This cohort study compares 3-year longitudinal changes in gait measures across the spectrum of baseline visual field damage in patients with glaucoma.
Key Points
Question
How have gait patterns changed across the spectrum of baseline visual field damage in glaucoma?
Findings
In this post hoc analysis of a cohort study of 241 adults with glaucoma, the walking speeds, including stride velocity and cadence detected through an electronic walkway, decreased at faster rates among participants with severe visual field damage compared with those with normal or mild visual field damage.
Meaning
Mobility function declined at a faster rate in adults with glaucoma; the declines in walking speeds were fairly substantial, suggesting that individuals with visual field damage experienced more rapid gait declines than their normally sighted peers.
Abstract
Importance
Gait dysfunction is common in older people with visual impairment and is a major cause of falls.
Objective
To compare 3-year longitudinal changes in gait measures across the spectrum of baseline visual field (VF) damage in glaucoma.
Design, Setting, and Participants
A post hoc analysis was designed on September 1, 2018, following a prospective cohort study, which enrolled older adults with glaucoma or suspected glaucoma from September 2013 to March 2015 and followed up for up to 3 years. Baseline VF damage was defined by integrated VF (IVF) sensitivity and categorized as normal/mild (IVF >28 dB), moderate (IVF, 23-28 dB), and severe (IVF, <23 dB). Each participant walked on an electronic walkway back and forth twice at normal pace each study year. Linear mixed-effects models evaluated longitudinal change in gait outcomes (1) stratified within each VF severity category and (2) across the range of IVF sensitivity. Analysis took place from October 2019 to October 2020.
Main Outcomes and Measures
Three-year changes in 7 gait assessments under usual-pace walking, including base support and its coefficient of variation, stride length and its coefficient of variation, stride velocity and its coefficient of variation, and cadence.
Results
Of 241 participants, the mean (SD) age was 70.8 (7.7) years, 116 (48.2%) were women, and 70 (29.0%) were African American. When comparing longitudinal gait changes over 3 years across the spectrum of IVF sensitivity, each 5-unit (dB) decrement was associated with more rapid declines in stride velocity (−0.05 z score unit/y; 95% CI, −0.09 to −0.01; P = .01) and cadence (−0.07 z score unit/y; 95% CI, −0.10 to −0.03; P < .001). When evaluating gait changes within each glaucoma severity group, shorter stride length was associated with persons with normal/mild (−0.06 z score unit/y; 95% CI, −0.10 to −0.03; P = .001), moderate (−0.08 z score unit/y; 95% CI, −0.12 to −0.04; P < .001), and severe VF damage (−0.16 z score unit/y; 95% CI, −0.24 to −0.07; P < .001), while stride velocity (−0.18 z score unit; 95% CI, −0.28 to −0.07; P = .002) and slower cadence (−0.15 z score unit; 95% CI, −0.25 to −0.04; P = .006) were associated with those with severe VF damage.
Conclusions and Relevance
At worse levels of baseline VF damage, patients with glaucoma in this study demonstrated an exacerbated decline in walking speeds (ie, stride velocity and cadence), indicating that mobility speeds decrease faster over time in older adults with glaucoma.
Introduction
Gait dysfunction is a well-established predictor for falls, functional decline, and diminished quality of life in the older population.1,2 The prevalence of gait dysfunction increases substantially with age, from around 10% in age 60 to 69 years to approximately 60% among those older than 85 years.3 Moreover, slower gait speed has been associated with several chronic disorders in older adults, such as diabetes, heart failure, and dementia4,5,6,7 and has been proposed as a marker of pathological aging.8 Gait dysfunction is more prevalent in individuals with vision impairment and may produce dangerous walking patterns leading to falls reflecting other consequences (ie, fear of falling) in this population.9,10
Changes in gait have been found to reflect specific health disorders and overall health as people age.11,12,13,14 Previous cross-sectional studies have shown gait is altered in persons with vision impairment compared with those with normal sight15,16,17; however, most of this research has focused on a limited number of gait measures, ie, gait speed and stride length,16,17,18,19 as opposed to base of support and gait variability, which have also been found to identify individuals at higher risk of falling.20 Moreover, prior work on gait assessments in persons with vision impairment has low generalizability owing to small sample sizes and focus on younger (midlife) populations.15,16,17 Our previous cross-sectional research showed that glaucoma severity was associated with several fall-relevant gait features, ie, stride-to-stride variability,9,10 yet there is a lack of longitudinal data to characterize the effects of visual field (VF) loss on the trajectory of gait dysfunction and poor functional performance in glaucoma. Increasing the understanding of gait changes in older people with visual impairment may direct future prevention and therapeutic strategies, allowing for minimizing the consequence of visual impairment.
We used data from the Falls in Glaucoma Study (FIGS), which was a prospective cohort study to characterize differences in fall rates across glaucoma severity, to identify reversable risk factors for falls and to examine longitudinal changes in mobility, particularly those experiencing falls. The present study was designed after the main results from the FIGS to quantify the longitudinal changes in 7 gait assessments under usual-paced walking associated with the severity of VF damage. We hypothesized that patients with glaucoma with worse baseline VF damage would experience a more rapid decline of gait function than those with normal/moderate VF damage over a 3-year period.
