25,26.
Adjunctive lesions
Mallory-Denk bodies (MDB). As previously mentioned, MDBs (Fig. 3) may be seen within the cytoplasm of ballooned hepatocytes as clumped eosinophilic plot of cytoskeleton, mainly consisting of intermediate filaments CK8/18. They may be seen in other conditions including alcoholic, cholestatic liver diseases, primary biliary cholangitis and hepatocellular carcinoma (HCC). MDBs are noted to be fewer and less developed in NASH than in alcoholic hepatitis. Their meaning as an epiphenomenon of hepatocyte injury, cytoprotective elements or a contributor to hepatocyte damage, is still unknown. MBDs formation is reversible and does not reduce hepatocyte viability 27.
Portal inflammation. Portal inflammation in adult NAFLD is usually minimal to mild and it is mainly composed of mononuclear cells, including lymphocytes, macrophages, and plasma cells. The presence of more than a mild portal inflammation should lead to considering the hypothesis of different diagnoses. However, more than mild portal inflammation can be seen in NASH and it correlates with a greater pathological severity of disease, including advanced stages of fibrosis 28.
Megamitochondria are described as round or needle-shape eosinophilic PAS-diastase resistant intracytoplasmic hepatocyte inclusions (Fig. 7). In NASH they are rarely seen, while they are more abundant in ALD where they have been associated with a better prognosis29. They can also be detected in other pathological conditions (Wilson’s disease, drug-induced liver damage) and in pregnancy 9.
Figure 7.

Megamitochondria: round eosinophilic intracytoplasmic hepatocyte inclusion (arrow). (H&E; original magnification 60x).
Glycogenated nuclei optically appear as empty vacuoles on H&E stain (Fig. 4). They are a normal finding in childhood, mainly in periportal areas, and may be seen even in young adults. When they are numerous in adult liver, glycogenated nuclei are to be considered as abnormal finding. Glycogenated nuclei are common in NAFLD and NASH, and they have been associated with diabetes. They do not have any clinical significance 27,30.
Centrilobular arterial branches. The presence of minute branches of the hepatic artery in perivenular zones has been described in NASH, especially when perivenular inflammation is present 21. This finding should not be misinterpreted as a portal tract. Centrizonal arteries are associated with the presence of CD34-positive microvessel formation within a high stage fibrosis, suggesting a possible association between neo-angiogenesis and NASH progression to cirrhosis 31.
Iron deposition: Approximately one-third of patients affected by NAFLD show signs of iron-overload, which may be exclusively mesenchymal, or parenchymal, or even a mixed mesenchymal/parenchymal pattern with different degrees of severity. The clinical impact of iron deposition in NAFLD is not yet entirely clear, since all available studies show conflicting results. A recent observational study provided evidence that a mesenchymal pattern of iron deposition may be of particular relevance for the clinical outcome, suggesting that the pattern of iron deposition in liver biopsy may identify patients at risk from vascular or hepatic events, who may need closer monitoring32. Therefore, it is good clinical practice to search for iron deposition and to report its eventual presence, pattern and severity in the pathological report. Hepatic siderosis can be assessed by Perls’ stain using a simple semi-quantitative score: score 0 as absent or barely discernible granules at a magnification of 40-fold (40×); score 1 as barely discernible granules at a magnification of 20× but easily confirmed at 40×; score 2 as discrete granules resolved at 10× magnification; score 3 as discrete granules resolved at a magnification of 2.5× and, finally, score 4 as massive granules visible even upon 1.0× magnification 9.
The non-alcoholic nature of disease is often a challenge to determine histologically. In alcoholic hepatitis, hepatocytes that contain Mallory-Denk bodies tend to be more pronounced and are often surrounded by polymorphonuclear leukocytes, a lesion referred to as satellitosis. Prominent bilirubinostasis and sclerosing hyaline necrosis are features of ALD. In case of well-compensated ALD distinction from NAFL or NASH may be impossible and should relies on careful clinical documentation of alcohol use.
Grading and staging of NASH
As in chronic hepatitis, there are several staging and grading systems available in clinical research as well as for the assessment in liver biopsy during the daily pathological practice. As in other settings, there should be a clear communication between the pathologist and clinical staff about the histological system used.
A first system was proposed by Brunt et al. in 1999 33. This was a 3-tiered grading system based on the evaluation of steatosis, ballooning and inflammation (lobular and portal) (Tab. II). Regarding the staging of fibrosis, a scale from 0 to 4 is used as reported in Table III. Staging was based on the characteristic pattern and evolution of fibrosis in NASH with an initial involvement of perisinusoidal spaces in zone 3 (stage 1) and subsequent development of portal/periportal fibrosis (stage 2), bridging fibrosis (stage 3), and, finally, cirrhosis (stage 4) (Tab. IV). A prerequisite for applying this system is a diagnosis of steatohepatitis.
