This randomized clinical trial examines the use of carboplatin and isotretinoin in children with high-risk medulloblastoma, with subgroup analysis by molecular classification.
Key Points
Question
Does carboplatin during radiotherapy or isotretinoin during maintenance chemotherapy improve survival in children with high-risk medulloblastoma?
Findings
In this randomized clinical trial including 261 children with medulloblastoma, isotretinoin was not found to improve survival. The addition of carboplatin during radiotherapy improved survival from 54% to 73% only for children with high-risk group 3 medulloblastoma.
Meaning
The findings of this randomized clinical trial indicate that prospective molecular and clinical risk stratification for medulloblastoma is necessary because the addition of carboplatin during radiotherapy is recommended for high-risk group 3 medulloblastoma, but not for other molecular subgroups.
Abstract
Importance
Brain tumors are the leading cause of disease-related death in children. Medulloblastoma is the most common malignant embryonal brain tumor, and strategies to increase survival are needed.
Objective
To evaluate therapy intensification with carboplatin as a radiosensitizer and isotretinoin as a proapoptotic agent in children with high-risk medulloblastoma in a randomized clinical trial and, with a correlative biology study, facilitate planned subgroup analysis according to World Health Organization consensus molecular subgroups of medulloblastoma.
Design, Setting, and Participants
A randomized clinical phase 3 trial was conducted from March 2007 to September 2018. Analysis was completed in September 2020. Patients aged 3 to 21 years with newly diagnosed high-risk medulloblastoma from Children’s Oncology Group institutions within the US, Canada, Australia, and New Zealand were included. High-risk features included metastasis, residual disease, or diffuse anaplasia.
Interventions
Patients were randomized to receive 36-Gy craniospinal radiation therapy and weekly vincristine with or without daily carboplatin followed by 6 cycles of maintenance chemotherapy with cisplatin, cyclophosphamide, and vincristine with or without 12 cycles of isotretinoin during and following maintenance.
Main Outcomes and Measures
The primary clinical trial end point was event-free survival, using the log-rank test to compare arms. The primary biology study end point was molecular subgroup classification by DNA methylation array.
Results
Of 294 patients with medulloblastoma, 261 were evaluable after central radiologic and pathologic review; median age, 8.6 years (range, 3.3-21.2); 183 (70%) male; 189 (72%) with metastatic disease; 58 (22%) with diffuse anaplasia; and 14 (5%) with greater than 1.5-cm2 residual disease. For all participants, the 5-year event-free survival was 62.9% (95% CI, 55.6%-70.2%) and overall survival was 73.4% (95% CI, 66.7%-80.1%). Isotretinoin randomization was closed early owing to futility. Five-year event-free survival was 66.4% (95% CI, 56.4%-76.4%) with carboplatin vs 59.2% (95% CI, 48.8%-69.6%) without carboplatin (P = .11), with the effect exclusively observed in group 3 subgroup patients: 73.2% (95% CI, 56.9%-89.5%) with carboplatin vs 53.7% (95% CI, 35.3%-72.1%) without (P = .047). Five-year overall survival differed by molecular subgroup (P = .006): WNT pathway activated, 100% (95% CI, 100%-100%); SHH pathway activated, 53.6% (95% CI, 33.0%-74.2%); group 3, 73.7% (95% CI, 61.9%-85.5%); and group 4, 76.9% (95% CI, 67.3%-86.5%).
Conclusions and Relevance
In this randomized clinical trial, therapy intensification with carboplatin improved event-free survival by 19% at 5 years for children with high-risk group 3 medulloblastoma. These findings further support the value of an integrated clinical and molecular risk stratification for medulloblastoma.
Trial Registration
ClinicalTrials.gov Identifier: NCT00392327
Introduction
Medulloblastoma is the most common embryonal brain tumor and occurs mainly in children (median age, 8 years).1 With multimodal therapy, including neurosurgical resection, craniospinal radiation, and combination chemotherapy, most children may be cured.2,3,4 There are few curative options for therapy following relapse.5 Evaluation of therapeutic interventions to prevent relapse are warranted.
Clinical and histologic characteristics associated with an increased risk of relapse in medulloblastoma include metastatic disease, diffuse anaplasia, or incomplete surgical resection; these factors define high-risk medulloblastoma.2,6 Based on preclinical evidence that carboplatin may potentiate radiotherapy to increase cancer cell death7 a phase 1/2 study of carboplatin during radiotherapy (CCG99701, NCT00003203) was conducted in patients with high-risk medulloblastoma between 1998 and 2004 that established a recommended dose of carboplatin of 35 mg/m2 for 30 doses before daily radiotherapy.4 This regimen resulted in a 5-year progression-free survival (SE) rate of 71% (6%) in patients with metastatic medulloblastoma, which compared favorably with previously reported outcomes for high-risk patients.2,3,6,8
The potential to improve intensive pediatric cancer therapy with isotretinoin as a differentiating agent was demonstrated in a phase 3 study of neuroblastoma, an extra-CNS embryonal cancer of childhood. In high-risk neuroblastoma, the addition of isotretinoin improved 3-year event-free survival (EFS) (SE) from 29% (5%) to 46% (6%) (P = .027).9 In preclinical studies of medulloblastoma, isotretinoin was demonstrated to induce apoptosis through BMP-2.10 Retinoids have been demonstrated to act synergistically with cisplatin in preclinical models of medulloblastoma and other cancers.11,12,13
Genomic studies have identified at least 4 distinct molecular subgroups of medulloblastoma that may improve diagnostic and prognostic specificity.14,15,16,17,18 The 2016 World Health Organization Classification of Tumors of the Central Nervous System defined an integrated diagnosis including WNT (WNT signaling pathway activated), SHH (SHH signaling pathway activated) with or without TP53 mutation, and non-WNT/non-SHH (provisionally designated group 3 and group 4) medulloblastoma.19
The Children’s Oncology Group conducted a 4-arm randomized clinical trial to evaluate the therapeutic benefit of carboplatin as a radiosensitizer and isotretinoin as a proapoptotic agent in children with high-risk medulloblastoma. This study sought to objectively evaluate these interventions as well as molecular subgroups and risk factors appropriate to incorporate into clinical risk stratification and future risk-adapted clinical trials for children with medulloblastoma.