Methods
Participants
Participants were enrolled from FIGS, a single-center cohort study conducted at Johns Hopkins Wilmer Eye Institute from September 2013 to March 2015 and followed up for up to 3 years. A post hoc study was designed on September 1, 2018, following prospective collection of FIGS data. The study eligibility criteria were described elsewhere in detail.21,22,23 Briefly, participants were included if they were 60 years and older, lived within 60 miles from Wilmer Eye Institute, and had primary glaucoma or suspected glaucoma. The institutional review board at Johns Hopkins approved this study. All participants signed written consent. There was no incentive or compensation for participation.
Vision Evaluation
Baseline VF damage was evaluated through Humphrey HFA-II perimeter (Carl Zeiss Meditec). Integrated VF (IVF) sensitivity was derived from 24-2 VF tests by combining pointwise sensitivities from each eye and creating a sensitivity at each spatial coordinate through the maximum sensitivity approach.10,24 Subsequently, we converted the decibel sensitivity values in the IVF to raw (unlogged) sensitivity values, then calculated the mean of all points in the full VF, and retransformed mean raw sensitivity back to decibel values to derive the mean IVF sensitivity. Normal VF was in the range of 31 dB or above, while lower than 31 dB reflected VF damage. The severity of VF damage was categorized as normal/mild (IVF, >28 dB), moderate (IVF, 23-28 dB), or severe (IVF, <23 dB), with better-eye sensitivities calculated by this method corresponding to the severity categories described by Hodapp et al.25
Gait Assessments
Gait measures were assessed at baseline clinical evaluation and 3 annual follow-up visits using the GAITRite Electronic Walkway (CIR Systems). Each study year, participants walked on the electronic walkway back and forth twice at their normal pace to determine gait patterns and allow for detection of changes in gait over time. The following 7 gait parameters were selected as primary outcomes of interest, given their associations with individuals’ risk of falling in prior literature26,27,28: (1) base of support: the distance (centimeters) between the heel center of the dominant foot and the line of progression generated by the prior heel and subsequent strikes of the nondominant foot; (2) stride length: the distance (centimeters) between heel centers of consecutive footsteps of the dominant foot; (3) stride velocity (centimeters per second): stride length (centimeters) of the dominant foot divided by ambulation time (seconds); and (4) cadence (steps per minute): mean number of steps per minute; and (5-8) coefficient of variation (CV) for base of support, stride length, and stride velocity (calculated as ratio of SD to the mean value multiplied by 100%).10 CVs were expressed as percentages to capture the stride-to-stride variability, with lower percentages indicating lower variability and greater consistency. All gait metrics were converted to standardized z score units as follows: (individual participant value – mean of sample values) / (sample SD), so that the mean value for study population was set at 0 with an SD of 1.
Covariates
Baseline demographic characteristics were collected with a standard questionnaire, including self-reported age, sex, race, living arrangement, and education level. Polypharmacy, ie, taking 5 or more noneyedrop medications, was generated through a questionnaire or direct observation of medication bottles. The number of comorbidities was quantified as the sum of comorbidities identified from a full list of comorbid illness known to affect physical function using a standardized questionnaire.29 The list of comorbidities included diabetes, stroke, arthritis, hip fracture, back problems, myocardial infarction, angina, congestive heart failure, peripheral vascular disease, hypertension, emphysema, asthma, Parkinson disease, nonskin cancer, and vertigo or Ménière disease. Cognitive impairment was examined by a Mini-Mental State Examination for visual impairment questionnaire.
Statistical Analysis
Differences in demographic and health characteristics of the study population by VF severity category were evaluated using t test for continuous variables and χ2 test for categorical variables. Fitted plots of gait outcomes, ie, stride length, stride velocity, and cadence, assessed at 4 annual intervals were generated to visualize the direction and magnitude of longitudinal gait changes in 3 VF groups (normal/mild, moderate, and severe VF damage) (Figure). Linear mixed-effects models were used to study the longitudinal change in all gait outcomes as a function of VF impairment by including VF damage, follow-up years, and interaction of VF damage and follow-up years as model elements. For all models, the unstructured correlation was used, and standardized residuals were compared with predicted estimates to warrant the goodness of fit. The covariates described above, including age, sex, race, living arrangement, education, polypharmacy, comorbidity, and cognitive function, were included in these models given their associations with vision and/or gait outcomes.30,31,32 This investigation was a post hoc analysis of data from the FIGS cohort. Two-sided P values were statistically significant at .05. All analyses were conducted using Stata version 16.0 (StataCorp LP). Analysis was conducted from October 2019 to October 2020.