Table II.
Brunt system to grade NASH activity (from Brunt et al., 1999) 33.
| Grade | Steatosis | Ballooning | Inflammation |
|---|---|---|---|
| Grade 1 (mild) | 1-3 (up to 66%) | Minimal | Lobular: 1-2 Portal: none/mild |
| Grade 2 (moderate) | 2-3 (> 33% up to 66%) | Present | Lobular: 2 Portal: mild-moderate |
| Grade 3 (severe) | 2-3 | Marked | Lobular: 3 Portal: mild-moderate |
Steatosis: grade 1 ≤ 33%; grade 2 > 33%, < 66%; grade 3 ≥ 66%.
Inflammation: Lobular (0-3): 0 (none), 1 (< 2 foci/20x field), 2 (2-4 foci/20x field); 3 (> 4 foci/20x field); Portal (0-3): 0 (none), 1 (mild), 2 (moderate), 3 (marked).
Table III.
Brunt system for staging NASH fibrosis (from Brunt et al., 1999) 33.
| Stage | Zone 3, Sinusoidal | Portal Based | Bridging | Cirrhosis |
|---|---|---|---|---|
| 1 | Focal or extensive | 0 | 0 | 0 |
| 2 | Focal or extensive | Focal or extensive | 0 | 0 |
| 3 | Bridging septa | Bridging septa | + | 0 |
| 4 | ± | ± | Extensive | + |
Evaluation of fibrosis is performed by Masson trichrome histochemical stain.
Table IV.
NASH Clinical Research Network (CRN) scoring system for nonalcoholic fatty liver disease (from Kleiner et al., 2005) 19.
| Steatosis grade | Lobular Inflammation | Hepatocellular ballooning | |
|---|---|---|---|
| 0: < 5% | 0: None | 0: None | |
| 1: 5-33% | 1: < 2 foci/20x field | 1: Mild, few | |
| 2: 34-66% | 2-4 foci/20x field | 2: Moderate-marked, many | |
| 3: > 66% | 3: > 4 foci/20x field | ||
| NAFLD activity score (NAS): 0-8 | |||
| Fibrosis (evaluated with Masson trichrome stain) | |||
| 0 | None | ||
| 1a | Mild zone 3 sinusoidal fibrosis (trichrome stain to be identified) | ||
| 1b | Moderate zone 3 sinusoidal fibrosis (could be detected on H&E examination) | ||
| 1c | Portal fibrosis only | ||
| 2 | Zone 3 sinusoidal fibrosis and periportal fibrosis | ||
| 3 | Bridging fibrosis | ||
| 4 | Cirrhosis | ||
NASH: nonalcoholic steatohepatitis; NAFLD, nonalcoholic fatty liver disease.
Kleiner et al., in 2005 19 proposed a novel NAFLD Activity Score (NAS) system. This is a modified Brunt system, which could be applicable to any patient (adult or children), and to the various histological spectrum of NAFLD. NAS was defined as the unweighted sum of steatosis, lobular inflammation, and ballooning. In the validation study, NAS scores 1 or 2 corresponded to negative for NASH, while a NAS scores 5-8 correlated with a definite diagnosis of NASH. Activity scores 3 and 4 were mainly observed in cases that did not fulfill the pathologists’ criteria for NASH. This led to the assessment of NAS score as a diagnostic tool. However, the scores obtained from NAS system cannot be used as a surrogate of the histopathological criteria for NASH diagnosis. Here, the histological diagnosis of NASH is a prerequisite to apply the NAS score. As for fibrosis, Kleiner et al. slightly modified the original Brunt scheme, as reported in Table V.
Table V.
Steatosis-Activity-Fibrosis (SAF) scoring system of NAFLD (from Bedossa et al., 2012) 34.
| Steatosis grade (S): 0-3 | Hepatocyte ballooning: 0-2 |
| Based on percentage of hepatocytes with large and/or medium size intracytoplasmic lipid droplets S0: < 5% S1: 5-33% S2: 34-66% S3: > 66% |
0: none 1: Cluster of rounded hepatocytes with pale/reticulated cytoplasm 2: Same as 1 with enlarged hepatocytes (more than twice of normal size) |
| Lobular inflammation: 0-2 | Activity grade (A): 0-4 |
| 0: None 1: ≤ 2 foci/20x field 2: > 2 foci/20x field |
Sum of score for ballooning and lobular inflammation A1 (A = 1): Mild activity A2 (A = 2): Moderate activity A3 and A4 (A > 2): Severe activity |
| Fibrosis stage (F) | SAF score |
| F0: no significant fibrosis F1: 1a Mild zone 3 sinusoidal fibrosis 1b Moderate zone 3 sinusoidal fibrosis 1c Portal fibrosis only F2: zone 3 sinusoidal fibrosis with periportal fibrosis F3: Bridging fibrosis F4: Cirrhosis |
S0-3; A0-4; F0-4 |
Steatosis-Activity-Fibrosis (SAF) scoring system was proposed by the European Fatty Liver Inhibition of Progression (FLIP) consortium in 2012 34. Based on their observation that liver function tests (including transaminases) did not differ when pure steatosis was compared to normal liver, in SAF-score the activity parameters include only ballooning and lobular inflammation, whereas steatosis is separately assessed (Tab. VI). Fibrosis is assessed in a 4-tiered system as proposed by Kleiner et al. 19 (Tab. V).
