Abstract
While immunotherapy with nivolumab is promising for patients with renal cell carcinoma (RCC), overactivation of the immune system can lead to serious side effects. Immune-related meningoencephalitis without a viral or microbial etiology is a rare complication that may occur in patients treated with checkpoint inhibitors (CPI). Herein, we report a 66-year-old man who underwent a partial nephrectomy which revealed a papillary RCC with clear cell component. Three years later, an abdomen and pelvic CT revealed metastatic lesions in the left psoas muscle and in the left 12th rib. The patient was treated with pazopanib which was discontinued after 2 weeks due to significant hepatic and renal toxicity. He subsequently started sunitinib. Two months later, a chest, abdomen, and pelvic CT demonstrated progressive metastatic RCC in the retroperitoneal mass of the left psoas muscle and paraspinal musculature as well as a left renal mass. The patient was treated with 7 cycles of the CPI nivolumab. He was subsequently hospitalized for 3 weeks after experiencing bilateral lower extremity weakness, lethargy, several falls, hyperthermia, confusion, and gait abnormalities. A CSF analysis demonstrated a lymphocyte pleocytosis with elevated protein and no bacterial or viral growth. The patient was treated with high-dose steroids after which his symptoms resolved. Chest, abdomen, and pelvic CT scans over the next 3 years revealed no evidence of metastatic disease, reflecting a progression-free survival of 40 months. We highlight the unique case of a patient with metastatic RCC who experienced immune-related meningoencephalitis following immunotherapy with nivolumab. Medical oncologists should be alert to the potential development of immune-related encephalitis in patients treated with nivolumab and should promptly diagnose and treat this concerning condition. The excellent oncologic outcome of this case emphasizes the need for continued aggressive measures for management of CNS toxicity resulting from CPI therapy.
Keywords: Oncology, Renal cell carcinoma, Immunotherapy, Meningoencephalitis, Meningitis, Nivolumab, Checkpoint inhibitor
Introduction
In 2020, the American Cancer Society projects that 73,750 new cases of renal cell cancer (RCC) will be diagnosed and that 14,830 people will die from this disease [1]. The lifetime risk for developing RCC is 2% for men and 1% for women. Approximately 25–30% of patients present with metastatic RCC at the time of diagnosis which is associated with a high mortality [2]. Nivolumab is a fully human immunoglobulin programmed death 1 (PD-1) immune checkpoint inhibitor (CPI) antibody that selectively blocks the interaction between PD-1 expressed on activated T cells with its ligands PD-L1 and PD-L2 expressed on antigen-presenting cells and cancer cells that result in significant enhancement of T-cell function [2, 3, 4, 5]. Nivolumab has been approved by the US Food and Drug Administration for non-small cell lung cancer, RCC, melanoma, hepatocellular carcinoma, and recurrent or metastatic head and neck cancer [5, 6]. It has demonstrated antitumor activity with improved overall survival and a manageable safety profile for metastatic RCC [5, 7, 8].
The heightened immune response due to nivolumab use may trigger a myriad of immune-related adverse events such as hepatitis, colitis, diabetes mellitus, pericarditis, pneumonitis, arthritis, acute interstitial nephritis, aplastic anemia, and uveitis [3, 9]. Immune-related neurotoxicity affects approximately 1% of patients treated with nivolumab and includes peripheral neuropathy, aseptic meningitis, encephalitis, myasthenia gravis, acute and chronic demyelinating polyradiculoneuropathy, Guillain-Barré syndrome, and multifocal central nervous system demyelination [3, 8, 9, 10, 11]. Immune-related adverse events are due to the inhibition of immune checkpoints that spur local and systemic autoimmune responses and most likely involve neuronal damage by T-cells, autoantibodies, and cytokine-mediated inflammation [4, 12].
We report the exceedingly rare case of a patient with metastatic clear cell RCC (CCRCC) who experienced immune-related meningoencephalitis following treatment with nivolumab. The distinctive characteristics, differential diagnosis, management, and prognosis of immune-related encephalitis are discussed.