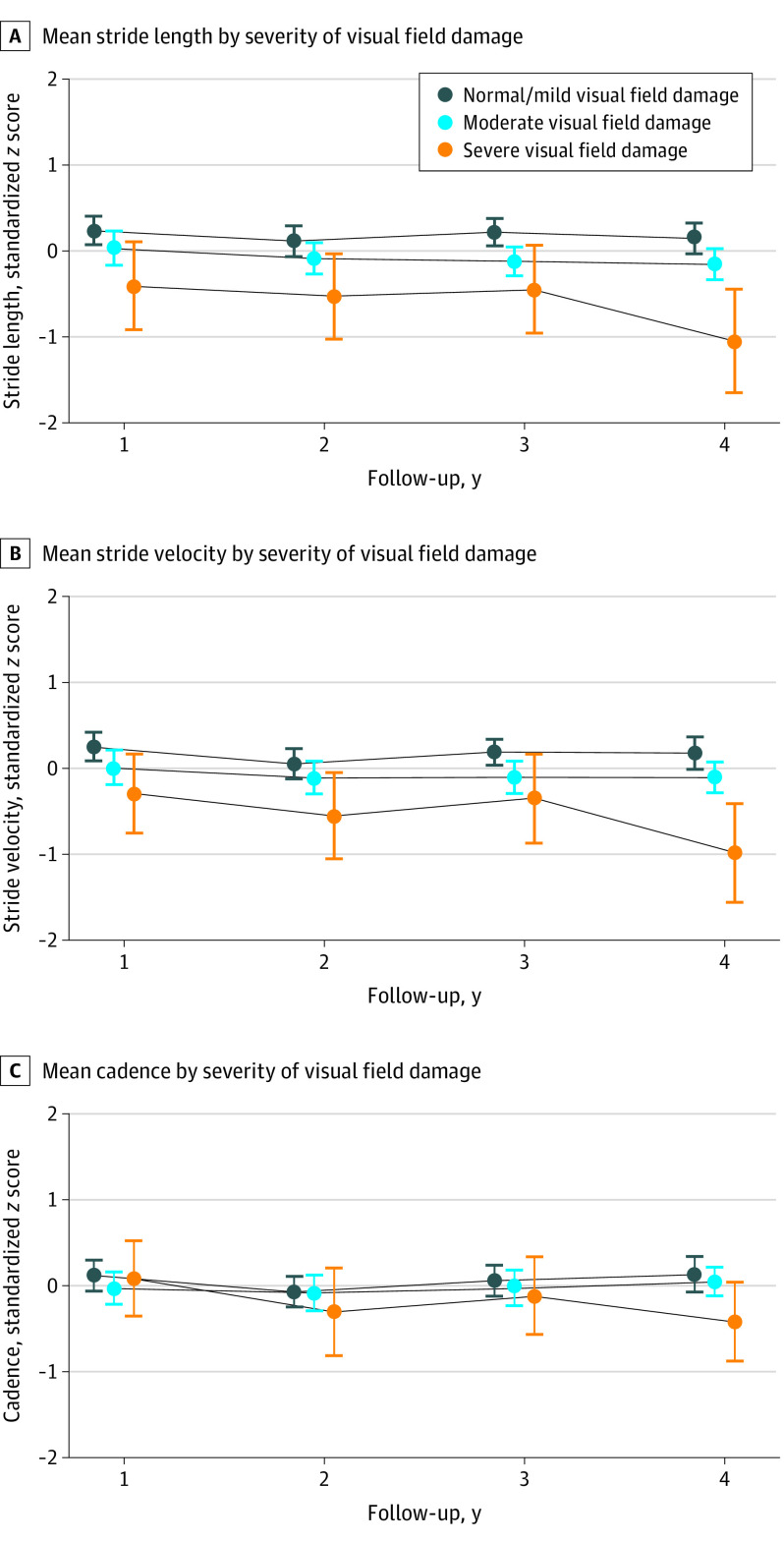
Figure. Stride Length, Velocity, and Cadency by Severity of Visual Field Damage Over 3 Years.
Normal/mild (integrated visual field sensitivity >28 dB), moderate (integrated visual field, 23-28 dB), or severe (integrated visual field, <23 dB). There were 241 individuals (119 in normal/mild, 98 in moderate, and 24 in severe groups) for year 1, 212 (104 in normal/mild, 87 in moderate, and 21 in severe groups) for year 2, 191 (94 in normal/mild, 76 in moderate, and 21 in severe groups) for year 3, and 171 (84 in normal/mild, 70 in moderate, and 17 in severe groups) for year 4.
Results
For 241 participants evaluated at baseline, the mean (SD) age was 70.8 (7.7) years, 116 (48.2%) were women, and 70 (29.0%) were African American.22 Approximately half (119 [49.4%]) of participants had normal/mild VF damage, while 98 (40.6%) and 24 (10.0%) had moderate and severe VF damage, respectively. Participant characteristics and gait metrics at baseline are given in Table 1.
Table 1. Participant Characteristics and Gait Metrics by Severity of Glaucoma Damage at Baseline (N = 241).
