Abstract
Background—
Readmission is responsible for a large proportion of inpatient care costs in adult congenital heart disease (ACHD). There are, however, few data available to identify at risk patients or to suggest strategies for intervention to prevent rehospitalization.
Methods and Results—
We conducted an analysis of admissions in patients over age 18 with a 3-digit ICD-9 code of 745–747 from the State Inpatient Databases of Arkansas (2008–2010), California (2003–2012), Florida (2005–2012), Hawaii (2006–2010), Nebraska (2003–2011), and New York (2005–2012). We investigated index admission diagnoses most commonly associated with 1-year readmission and the most common reasons for readmission. We then selected variables we believed would be associated with increased rates of 1-year readmission and constructed multivariable regression models grouping patients by congenital lesion, to examine the relative contribution of the specified variables to readmission risk for each lesion. A total of 64,420 patients were included in the final analysis. Thirty-nine percent of patients experienced a readmission within 12 months of an index admission. Compared to those who did not experience a readmission, those who did were more likely to have had a primary diagnosis of congestive heart failure (CHF) at the time of index admission, and the most common diagnoses at the time of readmission were CHF and arrhythmia. There is lesion-specific heterogeneity in risk factors for readmission.
Conclusions—
ACHD patients have high rates of readmission, predominantly for CHF and arrhythmia. Predictors of readmission are lesion specific, and future strategies aimed at decreasing readmission rate will likely need to be individualized.
Keywords: adult congenital heart disease, rehospitalization, congestive heart failure
Journal Subject Terms: Congenital Heart Disease, Quality and Outcomes
Hospital readmission is an important metric employed for in the evaluation of care quality and efficiency. Readmissions are a popular target for improvements in the systematic provision of care due to the fact that they are costly, and are believed to be largely preventable1–4. Although this belief remains controversial, reduction of readmissions has been widely adopted as the standard for comparing relative quality of care, and is now tied to federal reimbursement and penalties in the United States (US)5.
As with other heart disease, rehospitalization is fundamentally important in the evaluation of care quality in adult congenital heart disease (ACHD). Chronic cardiac diseases like ACHD are responsible for the majority of healthcare spending in the United States (US)6. Recent data have shown that spending for inpatient care in ACHD patients has been expanding rapidly7,8. Given that the recently published suggestions for improving quality of ACHD care included improvements in cost effectiveness and efficiency as outcomes, research is needed to investigate strategies to decrease readmission rate to improve these outcomes9,10. In addition, Gurvitz et al proposed quality of life as an important outcome to be tracked in providing optimal ACHD care. The strong link between quality of life and annual rate of hospitalization further supports the need for research in this area11.
There is very little information available on readmission rates in the ACHD population. In the present study, we sought to address this knowledge gap by investigating the frequency and character of unplanned readmissions among ACHD patients in the US. We also investigated potentially modifiable factors associated with increased risk for rehospitalization with the goal of identifying targets for improvement in care quality.
Methods
For this analysis, State Inpatient Databases (SID) were used which are part of the Healthcare Cost and Utilization Project (HCUP)12. We specifically used the SIDs for Arkansas (2008–2010), California (2003–2012), Florida (2005–2012), Hawaii (2006–2010), Nebraska (2003–2011), and New York (2005–2012). These SIDs were selected because they uniquely track hospitalizations in individual patients longitudinally, whereas data from other states track hospitalizations without tracking patients. The dates used were the most complete and up to date available at the time of analysis in April 2015. The primary outcome was readmission over a 12-month period for individual ACHD patients in the states investigated. The present study was approved by the institutional review board at Washington University School of Medicine.
As a first step, we identified patients in the databases with ACHD by selecting patients in the SIDs with an age of greater than 18, and with a 3-digit ICD-9 diagnosis code of 745, 746, or 747, the administrative codes that identify congenital cardiovascular anomalies. To this group of patients we applied a validated hierarchical algorithm described by Broberg et al to categorize patients based on anatomy13. Any patients who failed to be classified according to this algorithm were excluded to increase the probability that all the patients included for analysis in fact had ACHD. Patients were classified at the time of index hospitalization. We then excluded patients with an index hospitalization within the first or last 12 months of the investigated period, so that we were certain that: the index hospitalization was not a rehospitalization following one to which we were blind; and that a full 12 months of follow-up post index was available for every patient. We finally excluded patients with a readmission that was categorized as “elective” based on HCUP categorization, given that these readmissions were most likely unpreventable. After applying the specified inclusion and exclusion criteria, the final sample consisted of 66,023 patients who had been hospitalized at least once during the study period.
We first investigated differences in the primary admitting diagnosis at the time of index admission between patients who were and those who were not readmitted within 12 months of an index admission. We conducted a chi-squared test comparing each of the five most common admitting diagnoses at index admission and readmission status. All tests were assigned a p-value of ≤ 0.05 to determine significance.
We next investigated the relative frequencies of various admitting diagnoses at the time of readmission in each anatomical group.
We thereafter compared clinical characteristics and demographics between patients who were and were not readmitted within 12 months of an index admission using Student’s two-sample t-test and chi-squared tests for continuous and categorical data, respectively. We used these analyses to facilitate identification of variables for construction of multivariable regression models. After considering the group of variables identified by this method, we further narrowed the list based on clinical experience and literature review. The final list of independent model variables derived in this manner varied depending on underlying cardiac anatomy.
Using these independent variables, we built a series of hierarchical multivariable logistic regression models with readmission within 12 months of index hospitalization as the dependent variable. These models treated site of hospitalization as a random effect to account for inherent differences between hospitals. A different model for each anatomical group was constructed. The sample size of each model varied, due to the varying number of subjects in each diagnosis group. The odds ratios, 95% confidence intervals, and p-values are reported from these models and all significance tests were two-sided with type I error set to 5%, i.e. α = 0.05. Through these models we identified significant predictors of readmission for each anatomical group. As this was intended only as an exploratory analysis, the potential liability of multiple testing was not addressed.
Next, we built a similar series of hierarchical multivariable logistic regression models using the same sets of pre-identified variables with readmission as the dependent variable, but this time converted all independent variables to dichotomous variables. Any variables that were categorical were converted to multiple dichotomous indicators using dummy variables. We did this so that we would be able to rank odds ratios for the independent variables in the models based on their ability to predict readmission. Since our patients were grouped by hospital, we again treated hospital as a random effect in the model. The odds ratios, 95% confidence intervals, and p-values were obtained and ranks were derived. All significance tests were two-sided with type I error set to 5%, i.e. α = 0.05.
All analysis conducted in SAS v9.4 (SAS Institute Inc., Cary, NC).