Case Description
A 66-year-old African-American man (height: 6' 6” (1.981 m); weight: 258 lbs (117.028 kg); body mass index: 29.82 kg/m2) reported that a mass of the upper pole of the left kidney was incidentally discovered 4 years earlier while undergoing a lumbar MRI. He underwent a retroperitoneal partial nephrectomy which revealed a papillary renal cell carcinoma (RCC) with a clear cell component measuring 6.6 × 5.2 × 3.8 cm. There were no sarcomatoid features, and the histologic grade was Fuhrman nuclear grade 3/4. The tumor was limited to the kidney without vascular invasion. The tumor stage was pT1aNX. The patient underwent surveillance of his RCC with repeat chest, abdomen, and pelvic CT scans. Past medical history was significant for aortic aneurysm, diabetes mellitus, hypercholesterolemia, proteinuria, hypertension, kidney stones, cerebrovascular accident, atrial flutter with a history of ablation, warfarin use, renal failure, and neuropathy. The patient's mother was diagnosed with tuberculosis (TB).
Three years after the partial nephrectomy, an abdomen and pelvic CT revealed evidence of 2 metastatic lesions, with one measuring 4.0 cm in diameter involving the left psoas muscle in the posterior paraspinal musculature and retroperitoneal fat. The 2nd mass measuring 2.3 cm was located along the lateral aspect of the left 12th rib. The patient underwent a left retroperitoneal exploration and excisional biopsy. The pathology confirmed benign fibroadipose tissue with fat necrosis associated with dystrophic calcification of the retroperitoneal mass. The 12th rib mass showed a focus of metastatic clear cell carcinoma involving a 2.5-mm fibrous scar (2.5 mm). Immunostains supported metastatic CCRCC. The patient underwent close surveillance with chest, abdomen, and pelvic CT scans for the next year, all of which demonstrated no metastatic disease.
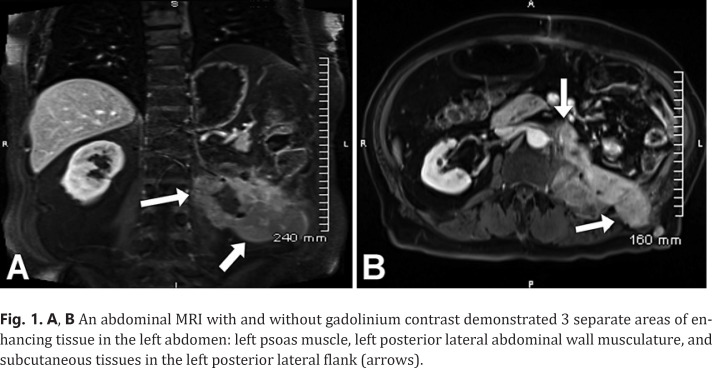
A PET scan 1 year following the excisional biopsy demonstrated activity at the 11–12 intercostal space, left psoas muscle, and quadratus lumberum below the lower pole of the left kidney. A biopsy of the mass confirmed recurrent RCC. The patient initiated the tyrosine kinase inhibitor pazopanib 800 mg which was discontinued after 2 weeks due to gout and elevated levels of bilirubin and creatinine. An abdominal MRI showed metastatic disease involving the left psoas muscle which extended out of the prior left lateral abdominal wall surgical tract. The patient subsequently started the tyrosine kinase inhibitor sunitinib 50 mg. Two months later a chest, abdomen, and pelvic CT revealed a retroperitoneal mass of the left psoas and paraspinal musculature and a left renal mass measuring 2.7 cm in diameter. A left radical nephrectomy and periaortic lymphadenectomy was performed which revealed a recurrent CCRCC measuring 8.0 × 3.0 × 2.6 cm with a Fuhrman grade 2. An abdominal MRI with and without gadolinium contrast demonstrated 3 separate areas of enhancing tissue in the left abdomen: left psoas muscle, left posterior lateral abdominal wall musculature, and subcutaneous tissues in the left posterior lateral flank (Fig. 1A, B).
Fig. 1.
A, B An abdominal MRI with and without gadolinium contrast demonstrated 3 separate areas of enhancing tissue in the left abdomen: left psoas muscle, left posterior lateral abdominal wall musculature, and subcutaneous tissues in the left posterior lateral flank (arrows).