Methods
Patients and Eligibility
Patients aged 3 to 21 years with newly diagnosed high-risk medulloblastoma were eligible. High-risk features included metastatic disease, diffuse anaplastic histologic characteristics, or incomplete surgical resection, defined as greater than 1.5 cm2 residual tumor. Other eligibility criteria included Karnofsky or Lansky performance score greater than or equal to 30, no prior therapy or other experimental therapy, and adequate organ function. Pregnant or breastfeeding patients were excluded. Staging included magnetic resonance imaging of the brain and spine and lumbar cerebrospinal fluid cytologic examination. Institutional review board approval was obtained at each institution and written informed consent and assent when appropriate was obtained for all participants. Self-declared race and ethnicity was reported by each institution according to National Institutes of Health–defined categories. Retrospective central pathologic review confirmed the diagnosis of medulloblastoma and anaplastic status. Retrospective central radiologic review confirmed initial staging, extent of resection, radiologic progression, relapse, or second malignant neoplasm. The trial protocol is available in Supplement 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
Study Design
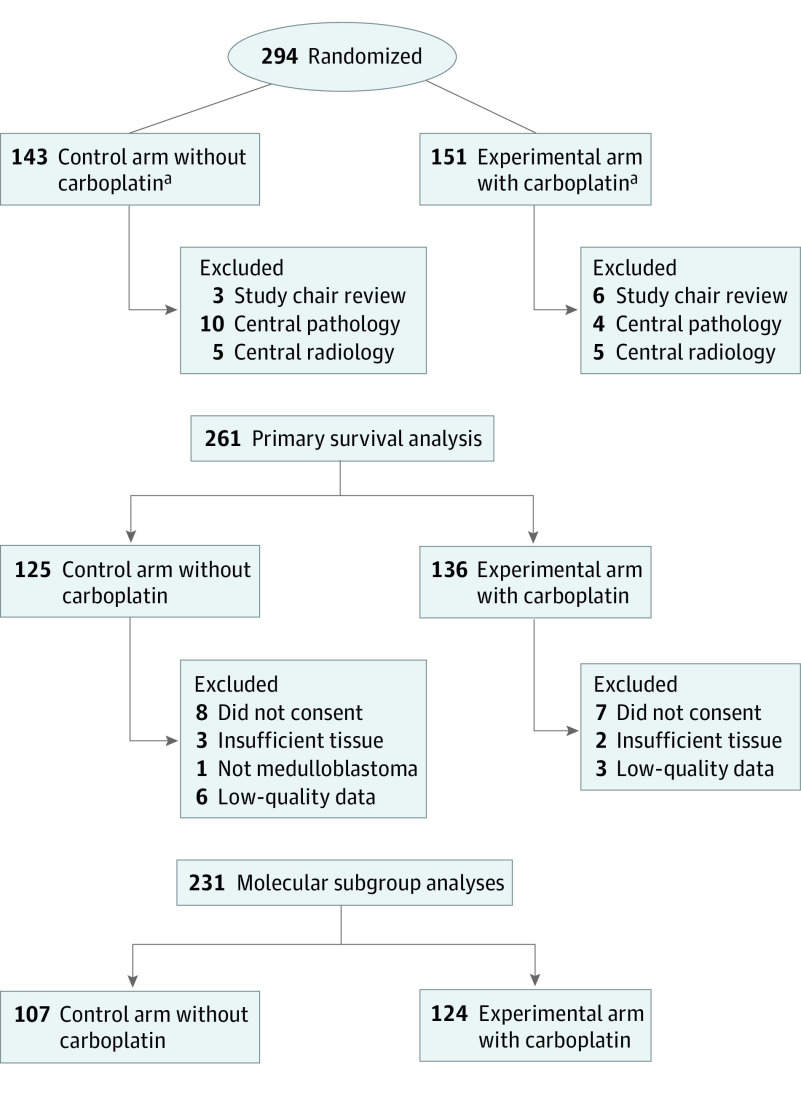
This was a randomized, phase 3, factorial-designed study with 4 arms (Figure 1). Patients were initially stratified based on tumor location (medulloblastoma vs supratentorial primitive neuroectodermal tumor [sPNET]). Owing to the emergence of new data on the biological differences between sPNET and medulloblastoma, the study was amended to adjust accrual goals to detect treatment effects within medulloblastoma alone and discontinue enrollment of patients with sPNET, which have been reported elsewhere.20 The original protocol required tissue submission so that clinical outcome results could be interpreted in the context of genomic information that was rapidly evolving in 2006. The study was amended in 2014 to clarify that the primary aim of the correlative biology study for medulloblastoma was to assign molecular subgroup status by DNA methylation classification to interpret randomization outcomes according to World Health Organization consensus molecular subgroups. The secondary biology study aim was to evaluate previously reported prognostic biomarkers in this clinical trial cohort. At the time of enrollment, clinical trial participants were stratified by clinical high-risk criteria, including (1) M0 medulloblastoma with greater than 1.5 cm2 residual, (2) M+ medulloblastoma, and (3) M0 diffusely anaplastic medulloblastoma. All patients received 36 Gy craniospinal radiotherapy with boost to the posterior fossa of 55.8 Gy cumulative dose with conventional fractionation of 1.8 Gy/d. Radiotherapy was required to be initiated within 31 days of diagnostic surgery, and 6 doses of weekly vincristine, 1.5 mg/m2, were given during radiotherapy. Patients randomized to receive carboplatin, 35 mg/m2 per dose, were given 30 doses administered daily before radiotherapy. Maintenance therapy consisted of six 28-day cycles, which included cisplatin, 75 mg/m2, on day 1; cyclophosphamide, 1000 mg/m2, on days 2 and 3; and vincristine, 1.5 mg/m2, on days 1 and 8. Patients randomized to receive isotretinoin received 80 mg/m2 twice a day on days 15 to 28 (14 days every 28 days) for 12 cycles during and following maintenance therapy.
Figure 1. All 294 Patients With Medulloblastoma Enrolled in the Children’s Oncology Group ACNS0332 Study.
aSecond randomization to isotretinoin maintenance included 56 patients in the arm without carboplatin and 58 patients in the arm with carboplatin before closure of the isotretinoin randomization based on futility analysis. Study chair exclusions were for patients deemed to be ineligible owing to timing of start of therapy (n = 6), staging/extent of disease (n = 2), and disease type/histologic characteristics (n = 1). Central pathologic review exclusions were for tumors determined not to be medulloblastoma (n = 6) or not to have diffuse anaplastic histologic characteristics (n = 8). Central radiologic review exclusion was for residual disease less than 1.5-cm2, including gross total resection (n = 7) or near-total resection (n = 3).