Results
Population
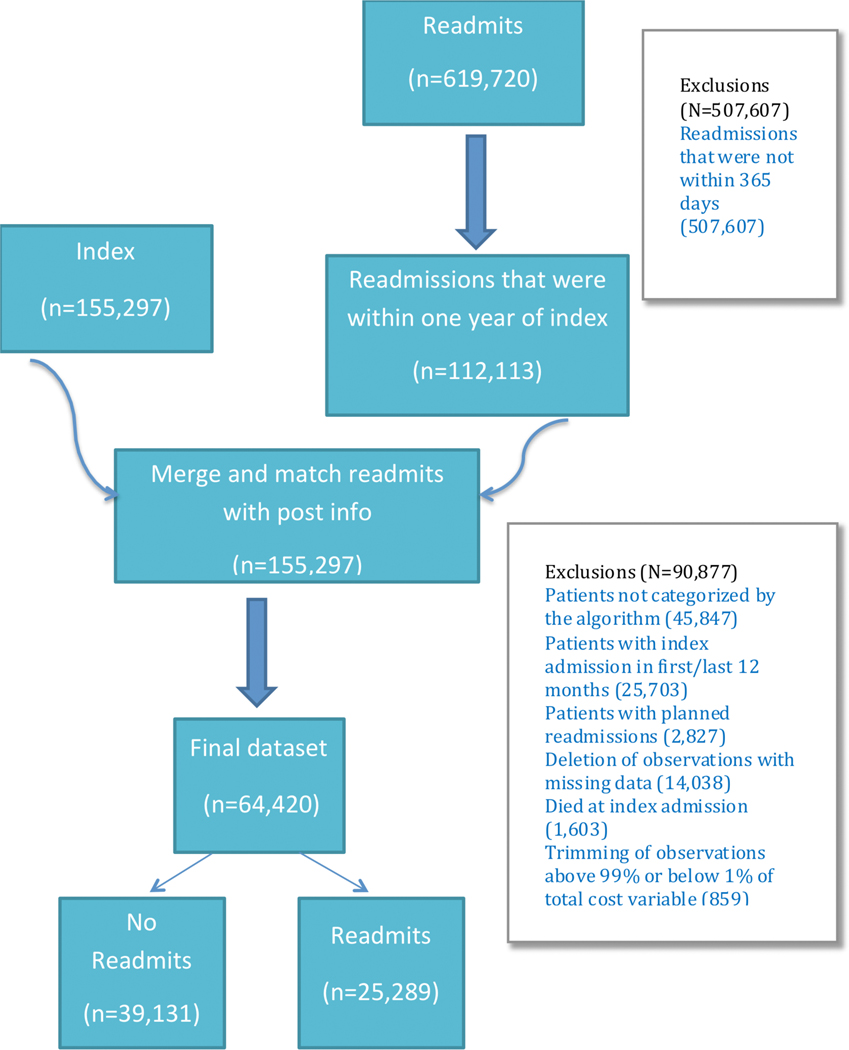
Our initial query of the SIDs resulted in 155,297 index admissions, and 619,720 readmissions among patients over 18 with a 3-digit ICD9 code of 745, 746, or 747. After excluding readmissions which fell outside the 365 day time window after an index admission, this left 112,113 readmission events which were linked to index admissions. After excluding patients who failed to be categorized according to the algorithm described by Broberg et al (n=45,847), eliminating patients with index hospitalizations within the first or last twelve months (n=25,703), patients with planned readmissions (n=2,827), patients for whom there was essential missing data (14,083), patients who died at the time of index admission (1,603), and trimming data to eliminate patients with annual costs above the top 99% or below the lower 1% of the total cost distribution (n=859), this left a final sample size of n=64,420 index admissions as graphically depicted in Figure 1.
Figure 1:
Inclusion and Exclusion Diagram: Graphical representation of the patients included in the present analysis. 155,297 index admissions were found with a total of 619,720 total readmissions. After excluding readmissions that took place longer than 1 year after the index admission, this left 112,113 readmissions which were then linked to their corresponding index admissions. The index admission data were then excluded if patients failed to be categorized by the algorithm to identify ACHD lesion, if a full 12-month follow-up period before and after index admission was not available, if patients died during index admission, if records were missing information required for analysis, and to remove outliers. This resulted in a final tally of 64,420 index admissions which were then used for analysis.
Overall, 39% of patients experienced a readmission within 12 months of an index admission. Patients who were readmitted were slightly older (59.4±18.7 versus 55.3±18.3, p<0.001) and were slightly more likely to be female (52% versus 50%, p<0.001) than those who were not. Readmitted patients also had a longer length of stay at the time of index admission (8.3±20.0 versus 6.3±13.0, p<0.001) and were less likely to have had a major operation performed during their index admission (31% versus 46%, p<0.001). Patients