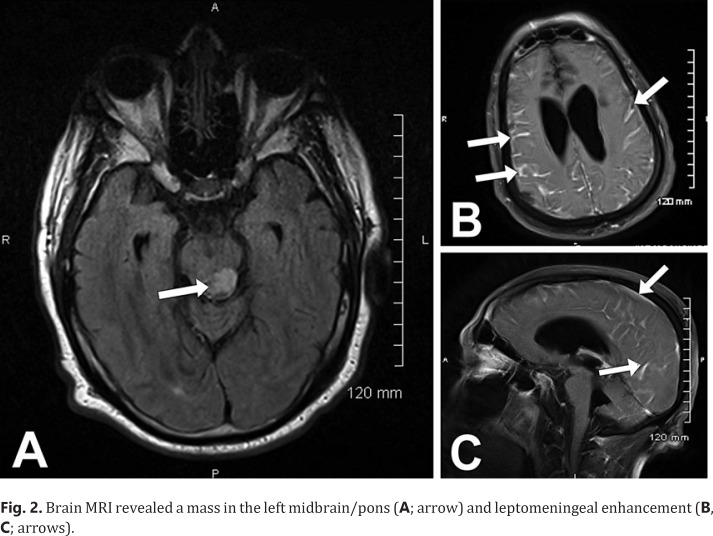
A QuantiFERON TB Gold test was positive, indicating the likelihood of Mycobacterium tuberculosis (MTB) infection due to the patient's exposure to TB 30 years earlier. Therefore, he was unable to participate in an immunotherapy trial. The patient tolerated 7 cycles of nivolumab 240 mg without any complaints. He was subsequently hospitalized for 3 weeks after experiencing bilateral lower extremity weakness, lethargy, several falls, hyperthermia, confusion, and gait abnormalities. Upon admission, he developed hypothermia and was admitted to the intensive care unit (ICU) and was intubated. A CSF analysis demonstrated a lymphocyte pleocytosis (white blood cell count 27, protein 70, lymphs 78%). A CSF virus culture and gram stain demonstrated no growth, the CSF pathogen panel was negative, and the cryptococcal antigen was negative. The blood culture demonstrated no growth, and there was no acid-fast bacilli by fluorochrome. A brain MRI revealed evidence of diffuse leptomeningeal enhancement as well as a 1.7 × 1.0 × 1.0 cm area of focal decreased T1 signal intensity involving the junction between the midbrain and pons on the left posteriorly, although the latter finding had been present for 4 years without any alterations (Fig. 2A–C). The MTB polymerase chain reaction (PCR) test was negative. The patient was treated with prednisone 90 mg for 6 days followed by a tapering dose. He also received isoniazide 300 mg and pyridoxine 50 mg for a latent TB infection as well as levetiracetam, since it was thought that his hyperthermia may have been due to a diencephalic seizure.
Fig. 2.
Brain MRI revealed a mass in the left midbrain/pons (A; arrow) and leptomeningeal enhancement (B, C; arrows).
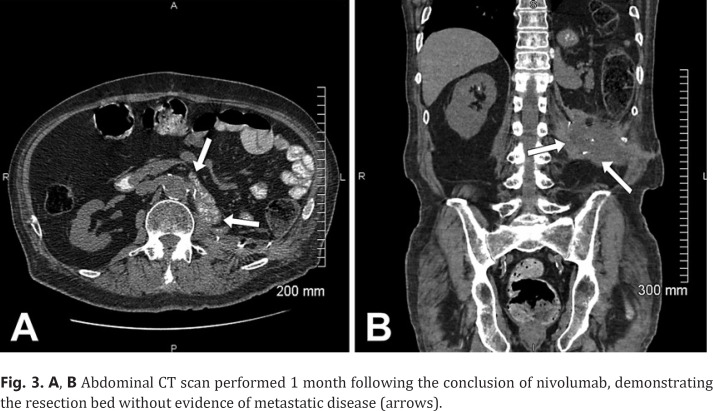
The patient's symptoms of immune-related meningoencephalitis resolved within 2 weeks of his hospitalization. He was successfully extubated after 14 days on a ventilator. He recovered fully after a short course of acute rehab admission. He did not receive any additional treatment with nivolumab or any other agent for his metastatic CCRCC. Chest, abdomen, and pelvic CT scans over the next 3 years revealed no evidence of metastatic disease, indicating a progression-free survival of 40 months (Fig. 3A, B).
Fig. 3.
A, B Abdominal CT scan performed 1 month following the conclusion of nivolumab, demonstrating the resection bed without evidence of metastatic disease (arrows).
Discussion
A thorough investigation is warranted in a patient who is treated with nivolumab and develops neurological adverse effects. Patients who present with confusion or delirium, headaches, altered behavior, short-term memory loss, speech abnormalities, fatigue, focal weakness, decreased level of consciousness, hallucinations, aspastic tremors, fever, or vomiting may have evidence of meningoencephalitis associated with nivolumab [3, 9, 10]. The diagnostic evaluation and treatment of immune-related meningoencephalitis is presented in Table 1. Special attention should rule out other conditions that may cause neurological impairment such as disease progression, seizure activity, infection (in particular herpes simplex virus), and metabolic alterations [3, 9]. In most cases, the immunotherapy must be permanently discontinued. Treatment for immune-related meningoencephalitis involves hospitalization with high-dose corticosteroids as well as intravenous immunoglobulins and plasmapheresis in severe cases [9, 12, 13]. Immune CPI neurotoxicity generally occurs within the first 6 weeks of treatment [6, 13] but theoretically can happen at any point during the treatment.
