Abstract
Positron Emission Tomography (PET) imaging with antibody-based contrast agents frequently uses the radioisotopes [64Cu]Cu2+ and [89Zr]Zr4+. The macrobicyclic chelator commonly known as sarcophagine (sar) is ideal for labeling receptor-targeted biomolecules with [64Cu]Cu2+. The siderophore chelator, desferrioxamine-B (dfo), has been widely used to incorporate [89Zr]Zr4+ into antibodies. Here, we describe new bifunctional chelators of sar and dfo: these chelators have been functionalized with dibromomaleimides (dbm), that enable site-specific and highly stable attachment of molecular cargoes to reduced, solvent-accessible, interstrand native disulfide groups. The new sar–dbm and dfo–dbm derivatives can be easily conjugated with the IgG antibody trastuzumab via reaction with reduced interstrand disulfide groups to give site-specifically modified dithiomaleamic acid (dtm) conjugates, sar–dtm–trastuzumab and dfo–dtm–trastuzumab, in which interstrand disulfides are rebridged covalently with a small molecule linker. Both sar– and dfo–dtm–trastuzumab conjugates have been radiolabeled with [64Cu]Cu2+ and [89Zr]Zr4+, respectively, in near quantitative radiochemical yield (>99%). Serum stability studies, in vivo PET imaging, and biodistribution analyses using these radiolabeled immunoconjugates demonstrate that both [64Cu]Cu-sar–dtm–trastuzumab and [89Zr]Zr-dfo–dtm–trastuzumab possess high stability in biological milieu. Dibromomaleimide technology can be easily applied to enable stable, site-specific attachment of radiolabeled chelators, such as sar and dfo, to native interstrand disulfide regions of antibodies, enabling tracking of antibodies with PET imaging.
Monoclonal IgG antibody therapies have been transformative in the treatment of many cancers and autoimmune diseases, and new antibody therapies are likely to have further clinical impact. The ability to quantitatively image antibody biodistribution at the whole-body level can help predict individual patient response, as well as aid in understanding treatment outcomes during clinical development.1,2 Positron-emitting radiometallic isotopes attached to antibodies via chelators have been widely used to image antibody biodistribution using Positron Emission Tomography (PET).
Conventional labeling strategies have attached chelators to antibodies using electrophilic groups such as isothiocyanates, N-hydroxysuccinimides, and anhydrides that react with solvent accessible primary amines of lysine side chains.3−5 These bioconjugation methods are simple, but as these reactions are not site-specific and lack stoichiometric control, the presence of multiple lysine side chains leads to heterogeneous product mixtures. The resulting chelator–antibody conjugates often demonstrate reduced affinity for target receptors, particularly at high chelator:antibody conjugation ratios.6−8 Several recent and elegant site-specific strategies include enzyme-mediated conjugation of chelators to (i) glycan regions of IgG antibodies using a combination of β-1,4-galactosidase/β-1,4-galactosyltransferase(Y289L),9−11 (ii) hinge region glutamine residues of IgG antibodies using a combination of deglycosylation with N-glycosidase followed by chelator coupling with bacterial transglutaminase,12 and (iii) modified C-termini of antibody fragment derivatives using the bacterial enzyme sortase A.13 Alternatively, incorporation of a non-natural amino acid containing an azide group in an IgG antibody has enabled orthogonal and site-selective conjugation of chelators using click chemistry.14 These approaches have resulted in radiolabeled chelator–protein conjugates that display improved PET imaging capabilities relative to radiolabeled conjugates prepared using conventional, non-site-specific methods. However, these enzyme-based or protein engineering-based conjugation methods are more difficult to implement than conventional, non-site-specific conjugation technologies. Furthermore, modification or removal of glycan regions can modify the pharmacokinetics of antibodies, as well as affect their Fc-receptor binding properties and antibody-dependent cell-mediated toxicity.15−17
Maleimide derivatives have been widely used to incorporate radiolabeled chelators into IgG antibodies and other proteins.18−21 Maleimides react selectively with reduced disulfides at near-neutral pH, and in the case of IgG antibodies, reduction of the four solvent-accessible, interstrand disulfide bonds leads to the presence of eight available thiol attachment points. While maleimides enable site-selective attachment of chelators and other cargoes to IgG antibodies, these conjugates are unstable: the resulting thioether can undergo a retro-Michael reaction in biological milieu, converting back to the starting thiol and maleimide.21−24In vivo, the starting maleimide motif, still tethered to its payload, can then react with endogenous molecules containing bioavailable thiols, such as glutathione and albumin. In radionuclide imaging, this can potentially result in accumulation of radioactivity at off-target sites, decreasing image contrast, sensitivity, and the ability to quantify antibody distribution.