Molecular Analysis
Molecular analysis was conducted retrospectively at a central laboratory. DNA was extracted from fresh-frozen and formalin-fixed, paraffin-embedded tissue samples. Samples were analyzed using arrays (Illumina Infinium Methylation EPIC BeadChip; Illumina Inc) in accordance with the manufacturer’s instructions. For tumor samples with sufficient genomic DNA following methylation array analysis, whole-exome sequencing was performed. Details of molecular analyses are provided in the eMethods in Supplement 2.21,22
Neuropsychological and Health-Related Quality of Life Evaluations
Coenrollment into the neuropsychological function study ALTE07C1,23,24 Neuropsychological, Social, Emotional, and Behavioral Outcomes in Children with Cancer, was encouraged. Patients were assessed at 3 points: 9 (±3 months) (T1), 30 (±6 months) (T2), and 60 months (±12 months) (T3) after diagnosis with age-appropriate standardized measures of intellectual functioning, attention, and memory. Parent-rated questionnaires assessed executive functions, social-emotional functioning, and quality of life. Neurocognitive end points included estimated IQ of the Wechsler Intelligence scales, the Wechsler Processing Speed Index, and the Global Executive Composite of the Behavior Rating Inventory of Executive Function.
Statistical Analysis
The primary objectives were to test whether the addition of (1) carboplatin to radiotherapy or (2) isotretinoin to maintenance chemotherapy improved EFS. An event included progression or recurrence, second malignant neoplasm, or death from any cause. Each primary objective was considered independent of the other. Interim analyses were conducted annually for efficacy and futility by an independent COG Data Safety Monitoring Committee. Based on a 1-sided log-rank test with 5% type I error, an accrual of 280 participants and 110 events was targeted to provide 80% power to detect a 15% increase in 5-year EFS (from 56% to 71%) for either intervention. Outcome distributions were estimated using the Kaplan-Meier method. One-sided log-rank tests were used to compare outcome by randomization arm, stratified by study stratum and the other randomization (isotretinoin or carboplatin assignment), for the entire study population and within each molecular subgroup. Two-sided log-rank tests were used to explore associations between molecular factors and outcome. Secondary end points included overall survival (OS). General linear models, including treatment group, sex, age at diagnosis, and method of payment (surrogate marker of socioeconomic status), were used to explore the association between the neuropsychological end points and randomization groups as well as change in neurocognitive functioning over time. Fisher exact test, χ2 tests, and Kruskal-Wallis tests were used to examine associations among categorical variables. Wilcoxon rank-sum tests were used to examine associations between continuous variables (eg, age) with carboplatin assignment. Findings were considered significant at P < .05. Data were analyzed using SAS statistical software, version 9.4 (SAS Institute).
Results
A total of 294 patients with medulloblastoma were enrolled between March 2007 and September 2018. Nine patients were ineligible owing to timing of the start of protocol therapy (n = 6), category (n = 2), or histologic characteristics (n = 1). Twenty-four additional patients were ineligible based on retrospective central pathologic (n = 14) or central radiologic (n = 10) review. Details and comparison of excluded patients are provided in eTable 1 and eTable 2 in Supplement 2. Median follow-up time was 6.7 years, and 15 participants were lost to follow-up before 5 years. To provide the most relevant baseline for future studies, outcome results given herein are restricted to patients eligible by both prospective and retrospective central review (n = 261) (Figure 1).
The 5-year EFS was 62.9% (95% CI, 55.6%-70.2%) and 5-year OS was 73.4% (95% CI, 66.7%-80.1%) (Figure 2A). Of the 261 patients included in the analysis, 183 (70.1%) were male, with median age, 8.6 years (range, 3.3-21.2) at enrollment, 189 (72.4%) with metastatic disease, 58 (22.2%) with diffuse anaplasia, and 14 (5.4%) with greater than 1.5-cm2 residual disease. Randomization to isotretinoin was discontinued in 2015 following an interim futility analysis demonstrating that the addition of isotretinoin was unlikely to lead to a significant EFS difference (eFigure 1 in Supplement 2). At that time, the 5-year EFS according to isotretinoin randomization was 68.6% (95% CI, 52.1%-85.1%) with isotretinoin compared with 67.8% (95% CI, 47.6%-88.0%) without isotretinoin. Of the 114 patients who received isotretinoin, 56 did not receive carboplatin; 58 received carboplatin.
Figure 2. Event-Free Survival (EFS) and Overall Survival (OS) (n = 261).
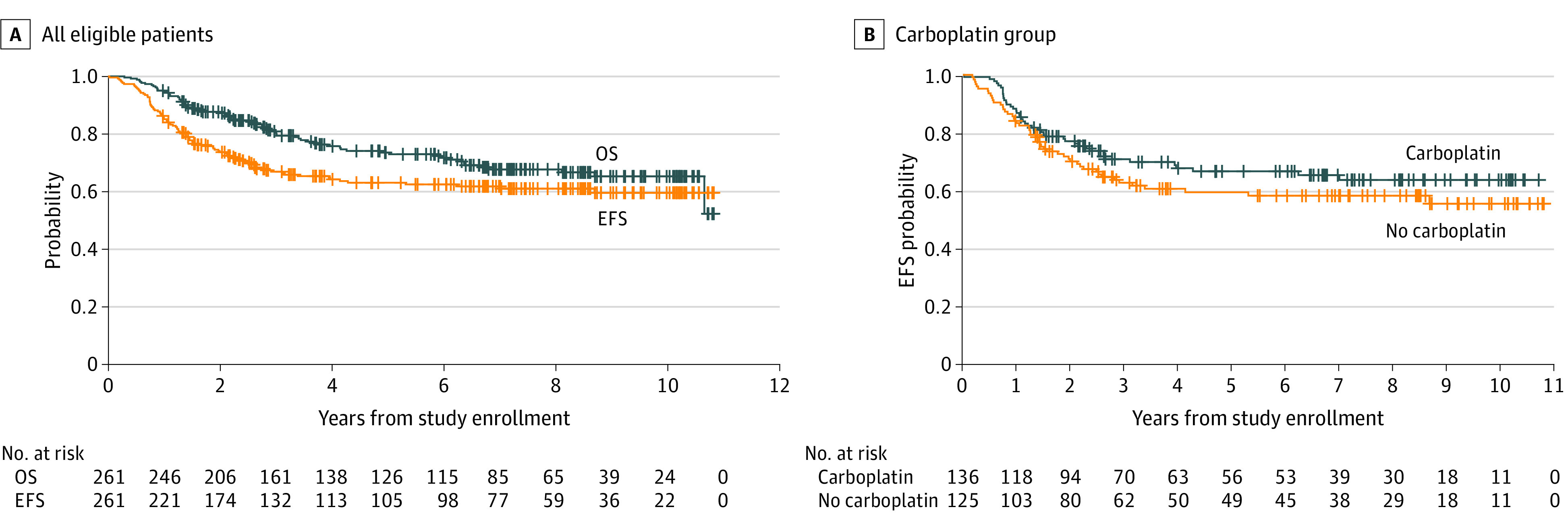
A, Survival for all eligible patients. B, Survival for eligible patients by randomization to carboplatin.