with Eisenmenger syndrome or cyanosis, single ventricle, and conotruncal abnormality were more likely than not to be admitted at least once in the 12 months following an index admission. All other lesions were more likely not to experience a readmission, with those having congenital aortic valve stenosis (AS) or a bicuspid aortic valve (BAV) being the least likely to be readmitted. Complete demographic data is depicted in Table 1.
Table 1.
Demographics.
| Variable | Not readmitted (n=39,131) | Readmitted (25,289) | p-value |
|---|---|---|---|
| Age (years) | 55.3±18.3 | 59.4±18.7 | <0.001 |
| Female gender % | 50 | 52 | <0.001 |
| LOS (days) | 6.3±13.0 | 8.3±20.0 | <0.001 |
| Major operation at index admission % | 46 | 31 | <0.001 |
| Anatomical group n (%) | |||
| Eisenmenger/ cyanotic | 91 (42) | 124 (58) | |
| Single ventricle | 644 (48) | 710 (52) | |
| TGA | 536 (51) | 515 (49) | |
| Conotruncal abnormality | 215 (44) | 277 (56) | |
| AVSD | 274 (56) | 217 (44) | |
| Ebstein’s | 254 (56) | 197 (44) | |
| Pulmonary valve stenosis | 876 (59) | 617 (41) | |
| Anomalous pulmonary venous return | 24 (56) | 19 (44) | |
| Coarctation | 564 (61) | 355 (39) | |
| Shunts | 25,249 (58) | 18,133 (42) | |
| Subaortic stenosis | 229 (55) | 187 (45) | |
| AS/BAV | 6998 (69) | 3114 (31) | |
| Anomalous coronary artery | 3674 (63) | 2175 (37) | |
| Other | 2373 (56) | 1895 (44) | |
| Race % | <0.001 | ||
| White | 72 | 68 | |
| Black | 8 | 12 | |
| Hispanic | 12 | 13 | |
| Asian/Pacific Islander | 4 | 4 | |
| Native American | 0 | 0 | |
| Other | 4 | 4 | |
| Median household income quartile % | <0.001 | ||
| 1st | 22 | 27 | |
| 2nd | 26 | 27 | |
| 3rd | 25 | 24 | |
| 4th | 28 | 23 | |
| Expected Payer % | <0.001 | ||
| Medicare | 35 | 51 | |
| Medicaid | 12 | 15 | |
| Private | 45 | 27 | |
| Self-pay | 4 | 3 | |
| No charge | 1 | 1 | |
| Other | 4 | 3 |
LOS: length of stay; TGA: transposition of the great arteries; AVSD: atrioventricular septal defect; AS/BAV: aortic stenosis/bicuspid aortic valve
Diagnosis at index admission
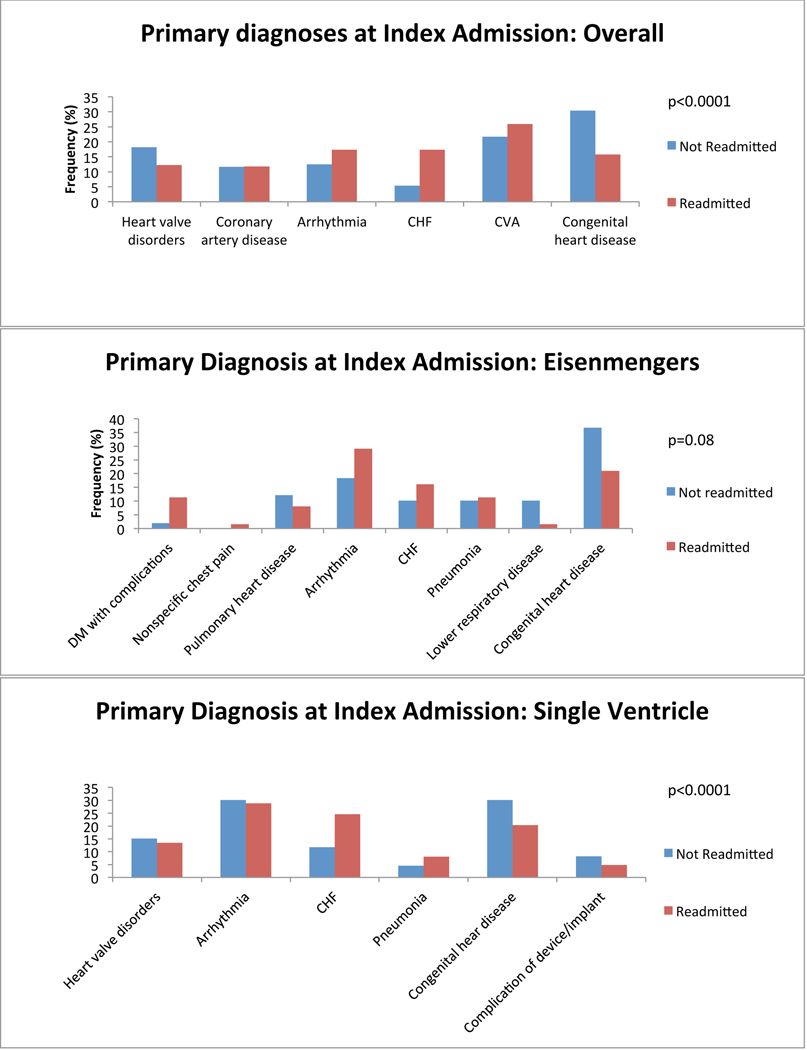
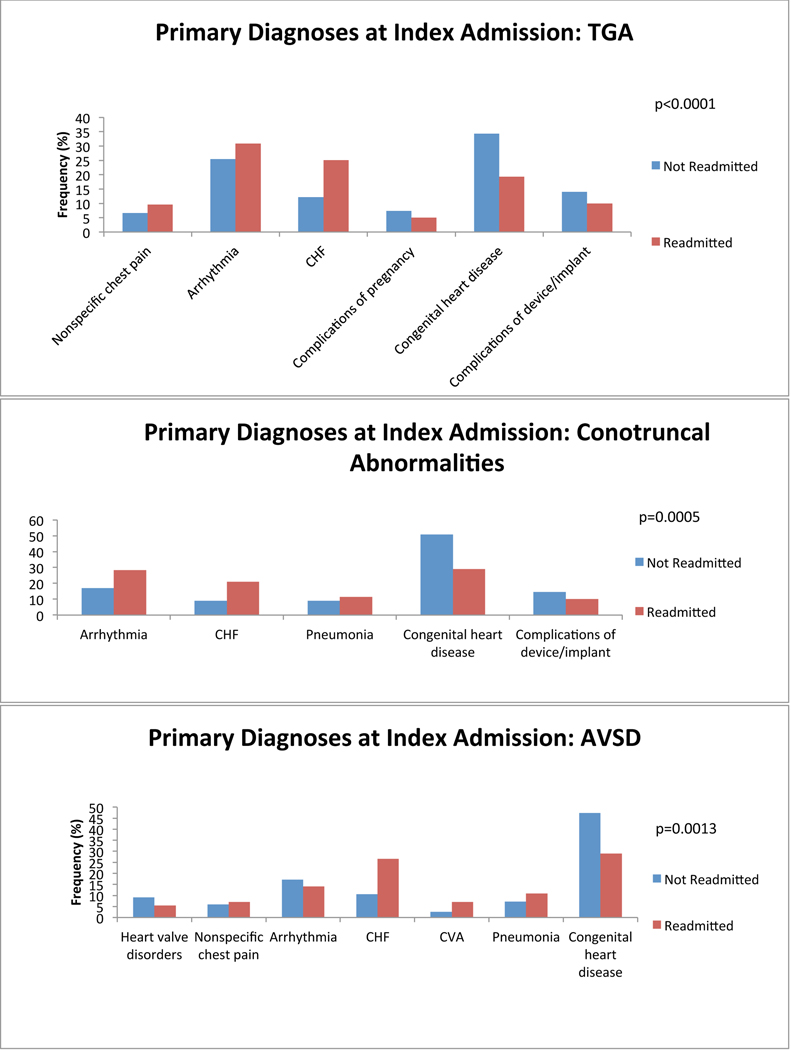
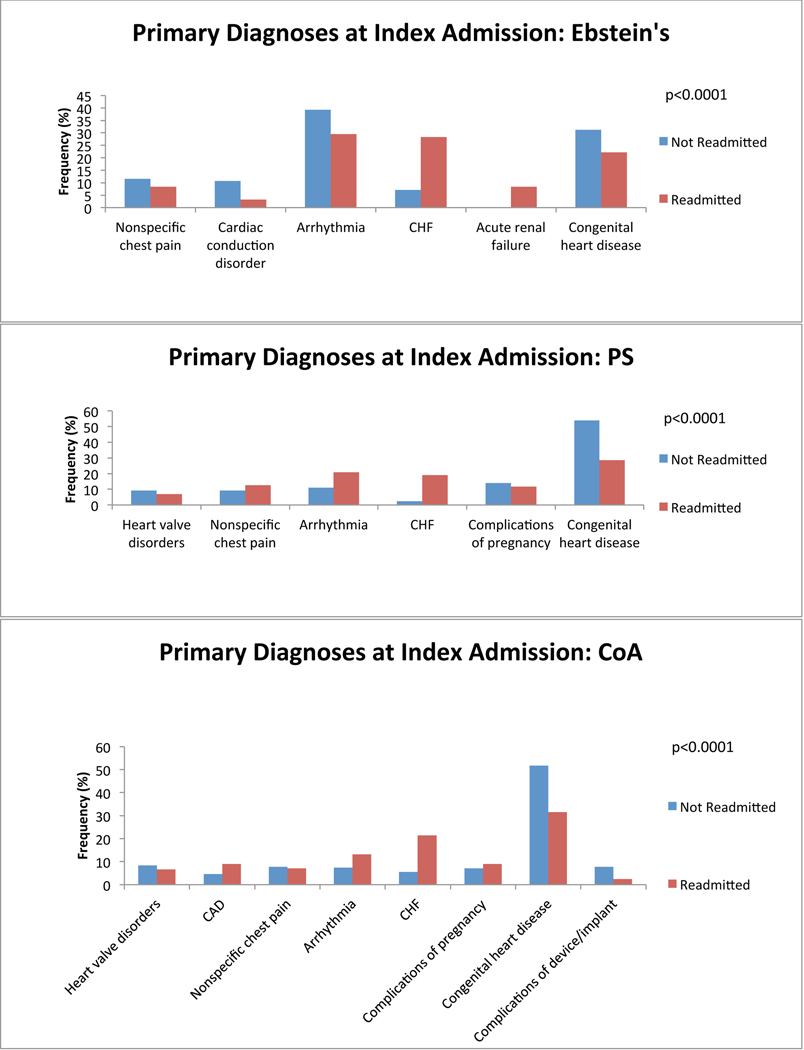
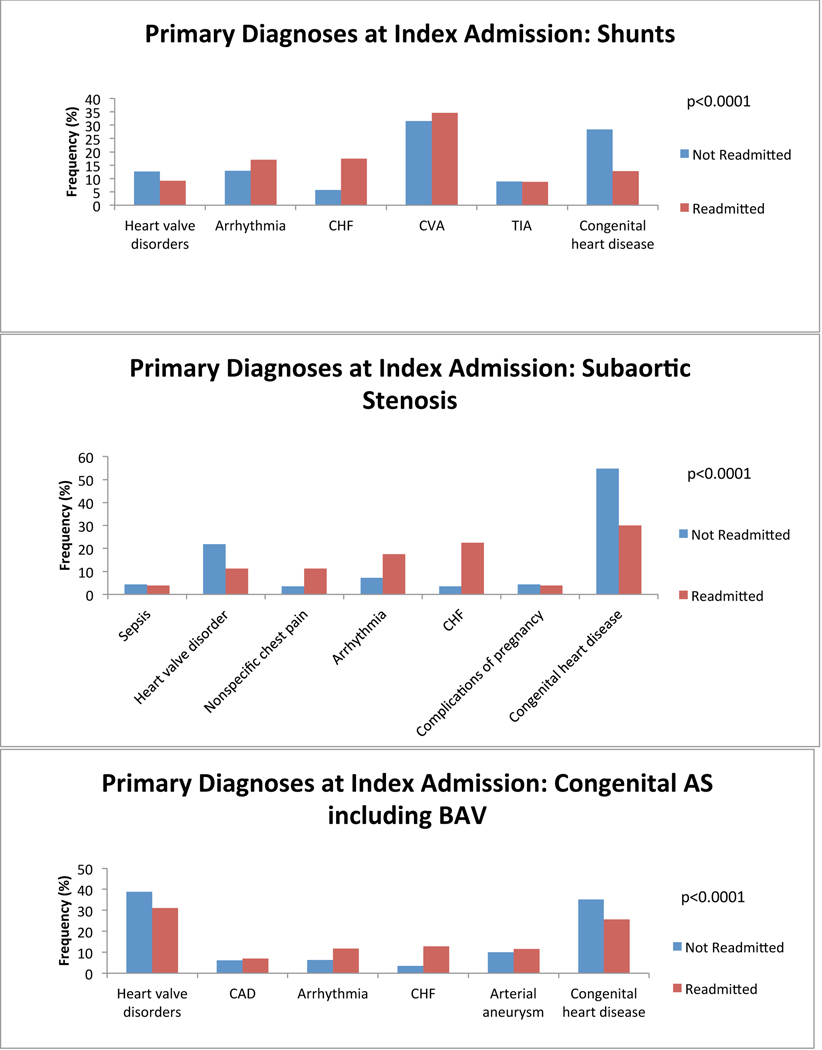
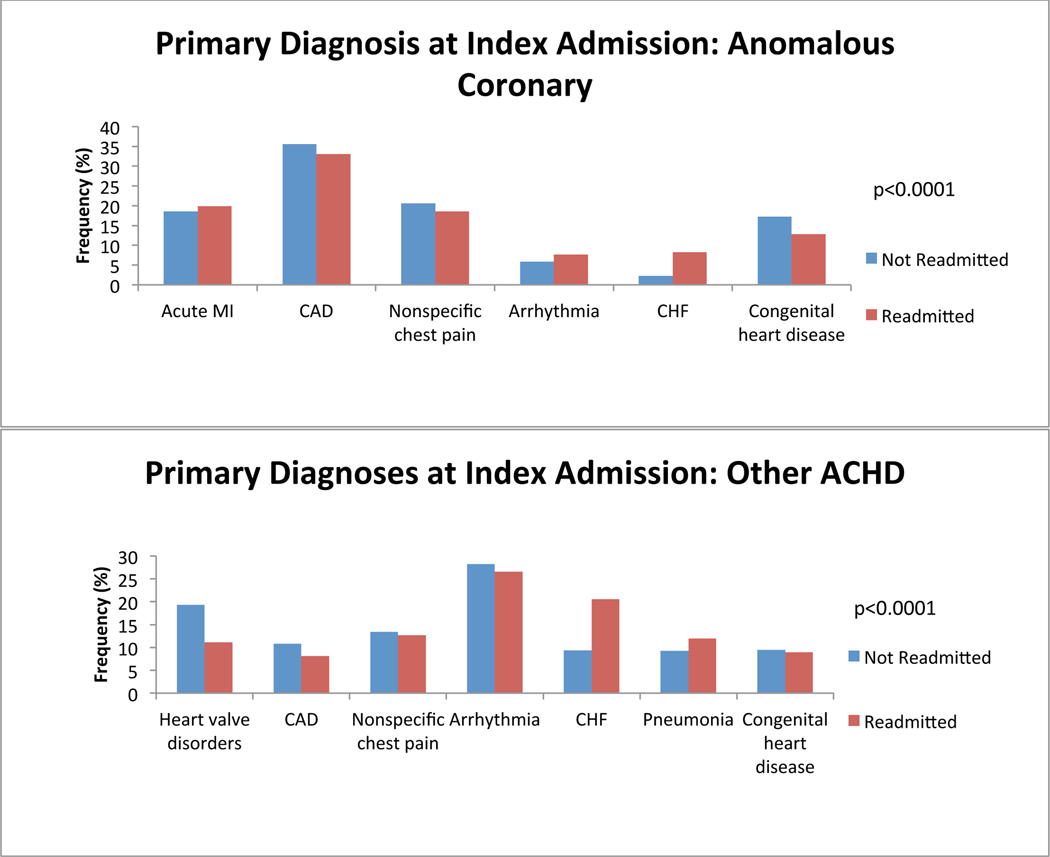