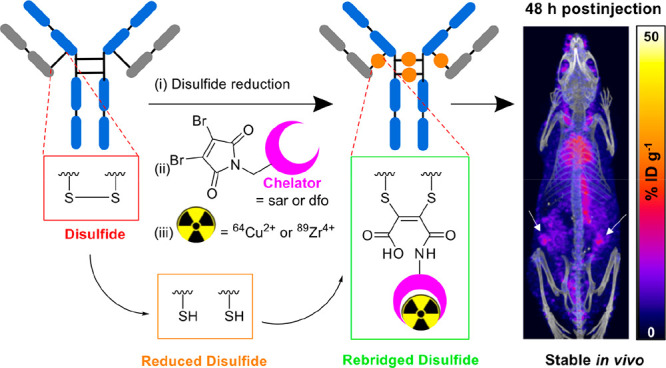
New conjugation platforms including bissulfone,25 divinylpyrimidine,26 divinyltriazine,27 dibromoalkyl oxetane,28 dibromopyridazinedione,29,30 and disubstituted maleimide31−36 derivatives enable site-specific attachment of cargo to dithiol groups of antibodies, including those at IgG hinge regions. Here, we focus on dibromomaleimide: this motif reacts specifically with two reduced thiol groups of antibodies, thus enabling concomitant attachment of cargo and rebridging of two cysteines. The resulting dithiomaleimide can be hydrolyzed to a dithiomaleamic acid under mildly basic conditions to give homogeneous antibody conjugates.32−35 Importantly, the dithiomaleamic acid conjugates are unreactive toward serum thiols and do not undergo retro-Michael reactions in biological media, unlike conventional maleimide derivatives. This dibromomaleimide platform has the potential to enable highly stable site-specific radiolabeling of antibodies at hinge region disulfides.
Zirconium-89 (t1/2 = 78 h, β+Emax = 897 keV, 23%) and copper-64 (t1/2 = 12.7 h, β+Emax = 656 keV, 18%) have both been used for imaging antibody distribution with PET: the half-lives of these isotopes match the time required for antibodies to clear circulation and accumulate in target tissue (1 day–1 week). Derivatives of a naturally occurring siderophore, desferrioxamine-B (dfo), are commonly used to incorporate [89Zr]Zr4+ into monoclonal IgG antibodies for clinical and preclinical PET imaging.1,2,4,11,18,19 The dfo chelator contains three hydroxamate groups, enabling hexadentate O6 coordination of Zr4+. Many chelators have been developed for [64Cu]Cu2+ over the past three decades. Peptide and protein conjugates of the hexaazamacrobicyclic chelator, 3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosane, known commonly as “sarcophagine” (sar), have proved to be simple to radiolabel and are highly stable to demetalation in vivo, leading to high quality PET images.3,10,13,37−39 Here we describe preparation of dibromomaleimide derivatives of dfo and sar chelators and show the usability and applicability of this dibromomaleimide platform for site-specific radiolabeling of an IgG1 antibody with [89Zr]Zr4+ and [64Cu]Cu2+.