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| Normal/mild VF damage (IVF, >28 dB) | Moderate VF damage (IVF, 23-28 dB) | Severe VF damage (IVF, <23 dB) | ||
| No. (%) | 119 (49.4) | 98 (40.6) | 24 (10.0) | NA |
| Age, mean (SD), y | 69.1 (6.5) | 72.3 (8.7) | 70.1 (7.1) | .007 |
| Female | 59 (50) | 45 (46) | 12 (50) | .85 |
| Male | 60 (50) | 53 (54) | 12 (50) | .85 |
| African American | 29 (24) | 24 (25) | 17 (71) | <.001 |
| Living alone | 19 (16) | 22 (24) | 6 (25) | .31 |
| Education | ||||
| ≤High school | 15 (13) | 16 (16) | 6 (25) | .48 |
| Some college | 15 (13) | 13 (13) | 5 (17) | |
| Bachelor | 34 (29) | 19 (19) | 6 (25) | |
| ≥Master’s degree | 55 (46) | 50 (51) | 8 (33) | |
| Polypharmacya | 47 (41) | 44 (46) | 16 (64) | .09 |
| No. of comorbidities | ||||
| ≤1 | 39 (32) | 37 (38) | 8 (33) | .89 |
| 2-3 | 52 (44) | 43 (44) | 11 (46) | |
| 4-5 | 28 (24) | 18 (18) | 5 (21) | |
| MMSE-VI, mean (SD) | 20.3 (1.5) | 19.7 (2.2) | 20.0 (1.7) | .06 |
| Any falls last year | 52 (44) | 36 (37) | 13 (54) | .26 |
| Gait, mean (SD) | ||||
| Base of support, cm | 9.8 (3.0) | 10.3 (2.5) | 12.2 (4.1) | .003 |
| Base of support CV, % | 24.4 (13.6) | 25.5 (12.5) | 20.1 (11.1) | .19 |
| Stride length, cm | 115.3 (15.1) | 111.8 (16.1) | 104.3 (21.1) | .008 |
| Stride length CV, % | 4.1 (1.9) | 5.1 (2.6) | 6.8 (5.1) | <.001 |
| Stride velocity, cm/s | 104.0 (1.9) | 99.4 (18.8) | 93.7 (21.9) | .02 |
| Stride velocity CV, % | 6.4 (2.8) | 7.3 (4.1) | 9.1 (6.1) | .005 |
| Cadence, steps/min | 107.5 (10.7) | 105.8 (10.2) | 107.1 (11.5) | .53 |
Abbreviations: CV, coefficient of variation; dB, decibel; IVF, integrated visual field; MMSE-VI, Mini-Mental State Examination for vision impairment (maximum score, 22); NA, not applicable; VF, visual field.
Polypharmacy: ≥5 systemic prescription medications.
Among 241 participants with baseline gait measures, 212 (88%), 191 (79%), and 171 (71%) had gait assessment at the end of first, second, and third follow-up years, respectively. When evaluating gait changes throughout the 3-year follow-up period for persons within each glaucoma severity group, stride length declined over time in those with severe (−0.16 z score unit/y; 95% CI, −0.24 to −0.07; P < .001), moderate (−0.08 z score unit/y; 95% CI, −0.12 to −0.04; P < .001), and normal/mild (−0.06 z score unit/y; 95% CI, −0.10 to −0.03; P = .001) VF damage (Figure, A). Additionally, declines in stride velocity (−0.18 z score unit/y; 95% CI, −0.28 to −0.07; P = .002) and cadence (−0.15 z score unit/y; 95% CI, −0.25 to −0.04; P = .006) were present in persons with severe VF damage. However, no significant changes in base of support (normal/mild: 0.02 z score unit/y; 95% CI, −0.02 to 0.06; P = .38; moderate: 0.05 z score unit/y; 95% CI, 0-0.09; P = .06; severe: 0.02 z score unit/y; 95% CI, −0.07 to 0.10; P = .75) and its CV (normal/mild: 0.01 z score unit/y; 95% CI, −0.04 to 0.07; P = .68; moderate: −0.05 z score unit/y; 95% CI, −0.11 to 0.01; P = .13; severe: −0.05 z score unit/y; 95% CI, −0.17 to 0.07; P = .43), stride length CV (normal/mild: 0 z score unit/y; 95% CI, −0.07 to 0.07; P = .99; moderate: −0.03 z score unit/y; 95% CI, −0.11 to 0.04; P = .41; severe: −0.08 z score unit/y; 95% CI, −0.23 to 0.07; P = .30), and stride velocity CV (normal/mild: −0.05 z score unit/y; 95% CI, −0.12 to 0.02; P = .16; moderate: −0.06 z score unit/y; 95% CI, −0.13 to 0.02; P = .16; severe: −0.11 z score unit/y; 95% CI, −0.26 to 0.05; P = .19) were identified within people with normal/mild, moderate, and severe VF damage (Table 2).
Table 2. Mean Changes in Gait Metrics Over a 3-Year Follow-up Period (in Standardized z Scores) for People Within Each Severity of Glaucoma Damage.
| Gait | VF damagea | |||||
|---|---|---|---|---|---|---|
| Normal/mild, β (95% CI) | P value | Moderate, β (95% CI) | P value | Severe, β (95% CI) | P value | |
| Base of support, cm | 0.02 (−0.02 to 0.06) | .38 | 0.05 (0 to 0.09) | .06 | 0.02 (−0.07 to 0.10) | .75 |
| Base of support CV, % | 0.01 (−0.04 to 0.07) | .68 | −0.05 (−0.11 to 0.01) | .13 | −0.05 (−0.17 to 0.07) | .43 |
| Stride length, cm | −0.06 (−0.10 to −0.03) | .001 | −0.08 (−0.12 to −0.04) | <.001 | −0.16 (−0.24 to −0.07) | <.001 |
| Stride length CV, % | 0 (−0.07 to 0.07) | .99 | −0.03 (−0.11 to 0.04) | .41 | −0.08 (−0.23 to 0.07) | .30 |
| Stride velocity, cm/s | −0.03 (−0.08 to 0.02) | .26 | −0.05 (−0.11 to 0) | .05 | −0.18 (−0.28 to −0.07) | .002 |
| Stride velocity CV, % | −0.05 (−0.12 to 0.02) | .16 | −0.06 (−0.13 to 0.02) | .16 | −0.11 (−0.26 to 0.05) | .19 |
| Cadence, steps/min | 0.03 (−0.02 to 0.08) | .25 | 0.01 (−0.04 to 0.06) | .69 | −0.15 (−0.25 to −0.04) | .006 |
Abbreviations: CV, coefficient of variation; VF, visual field.