We first investigated which primary admitting diagnoses at the time of index admission was associated with a greater probability of readmission. We found differences among the top 5 most common reasons for admission in all anatomical groups. Congestive heart failure (CHF) was among the most common reasons for index admission regardless of group, and was more common among ACHD patients who would be readmitted than among those who would not. Cardiac arrhythmia tended to be a more common admitting diagnosis among all ACHD patients who would be readmitted except in single ventricle patients (in whom it was equally as common among non-readmitted patients) and in patients with atrioventricular septal defect (AVSD), Ebstein’s and those who failed to be characterized by the algorithm (in whom it was less common among readmitted patients). Cerebrovascular accident (CVA) tended to be more common among ACHD patients who would be readmitted, attributable predominantly to patients with AVSD and shunts. We also found that ACHD patients who were not readmitted within 12 months were more likely to have a primary diagnosis of cardiac and congenital circulatory anomaly at the time of index admission. This finding was consistent across all anatomical groups. Nevertheless, there remained lesion specific variability in primary diagnosis at the time of index admission. Figure 2 depicts comparisons of the five most common primary diagnoses at the time of index admission among both patients who were and those who were not readmitted, divided by anatomical group. There was inadequate data available to perform this analysis in patients with anomalous pulmonary venous return.
Figure 2:
Diagnosis at index admission. Graphs of the 5 most common admitting diagnoses among patients who experienced a readmission (red) and those who did not (blue) within 12 months after an index admission, separated by anatomical lesion. P-values for statistically significant differences between the admitting diagnoses are indicated on the graph. CHF: congestive heart failure; CVA: cerebrovascular accident; Congenital heart disease: congenital abnormality of the heart and great vessels; DM: diabetes mellitus; TIA: transient ischemic attack; TGA: transposition of the great arteries; AVSD: atrioventricular septal defect; PS: pulmonary valve stenosis; CoA: coarctation of the aorta; AS: aortic stenosis; BAV: bicuspid aortic valve.