Table VI.
Simplified diagnostic algorithm for NAFLD diagnosis on liver biopsy (from Bedossa et al., 2012, mod.) 34.
| Steatosis > 5% | Lobular inflammation (any degree) |
Balloning (any degree) |
Diagnosis |
|---|---|---|---|
| No | Yes/No | Yes/No | No NAFLD |
| Yes | Yes | No | NAFL |
| Yes | No | Yes | NAFL |
| Yes | Yes | Yes | NASH |
NAFLD: nonalcoholic fatty liver disease; NAFL: nonalcoholic fatty liver; NASH: nonalcoholic steato-hepatitis.
Practical considerations
A simple diagnostic algorithm may be a useful tool to make a final differential diagnosis between NAFL and NASH in clinical practice, as suggested by Bedossa et al. 34 and simplified in Table VI. Steatosis more than 5% is the minimum required criteria to be categorized in the NAFLD group. Steatosis is then modulated by hepatocyte ballooning and lobular inflammation, which are key diagnostic features.
When not all the diagnostic criteria for NASH are reached, it is useful to underline in the concluding remarks of the histological report whether steatosis is associated with ballooning or lobular inflammation, due to possible sampling error, indeed, NASH cannot be entirely excluded.
The presence of sinusoidal fibrosis does not allow the diagnosis of NASH if both ballooning and lobular inflammation are not evident in liver biopsy assessment. A descriptive conclusion should be provided in these cases (i.e. NAFL with lobular inflammation and stage 1 fibrosis). NAFL with fibrosis in case of absence of any sign of activity is believed to represent a form of remission or inactive NASH 23,35.
Figures and tables
References
- 1.Mashek DG, Khan SA, Sathyanarayan A, et al. Hepatic lipid droplet biology: Getting to the root of fatty liver. Hepatology 2015;62:964-967. https://doi.org/10.1002/hep.27839 10.1002/hep.27839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202-209. https://doi.org/10.1016/j.jhep.2020.03.039 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. https://doi.org/10.1002/hep.28431 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-1402. https://doi.org/10.1016/j.jhep.2015.11.004 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 5.Sheka AC, Adeyi O, Thompson J, et al. Nonalcoholic steatohepatitis: a review. JAMA 2020;323:1175-1183. https://doi.org/10.1001/jama.2020.2298 10.1001/jama.2020.2298 [DOI] [PubMed] [Google Scholar]
- 6.Torbenson M, Washington K. Pathology of liver disease: advances in the last 50 years. Hum Pathol 2020;95:78-98. https://doi.org/10.1016/j.humpath.2019.08.023 10.1016/j.humpath.2019.08.023 [DOI] [PubMed] [Google Scholar]
- 7.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365(9468):1415-1428. https://doi.org/10.1016/S0140-6736(05)66378-7 10.1016/S0140-6736(05)66378-7 [DOI] [PubMed] [Google Scholar]
- 8.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330-344. https://doi.org/10.1038/nrgastro.2013.41 10.1038/nrgastro.2013.41 [DOI] [PubMed] [Google Scholar]
- 9.Mac Sween RNM BA, Portmann BC, Ishak KG, et al. Pathology of the liver. 4th ed. Churchill Livingstone: London: 2002. [Google Scholar]
- 10.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112(17):2735-2752. https://doi.org/10.1161/CIRCULATIONAHA.105.169404 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 11.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech 2009;2:231-237. https://doi.org/10.1242/dmm.001180 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding C, Chan Z, Magkos F. Lean, but not healthy: the ‘metabolically obese, normal-weight’ phenotype. Curr Opin Clin Nutr Metab Care 2016;19:408-417. https://doi.org/10.1097/MCO.0000000000000317 10.1097/MCO.0000000000000317 [DOI] [PubMed] [Google Scholar]
- 13.Das K, Chowdhury A. Lean NASH: distinctiveness and clinical implication. Hepatol Int. 2013;7 Suppl 2:806-13. https://doi.org/10.1007/s12072-013-9477-5 10.1007/s12072-013-9477-5 [DOI] [PubMed] [Google Scholar]
- 14.Dyson JK, McPherson S, Anstee QM. Non-alcoholic fatty liver disease: non-invasive investigation and risk stratification. J Clin Pathol 2013;66:1033-1045. https://doi.org/10.1136/jclinpath-2013-201620 10.1136/jclinpath-2013-201620 [DOI] [PubMed] [Google Scholar]