Normal/mild (integrated visual field sensitivity, >28 dB), moderate (integrated visual field, 23-28 dB), or severe (integrated visual field, <23 dB). Mixed-effects estimates adjusted for age, race, sex, living arrangement, education, comorbidity, polypharmacy, and cognitive function.
When comparing gait changes over the 3-year study period across severity of VF damage, each 5-dB decrement in IVF sensitivity was associated with more rapid decline of stride velocity (−0.05 z score unit/y; 95% CI, −0.09 to −0.01; P = .01) and cadence (−0.07 z score unit/y; 95% CI, −0.10 to −0.03; P < .001) per year (Table 3). Likewise, in comparisons across categories of VF damage, participants with more severe VF damage had more accelerated reduction of stride velocity (−0.15 z score unit/y; 95% CI, −0.27 to −0.03; P = .02) (Figure, B) and cadence (−0.17 z score unit/y; 95% CI, −0.29 to −0.06; P = .003) (Figure, C) per year compared with those with normal/mild VF damage; however, those with moderate VF group had a similar rate of changes for stride velocity (−0.02 z score unit/y; 95% CI, −0.10 to 0.05; P = .51) and cadence (−0.02 z score unit/y; 95% CI, −0.09 to 0.05; P = .63) (Table 3). No significant associations were noted between IVF sensitivity and longitudinal changes in base of support (0 z score unit/y; 95% CI, −0.02 to 0.03; P = .74) and its CV (−0.01 z score unit/y; 95% CI, −0.06 to 0.03; P = .57), stride length (−0.02 z score unit/y; 95% CI, −0.05 to 0.01; P = .12) and its CV (−0.05 z score unit/y; 95% CI, −0.11 to 0; P = .06), and stride velocity CV (−0.04 z score unit/y; 95% CI, −0.09 to 0.02; P = .16).
Table 3. Comparing Mean Changes in Gait Metrics Over a 3-Year Follow-up Period (in Standardized z Scores) Across the Severity of Glaucoma Damage.
| Gait | 5-Unit decrement in integrated VF, dB | VF damagea | |||||
|---|---|---|---|---|---|---|---|
| β (95% CI) | P value | Normal/mild | Moderate, β (95% CI) | P value | Severe, β (95% CI) | P value | |
| Base of support, cm | 0 (−0.02 to 0.03) | .74 | Reference | 0.03 (−0.03 to 0.09) | .30 | 0 (−0.10 to 0.09) | .94 |
| Base of support CV, % | −0.01 (−0.06 to 0.03) | .57 | Reference | −0.06 (−0.14 to 0.02) | .16 | −0.06 (−0.20 to 0.07) | .37 |
| Stride length, cm | −0.02 (−0.05 to 0.01) | .12 | Reference | −0.02 (−0.08 to 0.04) | .55 | −0.09 (−0.19 to 0) | .05 |
| Stride length CV, % | −0.05 (−0.11 to 0) | .06 | Reference | −0.03 (−0.14 to 0.07) | .55 | −0.08 (−0.25 to 0.09) | .35 |
| Stride velocity, cm/s | −0.05 (−0.09 to −0.01) | .01 | Reference | −0.02 (−0.10 to 0.05) | .51 | −0.15 (−0.27 to −0.03) | .02 |
| Stride velocity CV, % | −0.04 (−0.09 to 0.02) | .16 | Reference | 0 (−0.11 to 0.10) | .93 | −0.05 (−0.23 to 0.12) | .53 |
| Cadence, steps/min | −0.07 (−0.10 to −0.03) | <.001 | Reference | −0.02 (−0.09 to 0.05) | .63 | −0.17 (−0.29 to −0.06) | .003 |
Abbreviations: CV, coefficient of variation; VF, visual field.
Normal/mild (integrated VF sensitivity, >28 dB), moderate (integrated VF, 23-28 dB), or severe (integrated VF, <23 dB). Mixed-effects estimates adjusted for age, race, sex, living arrangement, education, comorbidity, polypharmacy, and cognitive function.
Discussion
In this prospective cohort study, we examined whether 3-year longitudinal changes in gait differed by the degree of baseline VF damage. At greater levels of VF damage, patients with glaucoma demonstrated a faster decline in walking speeds (ie, stride velocity and cadence) over time. However, stride length declined at a similar rate for people with normal/mild, moderate, and severe VF damage, and no associations were found between IVF sensitivity and longitudinal changes in the base of support and stride-to-stride variability. Collectively, our findings suggest that patients with glaucoma with advanced VF damage are more prone to future declines in specific gait measures and the associated health-related consequences. These data provide the first objective evidence of worse health trajectories in persons with more severe glaucoma.