Diagnosis at readmission
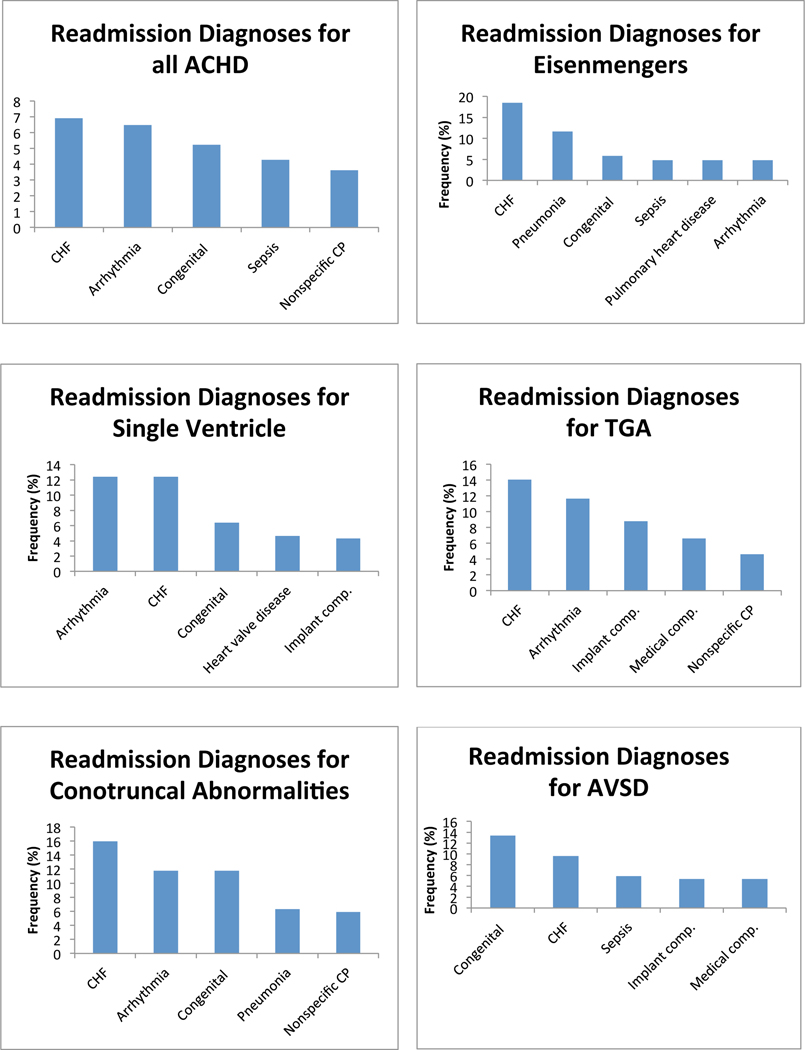
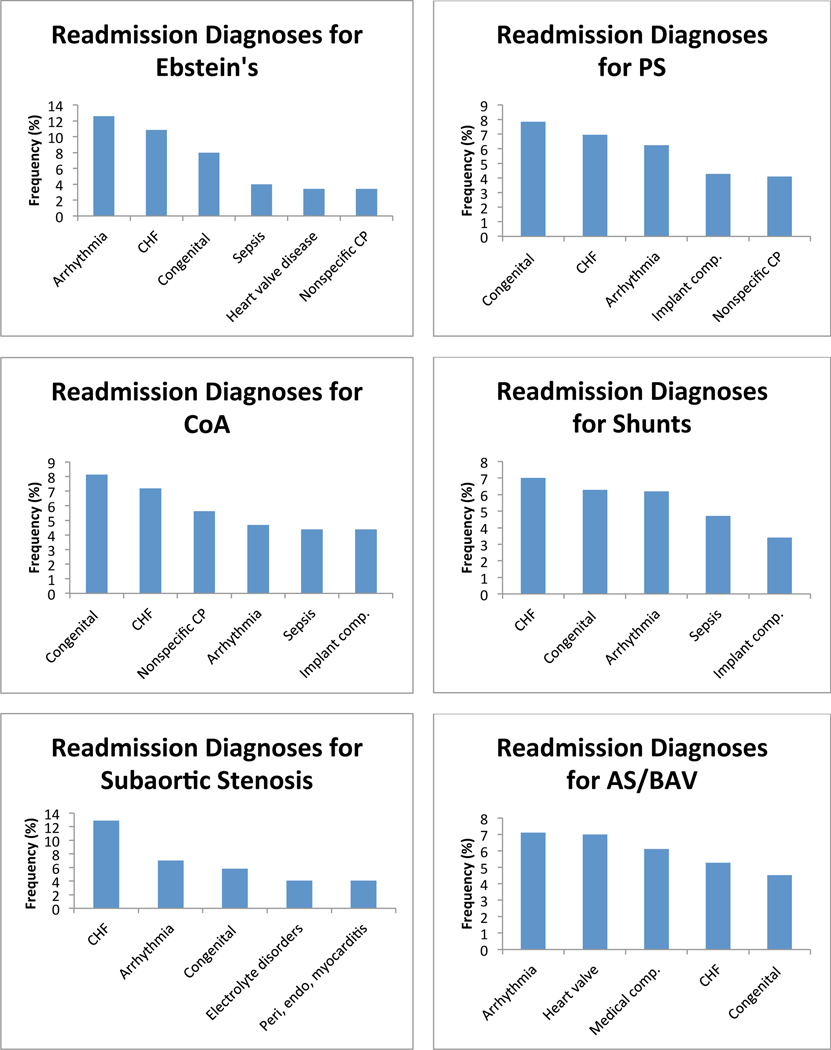
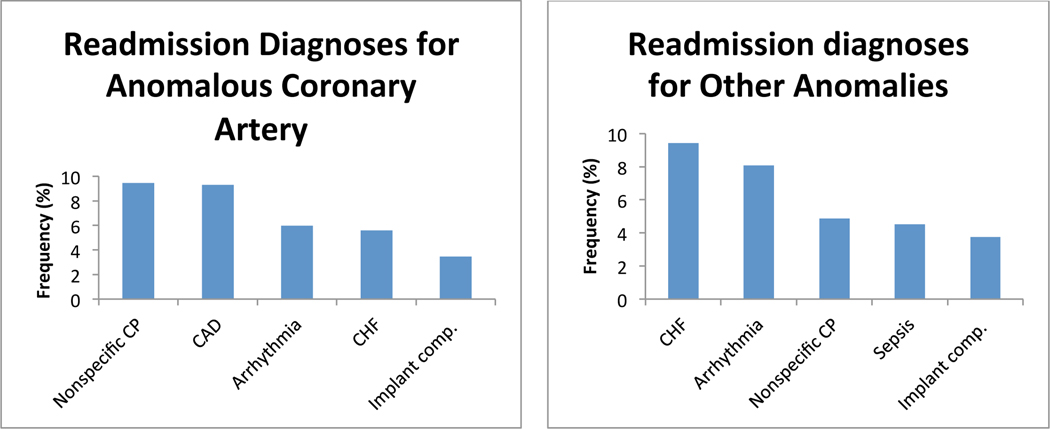
Next, we investigated the most common primary diagnoses at the time of readmission among ACHD patients who were readmitted within 12 months of an index hospitalization. CHF was the most common primary diagnosis at readmission for all ACHD, and was among the 5 most common diagnoses at readmission in every diagnostic group. Cardiac dysrhythmia was the second most common overall diagnosis, and was among the 5 most common diagnoses at readmission in every anatomical group except AVSD. Cardiac and circulatory congenital anomalies was the third most common overall readmission diagnosis, and was among the 5 most common diagnoses at readmission in all anatomical groups except transposition of the great arteries (TGA), anomalous coronary artery, and among patients who failed to be categorized by the algorithm. We found significant lesion specific variability in the diagnosis at the time of readmission. Figure 3 depicts the five most common diagnoses at readmission in each anatomical group. There was inadequate data available to perform this analysis in patients with anomalous pulmonary venous return.
Figure 3:
Diagnosis at readmission. Graphs of the 5 most common admitting diagnoses at the time of readmission separated by anatomical lesion. CHF: congestive heart failure; Congenital: congenital abnormality of the heart and great vessels; CP: chest pain; Implant comp.: complications of a device or implant; Medical comp.: complications of a medical or surgical procedure; TGA: transposition of the great arteries; AVSD: atrioventricular septal defect; PS: pulmonary valve stenosis; CoA: coarctation of the aorta; AS: aortic stenosis; BAV: bicuspid aortic valve.
Predictors of readmission
We next investigated risk factors for readmission based on multivariate regression models. For ACHD in general, we found that the two strongest predictors of readmission were chronic kidney disease (CKD) (OR 1.582, 95% CI 1.4–1.7, p<0.0001), and diabetes with complications (OR 1.523, 95% CI 1.4–1.6, p<0.0001). We also found that having private insurance and having an index admission for complications of a medical or surgical were the two strongest predictors of a decreased probability of readmission (OR 0.59, 95% CI 0.6–0.6, p<0.0001 and CI 0.75, 95% CI 0.7–0.8, p<0.0001 respectively). The seven variables having the greatest effect on probability of readmission and which were statistically significant on multivariate analysis, are listed in Table 2.