Patient characteristics were similar between the carboplatin randomization arms (Table). Five-year EFS according to carboplatin randomization was 66.4% (95% CI, 56.4%-76.4%) with carboplatin vs 59.2% (95% CI, 48.8%-69.6%) for the control arm (P = .11) (Figure 2B). Five-year OS was 77.6% (95% CI, 69.0%-86.2%) with carboplatin vs 68.8% (95% CI, 59.0%-78.6%) for the control arm (P = .28). Molecular subgroup classification was available for 89% (n = 231) of patients (Figure 3; eTable 3 and eFigure 2 in Supplement 2). Five-year survival by molecular subgroup (eFigure 3 in Supplement 2) was EFS, 92.9% (95% CI, 75.7%-100%) and OS, 100% (95% CI, 100%-100%) for WNT; EFS, 49.6% (95% CI, 27.8%-71.4%) and OS, 53.6% (95% CI, 33.0%-74.2%) for SHH; EFS, 64.2% (95% CI, 51.5%-76.9%) and OS, 73.7% (95% CI, 61.9%-85.5%) for group 3; and EFS, 65.6% (95% CI, 54.6%-76.6%) and OS, 76.9% (95% CI, 67.3%-86.5%) for group 4 (P = .06 for EFS; P = .006 for OS). An effect of carboplatin on outcome was observed exclusively in group 3, with 5-year EFS of 73.2% (95% CI, 56.9%-89.5%) and OS of 82.8% (95% CI, 68.7%-96.9%) with carboplatin compared with EFS, 53.7% (95% CI, 35.3%-72.1%) and OS, 63.7% (95% CI, 46.1%-81.3%) without carboplatin (P = .047 for EFS, P = .06 for OS) (Figure 4).
Table. Patient Characteristics by Carboplatin Randomization.
| Characteristic | No. (%)a | ||
|---|---|---|---|
| All patients (n = 261) | Carboplatin indicator | ||
| No (n = 125) | Yes (n = 136) | ||
| Sex | |||
| Male | 183 (70.1) | 93 (74.4) | 90 (66.2) |
| Female | 78 (29.9) | 32 (25.6) | 46 (33.8) |
| Race | |||
| White | 199 (76.2) | 89 (71.2) | 110 (80.9) |
| Black or African American | 24 (9.2) | 17 (13.6) | 7 (5.1) |
| Asian | 7 (2.7) | 2 (1.6) | 5 (3.7) |
| American Indian or Alaska Native | 2 (0.8) | 1 (0.8) | 1 (0.7) |
| Multiple races | 1 (0.4) | 1 (0.8) | 0 |
| Not reported | 3 (1.1) | 3 (2.4) | 0 |
| Unknown | 25 (9.6) | 12 (9.6) | 13 (9.6) |
| Age at enrollment, y | |||
| Median | 8.6 | 8.4 | 8.8 |
| Range | 3.3-21.2 | 3.3-20.1 | 3.4-21.2 |
| Stratum | |||
| M+ medulloblastoma | 189 (72.4) | 92 (73.6) | 97 (71.3) |
| M0 diffusely anaplastic medulloblastoma with <1.5 cm2 residual | 58 (22.2) | 26 (20.8) | 32 (23.5) |
| M0 medulloblastoma with >1.5 cm2 residual | 14 (5.4) | 7 (5.6) | 7 (5.1) |
| M category | |||
| M0 | 72 (27.6) | 33 (26.4) | 39 (28.7) |
| M1 | 33 (12.6) | 14 (11.2) | 19 (14.0) |
| M2 | 41 (15.7) | 23 (18.4) | 18 (13.2) |
| M3 | 115 (44.1) | 55 (44.0) | 60 (44.1) |
| Molecular subgroup | |||
| Not analyzed | 25 (9.6) | 14 (11.2) | 11 (8.1) |
| MB, G3 | 79 (30.3) | 36 (28.8) | 43 (31.6) |
| MB, G4 | 101 (38.7) | 48 (38.4) | 53 (39.0) |
| MB, SHH | 37 (14.2) | 18 (14.4) | 19 (14.0) |
| MB, WNT | 14 (5.4) | 5 (4.0) | 9 (6.6) |
| Inconclusive | 4 (1.5) | 3 (2.4) | 1 (0.7) |
| Pineoblastoma | 1 (0.4) | 1 (0.8) | 0 |
Percentages may not sum to 100 owing to rounding.
Figure 3. Molecular Classification of Medulloblastoma (MB).