- 15.Adams LA, Lindor KD, Angulo P. The prevalence of autoantibodies and autoimmune hepatitis in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol 2004;99:1316-1320. https://doi.org/10.1111/j.1572-0241.2004.30444.x 10.1111/j.1572-0241.2004.30444.x [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM, Kleiner DE, Carpenter DH, et al. Nonalcoholic fatty liver disease: Reporting histologic findings in clinical practice. Hepatology 2020. https://doi.org/10.1002/hep.31599 10.1002/hep.31599 [DOI] [PubMed] [Google Scholar]
- 17.Anstee QM, Lawitz EJ, Alkhouri N, et al. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials. Hepatology 2019;70:1521-1530. https://doi.org/10.1002/hep.30842 10.1002/hep.30842 [DOI] [PubMed] [Google Scholar]
- 18.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1264-1281 e4. https://doi.org/10.1053/j.gastro.2018.12.036 10.1053/j.gastro.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-1321. https://doi.org/10.1002/hep.20701 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 20.Hall A, Covelli C, Manuguerra R, et al. Transaminase abnormalities and adaptations of the liver lobule manifest at specific cut-offs of steatosis. Sci Rep 2017;7:40977. https://doi.org/10.1038/srep40977 10.1038/srep40977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis 2012;32:3-13. https://doi.org/10.1055/s-0032-1306421 10.1055/s-0032-1306421 [DOI] [PubMed] [Google Scholar]
- 22.Bedossa P, Consortium FP. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014;60:565-575. https://doi.org/10.1002/hep.27173 10.1002/hep.27173 [DOI] [PubMed] [Google Scholar]
- 23.Burt AD, Lackner C, Tiniakos DG. Diagnosis and Assessment of NAFLD: Definitions and Histopathological Classification. Semin Liver Dis 2015;35:207-220. https://doi.org/10.1055/s-0035-1562942 10.1055/s-0035-1562942 [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y, Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2014;20(42):15539-15548. https://doi.org/10.3748/wjg.v20.i42.15539 10.3748/wjg.v20.i42.15539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389-397 e10. https://doi.org/10.1053/j.gastro.2015.04.043 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547-1554. https://doi.org/10.1002/hep.27368 10.1002/hep.27368 [DOI] [PubMed] [Google Scholar]
- 27.Zatloukal K, French SW, Stumptner C, et al. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res 2007;313:2033-2049. https://doi.org/10.1016/j.yexcr.2007.04.024 10.1016/j.yexcr.2007.04.024 [DOI] [PubMed] [Google Scholar]
- 28.Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009;49:809-820. https://doi.org/10.1002/hep.22724 10.1002/hep.22724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014;146:1231-1239 e1-6. https://doi.org/10.1053/j.gastro.2014.01.018 10.1053/j.gastro.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis 2001;21:3-16. https://doi.org/10.1055/s-2001-12925 10.1055/s-2001-12925 [DOI] [PubMed] [Google Scholar]
- 31.Gill RM, Belt P, Wilson L, et al. Centrizonal arteries and microvessels in nonalcoholic steatohepatitis. Am J Surg Pathol 2011;35:1400-1404. https://doi.org/10.1097/PAS.0b013e3182254283 10.1097/PAS.0b013e3182254283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eder SK, Feldman A, Strebinger G, et al. Mesenchymal iron deposition is associated with adverse long-term outcome in non-alcoholic fatty liver disease. Liver Int 2020;40:1872-1882. https://doi.org/10.1111/liv.14503 10.1111/liv.14503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467-2474. https://doi.org/10.1111/j.1572-0241.1999.01377.x 10.1111/j.1572-0241.1999.01377.x [DOI] [PubMed] [Google Scholar]
- 34.Bedossa P, Poitou C, Veyrie N, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012;56:1751-1759. https://doi.org/10.1002/hep.25889 10.1002/hep.25889 [DOI] [PubMed] [Google Scholar]
- 35.Bedossa P. Diagnosis of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Why liver biopsy is essential. Liver Int. 2018;38 Suppl 1:64-66. https://doi.org/10.1111/liv.13653 10.1111/liv.13653 [DOI] [PubMed] [Google Scholar]