Table 2:
Lesion-specific predictors of readmission.
| Diagnosis | Risk Factor | Not Readmitted (%) | Readmitted (%) | Odds Ratio | 95% CI | p-value | Rank |
|---|---|---|---|---|---|---|---|
| Overall | CKD | 2187 (6) | 3753 (15) | 1.58 | 1.44–1.74 | <.0001 | 1 |
| DM with complications | 1305 (3) | 1910 (8) | 1.52 | 1.40–1.65 | <.0001 | 2 | |
| Private insurance | 17625 (45) | 6839 (27) | 0.59 | 056–0.62 | <.0001 | 3 | |
| CHF | 5916 (15) | 7120 (28) | 1.43 | 1.36–1.50 | <.0001 | 4 | |
| Liver disease | 1709 (4) | 2045 (8) | 1.42 | 1.32–1.53 | <.0001 | 5 | |
| Complications of surgery or medical care | 4261 (11) | 2405 (10) | 0.75 | 0.70–0.79 | <.0001 | 6 | |
| VTE | 1129 (3) | 1228 (5) | 1.38 | 1.25–1.51 | <.0001 | 7 | |
| Eisemenger Syndrome | CVD | 2 (2) | 17 (14) | 4.28 | 1.07–40.45 | 0.042 | 2 |
| Single Ventricle | DM with complications | 7 (1) | 30 (4) | 2.61 | 1.06–6.42 | 0.0372 | 1 |
| Complications of surgery or medical care | 69 (11) | 56 (8) | 0.57 | 0.37–0.87 | 0.0088 | 2 | |
| Liver disease | 45 (7) | 100 (14) | 1.90 | 1.27–2.86 | 0.0019 | 3 | |
| Reactive airway disease | 67 (10) | 145 (20) | 1.69 | 1.19–2.40 | 0.0034 | 4 | |
| Private insurance | 252 (39) | 188 (26) | 0.49 | 0.35–0.69 | 0.0003 | 5 | |
| CHF | 151 (23) | 279 (39) | 1.52 | 1.16–2.00 | 0.0026 | 6 | |
| Arrhythmia | 317 (49) | 393 (55) | 1.29 | 1.02–1.65 | 0.0377 | 7 | |
| TGA | DM with complications | 3 (1) | 26 (5) | 8.09 | 2.27–28.80 | 0.0013 | 1 |
| Mood disorders | 29 (5) | 70 (14) | 2.50 | 1.52–4.10 | 0.0003 | 2 | |
| Anxiety disorders | 13 (2) | 29 (6) | 2.40 | 1.09–5.25 | 0.0291 | 3 | |
| Age | 35.2±16.4 | 38.4±18.3 | 0.52 | 0.33–0.92 | 0.0057 | 4 | |
| Private insurance | 251 (47) | 189 (37) | 0.59 | 0.39–0.89 | 0.0362 | 5 | |
| CHF | 107 (20) | 173 (34) | 1.70 | 1.22–2.38 | 0.0017 | 6 | |
| Conotruncal abnormality | CHF | 35 (16) | 103 (37) | 2.27 | 1.37–3.74 | 0.0015 | 1 |
| Arrhythmia | 67 (31) | 118 (43) | 1.57 | 1.02–2.41 | 0.0397 | 2 | |
| AVSD | CKD | 10 (4) | 26 (12) | 2.93 | 1.28–6.68 | 0.011 | 1 |
| Respiratory failure | 38 (14) | 21 (10) | 0.46 | 0.24–0.87 | 0.018 | 2 | |
| CHF | 64 (23) | 79 (36) | 1.64 | 1.05–2.56 | 0.0312 | 3 | |
| Ebstein’s | CHF | 45 (18) | 77 (39) | 2.29 | 1.36–3.86 | 0.0019 | 1 |
| PS | HTN with complications or secondary | 22 (3) | 60 (10) | 2.41 | 1.02–5.68 | 0.0437 | 1 |
| Private insurance | 427 (49) | 159 (26) | 0.39 | 0.28–0.56 | <0.0001 | 2 | |
| CHF | 100 (11) | 146 (24) | 1.58 | 1.14–2.18 | 0.0064 | 3 | |
| Bacterial infection | 103 (12) | 127 (21) | 1.55 | 1.13–2.14 | 0.0073 | 4 | |
| Reactive airway disease | 117 (13) | 143 (23) | 1.39 | 1.03–1.88 | 0.033 | 5 | |
| CoA | Liver disease | 16 (3) | 34 (10) | 2.90 | 1.40–6.03 | 0.0044 | 1 |
| HTN with complications or secondary | 34 (6) | 64 (18) | 2.48 | 1.32–4.66 | 0.0048 | 2 | |
| Complications of surgery or medical care | 73 (13) | 30 (8) | 0.40 | 0.22–0.73 | 0.0027 | 3 | |
| Pulmonary heart disease | 30 (5) | 49 (14) | 2.65 | 1.49–4.73 | 0.001 | 4 | |
| Device related complication | 36 (6) | 10 (3) | 0.40 | 0.17–0.98 | 0.0451 | 5 | |
| Reactive airway disease | 69 (12) | 90 (25) | 1.96 | 1.27–3.01 | 0.0023 | 6 | |
| Shunts | CKD | 1615 (6) | 3077 (17) | 1.72 | 1.55–1.92 | <.0001 | 1 |
| DM with complications | 943 (4) | 1530 (8) | 1.53 | 1.40–1.68 | <.0001 | 2 | |
| Private insurance | 10492 (42) | 4450 (25) | 0.60 | 0.56–0.64 | <.0001 | 3 | |
| Reactive airway disease | 3632 (14) | 4319 (24) | 1.40 | 1.33–1.48 | <.0001 | 4 | |
| CHF | 3893 (15) | 5266 (29) | 1.39 | 1.31–1.47 | <.0001 | 5 | |
| Mood disorders | 1942 (8) | 2058 (11) | 1.37 | 1.28–1.47 | <.0001 | 6 | |
| Bacterial infection | 3664 (15) | 4322 (24) | 1.35 | 1.28–1.43 | <.0001 | 7 | |
| Subaortic stenosis | CAD | 34 (15) | 54 (29) | 2.36 | 1.32–4.28 | 0.0055 | 1 |
| CHF | 40 (17) | 67 (36) | 2.12 | 1.16–3.72 | 0.0086 | 2 | |
| Pulmonary heart disease | 19 (8) | 35 (19) | 2.10 | 0.96–4.41 | 0.0486 | 3 | |
| HTN | 87 (38) | 64 (34) | 0.56 | 0.34–0.97 | 0.0468 | 4 | |
| Private insurance | 105 (46) | 42 (22) | 0.45 | 0.23–0.94 | 0.0472 | 5 | |
| AS/BAV | CKD | 265 (4) | 334 (11) | 1.90 | 1.44–2.50 | <.0001 | 1 |
| Bacterial infection | 839 (12) | 694 (22) | 1.73 | 1.52–1.97 | <.0001 | 2 | |
| Liver disease | 277 (4) | 278 (9) | 1.62 | 1.34–1.97 | <.0001 | 3 | |
| Private insurance | 4143 (59) | 1335 (43) | 0.64 | 0.57–0.73 | <.0001 | 4 | |
| Device related complications | 225 (3) | 186 (6) | 1.62 | 1.29–2.02 | <.0001 | 5 | |
| Substance abuse | 305 (4) | 283 (9) | 1.55 | 1.28–1.87 | <.0001 | 6 | |
| Complications of surgery or medical care | 1400 (20) | 482 (15) | 0.72 | 0.63–0.82 | <0.0001 | 7 | |
| Coronary anomaly | CHF | 360 (10) | 474 (22) | 1.66 | 1.39–1.97 | <.0001 | 1 |
| Mood disorders | 271 (7) | 283 (13) | 1.59 | 1.31–1.93 | <.0001 | 2 | |
| Private insurance | 1656 (45) | 605 (28) | 0.60 | 0.53–0.69 | <.0001 | 3 | |
| Conduction system disease | 368 (10) | 343 (16) | 1.46 | 1.23–1.74 | <.0001 | 4 | |
| DM with complications | 114 (3) | 134 (6) | 1.49 | 1.12–1.97 | 0.0059 | 5 | |
| Reactive airway disease | 569 (15) | 551 (25) | 1.42 | 1.23–1.64 | <.0001 | 6 | |
| Hypertension with complications or secondary | 294 (8) | 313 (14) | 1.32 | 1.01–1.72 | 0.0397 | 7 | |
| Other | CKD | 94 (5) | 199 (11) | 2.12 | 1.43–3.15 | 0.0002 | 1 |
| Device related complication | 71 (3) | 110 (6) | 1.81 | 1.30–2.53 | 0.0005 | 2 | |
| Private insurance | 874 (37) | 424 (22) | 0.56 | 0.47–0.66 | <.0001 | 3 | |
| DM with complications | 51 (2) | 87 (5) | 1.62 | 1.09–2.40 | 0.0168 | 4 | |
| Complications of surgery or medical care | 191 (8) | 116 (6) | 0.63 | 0.48–0.82 | 0.0005 | 5 | |
| CHF | 427 (18) | 593 (31) | 1.56 | 1.32–1.84 | <.0001 | 6 | |
| Anxiety disorder | 102 (4) | 136 (7) | 1.55 | 1.16–2.06 | 0.0027 | 7 |
Not Readmitted: number of patients in the indicated group with the indicated risk factor who were not readmitted; Readmitted: number of patients in the indicated group with the indicated risk factor who were readmitted; Rank: indicates the relative importance of the identified variable in predicting risk of readmission for patients with the indicated congenital diagnosis based on regression analysis. Only variables found to have a statistically significant effect are reported. In certain cases, variables with large effects on regression failed to achieve statistical significance, due to low prevalence. As there was uncertainty about the validity of these effects, these variables were not reported and their ranks were bypassed. The seven factors having the greatest effect on overall model variability are reported. DM: diabetes mellitus; CKD: Chronic kidney disease; VTE: venous thromboembolism; CVD cerebrovascular disease; CHF: congestive heart failure; HTN: hypertension; TGA: transposition of the great arteries; AVSD: atrioventricular septal defect; PS: congenital pulmonary stenosis; CoA: coarctation of the aorta; BAV: bicuspid aortic valve, AS: congenital aortic stenosis