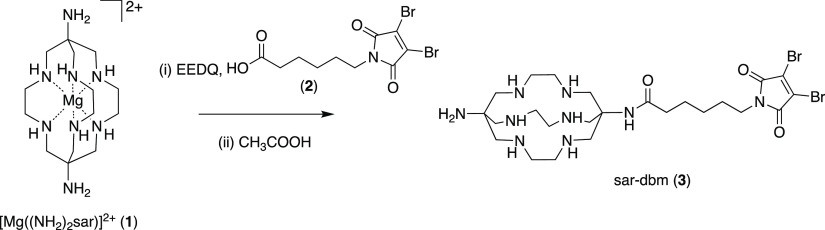
Preparation of sar–dbm and dfo–dbm Bifunctional Chelators
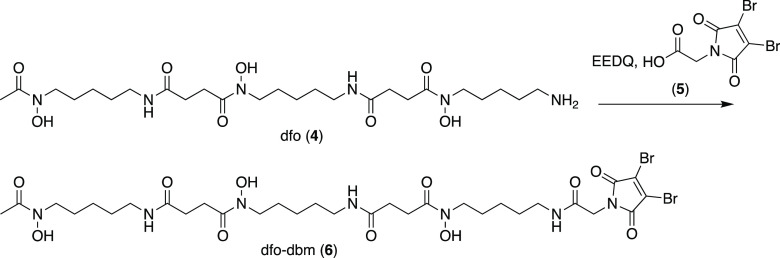
The 1,8-diamino-3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosane, or “(NH2)2sar”, chelator is a useful precursor for preparation of bioconjugates. It contains two primary amines and six secondary amines, all of which are reactive toward electrophiles.40 Prior studies have achieved selective functionalization of primary amines by “protecting” secondary amine groups through metal ion (Cu2+ or Mg2+) complexation.37−39 We elected to use [Mg((NH2)2sar)]2+ (1) as a precursor,39 as following derivatization, Mg2+ can be easily removed from the chelator by acidification. [Mg((NH2)2sar)]2+ was reacted with 3,4-dibromomaleimide-N-hexanoic acid (2) and coupling agent N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) in acetonitrile (Scheme 1). The resulting product mixture was treated with acetic acid, prior to purification using reverse-phase HPLC, to yield sar–dbm (3) in 8% yield. Similarly, dfo (4) was reacted with dibromomaleimide-N-glycine (5) and EEDQ in dimethyl sulfoxide, followed by purification using reverse-phase HPLC to give dfo–dbm (6, Scheme 2) in 22% yield.
Scheme 1. Synthesis of sar–dbm.
Scheme 2. Synthesis of dfo–dbm.
The inclusion of a C6 aliphatic linker group in sar–dbm aided reverse-phase HPLC purification and isolation of sar–dbm: the sar chelator motif is very hydrophilic and is poorly retained on reverse-phase columns in the absence of hydrophobic appendages. In contrast, the dfo chelator had sufficient adsorption on reverse-phase stationary phases, and so a C2 linker group, which has demonstrated improved conjugation properties relative to a C6 linker (vide infra),34 was utilized.
The yield of sar–dbm (8%) was significantly lower than the yield of dfo–dbm (22%). We attribute this to the lower reactivity of the apical primary amine groups of (NH2)2sar and its complexes relative to other primary amines, possibly due to steric encumbrance of the macrobicyclic hexaamine rings. Previously reported syntheses, in which (NH2)2sar complexes have been reacted with electrophilic motifs, have required significant heating.37−40 Here, the reaction was not heated, as the dibromomaleimide group is sensitive to heat. Although the yield of sar–dbm was low under these reaction conditions, it could be obtained in a one-pot synthesis from [Mg((NH2)2sar)]2+.
The compound sar–dbm2, in which both apical primary amines of (NH2)2 sar react with 2 equiv of 3,4-dibromomaleimide-N-hexanoic acid to yield a sarcophagine chelator containing two dibromomaleimide groups, was also observed. Although we have detected and even isolated small amounts of sar–dbm2 and its Mg2+ complex, [Mg(sar–dbm2)]2+, analyses of crude reaction mixtures revealed that it was present in low yield (see the Supporting Information).
Radiolabeled sar–dtm–Trastuzumab and dfo–dtm–Trastuzumab
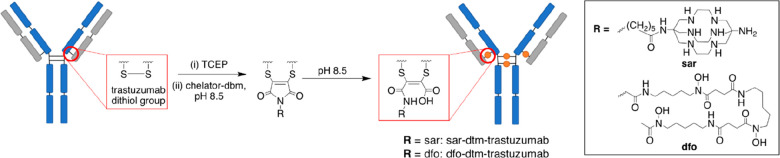
The monoclonal antibody trastuzumab was chosen as a model antibody for assessing conjugation of sar–dbm and dfo–dbm: trastuzumab targets the human epidermal growth factor receptor 2 (HER2) and is widely used for treatment of HER2-positive breast cancer. The four solvent-accessible interstrand disulfide bonds of trastuzumab were reduced with tris(2-carboxyethyl)phosphine) (TCEP), the reduced antibody was reacted with 8 mol equiv of either sar–dbm or dfo–dbm at pH 8.5, and the conjugation reactions were monitored over time by SDS-PAGE (vide infra). These reaction conditions were adapted from well-established methods for coupling drug-dibromomaleimide or fluorophore-dibromomaleimides to IgG1 constructs.31,34,41 Notably, increasing the molar equivalents of chelator–dbm relative to the trastuzumab antibody, for example, from 8 mol equiv of sar–dbm to either 16 or 24 mol equiv of sar–dbm, did not significantly affect the efficacy of the conjugation reaction (Figure S1).