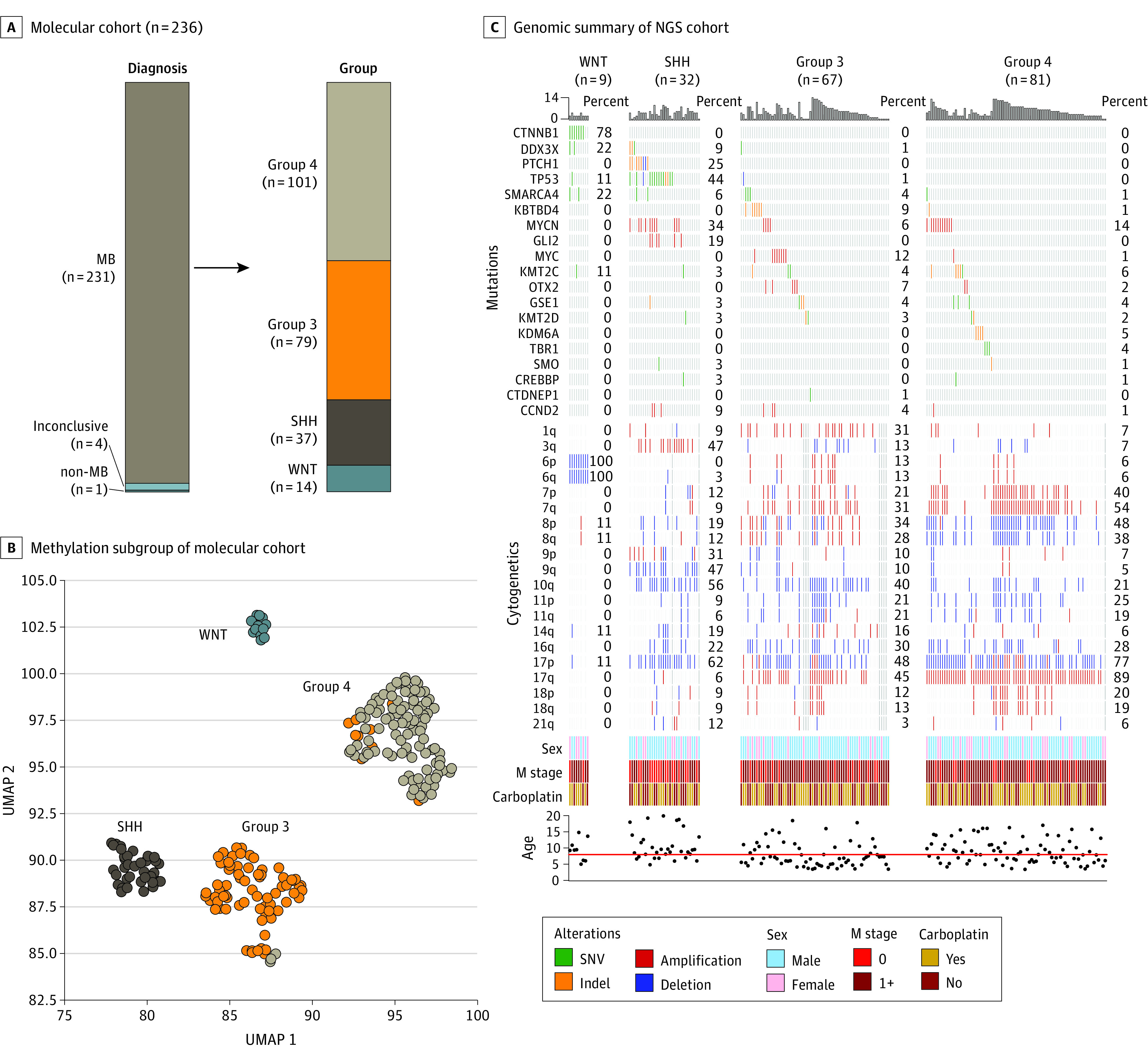
A, Molecular classification of patients with MB. A single patient clinically and histologically classified as having MB was found to have molecular subgrouping consistent with pineoblastoma. B, Uniform manifold approximation and projection (UMAP) plot depicting molecular classification of evaluated MB tumors (n = 231) according to subgroup. C, Heatmap (OncoPrint) summary of recurrently altered genes, chromosomal alteration, clinical characteristics, and randomization of patients with available whole-exome sequencing data (n = 189). NGS, next-generation sequencing; SHH, medulloblastoma, SHH pathway activated; SNV, single-nucleotide variant; WNT, medulloblastoma, WNT pathway activated.
Figure 4. Event-Free Survival (EFS) of 231 Patients With Medulloblastoma Receiving vs Not Receiving Carboplatin.
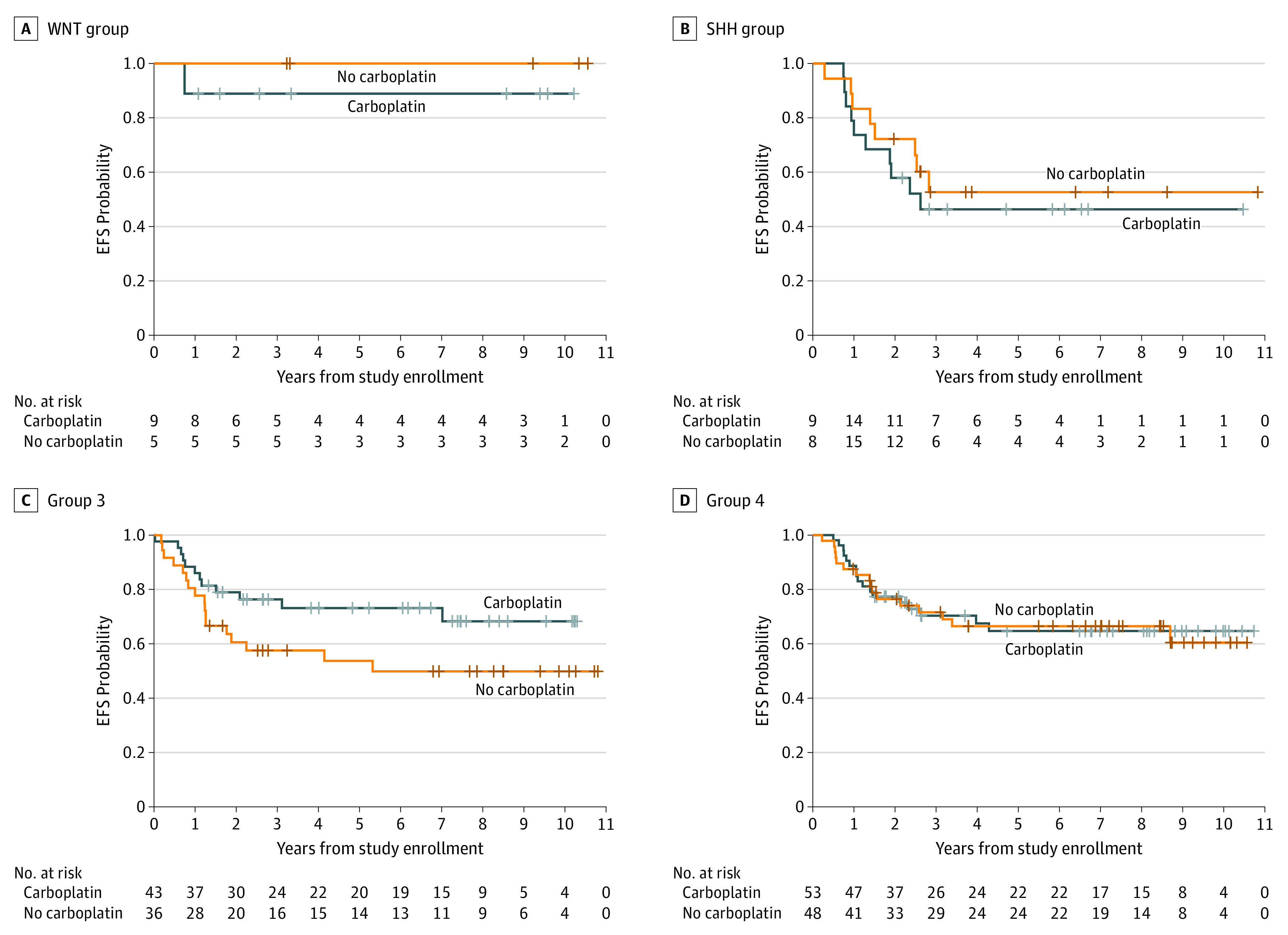
Classification of subgroups WNT pathway activated, log-rank P = .50 (A); SHH pathway activated, log-rank P = .28; (B); group 3, log-rank P = .047; (C); and group 4, log-rank P = .45 (D).