Given the heterogeneity of ACHD, we next divided the study population into anatomical groups using the algorithm described by Broberg et al and performed a similar analysis13. Up to seven variables for each anatomical subgroup having the greatest effect on probability of readmission and which were statistically significant on multivariate analysis, are listed in Table 2.
Discussion
The present study is an essential next-step in deriving strategies to improve the quality of care for ACHD patients. We recently investigated the annual cost of inpatient care for patients with ACHD and found that readmission was associated with the majority of variability in inpatient care cost. In the present study we found that ACHD patients were readmitted at very high rates with over 39% of patients readmitted within 12 months, most commonly for congestive heart failure and cardiac dysrhythmia. Furthermore, patients admitted with congestive heart failure once were more likely to be readmitted again within 12 months than patients admitted with other diagnoses, indicating that these groups of patients are high-yield targets for intervention. We identified lesion-specific risk factors for readmission, and found that these clinical variables are dependent on cardiac anatomy, suggesting that it will require individualized lesion-specific strategies to improve care quality in ACHD.
The rate of rehospitalization in the present ACHD population compared to control or at risk populations is difficult to assess based on the current analysis. We selected a 12-month follow up for the present study because we recently demonstrated that rehospitalization within 12 months was a major driver of increased cost of inpatient care in ACHD. Given that one of our primary goals in the present analysis was to identify hypotheses to direct strategies aimed at improving healthcare efficiency, the 12-month time point was therefore logical. It is almost certain that readmission rate in ACHD is lower than that for CHF among patients with normal cardiac anatomy, where readmission rate among medicare beneficiaries has been documented to be 44% at 6 months14. Nevertheless direct comparison is difficult given differences in age and comorbidities between the two groups. We found a higher rate of rehospitalization than that suggested in a recent cohort from Quebec and the Netherlands which documented rates of hospitalization among ACHD patients who had been previously hospitalized at approximately 0.3 per patient-year 15. This analysis was not focused on readmissions specifically however, so explanation of the reasons for the higher rates in the present cohort are difficult to identify, and may simply be related to any of a number of factors leading to elevated rates of rehospitalization in the US as compared to Canada and European countries16.
The relative rates of rehospitalization for particular diagnoses are as expected, and suggest the promise of disease-specific management to improve health outcomes in ACHD. Subjects with simple lesions for which proven and effective treatment strategies exist such as AS, PS and shunts had lower likelihoods of readmission than those with complex lesions such as Eisenmengers syndrome or single ventricle for which no therapy reliably alters disease progression. In the absence of good data to direct management patients with these latter lesions would be expected to have an elevated probability of frequent hospitalization, in particular after the advent of disease-related sequelae such as arrhythmia or CHF. In this regard, the present data may be useful in identifying patients with more complex disease at elevated risk of readmission as targets for enrollment in clinical trials with the goal of modifying the natural history of the disease, or of identifying thresholds for advanced therapy referral such as transplant.
The fact that the majority of rehospitalizations were attributable to either CHF or cardiac arrhythmia is not surprising. Previous studies have documented CHF and atrial arrhythmia in particular to be common reasons for hospitalization in general, and unplanned hospitalization specifically in ACHD patients7,17,18. It is also not surprising that hospitalization once for either of these diagnoses was associated with a risk for rehospitalization. Readmission rates are known to be high among patients with a diagnosis of either CHF or atrial fibrillation in the general adult population although this has not previously been demonstrated to be the case in ACHD patients in particular 14,19–22. These data suggest a high-risk population of ACHD patients to target for improving care quality. They also indicate that strategies proven to improve outcomes in CHF and atrial fibrillation may provide a reasonable framework on which to build care paradigms to decrease readmissions in ACHD. In deriving these paradigms disease-specific interventions will need to be implemented in recognition of the fact that standard therapy for CHF has not yet been found to be beneficial in ACHD, and that the most common arrhythmia for certain ACHD lesions is not atrial fibrillation.
Nevertheless risk factors for global rehospitalization vary widely depending on underlying anatomy, suggesting that individualized paradigms will be required to decrease readmission rates in ACHD patients. This finding is not surprising given the great heterogeneity of ACHD, but will complicate identification and implementation of strategies to decrease rates of readmission. Based on the present data, we would propose the following approach as a starting point for this process: Given the high incidence of hospitalization for congestive heart failure, and data indicating that patient education, careful care coordination and early follow-up may decrease rates of rehospitalization in the general CHF population, we should attempt to reproduce these results using similar strategies in the ACHD population23–26. The goals of predischarge and early post-discharge follow-up and education would be individualized based on the lesion-specific risk-factors for readmission identified here. Follow-up should include early arrangement of consultation with other medical specialties such as nephrology in cases where CKD is present, or endocrinology in cases of diabetes. We anticipate that the present data might be used to develop these disease specific paradigms to be investigated in future prospective trials with the goal of identifying successful lesion-specific strategies to decrease readmission rate.
