Abstract
Background:
Lower exercise capacity, as measured by 6-minute walk distance (6MWD), is associated with incident heart failure (HF). Among those without HF, the associations of measures of cardiac structure and function with 6MWD are unclear, and may provide insight regarding the risk of incident HF.
Objectives:
To understand the relationships between cardiac function and exercise capacity.
Methods:
We evaluated the associations of cardiac mechanics with 6MWD in the 6th exam of the Multi-Ethnic Study of Atherosclerosis. Two-dimensional, Doppler and speckle-tracking echocardiography was performed at rest and after passive leg raise to evaluate functional reserve after intravascular volume challenge.
Results:
Of 2,096 participants without HF (mean age: 73, 48% male, 58% non-White), individuals with lower (worse) left atrial (LA) reservoir strain were older and had higher blood pressure. Lower resting LA reservoir strain (β coefficient per SD decrease: −5.0, 95% CI: −8.8, −1.3 meters; P=0.009), inability to augment LA reservoir strain after passive leg raise (β coefficient per SD decrease: −5.8, 95% CI: −9.1, −2.5 meters; P<0.001), and lower right atrial reservoir strain (β coefficient per SD decrease: −4.4, 95% CI: −7.8, −1.1 meters; P=0.01) were associated with shorter 6MWD. Worse left ventricular (LV) diastolic function was also associated with lower 6MWD. There were no independent associations of indices of LV systolic function (global longitudinal strain, circumferential strain, ejection fraction) with 6MWD.
Conclusion:
Among individuals without HF, worse biatrial function, lack of LA functional reserve, and worse LV diastolic function were associated with reduced submaximal exercise capacity. Therapies aimed to improve these functional domains may increase exercise capacity and prevent HF.
Keywords: 6-minute walk test, echocardiography, cardiac structure and function, heart failure, subclinical
CONDENSED ABSTRACT
Among individuals without heart failure (HF), the associations of measures of cardiac function with exercise capacity as measured by the validated test of 6-minute walk distance (6MWD) are unclear. In a community-based cohort of the Multi-Ethnic Study of Atherosclerosis (MESA) without HF, we evaluated the associations of cardiac mechanics at rest and after passive leg raise with 6MWD. Among 2,096 participants, reduced biatrial reservoir strain, inability to augment LA strain after leg raise (i.e, intravascular volume challenge), and reduced diastolic function were significantly associated with worse exercise capacity. Improvement in these domains of cardiac function may prevent progression to HF.
The 6-minute walk distance (6MWD) test is a comprehensive measurement of submaximal exercise capacity, which globally integrates and assesses multiple organ systems to provide an objective measure of functional status (1). Lower 6MWD is a powerful prognostic tool for mortality in multiple chronic diseases, including heart failure (HF) (2,3). Notably, lower 6MWD has been independently associated with incident HF among individuals with stable ischemic heart disease (4). Thus, in high-risk individuals, 6MWD may be a useful tool to evaluate for subclinical reduction in exercise capacity that precedes the development of clinically manifest HF.
While the determinants of reduced exercise capacity are diverse, derangements of cardiac structure and function may play particularly important roles, as such abnormalities precede the development of clinical HF (5,6). In chronic HF, several traditional measures of left ventricular (LV), left atrial (LA), and right ventricular (RV) dysfunction on echocardiography have been associated with reduced 6MWD (3). However, the association of measures of cardiac structure and function with 6MWD in individuals free of HF are less clear, and such information may provide insight into potential therapeutic targets to prevent further deterioration in exercise capacity and progression to overt HF. Additionally, the associations of sensitive measures of cardiac function as assessed by speckle-tracking strain echocardiography with 6MWD are not known. We therefore evaluated the associations of cardiac function on echocardiography with 6MWD among individuals without prevalent HF.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort of US adults aged 45 to 84 at study recruitment who identified themselves as 1 of 4 race/ethnic groups: Black, White, Chinese, or Hispanic. Study investigators initially recruited 6,814 participants from 2000 to 2002 across 6 field centers (Baltimore, MD; Chicago, IL; St Paul, MN; Forsyth County, NC; New York, NY; and Los Angeles, CA). The comprehensive details regarding MESA study design have been previously described (7). At the time of recruitment, participants were free of cardiovascular diseases, including coronary heart disease, stroke/transient ischemic attack, HF, atrial fibrillation (AF), implantable cardiac devices, or history of cardiac surgery. After the initial in-person examination (2000 to 2002), participants were invited to 5 subsequent in-person visits, which occurred every 2–5 years. Additionally, study personnel contacted participants every 9–12 months to assess their clinical status. In this analysis, we evaluated participants who 1) attended the 6th in-person examination (September 2016 to June 2018) with available covariate data, 2) were free of clinical HF prior to Exam 6, and 3) underwent both echocardiography and 6-minute walk test. HF was defined as hospitalization for HF, which was ascertained through independent adjudication of hospitalization medical records of participants by 2 study physicians who were otherwise blinded to participant study data. Adjudication of HF required both documentation of symptoms of HF and either imaging suggestive of HF or medical treatment for HF. In MESA, history of atrial fibrillation (AF) at Exam 6 was defined by inpatient or outpatient Medicare claims for AF, International Classification of Disease hospital discharge diagnosis codes for AF, documented AF on 12-lead ECGs obtained at Exam 1 or Exam 5, self-report at Exam 6, or AF on ambulatory ECG monitoring (available in a subset of 1,557 participants who wore Zio Patch XT [iRhythm Technologies, San Francisco, CA] at Exam 6 as part of an ancillary study) (8). Estimated glomerular filtrate rate (eGFR) was calculated using the CKD-EPI equation (9). N-terminal pro-B- type natriuretic peptide (NT-proBNP) levels were obtained through the Olink Cardiovascular III panel (Uppsala, Sweden) and were subsequently expressed as normalized protein units on a log2 scale. The correlation between NT-proBNP measured by the Olink panel and by a commercially available ELISA immunoassay is strong (r=0.89) (10), and in MESA Exam 6, in a subset of n=993 participants, the Olink NT-proBNP correlated closely with log-transformed ELISA NT-proBNP (r=0.93). The study protocol was approved by the institutional review board of each field center; all participants provided written informed consent.
Echocardiography Protocol
Comprehensive resting 2-dimensional, Doppler, and speckle-tracking echocardiography was performed at Exam 6. One sonographer per study site was designated as the dedicated field center sonographer for all MESA echocardiograms. Each sonographer attended centralized training that was conducted by the Northwestern University Echocardiography Core Lab (NUECL, Chicago, IL). All echocardiograms were sent to NUECL in DICOM format for formal analysis using GE EchoPAC software (version 201; GE Healthcare; Waukesha, WI). All 2D images were obtained at a frame rate of 50–80 frames per second.