Considering both metastatic status and molecular subgroup, 20 group 3 patients with M0 stage (19 with anaplasia, 1 with residual) had 5-year EFS of 95.0% (95% CI, 84.2%-100%) and OS of 100% (95% CI, 100%-100%) compared with EFS, 53.6% (95% CI, 37.9%-69.3%) and OS, 64.9% (95% CI, 50.2%-79.6%) for those with metastatic disease (P = .002 for EFS; P = .003 for OS). For 19 M0 group 4 patients, 5-year EFS was 84.2% (95% CI, 66.0%-100%) and OS was 83.5% (95% CI, 65.1%-100%) compared with 5-year EFS of 60.9% (95% CI, 48.4%-73.4%) and OS of 75.0% (95% CI, 63.8%-86.2%) (P = .08 for EFS; P = .31 for OS). No difference associated with stage was observed in SHH or WNT subgroups. The effect of carboplatin was maintained when considering the 59 patients with metastatic group 3; 5-year EFS was 64.8% (95% CI, 43.8%-85.8%) and OS was 77.4% (95% CI, 58.8%-96.0%) with carboplatin compared with EFS, 40.3% (95% CI, 19.9%-60.7%) and OS, 51.2% (95% CI, 31.0%-71.4%) without carboplatin (P = .045 for EFS; P = .046 for OS) (eFigure 3 in Supplement 2).
Analysis of other previously described molecular risk factors found MYC amplification or isochromosome 17 to be associated with inferior survival in group 3 (5-year EFS: 49.3% [95% CI, 29.5%-69.1%] for either vs 73.6% [95% CI, 56.9%-90.3%] for no MYC amp or isochromosome 17; P = .01; 5-year OS: 62.8% [95% CI, 42.0%-83.6%] for either vs 83.4% [95% CI, 69.9%-96.9%] for no MYC amp or isochromosome 17; P = .03). Chromosome 11 loss or chromosome 17 gain was associated with superior survival in group 4 (5-year EFS: 91.7% [95% CI, 78.8%-100%] for either vs 56.5% [95% CI, 43.4%-69.6%] for neither; P = .003; 5-year OS: 100% [95% CI, 100%-100%] for either vs 69.2% [95% CI, 57.2%-81.2%] for neither; P = .001). TP53 mutation was observed in 14 of 32 (44%) of patients with SHH, but no association with outcome was observed among this cohort of high-risk patients (5-year EFS: 35.7% [95% CI, 7.7%-63.7%] for TP53 mutation vs 52.5% [95% CI, 20.7%-84.3%] for no TP53 mutation; P = .49; 5-year OS: 43.3% [95% CI, 14.7%-71.9%; for TP53 mutation vs 56.3% [95% CI, 26.5%-86.1%] for no TP53 mutation; P = .77).
Hematologic toxic effects were greater in the carboplatin arm during the induction phase of therapy, and an increased risk of thrombocytopenia and febrile neutropenia appeared to persist into the first cycles of maintenance therapy. With the exception of febrile neutropenia, there was no difference observed in the rate of high-grade toxicity reported in the latter cycles of maintenance therapy according to carboplatin randomization. High-grade toxicity (grade ≥3) occurring in more than 5% of the participants is summarized in eTable 4 in Supplement 2. Ototoxicity was not observed to be different according to carboplatin randomization, with cumulative toxicity by the later cycles of maintenance therapy of 9.2% in the carboplatin arm, compared with 11.1% in the control arm. Grade 3 anaphylaxis to carboplatin was reported in 4 patients in the carboplatin arm.
Neurocognitive assessments (n = 215) were obtained from 116 participants, 97 at time point 1 (T1), 71 at T2, and 47 at T3; of these patients, 66 (56.9%) were randomized to carboplatin. At T1, mean (SD) estimated group IQ scores (91.6 [18.1]) and parent ratings of global executive functioning (50.3 [11.4]) were within the average range, although processing speed scores were much weaker, averaging more than a full SD below the mean (78.0 [15.5]). There were no statistically significant differences in change over time in estimated IQ (T1-T2 estimate, −0.34; P = .93; T1-T3 estimate, 0.85; P = .89; T2-T3 estimate,0.19; P = .95) or processing speed (T1-T2 estimate, −2.97; P = .48; T1-T3 estimate, 1.75; P = .68; T2-T3 estimate, 1.43; P = .67). However, global executive functioning worsened over time, from T2 to T3 specifically (BRIEF global executive functioning estimate, 8.06; P = .03) after adjusting for age at diagnosis, sex, and method of payment. Considering the comparison of neurocognitive outcomes by carboplatin randomization, no detrimental effect of carboplatin was observed (eTable 5 in Supplement 2).