Limitations
There are multiple limitations to the present study related to the use of administrative data. The completeness and accuracy of the data is dependent on the care with which data was entered, which is variable from institution to institution. In particular, the frequency with which the admitting diagnosis was “cardiac and circulatory congenital anomaly” is frustrating and uninformative, likely indicating that the coder employed an ICD9 code for the patient’s primary cardiac lesion when the patient was admitted for a cardiac reason related to that primary lesion. This finding highlights the limitations imposed on the utility of administrative data by suboptimal coding. Differentiation between differing congenital cardiac lesions is difficult given institutional variability in coding for similar lesions. Although we made every effort to include all ACHD patients, to exclude patients without ACHD from analysis, and to correctly characterize patients based on underlying anatomy, no algorithm is fool-proof, and it is almost certain that some patients in the present study were mischaracterized. In addition, given that ICD9 does not distinguish between PFO and ASD, some proportion of the present population classified as having shunt lesions may in fact have a PFO. One of the unique components of the present study was the exclusive use of SIDs which permitted tracking of individual patients to detect rates of readmission. Tracking of individual patients however does not cross state lines. Even within states, the same patient admitted at different hospitals may have failed to be recognized as the same due to faulty data entry. In addition, SIDs do not fully track deaths, and it is possible that some patients succumbed prior to a full 12 month follow-up after an index hospitalization. Thus the rehospitalization rate reported in the present study may be an underestimate.
Conclusions
ACHD patients have high readmission rates, predominantly for CHF and arrhythmia. Predictors of readmission are lesion specific, and future strategies aimed at decreasing readmission rate will likely need to be individualized. Initial trials aimed at reducing readmissions in ACHD might optimally target patients admitted with CHF and build upon the paradigms found to be successful in decreasing readmissions in CHF.
What Is Known:
The rates of hospitalization and the costs of inpatient care for adult congenital heart disease (ACHD) are increasing in parallel with increasing disease prevalence and aging of the population with ACHD.
Readmission is a primary driver of inpatient spending in ACHD.
What The Study Adds:
This study quantifies the rate of readmission among patients with ACHD in a 5-state database.
The most common reasons for readmission in this population were heart failure and arrhythmias.
In addition, this study identifies lesion-specific clinical variables associated with increased risk of readmission in this population.
Acknowledgments
Funding Sources: Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH
Footnotes
Conflict of Interest Disclosures: None.
References:
- 1.Joynt KE, Orav EJ, Jha AK. The Association Between Hospital Volume and Processes, Outcomes, and Costs of Care for Congestive Heart Failure. Ann Intern Med. 2011;154:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krumholz HM, Peterson ED, Ayanian JZ, Chin MH, DeBusk RF, Goldman L, Kiefe CI, Powe NR, Rumsfeld JS, Spertus JA, Weintraub WS. Report of the National Heart, Lung, and Blood Institute working group on outcomes research in cardiovascular disease. Circulation. 2005;111:3158–3166. [DOI] [PubMed] [Google Scholar]
- 3.Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, Normand S-LT, Schreiner G, Spertus JA, Vidán MT, Wang Y, Wang Y, Krumholz HM. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Wang Y, Lin Z, Straube BM, Rapp MT, Normand S-LT, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. [DOI] [PubMed] [Google Scholar]
- 5.Joynt KE, Jha AK. Who Has Higher Readmission Rates for Heart Failure, and Why?: Implications for Efforts to Improve Care Using Financial Incentives. Circ Cardiovasc Qual Outcomes. 2010;4:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Heal Aff. 2007;26:38–48. [DOI] [PubMed] [Google Scholar]
- 7.Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54:460–467. [DOI] [PubMed] [Google Scholar]
- 8.O’Leary JM, Siddiqi OK, de Ferranti S, Landzberg MJ, Opotowsky AR. The Changing Demographics of Congenital Heart Disease Hospitalizations in the United States, 1998 Through 2010. JAMA. 2013;309:984–986. [DOI] [PubMed] [Google Scholar]
- 9.Gurvitz M, Marelli A, Mangione-Smith R, Jenkins K. Building quality indicators to improve care for adults with congenital heart disease. J Am Coll Cardiol. 2013;62:2244–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marelli AJ, Gurvitz M. From numbers to guidelines. Prog Cardiovasc Dis. 2011;53:239–246. [DOI] [PubMed] [Google Scholar]
- 11.Konstam V, Salem D, Pouleur H, Kostis J, Gorkin L, Shumaker S, Mottard I, Woods P, Konstam MA, Yusuf S. Baseline Quality of Life as a Predictor of Mortality and Hospitalization in 5,025 Patients With Congestive Heart Failure. Am J Cardiol. 1996;78:890–895. [DOI] [PubMed] [Google Scholar]
- 12.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract. 2002;5:143–51. [PubMed] [Google Scholar]
- 13.Broberg C, McLarry J, Mitchell J, Winter C, Doberne J, Woods P, Burchill L, Weiss J. Accuracy of administrative data for detection and categorization of adult congenital heart disease patients from an electronic medical record. Pediatr Cardiol. 2015;36:719–725. [DOI] [PubMed] [Google Scholar]
- 14.Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, Hennen J. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 15.Zomer AC, Ionescu-Ittu R, Vaartjes I, Pilote L, Mackie AS, Therrien J, Langemeijer MM, Grobbee DE, Mulder BJM, Marelli AJ. Sex differences in hospital mortality in adults with congenital heart disease: the impact of reproductive health. J Am Coll Cardiol. 2013;62:58–67. [DOI] [PubMed] [Google Scholar]
- 16.Eapen ZJ, Reed SD, Li Y, Kociol RD, Armstrong PW, Starling RC, McMurray JJ, Massie BM, Swedberg K, Ezekowitz JA, Fonarow GC, Teerlink JR, Metra M, Whellan DJ, O’Connor CM, Califf RM, Hernandez AF. Do countries or hospitals with longer hospital stays for acute heart failure have lower readmission rates?: Findings from ASCEND-HF. Circ Heart Fail. 2013;6:727–732. [DOI] [PubMed] [Google Scholar]
- 17.Negishi J, Ohuchi H, Yasuda K, Miyazaki A, Norifumi N, Yamada O. Unscheduled hospitalization in adults with congenital heart disease. Korean Circ J. 2015;45:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaemmerer H, Bauer U, Pensl U, Oechslin E, Gravenhorst V, Franke A, Hager A, Balling G, Hauser M, Eicken A, Hess J. Management of emergencies in adults with congenital cardiac disease. Am J Cardiol. 2008;101:521–525. [DOI] [PubMed] [Google Scholar]
- 19.van Diepen S, Bakal JA, McAlister FA, Ezekowitz JA. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation. 2011;124:289–296. [DOI] [PubMed] [Google Scholar]
- 20.Johnson BH, Smoyer-Tomic KE, Siu K, Walker DR, Sander S, Huse D, Smith DM, Song X, Amin A. Readmission among hospitalized patients with nonvalvular atrial fibrillation. Am J Health Syst Pharm. 2013;70:414–422. [DOI] [PubMed] [Google Scholar]
- 21.Amin AN, Jhaveri M, Lin J. Hospital readmissions in US atrial fibrillation patients: occurrence and costs. Am J Ther. 2013;20:143–150. [DOI] [PubMed] [Google Scholar]
- 22.Khazanie P, Liang L, Qualls LG, Curtis LH, Fonarow GC, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Hernandez AF, Piccini JP. Outcomes of medicare beneficiaries with heart failure and atrial fibrillation. JACC Heart Fail. 2014;2:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179–185. [DOI] [PubMed] [Google Scholar]
- 24.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA. 2004;291:1358–1367. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. [DOI] [PubMed] [Google Scholar]
- 26.Lee DS, Stukel TA, Austin PC, Alter DA, Schull MJ, You JJ, Chong A, Henry D, Tu J V. Improved outcomes with early collaborative care of ambulatory heart failure patients discharged from the emergency department. Circulation. 2010;122:1806–1814. [DOI] [PubMed] [Google Scholar]