Cardiac chamber quantification was performed in accordance with previous societal guideline recommendations (11). LV volumes, LV ejection fraction, and LA volumes were measured using the biplane method of disks from apical 4- and 2-chamber views. LV mass was calculated using the Devereux formula. RV fractional area change (FAC) was calculated using the following equation: 100 x (RV end-diastolic area – RV end-systolic area) / (RV end-diastolic area). Tissue doppler was performed at the septal and lateral mitral annulus during early diastole (e’ velocities) and atrial contraction (a’ velocities), and at the tricuspid annulus (RV s’ velocity). Tricuspid annular plane systolic excursion (TAPSE) was measured by M-mode. LV stroke volume (SV) was calculated by the hydraulic orifice formula using LV outflow tract velocity time integral and LV outflow tract diameter. Pulmonary artery systolic pressure (PASP) was estimated using the simplified Bernoulli equation.
Speckle-tracking echocardiography of the LA, LV, RV, and RA was performed by an experienced sonographer blinded to other clinical data using GE EchoPAC software. All strain curves were verified by 2 cardiologists with expertise in echocardiography. The endocardial border of each cardiac chamber was traced and regions of interest spanning epicardium to endocardium were defined. Subsequently, standard anatomic segments of each cardiac chamber are defined by the program to perform regional speckle tracking analysis. Speckle-tracking software subsequently generates curvilinear graphs of strain measurements over time, corresponding to the anatomic segments of the chamber of interest, along with a curve representing the average of the segments. The absolute values of all strain measurements are reported. As such, lower strain values correspond to worse function. Strain measurements from apical 4- and 2-chamber views were used to calculate LA strain. The apical 4-chamber view was used for calculation of RA strain values. Among participants in sinus rhythm, P-wave ECG gating was used to measure LA and RA strain. Three atrial strain parameters were measured: booster strain was defined as the average of the peak negative strain values across the 6 segments between onset of P-wave and onset of QRS complex, conduit strain was the average of the peak positive strain values across the 6 segments between the QRS complex and onset of subsequent P-wave, and reservoir strain was defined as the sum of booster and conduit strain (Supplemental Figure 1). Among participants in AF at time of echocardiogram, R wave ECG gating was used to measure atrial strain. In AF, atrial reservoir strain equals atrial conduit strain. This method has been previously validated in AF (12). LV global longitudinal strain (GLS) was calculated as the average strain from apical 4-, 3-, and 2-chamber views. LV circumferential strain was obtained from the LV mid-chamber short axis view.
Participants also underwent image acquisition after passive leg raise using a wedge-shaped pillow as a method of intravascular volume challenge. Participants were instructed to keep their legs straight and to keep their leg muscles relaxed. During passive leg raise, the apical 4-chamber view was utilized to collect pulsed-wave Doppler (at mitral valve leaflet tips and LV outflow tract), tissue Doppler (at septal and lateral mitral annulus), and 2-dimensional images of the LA for speckle-tracking.
6-Minute Walk Test
Participants who attended Exam 6 were invited to undergo the test of 6MWD. Individuals who were unable to ambulate (e.g., in a wheelchair), or with elevated pulse, blood pressure, or recent chest pain were excluded from the 6MWD test. A standardized protocol was followed across study sites according to societal recommendations (1). In a flat, indoor corridor, markers were placed 20 meters apart. Participants were instructed to walk laps around the markers along the corridor for as long as possible over the course of 6 minutes without running or jogging. Participants were notified of time remaining after each minute passed, and standardized phrases of encouragement were used. A practice test was not performed prior to actual test of 6MWD for each participant. 6MWD was defined as the total distance walked (in meters) in 6 minutes or until the participant asked to stop the test.
Statistical Analysis
Clinical characteristics at Exam 6 by quartile of resting LA reservoir strain were compared using χ2 tests for categorical variables and one-way analysis of variance or Kruskal-Wallis tests (depending on distribution) for continuous variables. The relationships between each echocardiographic variable of cardiac function and 6MWD were assessed for non-linearity through generalized additive models. If there were no departures from linearity among these various relationships, multivariable linear regression models were used to evaluate the associations of cardiac function with 6MWD. The resting echocardiographic measures of cardiac function of interest for these analyses were categorized into the following domains: LA function (LA booster and reservoir strain), LV systolic function (ejection fraction, GLS, circumferential strain), LV diastolic function (lateral e’ velocity, E/e’ average ratio, early diastolic strain rate), RA function (RA booster and reservoir strain), RV function (TAPSE, FAC, s’ velocity, RV free wall strain), and hemodynamics (PASP, SV, cardiac output [CO]). RV s’ velocity demonstrated a non-linear (i.e., curvilinear) relationship with 6MWD. We thus summarized the 2 dominant linear features of RV s’ with 6MWD with linear models using median value as the cutpoint based on overall shape of association. Measures of cardiac function were standardized in all models and reported as per 1-SD decrease. All covariates were obtained at the time of echocardiography (Exam 6). Model 1 adjusted for age, race/ethnicity, sex, study site, and education status. Model 2 further adjusted for smoking status, systolic blood pressure, heart rate, body mass index, diabetes, eGFR, anti-hypertensive medication, NT-proBNP, history of AF, coronary heart disease, stroke, LV mass, and LA volume. We subsequently evaluated associations of changes in the following measures of cardiac function after passive leg raise with 6MWD: LA reservoir strain, LA booster strain, lateral e’ velocity, E/e’ average, and SV. These models further adjusted for respective resting levels of cardiac function in addition to Model 1 and Model 2 covariates. Given the known associations of AF with LA reservoir strain and with 6MWD, we performed interaction analyses of history of AF on the associations of resting LA reservoir strain and change in LA reservoir strain after leg raise with 6MWD using an interaction term for AF. There was no evidence of multi-collinearity in fully adjusted models.
We performed several sensitivity analyses. First, we evaluated the association of LA reservoir strain with 6MWD after adjustment for AF at time of echocardiogram in place of history of AF, and we assessed interaction by AF at time of echo on the association of LA reservoir strain with 6MWD. Second, we evaluated the associations of resting LA and RA reservoir strain and change in LA strain after leg raise with 6MWD after further adjustment for average E/e’ ([septal E/e’ + lateral E/e’]/2). Third, to account for frailty of participants, we evaluated the associations of cardiac mechanics after further adjustment for patient-reported total volitional exercise (METS-minutes per week). In exploratory analysis, we assessed the incremental contribution of LA reservoir strain to clinical covariates and E/e’ average in predicting 6MWD using the analysis of variance test.