Discussion
In this prospective, phase 3 randomized clinical trial, we did not observe a sufficient benefit to recommend either carboplatin or isotretinoin for all children with high-risk medulloblastoma. Although the study was initially powered to evaluate medulloblastoma as a single disease, biologic insights have led to a contemporary view of medulloblastoma as 4 molecularly distinct subgroups that may determine response to therapy and prognosis.17,18 This study was therefore amended to include a critical planned molecular subgroup analysis 4 years before study completion and before release of any data to the study team, which enabled us to identify a subgroup of patients who benefit from intensified therapy. We observed 19% higher survival exclusively in group 3 medulloblastoma patients who were randomized to receive intensified chemoradiotherapy with concurrent carboplatin, and 25% higher survival for metastatic group 3 medulloblastoma patients. However, improved survival comes at a cost of increased toxic effects. It is therefore appropriate to avoid therapy intensification in groups of patients who would not be expected to benefit, specifically in those with WNT, SHH, and group 4 medulloblastoma.
This study also identified clinically designated high-risk patient populations with near 100% survival receiving this regimen who may be appropriate candidates for therapy reduction, including the WNT subgroup with metastatic disease, group 4 subgroup with chromosome 11 loss and/or chromosome 17 gain, and group 3 subgroup with localized (stage M0) diffusely anaplastic disease. However, this excellent survival is in the context of intense cytotoxic radiotherapy and chemotherapy. A good prognosis molecular subtype may not necessarily maintain an excellent outcome in the context of therapy reduction, as dose-reduction studies have demonstrated in medulloblastoma.25,26 Therapy reduction is therefore best undertaken in the context of a clinical trial, and consideration may be given to trials of therapy substitution with novel noncytotoxic agents rather than therapy reduction alone.
These results emphasize the importance of an integrated molecular diagnosis of medulloblastoma beyond what is described by the 2016 revision to the World Health Organization classification.19 Although immunohistochemistry panels27 and targeted sequencing28 may differentiate WNT and SHH subgroups, additional molecular profiling tests, such as nanoString29 or DNA methylation platforms,21 are needed to confidently distinguish group 3 from group 4 medulloblastoma. Although widely used as a research tool,30 there is a need to increase the clinically certified laboratories offering medulloblastoma subtyping.
Limitations
The study has limitations. Although this is one of the largest prospective clinical trials of high-risk medulloblastoma, the ability to conduct multiple subset analyses remains limited. Randomization was not stratified by molecular group and the balance between arms was by chance. It will be challenging to power future trials to evaluate medulloblastoma therapy by molecular subgroup, necessitating prospective molecular diagnosis and even broader international collaboration. At the time of this study design, few preclinical models of medulloblastoma were available; future clinical trials should benefit from subgroup-specific preclinical data using improved disease models.31 Only 5% of participants enrolled with incomplete surgical resection as an exclusive high-risk feature, and we cannot make confident conclusions about the effect of incomplete resection on survival compared with other risk factors in this small cohort. Surgical resection remains the only potentially modifiable factor for risk stratification. Considering the toxic effects of high-risk therapy, second-look surgery should be considered if there is a possibility that gross total resection may be achieved.
Prospective central radiologic and pathologic review will be incorporated into the next generation of medulloblastoma studies. The importance of prospective central imaging review for proper staging has been well demonstrated for standard-risk medulloblastoma, with central review revealing 10% of patients with missed metastatic disease and more with technically inadequate imaging who had inferior survival.2 This study additionally demonstrates that standard-risk patients may be inappropriately assigned to high-risk treatment without prospective central radiologic review.
We observed discrepancy between local institutional pathologic diagnosis and retrospective central pathologic review in 5% of the patients in this study. It is unfortunate that several of the participants enrolled for anaplasia did not have anaplastic medulloblastoma based on central pathologic review, which may have resulted in unnecessarily toxic treatment in patients who would have had an excellent prognosis with standard-risk therapy.18 Given the subjective nature of diffuse anaplasia and the toxic effects associated with 36-Gy craniospinal radiotherapy, we recommend against using anaplasia as an independent high-risk indicator in medulloblastoma when molecular subgrouping is available.
Neurocognitive outcome data were limited to a small subset of patients, and results should be interpreted with caution, particularly for the latter time point. Consistent with earlier studies32 including 36-Gy craniospinal radiotherapy, we observed below-average cognitive functioning across the entire cohort regardless of the addition of carboplatin, particularly for processing speed, for which performance was well below expected levels at the first point 6 to 12 months post diagnosis and remained poor. We did not observe any significant difference in neurocognitive toxic effects associated with randomization to carboplatin.
Conclusions
Based on the results of this prospective randomized clinical trial, concurrent carboplatin treatment during radiotherapy is recommended for pediatric patients with high-risk group 3 medulloblastoma and not recommended in other populations. A rapid, comprehensive integrated molecular diagnosis is necessary for medulloblastoma risk stratification. The toxic effects of standard therapy for medulloblastoma remain significant, particularly neurocognitive toxic effects in children. In addition to improved risk stratification, the development of novel therapy for high-risk medulloblastoma remains a priority to improve the quality of survival.