Gait measures, particularly gait speed, are highly associated with functionality, disability, and mortality. As such, gait speed represents one of the central elements of overall health that can be objectively measured. The present study is an important adjunct to prior research that examined VF damage and the changes in quality of life as their primary outcome,33 complementing this work by demonstrating faster declines in an important objective measure of health (gait speed) in persons with greater baseline levels of VF damage.
Our results add to prior research that reports vision impairment to be associated with worse mobility in patients with glaucoma10,34 and extend these findings with longitudinal data aimed at evaluating the association of glaucoma severity on gait changes over time. Previous cross-sectional studies have shown that people with glaucoma walk slower, and walking speed is negatively correlated with VF damage.35 However, the effect of VF loss on within-person changes in gait measures over time has not been fully investigated. A 5-year longitudinal Beaver Dam study did not find associations between vision parameters (eg, visual acuity and contrast sensitivity) and change in time of walking a 3-meter course at usual pace36; however, alternative gait measures including base of support and stride-to-stride velocity, which are associated with higher rates of falling in older adults, were not assessed objectively using appropriate technology.10 Our longitudinal study differed in that it specifically focused on patients with glaucoma and examined how VF damage affected changes in 7 walking metrics using a gait mat, allowing for a more detailed, quantitative understanding of mobility changes over time.
To our knowledge, this is the first study to demonstrate a worse functional trajectory in persons with greater glaucoma damage using objectively measured outcomes. Medeiros et al33 studied longitudinal changes in glaucomatous VF loss and patient-reported quality of life and found that severe baseline VF and progressive VF loss were associated with faster decline in National Eye Institute Visual Function Questionnaire-25 scores. Our study adds complementary evidence to these findings by quantifying functional decline with objective measures of gait parameters over time and linking these changes to the degree of VF damage. In routine geriatric assessments, gait speed represents a quick, inexpensive, and reliable measure of functional capacity with well-documented predictive ability for major adverse outcomes,37 such as disability, dysfunctionality, and mortality.1,4,13,38 Our findings suggest that patients with glaucoma may also be prone to secondary health-related consequences as judged by their changes in gait over time. Together, these studies detail the evidence that individuals with worse glaucoma have more rapid declines in function, which is a well-established link to future health and well-being.
Our study found that patients with glaucoma with worse levels of VF damage experienced more rapid declines in walking speed. Consistent with previous research that has demonstrated that age-associated decrease of stride length in older population,27 our study showed that stride length declined over time at a similar rate for people regardless of VF severity, although cadence slowed more at greater levels of VF damage. We conclude that walking speed slows more in advanced VF damage mainly because of fewer steps taken per minute, not because of shorter steps. Of note, slower gait velocity is linked with more efficient energy expenditure when performing a standardized task (at the expense of longer duration)39; thus, it is plausible that patients with advanced glaucoma seem to lower their step frequency (without shortening the stride length) as a self-selected walking rhythm, which may reflect an adaption of minimizing energy expenditure and adapting to mitochondrial inefficiency to preserve energy reserves.40,41 Future research should investigate the link between walking efficiency and speed in older adults with glaucoma.
Our study found that the base of support and stride-to-stride variability (ie, CVs of base support, stride length, and stride velocity) remained unchanged over the study for all severity levels of glaucoma damage. Prior research has reported that older adults with a broader base of support and higher stride-to-stride variability have a greater fear of falling, poorer balance, and greater risk of falling.20,42,43 Our findings suggest that people with worse VF damage are irregular in their gait patterns to adopt safer walking to maintain stability and prevent falls, although this abnormal gait pattern remained unlikely to change over a 3-year period regardless of disease severity.
Limitations
Limitations of our study include the fact that participants were enrolled from a single clinical center, such that our findings may not be generalizable to all older adults with visual impairment in the US. Second, the performance-based gait measures examined by walking on the flat surface with ambient lighting may not reflect the walking in the real world, such as walking up or down the stairs, making turns, or navigating hazards. Third, although some confounders such as polypharmacy and number of comorbidities might change over time, our examination of within-individual changes in gait measures is unlikely to be differential given the limited period of study and the low likelihood that these changes would correlate with disease severity (ie, nondifferential changes in proportions of polypharmacy and number of comorbidities across spectrum of VF damage). While individual pharmacy might provide a more precise method for judging the effect of medications on gait dysfunction, we considered polypharmacy as a measure of medication use, incorporating interactions between medications and avoiding uncertainty of few given types of medications. Fourth, although examining VF damage remains an important clinical tool for glaucoma, a more comprehensive evaluation of vision should be obtained in future research aiming to capture the full effect of glaucomatous visual impairment on mobility. Fifth, these findings are limited by the post hoc design, which increases the risk of detecting associations that may have occurred by chance. Sixth, it was our original hypothesis that baseline VF damage would have implications for gait changes over the full 3-year period (ie, expressed as changes per year); however, it is possible that IVF sensitivity varies over time. As this was a treated cohort population, changes in glaucoma severity were infrequent and typically mild (mean rate of change in visual field mean deviation = −0.09 dB/y), making it difficult to judge the changes in IVF sensitivity and changes in gait. Seventh, it is possible that other gait measures might have changed as well, but our study was underpowered to detect the differences because of the short period of follow-up.