To better understand the relationships between cardiovascular risk factors, cardiac mechanics, and submaximal exercise capacity, we performed formal mediation analyses of 2 measures of cardiac function (LA reservoir strain and e’ lateral tissue velocity) on the associations of cardiovascular risk factors (age, race, sex, and BMI) with 6MWD. Mediation analyses were performed using the mediation package in R version 4.0.2. First, linear regression models evaluated associations of the 4 prespecified cardiovascular risk factors with measures of cardiac function (potential mediator). Second, additional linear regression models evaluated associations of cardiovascular risk factors with 6MWD (outcome). Cardiovascular risk factors significantly associated with both potential mediator and outcome were assessed in formal mediation analyses. Multivariable-adjusted direct and indirect effects (i.e., mediation effect) were reported, with calculation of 95% confidence intervals (CIs) using bootstrapping with 1000 resamples. Statistically significant mediation was determined if the indirect effect was significantly different from zero. P values and 95%-confidence intervals presented in this report have not been adjusted for multiplicity, and therefore inferences drawn from these statistics may not be reproducible. All analyses were carried out using R version 4.0.2 (R Foundation for Statistical Computing).
Results
Characteristics of Study Participants
Of 6,814 participants in MESA, 4,718 were excluded from the analysis: 3,526 participants did not attend Exam 6 (1552 died prior to Exam 6), 757 did not perform the test of 6MWD, 53 did not have echocardiography performed, 42 had prevalent HF at Exam 6, and 340 had missing covariate data. The final cohort for this analysis was 2,096 participants (Supplemental Figure 2). The median 6MWD was 414 (25th-75th percentile: 350–476) meters. Compared with participants included in the analytic cohort, participants excluded from this analysis who attended Exam 6 were older, more likely Black race/ethnicity and had higher blood pressure and BMI (Supplemental Table 1). Participants with lower LA reservoir strain were older, more likely Hispanic race/ethnicity, and more likely to have diabetes, CHD, and AF (Table 1). Additionally, individuals with lower LA reservoir strain had higher BMI, systolic blood pressure, glucose, and NT-proBNP levels. The association between lower LA reservoir strain and higher NT-proBNP was stronger among participants with history of AF (Supplemental Figure 3). Lower LA reservoir strain was associated with higher LV mass, lower LV systolic function, worse LV diastolic function, higher LA volume, lower LA booster strain, reduced RA/RV function, and higher PASP (Table 2).
Table 1.
Clinical Characteristics (Exam 6) by Quartile of Resting Left Atrial Reservoir Strain.
| Quartile 1 (n= 509) (≤20.9%) |
Quartile 2 (n= 509) (>20.9–23.8%) |
Quartile 3 (n= 509) (>23.8–26.7%) |
Quartile 4 (n=508) (>26.7%) |
P value | |
|---|---|---|---|---|---|
| Age, y | 75.8±8.1 | 72.7±8.0 | 71.8±7.3 | 70.5±7.4 | <0.001 |
| Male | 250 (49.1) | 237 (46.6) | 244 (47.9) | 246 (48.4) | 0.87 |
| Race | 0.07 | ||||
| Black | 117 (23.0) | 127 (25.0) | 120 (23.6) | 123 (24.2) | |
| Chinese | 65 (12.8) | 71 (13.9) | 77 (15.1) | 68 (13.4) | |
| Hispanic | 129 (25.3) | 116 (22.8) | 97 (19.1) | 86 (16.9) | |
| White | 198 (38.9) | 195 (38.3) | 215 (42.2) | 231 (45.5) | |
| Smoking Status | 0.48 | ||||
| Current | 31 (6.1) | 35 (6.9) | 22 (4.3) | 33 (6.5) | |
| Former | 247 (48.5) | 242 (47.5) | 244 (47.9) | 225 (44.3) | |
| Never | 231 (45.4) | 232 (45.6) | 243 (47.7) | 250 (49.2) | |
| Diabetes mellitus | 143 (28.1) | 121 (23.8) | 114 (22.4) | 88 (17.3) | 0.001 |
| Coronary heart disease | 22 (4.3) | 14 (2.8) | 5 (1.0) | 8 (1.6) | 0.003 |
| Atrial fibrillation | 125 (24.6) | 41 (8.1) | 30 (5.9) | 29 (5.7) | <0.001 |
| Anti-hypertensive medication use | 358 (70.3) | 305 (59.9) | 283 (55.6) | 236 (46.5) | <0.001 |
| Physical Exam | |||||
| BMI, kg/m2 | 29.1±5.7 | 28.8±5.9 | 28.4±5.2 | 26.7±5.1 | <0.001 |
| Systolic blood pressure, mmHg | 127.9±20.2 | 126.9±19.1 | 126.0±18.3 | 122.7±18.4 | <0.001 |
| Diastolic blood pressure, mmHg | 68.4±10.3 | 69.2±9.8 | 69.0±9.2 | 68.2±8.6 | 0.26 |
| Heart rate, bpm | 65±10 | 65±10 | 65±9 | 65±9 | 0.97 |
| Laboratory | |||||
| LDL cholesterol, mg/dL | 103.1±36.4 | 106.8±34.1 | 108.2±33.0 | 109.8±36.2 | 0.02 |
| Triglycerides, mg/dL | 89 (64–129) | 85 (59–128) | 88 (65–121) | 79 (57–117) | 0.005 |
| Glucose, mg/dL | 100 (91–114) | 98 (90–113) | 98 (90–109) | 95 (89–104) | <0.001 |
| eGFR, mL/min/1.73 m2 | 73.2±17.8 | 75.7±17.1 | 76.3±17.0 | 77.5±16.7 | 0.001 |
| Log-transformed NTproBNP, pg/mL | 5.14±1.43 | 4.50±1.21 | 4.20±1.16 | 4.14±1.14 | <0.001 |
Categorical variables are reported as n (%). Continuous variables are reported as mean±SD or median (interquartile range) depending on distribution. BMI = body mass index; eGFR = estimated glomerular filtration rate; LDL = low-density lipoprotein; MI = myocardial infarction; NT-proBNP = N-terminal pro-B-type natriuretic peptide
Table 2.