Table 1.
Diagnostic evaluation for immune-related meningoencephalitis associated with nivolumab
| Symptoms − Confusion or delirium, headaches, altered behavior, short-term memory loss, speech abnormalities, fatigue, focal weakness, seizures, decreased level of consciousness, hallucinations, aspastic tremors, fever, or vomiting |
| Brain MRI with and without gadolinium contrast − Diffuse dural enhancement without parenchymal abnormalities − Abnormal leptomeningeal enhancement − Rule out stroke/ischemia and brain metastasis |
| CSF analysis − Lymphocytic pleocytosis, normal glucose, increased protein level |
| EEG − Diffuse non-specific slowing − Rule out seizure activity |
| Nerve conduction studies/electromyography − Confirms sensory or weakness symptoms |
| Toxicity screen |
| Blood and CSF paraneoplastic panel (anti-N-methyl-d-aspartate receptor and anti-Ma2 antibodies) |
| Thyroid panel |
| Complete blood chemistry with differential panel |
| Neurology consult Treatment − Empirical broad-spectrum antibiotics and antiviral therapy until microbiological findings confirmed − High-dose corticosteroids (methylprednisolone, prednisone, dexamethasone) − Intravenous immunoglobulins and plasmapheresis in severe cases |
Differentiating between aseptic meningitis and immune-related encephalitis or meningoencephalitis following nivolumab necessitates close scrutiny. These conditions often reveal a lymphocyte pleocytosis, elevated protein, and negative bacterial and viral infection by CSF analysis, abnormal leptomeningeal enhancement on brain MRI, and improvement with high-dose corticosteroids [9]. Patients with these conditions may present with headaches, vomiting, and fever; however, patients with aseptic meningitis often have a normal mental status unlike immune-related encephalitis. Drug-induced meningitis should also be considered in the differential diagnosis, as is reported with certain antibiotics, IVIG, refocoxib, and infliximab. Considering that our patient was not on any of these drugs, this diagnosis may be ruled out.
The patient in the present case had metastatic CCRCC and had been treated with nivolumab for 7 cycles prior to the onset of bilateral lower extremity weakness, lethargy, several falls, hyperthermia, confusion, and gait abnormalities. Due to the patient's positive QuantiFERON TB Gold test, there was consideration that the patient had a suspected latent TB infection.
A total of 1.5 million people died from TB in 2018, and multidrug-resistant TB is a significant issue worldwide [14]. Patients with a positive QuantiFERON TB Gold test are generally excluded from enrollment in clinical trials involving treatment with CPI. This is largely due to the potential risk of TB reactivation. Moreover, in case of immune-related side effects and need for high-dose steroids, there is a concern for reactivation of TB. Additionally, metastatic kidney cancer has a poor prognosis especially after treatment failure with tyrosine kinase inhibitors. In the current case, treatment with nivolumab led to an excellent outcome with durable complete response. Ongoing trials are evaluating the efficacy and safety of nivolumab in patients with metastatic cancer and significant autoimmune diseases. This patient population was excluded from the initial trial with CPIs. Similar trials in patients with positive QuantiFERON TB Gold test may be helpful in assessing the safety and efficacy of CPIs and providing guidance in using antitubercular agents in this setting.
In our case, the CSF culture and the MTB PCR test were negative, and there were no findings consistent with TB on chest X-ray. Additionally, the left midbrain/pons lesion was unlikely to be a tuberculoma as the mass had been present for 4 years without changes and remained unchanged for the next several years after receiving treatment for latent TB. He received isoniazide and pyridoxine for the suspected latent TB infection as he was at risk for TB reactivation on a high-dose steroid. He started levetiracetam, as the patient's hyperthermia may have been due to a diencephalic seizure.