Conclusions
In summary, our study found that at worse levels of VF damage, patients with glaucoma demonstrate a greater decline in walking speeds over time. The declines in walking speeds from this post hoc analysis were fairly substantial, suggesting that individuals with VF damage would have their gait declined more rapidly than normally sighted peers. However, stride length declined at a similar rate for people with normal/mild, moderate, and severe VF damage, and no associations were found between IVF sensitivity and longitudinal changes in the base of support and stride-to-stride variability.
Reference
- 1.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55(1):M43-M52. doi: 10.1093/gerona/55.1.M43 [DOI] [PubMed] [Google Scholar]
- 2.Mahlknecht P, Kiechl S, Bloem BR, et al. Prevalence and burden of gait disorders in elderly men and women aged 60-97 years: a population-based study. PLoS One. 2013;8(7):e69627-e69627. doi: 10.1371/journal.pone.0069627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloem BR, Haan J, Lagaay AM, van Beek W, Wintzen AR, Roos RAC. Investigation of gait in elderly subjects over 88 years of age. J Geriatr Psychiatry Neurol. 1992;5(2):78-84. doi: 10.1177/002383099200500204 [DOI] [PubMed] [Google Scholar]
- 4.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alam U, Riley DR, Jugdey RS, et al. Diabetic neuropathy and gait: a review. Diabetes Ther. 2017;8(6):1253-1264. doi: 10.1007/s13300-017-0295-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies SW, Greig CA, Jordan SL, Grieve DW, Lipkin DP. Short-stepping gait in severe heart failure. Br Heart J. 1992;68(5):469-472. doi: 10.1136/hrt.68.11.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beauchet O, Allali G, Berrut G, Hommet C, Dubost V, Assal F. Gait analysis in demented subjects: interests and perspectives. Neuropsychiatr Dis Treat. 2008;4(1):155-160. doi: 10.2147/NDT.S2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrucci L, Penninx BW, Leveille SG, et al. Characteristics of nondisabled older persons who perform poorly in objective tests of lower extremity function. J Am Geriatr Soc. 2000;48(9):1102-1110. doi: 10.1111/j.1532-5415.2000.tb04787.x [DOI] [PubMed] [Google Scholar]
- 9.Mihailovic A, De Luna RM, West SK, Friedman DS, Gitlin LN, Ramulu PY. Gait and balance as predictors and/or mediators of falls in glaucoma. Invest Ophthalmol Vis Sci. 2020;61(3):30. doi: 10.1167/iovs.61.3.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihailovic A, Swenor BK, Friedman DS, West SK, Gitlin LN, Ramulu PY. Gait implications of visual field damage from glaucoma. Transl Vis Sci Technol. 2017;6(3):23. doi: 10.1167/tvst.6.3.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrack JA, Simonsick EM, Chaves PHM, Ferrucci L. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc. 2012;60(10):1811-1816. doi: 10.1111/j.1532-5415.2012.04153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shumway-Cook A, Guralnik JM, Phillips CL, et al. Age-associated declines in complex walking task performance: the Walking InCHIANTI toolkit. J Am Geriatr Soc. 2007;55(1):58-65. doi: 10.1111/j.1532-5415.2006.00962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221-M231. doi: 10.1093/gerona/55.4.M221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility—giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. 2014;311(20):2061-2062. doi: 10.1001/jama.2014.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iosa M, Fusco A, Morone G, Paolucci S. Effects of visual deprivation on gait dynamic stability. ScientificWorldJournal. 2012;2012:974560. doi: 10.1100/2012/974560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura T. Quantitative analysis of gait in the visually impaired. Disabil Rehabil. 1997;19(5):194-197. doi: 10.3109/09638289709166526 [DOI] [PubMed] [Google Scholar]
- 17.Tomomitsu MSV, Alonso AC, Morimoto E, Bobbio TG, Greve JMD. Static and dynamic postural control in low-vision and normal-vision adults. Clinics (Sao Paulo). 2013;68(4):517-521. doi: 10.6061/clinics/2013(04)13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hay L, Bard C, Fleury M, Teasdale N. Availability of visual and proprioceptive afferent messages and postural control in elderly adults. Exp Brain Res. 1996;108(1):129-139. doi: 10.1007/BF00242910 [DOI] [PubMed] [Google Scholar]
- 19.Osoba MY, Rao AK, Agrawal SK, Lalwani AK. Balance and gait in the elderly: a contemporary review. Laryngoscope Investig Otolaryngol. 2019;4(1):143-153. doi: 10.1002/lio2.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050-1056. doi: 10.1053/apmr.2001.24893 [DOI] [PubMed] [Google Scholar]