Resting Echocardiographic Measures by Quartile of Resting LA Reservoir Strain.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P value | |
|---|---|---|---|---|---|
| LV structure | |||||
| LVMI, g/m2 | 85.9 (74.8–102.4) | 82.1 (70.0–96.5) | 76.4 (66.5–89.8) | 75.7 (64.8–88.7) | <0.001 |
| LV EDVI, mL/m2 | 39.5 (34.9–45.3) | 40.4 (35.7–47.1) | 39.0 (34.4–45.3) | 40.6 (36.3–46.8) | 0.003 |
| LV function | |||||
| LVEF, % | 62 (58–65) | 62 (60–66) | 63 (60–66) | 64 (61–66) | <0.001 |
| GLS, % | 18.6±2.9 | 19.8±2.5 | 20.4±2.4 | 21.0±2.5 | <0.001 |
| Circumferential strain, % | 17.9±3.7 | 18.3±3.2 | 18.4±3.4 | 18.6±3.2 | 0.01 |
| E velocity, m/sec | 0.78±21.6 | 0.73±0.18 | 0.73±0.15 | 0.76±0.16 | <0.001 |
| A velocity, m/sec | 0.84±0.22 | 0.86±0.22 | 0.85±0.19 | 0.83±0.20 | 0.05 |
| E/A ratio | 0.86 (0.73–1.09) | 0.82 (0.71–0.99) | 0.84 (0.74–1.02) | 0.89 (0.77–1.11) | <0.001 |
| Deceleration time, msec | 199±44 | 205±43 | 200±43 | 203±44 | 0.11 |
| Medial e’ velocity, cm/s | 6.7±1.9 | 7.1±1.8 | 7.6±1.8 | 8.4±2.0 | <0.001 |
| Lateral e’ velocity cm/s | 8.2±2.3 | 8.6±2.1 | 9.1±2.1 | 10.0±2.3 | <0.001 |
| E/e’ average | 10.0 (8.4–12.4) | 9.2 (7.6–11.0) | 8.7 (7.4–10.3) | 8.1 (6.7–9.9) | <0.001 |
| LV early diastolic strain rate, 1/sec | 1.02±0.32 | 1.02±0.28 | 1.09±0.29 | 1.20±0.33 | <0.001 |
| LA structure | |||||
| LAVI, mL/m2 | 31 (26–37) | 27 (23–32) | 25 (21–29) | 24 (21–28) | <0.001 |
| LA function | |||||
| a’ lateral velocity cm/s | 10.5±3.0 | 11.7±2.6 | 12.2±2.6 | 12.1±2.8 | <0.001 |
| a’ septal velocity cm/s | 9.5±2.3 | 10.6±2.0 | 11.3±1.9 | 11.5±1.9 | <0.001 |
| LA booster strain, % | 10.5±2.5 | 12.7±2.2 | 13.9±2.5 | 14.9±2.6 | <0.001 |
| RV structure | |||||
| RV EDA, cm2 | 18.7±4.1 | 18.6±4.1 | 18.4±4.0 | 18.4±3.7 | 0.47 |
| RV ESA, cm2 | 11.4±2.8 | 11.2±2.7 | 11.0±2.7 | 10.9±2.4 | 0.01 |
| RV function | |||||
| TAPSE, cm | 2.05±0.34 | 2.12±0.34 | 2.13±0.34 | 2.21±0.34 | <0.001 |
| RV s’, cm/s | 13.8±3.4 | 13.9±3.0 | 14.1±3.0 | 14.4±2.7 | 0.03 |
| FAC, % | 39.3±4.5 | 40.0±3.9 | 40.3±4.1 | 40.9±4.0 | <0.001 |
| Free wall strain, % | 23.5±4.8 | 24.8±4.9 | 25.2±4.7 | 26.3±4.6 | <0.001 |
| RA structure | |||||
| RA area, cm2 | 17.0 (14.3–20.2) | 16.2 (13.8–18.5) | 15.6 (13.3–18.2) | 15.5 (13.2–17.7) | <0.001 |
| RA function | |||||
| RA reservoir strain, % | 24.3±6.3 | 27.4±5.3 | 28.8±5.5 | 31.4±5.4 | <0.001 |
| RA booster strain, % | 13.4±3.6 | 14.5±3.4 | 15.1±3.6 | 15.4±3.5 | <0.001 |
| Hemodynamics | |||||
| PASP, mmHg | 33.5 (29.6–38.2) | 31.9 (27.9–36.6) | 31.6 (27.5–35.2) | 31.4 (27.5–35.7) | <0.001 |
| SV, mL | 75.1 (63.2–86.7) | 77.4 (65.5–90.5) | 79.1 (66.2–93.0) | 75.4 (65.6–89.6) | 0.007 |
| CO, L/min | 4.6 (3.9–5.4) | 4.8 (4.0–5.7) | 5.0 (4.1–5.9) | 4.8 (4.1–5.8) | <0.001 |
Categorical variables are reported as n (%). Continuous variables are reported as mean±SD or median (interquartile range) depending on distribution. CO = cardiac output; EDA = end diastolic area; EDV = end diastolic volume; ESA = end systolic area; EF = ejection fraction; FAC = fractional area change; GLS = global longitudinal strain; LA = left atrial; LV = left ventricular; PASP = pulmonary artery systolic pressure; RA = right atrial; RV = right ventricular; TAPSE = tricuspid annular systolic plane excursion; SV = stroke volume.
Resting Cardiac Mechanics and 6MWD
After full covariate adjustment, lower LA and RA reservoir strain were each significantly associated with lower 6MWD (Table 3). The association of worse LA reservoir strain with lower 6MWD was consistent among participants with and without history of AF (Figure 1), and there was no interaction by history of AF on the association of RA reservoir strain and 6MWD (Pinteraction = 0.10). In sensitivity analyses, the association of worse LA reservoir strain with lower 6MWD was consistent upon adjustment for AF at the time of echocardiography (β coefficient per SD decrease = −6.1, 95% CI: −9.9, −2.2 meters; P = 0.002), and there was no interaction by AF at echocardiography on this association (P-interaction = 0.91). Additionally, the associations of lower LA reservoir strain (β coefficient per SD decrease = −4.5, 95% CI: −8.4, −0.6 meters; P = 0.02) and RA reservoir strain (β coefficient per SD decrease = −4.4, 95% CI: −7.9, −1.0 meters; P = 0.01) with lower 6MWD remained significant after further adjustment for E/e’. Finally, lower lateral e’ tissue velocity, higher E/e’ ratio, and lower TAPSE were independently associated with reduced 6MWD after full covariate adjustment. In sensitivity analysis, the associations of cardiac mechanics with 6MWD were consistent after further adjustment for total volitional exercise (METS-min per week), a marker of frailty (Supplemental Table 2). In exploratory analysis, there was a modest, statistically significant improvement in prediction of 6MWD by adding LA reservoir strain to a model using Model 2 covariates and E/e’ average (Model R2: 0.46 vs 0.45, P =0.02).
Table 3.