While aseptic meningitis and tuberculous meningitis were in the differential diagnosis, the patient's presenting symptoms including an altered mental status, the CSF findings of a lymphocyte pleocytosis, elevated protein, and negative bacterial and viral infection, his 3-week hospital stay including his need for ICU admission and ventilator use, as well as leptomeningeal enhancement on brain MRI strongly supported the diagnosis of immune-related meningoencephalitis. This diagnosis was further substantiated by clinical improvement with immunosuppression and lack of empiric antibiotic therapy for meningoencephalitis. He attained complete resolution of his immune-related encephalitis within 2 weeks following discontinuation of nivolimab and a course of high-dose corticosteroids. Furthermore, he did not have any evidence of disease progression for the subsequent 40 months with respect to his metastatic CCRCC. Ruling out the other possibilities in the differential diagnosis, the most probable diagnosis of the present case is drug-induced autoimmune meningoencephalitis caused by nivolumab.
Conclusion
Immune-related meningoencephalitis is a rare phenomenon following treatment with nivolumab for metastatic RCC. The American Society of Clinical Oncology strongly recommends that patients are educated about the adverse effects that may arise with immunotherapy [9]. Due to the morbidity and potential mortality that may be associated with nivolumab, a high index of suspicion is warranted for immune-related neurotoxicities. Early diagnosis and prompt treatment are necessary to curtail the devastating sequelae that may ensue. Communication between different medical specialties including medical oncologists, neurologists, infectious disease specialists, and primary care physicians is of utmost importance in minimizing the risk of immune-related meningoencephalitis.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. The University of Louisville Institutional Review Board has determined that our project does not meet the “Common Rule” definition of human subjects' research and does not require IRB review. The Institutional Review Board number is 20.0710.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
None.
Author Contributions
Lisa B.E. Shields: Data conception, design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript and gave final approval. Mohammad S. Alsorogi: Data conception, design, acquisition, analysis, and interpretation; critically revised the manuscript and gave final approval. Nataliya Mar: Data conception, design, acquisition, analysis, and interpretation; critically revised the manuscript and gave final approval. Arash Rezazadeh Kalebasty: Data conception, design, acquisition, analysis, and interpretation; critically revised the manuscript and gave final approval.
Acknowledgment
We acknowledge Norton Healthcare for their continued support.
References
- 1.American Cancer Society. Key statistics for lung cancer. Accessed October 29, 2020. https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html.
- 2.Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol. 2017;35:JCO2016721985–8. doi: 10.1200/JCO.2016.72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baraibar I, Melero I, Ponz-Sarvise M, Castanon E. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf. 2019;42((2)):281–94. doi: 10.1007/s40264-018-0774-8. [DOI] [PubMed] [Google Scholar]
- 4.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50((12)):1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Giorgi U, Cartenì G, Giannarelli D, Basso U, Galli L, Cortesi E, et al. Safety and efficacy of nivolumab for metastatic renal cell carcinoma: real-world results from an expanded access programme. BJU Int. 2019;123((1)):98–105. doi: 10.1111/bju.14461. [DOI] [PubMed] [Google Scholar]
- 6.Weber JS, Postow M, Lao CD, Schadendorf D. Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist. 2016;21((10)):1230–40. doi: 10.1634/theoncologist.2016-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33((13)):1430–7. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah S, Dunn-Pirio A, Luedke M, Morgenlander J, Skeen M, Eckstein C. Nivolumab-induced autoimmune encephalitis in two patients with lung adenocarcinoma. Case Rep Neurol Med. 2018;2018:2548528. doi: 10.1155/2018/2548528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36((17)):1714–68. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin J, Chmielowski B, Lao CD, Hodi FS, Sharfman W, Weber J, et al. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist. 2017;22((6)):709–18. doi: 10.1634/theoncologist.2016-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams TJ, Benavides DR, Patrice KA, Dalmau JO, de Ávila AL, Le DT, et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol. 2016;73((8)):928–33. doi: 10.1001/jamaneurol.2016.1399. [DOI] [PubMed] [Google Scholar]
- 12.Kopecky J, Kubecek O, Geryk T, Slovackova B, Hoffmann P, Ziaran M, et al Nivolumab induced encephalopathy in a man with metastatic renal cell cancer: a case report. J Med Case Rep. 2018;12:262. doi: 10.1186/s13256-018-1786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke M, Hardesty M, Downs W. A case of severe encephalitis while on PD-1 immunotherapy for recurrent clear cell ovarian cancer. Gynecol Oncol Rep. 2018;24:51–3. doi: 10.1016/j.gore.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Tuberculosis. https://www.who.int/news-room/fact-sheets/detail/tuberculosis. Accessed October 29, 2020.