- 21.E JY, Mihailovic A, Schrack JA, et al. Characterizing longitudinal changes in physical activity and fear of falling after falls in glaucoma. J Am Geriatr Soc. 2021;69(5):1249-1256. doi: 10.1111/jgs.17014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.E JY, Schrack JA, Mihailovic A, et al. Patterns of daily physical activity across the spectrum of visual field damage in glaucoma patients. Ophthalmology. 2021;128(1):70-77. doi: 10.1016/j.ophtha.2020.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odden JL, Mihailovic A, Boland MV, Friedman DS, West SK, Ramulu PY. Evaluation of central and peripheral visual field concordance in glaucoma. Invest Ophthalmol Vis Sci. 2016;57(6):2797-2804. doi: 10.1167/iovs.15-19053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe RY, Diniz-Filho A, Costa VP, Gracitelli CP, Baig S, Medeiros FA. The impact of location of progressive visual field loss on longitudinal changes in quality of life of patients with glaucoma. Ophthalmology. 2016;123(3):552-557. doi: 10.1016/j.ophtha.2015.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodapp E, Parrish PK, Anderson DR. Clinical Decisions in Glaucoma. The CV Mosby Co; 1993. [Google Scholar]
- 26.Guimaraes RM, Isaacs B. Characteristics of the gait in old people who fall. Int Rehabil Med. 1980;2(4):177-180. doi: 10.3109/09638288009163984 [DOI] [PubMed] [Google Scholar]
- 27.Shimada H, Kim H, Yoshida H, et al. Relationship between age-associated changes of fait and falls and life-space in elderly people. J Phys Ther Sci. 2010;22(4):419-424. doi: 10.1589/jpts.22.419 [DOI] [Google Scholar]
- 28.Wolfson L, Whipple R, Amerman P, Tobin JN. Gait assessment in the elderly: a gait abnormality rating scale and its relation to falls. J Gerontol. 1990;45(1):M12-M19. doi: 10.1093/geronj/45.1.M12 [DOI] [PubMed] [Google Scholar]
- 29.Turano KA, Broman AT, Bandeen-Roche K, Munoz B, Rubin GS, West S; SEE Project Team . Association of visual field loss and mobility performance in older adults: Salisbury Eye Evaluation Study. Optom Vis Sci. 2004;81(5):298-307. doi: 10.1097/01.opx.0000134903.13651.8e [DOI] [PubMed] [Google Scholar]
- 30.Pirker W, Katzenschlager R. Gait disorders in adults and the elderly: a clinical guide. Wien Klin Wochenschr. 2017;129(3-4):81-95. doi: 10.1007/s00508-016-1096-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramulu PY, van Landingham SW, Massof RW, Chan ES, Ferrucci L, Friedman DS. Fear of falling and visual field loss from glaucoma. Ophthalmology. 2012;119(7):1352-1358. doi: 10.1016/j.ophtha.2012.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tibaek S, Holmestad-Bechmann N, Pedersen TB, Bramming SM, Friis AK. Reference values of maximum walking speed among independent community-dwelling Danish adults aged 60 to 79 years: a cross-sectional study. Physiotherapy. 2015;101(2):135-140. doi: 10.1016/j.physio.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 33.Medeiros FA, Gracitelli CP, Boer ER, Weinreb RN, Zangwill LM, Rosen PN. Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology. 2015;122(2):293-301. doi: 10.1016/j.ophtha.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sotimehin AE, Ramulu PY. Measuring disability in glaucoma. J Glaucoma. 2018;27(11):939-949. doi: 10.1097/IJG.0000000000001068 [DOI] [PubMed] [Google Scholar]
- 35.Friedman DS, Freeman E, Munoz B, Jampel HD, West SK. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology. 2007;114(12):2232-2237. doi: 10.1016/j.ophtha.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 36.Klein BE, Moss SE, Klein R, Lee KE, Cruickshanks KJ. Associations of visual function with physical outcomes and limitations 5 years later in an older population: the Beaver Dam eye study. Ophthalmology. 2003;110(4):644-650. doi: 10.1016/S0161-6420(02)01935-8 [DOI] [PubMed] [Google Scholar]
- 37.Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68(1):39-46. doi: 10.1093/gerona/gls174 [DOI] [PubMed] [Google Scholar]
- 38.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881-889. doi: 10.1007/s12603-009-0246-z [DOI] [PubMed] [Google Scholar]
- 39.Schrack JA, Simonsick EM, Ferrucci L. The relationship of the energetic cost of slow walking and peak energy expenditure to gait speed in mid-to-late life. Am J Phys Med Rehabil. 2013;92(1):28-35. doi: 10.1097/PHM.0b013e3182644165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters RL, Mulroy S. The energy expenditure of normal and pathologic gait. Gait Posture. 1999;9(3):207-231. doi: 10.1016/S0966-6362(99)00009-0 [DOI] [PubMed] [Google Scholar]
- 41.Lascaratos G, Chau KY, Zhu H, et al. Resistance to the most common optic neuropathy is associated with systemic mitochondrial efficiency. Neurobiol Dis. 2015;82:78-85. doi: 10.1016/j.nbd.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 42.Chamberlin ME, Fulwider BD, Sanders SL, Medeiros JM. Does fear of falling influence spatial and temporal gait parameters in elderly persons beyond changes associated with normal aging? J Gerontol A Biol Sci Med Sci. 2005;60(9):1163-1167. doi: 10.1093/gerona/60.9.1163 [DOI] [PubMed] [Google Scholar]
- 43.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45(3):313-320. doi: 10.1111/j.1532-5415.1997.tb00946.x [DOI] [PubMed] [Google Scholar]