Associations of Resting Cardiac Mechanics with 6MWD.
| Echocardiographic Variable (independent variable) | β coefficient (meters) per SD decrease (95% CI) | P value |
|---|---|---|
| LA Function | ||
| LA reservoir strain | ||
| Model 1* | −13.7 (−17.3, −10.2) | <0.001 |
| Model 2† | −5.0 (−8.8, −1.3) | 0.009 |
| LA booster strain | ||
| Model 1* | −5.1 (−8.6, −1.7) | 0.004 |
| Model 2† | −3.0 (−6.4, 0.5) | 0.10 |
| LV Systolic Function | ||
| Ejection Fraction | ||
| Model 1* | −2.5 (−6.0, 1.1) | 0.17 |
| Model 2† | −0.6 (−3.9, 2.7) | 0.72 |
| GLS | ||
| Model 1* | −8.6 (−12.2, −5.0) | <0.001 |
| Model 2† | −2.5 (−6.0, 1.1) | 0.17 |
| Circumferential strain | ||
| Model 1* | −8.6 (−11.4, −4.1) | <0.001 |
| Model 2† | −1.4 (−5.0, 2.1) | 0.43 |
| LV Diastolic Function | ||
| Lateral e’ velocity | ||
| Model 1* | −8.4 (−12.0, −4.8) | <0.001 |
| Model 2† | −4.8 (−8.3, −1.4) | 0.006 |
| E/e’ average | ||
| Model 1* | 12.4 (8.8, 16.1) | <0.001 |
| Model 2† | 4.9 (1.2, 8.5) | 0.008 |
| Early diastolic strain rate | ||
| Model 1* | −5.9 (−9.7, −2.2) | 0.002 |
| Model 2† | −3.3 (−6.9, 0.4) | 0.08 |
| RA Function | ||
| RA reservoir strain | ||
| Model 1* | −11.3 (−14.7, −7.8) | <0.001 |
| Model 2† | −4.4 (−7.8, −1.1) | 0.01 |
| RA booster strain | ||
| Model 1* | −5.7 (−9.1, 2.2) | 0.001 |
| Model 2† | −2.2 (−5.5, 1.1) | 0.19 |
| RV Function | ||
| TAPSE | ||
| Model 1* | −5.0 (−8.4, −1.6) | 0.004 |
| Model 2† | −3.9 (−7.1, −0.6) | 0.02 |
| FAC | ||
| Model 1* | −3.8 (−7.3, −0.4) | 0.03 |
| Model 2† | −1.1 (−4.3, 2.0) | 0.48 |
| s’ velocity | ||
| >13.7 cm/s | ||
| Model 1* | −1.1 (−6.1, 3.9) | 0.67 |
| Model 2† | −3.7 (−8.3, 0.9) | 0.11 |
| ≤13.7 cm/s | ||
| Model 1* | −4.8 (−9.7, 0.1) | 0.06 |
| Model 2† | −1.4 (−5.9, 3.2) | 0.56 |
| Free wall strain | ||
| Model 1* | −9.9 (−13.4, −6.3) | <0.001 |
| Model 2† | −2.9 (−6.3, 0.5) | 0.10 |
| Hemodynamics | ||
| PASP | ||
| Model 1* | 7.9 (3.9, 11.9) | <0.001 |
| Model 2† | 2.1 (−1.8, 6.0) | 0.29 |
| SV | ||
| Model 1* | 5.9 (2.2, 9.6) | 0.002 |
| Model 2† | −1.9 (−5.8, 2.0) | 0.33 |
| CO | ||
| Model 1* | 10.6 (7.0, 14.1) | <0.001 |
| Model 2†† | 1.9 (−1.7, 5.5) | 0.30 |
Adjusted for age, race/ethnicity, sex, study site, and education status.
Adjusted for Model 1 variables plus smoking status, systolic blood pressure, heart rate, body mass index, diabetes, estimated glomerular filtration rate, anti-hypertensive medication, NT-proBNP, atrial fibrillation, coronary heart disease, stroke, LV mass, and LA volume.
Adjusted for all Model 2 variables except heart rate
CO = cardiac output; FAC = fractional area change; LA = left atrial; LV = left ventricular; RV = right ventricular; SV = stroke volume; TAPSE = tricuspid annular plane systolic excursion
Figure 1. Association of LA Reservoir Strain with 6MWD by AF Status.

Shown are linear models of resting LA reservoir strain and 6MWD by AF status. The rug plot shows distribution of LA reservoir strain. 6MWD = 6-minute walk distance; AF = atrial fibrillation; LA = left atrial
After covariate adjustment, there were no significant associations of LA and RA booster strain, measures of LV systolic function (GLS, circumferential strain, ejection fraction), RV free wall strain, or hemodynamics (PASP, CO, SV) with 6MWD (Table 3). While the relationship between RV s’ velocity and 6MWD was non-linear (Supplemental Figure 4), there was no significant association after covariate adjustment.
Changes in Cardiac Mechanics after Passive Leg Raise and 6MWD
Across the analytic cohort, passive leg raise resulted in modest increases in LA reservoir strain (+2.9% ± 3.5%), lateral e’ tissue velocity (1.3 cm/s ± 1.9 cm/s), and SV (0.6 mL ± 9.2 mL) along with modest decreases in E/e’ average ratio (−1.0±2.1) and LA booster strain (−0.3% ± 3.4%). After full covariate adjustment, less augmentation of LA reservoir strain after passive leg raise was significantly associated with lower 6MWD, independent of resting LA reservoir strain levels (Table 4). The association of less augmentation of LA reservoir strain post-leg raise with lower 6MWD was consistent among participants with and without history of AF (Figure 2). In sensitivity analysis, the association of change in LA reservoir strain with lower 6MWD remained significant after further adjustment for resting E/e’ (β coefficient per SD decrease = −6.0, 95% CI: −9.4, −2.6 meters; P <0.001). There were no associations between changes of the other measures of cardiac mechanics with 6MWD after full covariate adjustment.
Table 4.