Trial Protocol
eMethods. Detailed Methods
eTable 1. Randomization Assignment to All Arms, Including Prior to and Following Closure of Isotretinoin Randomization Owing to Futility
eTable 2. Patient Characteristics by Carboplatin Randomization (n = 285 Eligible Before Retrospective Central Review)
eTable 3. Comparison of Subjects Included in Molecular Analyses vs Subjects Not Included in Molecular Analyses
eTable 4. Grade 3+ Toxicity According to Treatment Phase and Carboplatin Assignment
eTable 5. Neurocognitive Outcomes Across Three Timepoints and According to Carboplatin Randomization
eFigure 1. Event-free Survival According to Isotretinoin Randomization
eFigure 2. UMAP Plot and Molecular Classification
eFigure 3. EFS for All Patients and OS in Metastatic Group 3 Patients
Data Sharing Statement
References
- 1.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17(suppl 4):iv1-iv62. doi: 10.1093/neuonc/nov189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202-4208. doi: 10.1200/JCO.2006.06.4980 [DOI] [PubMed] [Google Scholar]
- 3.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813-820. doi: 10.1016/S1470-2045(06)70867-1 [DOI] [PubMed] [Google Scholar]
- 4.Jakacki RI, Burger PC, Zhou T, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children’s Oncology Group phase I/II study. J Clin Oncol. 2012;30(21):2648-2653. doi: 10.1200/JCO.2011.40.2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koschmann C, Bloom K, Upadhyaya S, Geyer JR, Leary SE. Survival after relapse of medulloblastoma. J Pediatr Hematol Oncol. 2016;38(4):269-273. doi: 10.1097/MPH.0000000000000547 [DOI] [PubMed] [Google Scholar]
- 6.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17(3):832-845. doi: 10.1200/JCO.1999.17.3.832 [DOI] [PubMed] [Google Scholar]
- 7.Douple EB, Richmond RC, O’Hara JA, Coughlin CT. Carboplatin as a potentiator of radiation therapy. Cancer Treat Rev. 1985;12(suppl A):111-124. doi: 10.1016/0305-7372(85)90026-X [DOI] [PubMed] [Google Scholar]
- 8.Kortmann RD, Kühl J, Timmermann B, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT ’91. Int J Radiat Oncol Biol Phys. 2000;46(2):269-279. doi: 10.1016/S0360-3016(99)00369-7 [DOI] [PubMed] [Google Scholar]
- 9.Matthay KK, Villablanca JG, Seeger RC, et al. ; Children’s Cancer Group . Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341(16):1165-1173. doi: 10.1056/NEJM199910143411601 [DOI] [PubMed] [Google Scholar]
- 10.Hallahan AR, Pritchard JI, Chandraratna RA, et al. BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med. 2003;9(8):1033-1038. doi: 10.1038/nm904 [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Guo L, Luo Y, Li JW, Li H. All trans-retinoic acid suppresses in vitro growth and down-regulates LIF gene expression as well as telomerase activity of human medulloblastoma cells. Anticancer Res. 2000;20(4):2659-2664. [PubMed] [Google Scholar]
- 12.Shalinsky DR, Bischoff ED, Gregory ML, et al. Enhanced antitumor efficacy of cisplatin in combination with ALRT1057 (9-cis retinoic acid) in human oral squamous carcinoma in nude mice. Clin Cancer Res. 1996;2(3):511-520. [PubMed] [Google Scholar]
- 13.Aebi S, Kröning R, Cenni B, et al. All-trans retinoic acid enhances cisplatin-induced apoptosis in human ovarian adenocarcinoma and in squamous head and neck cancer cells. Clin Cancer Res. 1997;3(11):2033-2038. [PubMed] [Google Scholar]
- 14.Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24(12):1924-1931. doi: 10.1200/JCO.2005.04.4974 [DOI] [PubMed] [Google Scholar]
- 15.Kool M, Koster J, Bunt J, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3(8):e3088. doi: 10.1371/journal.pone.0003088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho YJ, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424-1430. doi: 10.1200/JCO.2010.28.5148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408-1414. doi: 10.1200/JCO.2009.27.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465-472. doi: 10.1007/s00401-011-0922-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820. doi: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 20.Hwang EI, Kool M, Burger PC, et al. Extensive molecular and clinical heterogeneity in patients with histologically diagnosed CNS-PNET treated as a single entity: a report from the Children’s Oncology Group randomized ACNS0332 trial. J Clin Oncol. 2018;JCO2017764720. doi: 10.1200/JCO.2017.76.4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469-474. doi: 10.1038/nature26000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547(7663):311-317. doi: 10.1038/nature22973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Embry L, Annett RD, Kunin-Batson A, et al. Implementation of multi-site neurocognitive assessments within a pediatric cooperative group: can it be done? Pediatr Blood Cancer. 2012;59(3):536-539. doi: 10.1002/pbc.24139 [DOI] [PubMed] [Google Scholar]
- 24.Walsh KS, Noll RB, Annett RD, Patel SK, Patenaude AF, Embry L. Standard of care for neuropsychological monitoring in pediatric neuro-oncology: lessons from the Children’s Oncology Group (COG). Pediatr Blood Cancer. 2016;63(2):191-195. doi: 10.1002/pbc.25759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson GW, Rudneva VA, Buchhalter I, et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol. 2018;19(6):768-784. doi: 10.1016/S1470-2045(18)30204-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafay-Cousin L, Bouffet E, Strother D, et al. Phase II study of nonmetastatic desmoplastic medulloblastoma in children younger than 4 years of age: a report of the Children’s Oncology Group (ACNS1221). J Clin Oncol. 2020;38(3):223-231. doi: 10.1200/JCO.19.00845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellison DW, Dalton J, Kocak M, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121(3):381-396. doi: 10.1007/s00401-011-0800-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramkissoon SH, Bandopadhayay P, Hwang J, et al. Clinical targeted exome-based sequencing in combination with genome-wide copy number profiling: precision medicine analysis of 203 pediatric brain tumors. Neuro Oncol. 2017;19(7):986-996. doi: 10.1093/neuonc/now294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Northcott PA, Shih DJ, Remke M, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615-626. doi: 10.1007/s00401-011-0899-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar R, Liu APY, Orr BA, Northcott PA, Robinson GW. Advances in the classification of pediatric brain tumors through DNA methylation profiling: From research tool to frontline diagnostic. Cancer. 2018;124(21):4168-4180. doi: 10.1002/cncr.31583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brabetz S, Leary SES, Gröbner SN, et al. A biobank of patient-derived pediatric brain tumor models. Nat Med. 2018;24(11):1752-1761. doi: 10.1038/s41591-018-0207-3 [DOI] [PubMed] [Google Scholar]
- 32.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511-5519. doi: 10.1200/JCO.2005.00.703 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Detailed Methods
eTable 1. Randomization Assignment to All Arms, Including Prior to and Following Closure of Isotretinoin Randomization Owing to Futility
eTable 2. Patient Characteristics by Carboplatin Randomization (n = 285 Eligible Before Retrospective Central Review)
eTable 3. Comparison of Subjects Included in Molecular Analyses vs Subjects Not Included in Molecular Analyses
eTable 4. Grade 3+ Toxicity According to Treatment Phase and Carboplatin Assignment
eTable 5. Neurocognitive Outcomes Across Three Timepoints and According to Carboplatin Randomization
eFigure 1. Event-free Survival According to Isotretinoin Randomization
eFigure 2. UMAP Plot and Molecular Classification
eFigure 3. EFS for All Patients and OS in Metastatic Group 3 Patients
Data Sharing Statement