Associations of Changes in Cardiac Mechanics after Passive Leg Raise with 6MWD.
| Echocardiographic Variable (independent variable) | β coefficient (meters) per SD decrease (95% CI) | P value |
|---|---|---|
| LA Function | ||
| ΔLA reservoir strain | ||
| Model 1* | −9.9 (−13.5, −6.4) | <0.001 |
| Model 2† | −5.8 (−9.1, −2.5) | <0.001 |
| ΔLA booster strain | ||
| Model 1* | −3.4 (−7.3, 0.4) | 0.08 |
| Model 2† | −2.4 (−6.0, 1.2) | 0.19 |
| LV Diastolic Function | ||
| ΔLateral e’ velocity | ||
| Model 1* | −4.7 (−8.4, −0.9) | 0.02 |
| Model 2† | −2.7 (−6.2, 0.8) | 0.13 |
| ΔE/e’ average | ||
| Model 1* | 6.7 (2.7, 10.8) | 0.001 |
| Model 2† | 3.4 (−0.4, 7.2) | 0.08 |
| Hemodynamics | ||
| ΔSV | ||
| Model 1* | 6.1 (−1.6, 13.8) | 0.12 |
| Model 2† | −3.2 (−10.4, 4.0) | 0.38 |
Adjusted for age, race/ethnicity, sex, study site, education status, and respective resting measure of cardiac mechanics.
Adjusted for Model 1 variables plus smoking status, systolic blood pressure, heart rate, body mass index, diabetes, estimated glomerular filtration rate, anti-hypertensive medication, NT-proBNP, atrial fibrillation, coronary heart disease, stroke, LV mass, and LA volume.
LA = left atrial; LV = left ventricular; SV = stroke volume
Figure 2. Association of Change in LA Reservoir Strain with 6MWD.

Shown are linear models of change in LA reservoir strain after passive leg raise (intravascular volume challenge) and 6MWD by AF status. The rug plot shows distribution of change in LA reservoir strain. 6MWD = 6-minute walk distance; AF = atrial fibrillation; LA = left atrial
Cardiac Mechanics as Mediators of Associations between Cardiovascular Risk Factors and 6MWD
After multivariable adjustment, age, BMI, and non-White race/ethnicity were independently associated with LA reservoir strain, while age, BMI, and sex were independently associated with lateral e’ velocity. All 4 clinical variables (age, BMI, sex, and race) were significantly associated with 6MWD. LA reservoir strain was a modest, partial mediator of the relationships between 1) age and 6MWD, 2) BMI and 6MWD, and 3) non-White race and 6MWD (Figure 3). Specifically, LA reservoir strain appeared to mediate 3% (95% CI: 0.6–5%) of the association between age and 6MWD, 4% (95% CI: 1–8%) of the association between BMI and 6MWD, and 3% (95% CI: 0.6–8%) of the association between non-White race/ethnicity and 6MWD. Lateral e’ velocity was a modest, partial mediator of the relationships between 1) age and 6MWD, 2) BMI and 6MWD, and 3) male sex and 6MWD. Specifically, lateral e’ velocity appeared to mediate 6% (95% CI: 3–9%) of the association between age and 6MWD, 1% (95% CI 0.08–3%) of the association between BMI and 6MWD, and 3% (95% CI: 1–6%) of the association between male sex and 6MWD.
Figure 3. Mediation Analysis of Risk Factors, Cardiac Mechanics, and 6MWD.
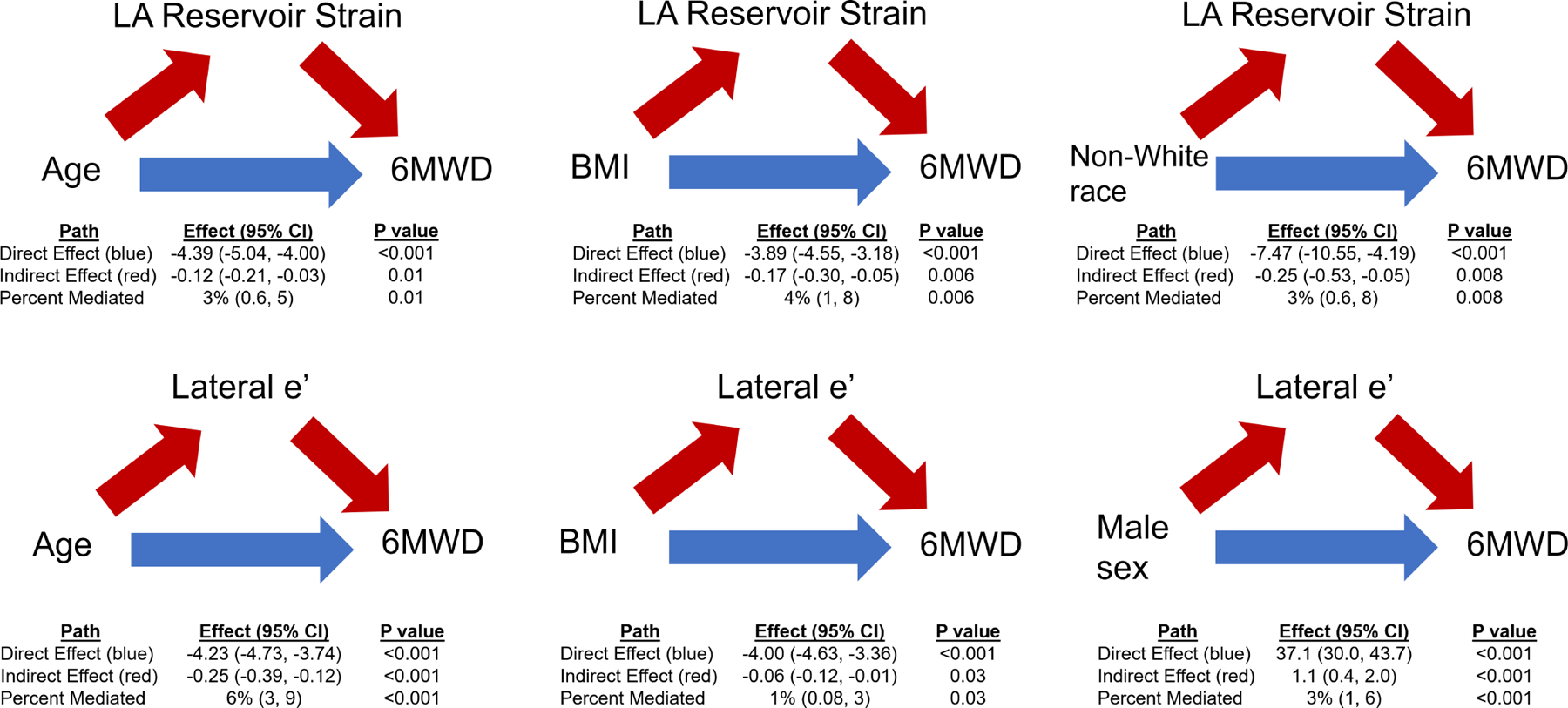
The path models and mediation analyses describing mediation by LA reservoir strain or e’ lateral velocity on the associations between demographics and 6MWD are displayed. Red arrows display the indirect (mediation) effect. Blue arrows display the direct effect.
Discussion
In this analysis, we evaluated the associations of cardiac mechanics both at rest and after a provocative hemodynamic maneuver with exercise capacity, as assessed by 6MWD. Lower resting biatrial reservoir strain and an inability to augment LA strain to intravascular volume challenge (i.e., lack of functional reserve) were each independently associated with lower 6MWD. The associations of worse biatrial function with lower 6MWD remained significant even after adjustment for LV diastolic functional parameters and were consistent among individuals with and without AF. Resting measures of LV diastolic dysfunction were also significantly associated with lower submaximal exercise capacity. Finally, LA reservoir strain and e’ velocity were modest mediators of associations between certain cardiovascular risk factors and reduced exercise capacity.
HF is a syndrome that is defined by marked limitations in physical activity. However, subclinical deterioration in exercise capacity typically occurs secondary to aging and cumulative burden of cardiovascular risk factors and is associated with increased risk of future HF (2,13,14). There is a multidimensional pathogenesis that drives reduced physical fitness secondary to aging, including abnormalities in skeletal muscle bioenergetics, respiratory mechanics, and cardiovascular function (15,16). Evidence has suggested that decreases in exercise capacity with age among individuals without HF is driven primarily by reduced cardiovascular reserve (16–20). Among individuals with prevalent HF, LV diastolic dysfunction, LA size and LA function, but not LV systolic dysfunction, are associated with reduced exercise capacity as measured by cardiopulmonary exercise testing (21,22). Diastolic dysfunction and LA reservoir strain have been previously associated with reduced exercise capacity, as defined by metabolic equivalents, in individuals without HF (20,23–25). Notably, these previous investigations have evaluated study populations with clinical indications for stress testing (20,25) or of relatively small size (23,24). Additionally, such prior analyses investigated limited measures of LA/LV function and did not use 6MWD as a measure of exercise capacity. In the current investigation, we provide further insight into the cardiovascular pathogenesis related to reduced exercise capacity through evaluation of comprehensive, sensitive measures of cardiac mechanics both at rest and after hemodynamic provocation in a large, community-based cohort.
Our findings suggest a specific role of biatrial mechanical dysfunction in driving reduced exercise capacity and HF risk (Central Illustration). While evaluation of LA reservoir strain is not part of standard clinical practice, it has been associated with risk of cardiovascular disease and offers prognostic indication in the setting of HF, and may be incorporated in future clinical practice for diastolic function assessment. Lower LA reservoir function has been associated with increased risk of AF development in older adults and with worse clinical outcomes in those with prevalent HF with preserved ejection fraction (12,26). In our study, reduced resting LA and RA reservoir strain, but not booster strain, were independently associated with lower 6MWD. As such, exercise capacity appears to be more strongly driven by the ability of the atria to stretch and deform as they fill with blood from the systemic venous (RA) and pulmonary venous circulations (LA) during ventricular systole. Reduced LA reservoir strain may thus lead to impaired pulmonary venous drainage and pulmonary edema with exertion that may limit functional capacity. Reduced RA reservoir strain may lead to increased systemic venous congestion and decreased pulmonary vascular flow, leading to poor exercise capacity. Furthermore, an inability to augment LA reservoir strain after passive leg raise was also significantly associated with lower 6MWD. Thus, the lack of functional reserve of the LA to tolerate an intravascular volume challenge further suggests a mechanistic role of LA reservoir dysfunction in driving reduced exercise capacity, and potentially, risk of HF. Taken together, our findings provide insight into the pathophysiologic role of both LA and RA dysfunction in driving reduced exercise capacity, and lend credence to the potential clinical utility of biatrial functional assessment at rest and after leg raise to identify individuals at high risk for HF.
Central Illustration: LA Function, LA Functional Reserve, and 6MWD.

Reduced resting LA reservoir strain and lack of augmentation of LA reservoir strain after passive leg raise (i.e. intravascular hemodynamic challenge) were independently associated with reduced exercise capacity. Additionally, reduced resting RA reservoir strain was associated with lower 6MWD.
Measures of ventricular function provide additional insight into cardiovascular mechanisms of reduced physical fitness. LV diastolic dysfunction (i.e., lower e’ tissue velocity, higher E/e’ ratio) was associated with reduced 6MWD in our study. These findings extend upon previous investigations that demonstrated comparable results among patients with and without HF (3,20,21,23,24). Additionally, we confirm a lack of association between LV ejection fraction and exercise capacity, and extend this finding to more sensitive measures of LV systolic function (LV GLS and circumferential strain). Load-dependent indices of LV systolic function do not appear to be associated with reduced exercise capacity; further investigation is required to understand associations of load-independent indices of LV systolic function with exercise capacity. Finally, reduced RV systolic function as measured by TAPSE was associated with lower 6WMD, while other measures of RV systolic function (FAC, RV s’ velocity, free wall strain), were not. Quantification of RV function remains challenging, and these results suggest that RV systolic dysfunction may be associated with reduced exercise capacity and thus contribute to risk of HF. The association between reduced RV systolic function and lower 6MWD appears to be independent of RV afterload, as there was no significant association of PASP with exercise capacity. Further investigations are required to confirm these findings.
Indices of cardiovascular function modestly mediated the associations between certain demographics and exercise capacity. LA dysfunction appeared to mediate the associations of higher age, higher BMI, and non-White race/ethnicity with lower 6MWD. Diastolic dysfunction appeared to mediate the associations of higher age, higher BMI, and female sex with lower 6MWD. Despite reaching statistical significance, the mediation effect sizes were small, accounting for at most 6% of the association between cardiovascular risk factors and reduced exercise capacity. These findings suggest that cardiac mechanics may not fully explain the association between demographics and reduced functional capacity. However, as opposed to certain risk factors (age, sex), cardiac mechanics may be potentially modifiable. Thus, therapies targeting improvement in these specific domains of cardiovascular function may prevent further decline in exercise capacity and progression to HF.
Our study has strengths and limitations. To our knowledge, this is the first study to assess the associations of comprehensive measures of cardiac mechanics at rest and after leg raise with exercise capacity among individuals without HF. The echocardiographic protocol was standardized across multiple sites, required centralized training, and all images were analyzed an experienced core laboratory. Finally, while cardiopulmonary exercise testing was not systematically performed in this cohort, we utilized an objective, standardized, well-validated, and prognostic measure of exercise capacity (6MWD). Due to the cross-sectional study design, we cannot determine causation or temporal relationships between impaired cardiac mechanics and reduced exercise capacity; it is possible that decreased fitness results in abnormalities in cardiovascular function. However, reduction in cardiac mechanics is known to precede the development of heart failure (6) and is associated with worsening exercise capacity in prevalent HF (3). The MESA Exam 6 cohort is an older and relatively high-risk cohort for HF, as evidenced by the prevalence of comorbidities and relatively low 6MWD of the overall cohort. Further investigation is required to evaluate our findings in cohorts at lower risk for HF. Although it is possible that LA reservoir strain may reflect LV long-axis function given LA-LV coupling, the independent association of LA reservoir strain, but not LV GLS, with 6MWD suggests LA dysfunction itself may be associated with worse exercise capacity. While our study was relatively large in sample size, several participants in MESA were excluded from our study. Those participants who were excluded had higher prevalence of cardiovascular risk factors, suggesting our findings may underestimate associations of cardiac mechanics with exercise capacity.
In a community-based cohort, several domains of cardiovascular function were associated with reduced exercise capacity. Biatrial reservoir dysfunction, lack of LA functional reserve to volume challenge, and LV diastolic dysfunction were consistently associated with lower exercise capacity. These findings highlight a multifaceted cardiovascular pathogenesis of worsening exercise capacity. These domains of cardiac mechanics hold promise in identifying individuals at highest risk for progression, who may benefit from more aggressive preventive measures and clinical monitoring for deterioration. Finally, therapies aimed to improve those domains of cardiovascular function with most robust associations with reduced physical function may prevent progression to clinically manifest HF syndromes.
Supplementary Material
Perspectives.
Competency in Medical Knowledge:
Atrial reservoir function, left atrial functional reserve, and LV diastolic function are associated with exercise capacity and abnormalities identify individuals at risk of developing heart failure (HF).
Translational Outlook:
Better understanding of the relationship between atrial dysfunction and HF may lead to development of new preventive strategies.
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), by grants KL2TR001424, UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS), and by grants R01 HL127028, R01 HL127659, and R01 AG18915 from the NIH.
ABBREVIATIONS
- 6MWD
6-minute walk distance
- AF
atrial fibrillation
- CO
cardiac output
- GLS
global longitudinal strain
- LA
left atrial
- LV
left ventricular
- MESA
Multi-Ethnic Study of Atherosclerosis
- PASP
pulmonary artery systolic pressure
- RV
right ventricular
- SV
stroke volume
- TAPSE
tricuspid annular plane systolic excursion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Sanjiv Shah has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapies, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardiora, CVRx, Cytokinetics, Eisai, GSK, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Sanofi, Shifamed, Tenax, and United Therapeutics. The remaining authors have nothing to disclose.
References
- 1.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 2.Bittner V, Weiner DH, Yusuf S et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA 1993;270:1702–7. [PubMed] [Google Scholar]
- 3.Zotter-Tufaro C, Mascherbauer J, Duca F et al. Prognostic Significance and Determinants of the 6-Min Walk Test in Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail 2015;3:459–466. [DOI] [PubMed] [Google Scholar]
- 4.Beatty AL, Schiller NB, Whooley MA. Six-minute walk test as a prognostic tool in stable coronary heart disease: data from the heart and soul study. Arch Intern Med 2012;172:1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J 2008;29:741–7. [DOI] [PubMed] [Google Scholar]
- 6.Choi EY, Rosen BD, Fernandes VR et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J 2013;34:2354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 8.Heckbert SR, Austin TR, Jensen PN et al. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: The Multi-Ethnic Study of Atherosclerosis. J Electrocardiol 2018;51:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inker LA, Schmid CH, Tighiouart H et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders-van Wijk S, Tromp J, Beussink-Nelson L et al. Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure With Preserved Ejection Fraction: Results From the PROMIS-HFpEF Study. Circulation 2020;142:2029–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 12.Freed BH, Daruwalla V, Cheng JY et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imaging 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florido R, Kwak L, Lazo M et al. Six-Year Changes in Physical Activity and the Risk of Incident Heart Failure: ARIC Study. Circulation 2018;137:2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey A, Cornwell WK 3rd, Willis B et al. Body Mass Index and Cardiorespiratory Fitness in Mid-Life and Risk of Heart Failure Hospitalization in Older Age: Findings From the Cooper Center Longitudinal Study. JACC Heart Fail 2017;5:367–374. [DOI] [PubMed] [Google Scholar]
- 15.Upadhya B, Pisani B, Kitzman DW. Evolution of a Geriatric Syndrome: Pathophysiology and Treatment of Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc 2017;65:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correia LC, Lakatta EG, O’Connor FC et al. Attenuated cardiovascular reserve during prolonged submaximal cycle exercise in healthy older subjects. J Am Coll Cardiol 2002;40:1290–7. [DOI] [PubMed] [Google Scholar]
- 17.Pandey A, Kraus WE, Brubaker PH, Kitzman DW. Healthy Aging and Cardiovascular Function: Invasive Hemodynamics During Rest and Exercise in 104 Healthy Volunteers. JACC Heart Fail 2020;8:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuire DK, Levine BD, Williamson JW et al. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation 2001;104:1350–7. [PubMed] [Google Scholar]
- 19.Wolsk E, Bakkestrom R, Thomsen JH et al. The Influence of Age on Hemodynamic Parameters During Rest and Exercise in Healthy Individuals. JACC Heart Fail 2017;5:337–346. [DOI] [PubMed] [Google Scholar]
- 20.Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA 2009;301:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardin JM, Leifer ES, Fleg JL et al. Relationship of Doppler-Echocardiographic left ventricular diastolic function to exercise performance in systolic heart failure: the HF-ACTION study. Am Heart J 2009;158:S45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Roeder M, Rommel KP, Kowallick JT et al. Influence of Left Atrial Function on Exercise Capacity and Left Ventricular Function in Patients With Heart Failure and Preserved Ejection Fraction. Circ Cardiovasc Imaging 2017;10. [DOI] [PubMed] [Google Scholar]
- 23.Skaluba SJ, Litwin SE. Mechanisms of exercise intolerance: insights from tissue Doppler imaging. Circulation 2004;109:972–7. [DOI] [PubMed] [Google Scholar]
- 24.Okura H, Inoue H, Tomon M et al. Impact of Doppler-derived left ventricular diastolic performance on exercise capacity in normal individuals. Am Heart J 2000;139:716–22. [DOI] [PubMed] [Google Scholar]
- 25.Kusunose K, Motoki H, Popovic ZB, Thomas JD, Klein AL, Marwick TH. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart 2012;98:1311–7. [DOI] [PubMed] [Google Scholar]
- 26.Patel RB, Delaney JA, Hu M et al. Characterization of cardiac mechanics and incident atrial fibrillation in participants of the Cardiovascular Health Study. JCI Insight 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