For reaction of either sar–dbm or dfo–dbm with reduced trastuzumab, thiol substitution to give dithiomaleimide conjugates proceeded rapidly (within 5 min). Subsequent hydrolysis of the dithiomaleimide to the dithiomaleamic acid (dtm) was achieved by further incubation of the reaction solutions at pH 8.5 for 2–48 h (Scheme 3). Prior studies on this class of compounds have shown that dithiomaleimide hydrolysis proceeds faster in the presence of electron-withdrawing imide substituents: significantly, the proximity of the amide bond to the maleimide motif increases the rate of hydrolysis.34 For dfo–dtm–trastuzumab, with a C2 glycine-derived linker, hydrolysis (as monitored by SDS-PAGE) was complete within 2 h (Figure S2). The sar–dtm–sarcophagine conjugate, with a longer C6 aminohexanoate linker, was incubated for 48 h at 37 °C to ensure complete hydrolysis (Figure S1).
Scheme 3. Preparation of Chelator–dtm–Trastuzumab Immunoconjugates.
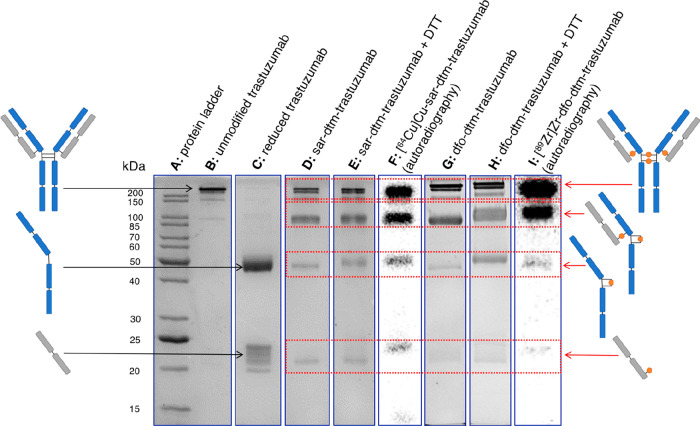
As expected, the SDS-PAGE of TCEP-reduced trastuzumab showed the presence of separate heavy and light chains under the denaturing analysis conditions (Figure 1, lane C). Following dithiol rebridging reactions with chelator–dbm (for example sar–dbm, Figure 1, lane D, and dfo–dbm, Figure 1, lane G) and subsequent hydrolysis, the two major products observed corresponded to the “full antibody”, in which the maleamic acid groups bridge inter chain dithiols at the hinge region, and the “half antibody”, in which the maleamic acid groups bridge intra chain dithiols at the hinge region. The “full antibody” rebridging pattern mirrors that of the native trastuzumab antibody. The “half antibody” corresponds to a covalently linked single heavy chain + single light chain and is typical of this type of reaction.31,34 It arises as a result of “disulfide scrambling” and is only observed by harsh denaturing analysis. Less intense bands, corresponding to antibody heavy chains and light chains that remained “unbridged”, were also discernible, and these were more prominent for sar–dtm–trastuzumab than dfo–dtm–trastuzumab.
Figure 1.
SDS-PAGE of chelator–dtm–trastuzumab conjugates. The full gels are included in Figures S3–S5.
The same conjugation products were also analyzed using reducing SDS-PAGE conditions: dithiothreitol (DTT) was incubated with the antibody conjugate prior to running the SDS-PAGE. The same two “full antibody” and “half antibody bands” were observed (Figure 1, lanes E and H), indicating that (i) the conjugation and subsequent hydrolysis reactions to yield maleamic acid-conjugated motifs were complete, and (ii) the rebridged products were stable in the presence of excess thiols, consistent with prior reports detailing the high stability of such conjugates.31,34
The bioconjugates sar–dtm–trastuzumab and dfo–dtm–trastuzumab were also characterized by ESI-HRMS, which showed that both sar–dtm–trastuzumab and dfo–dtm–trastuzumab contain two–four copies of each chelator. The most intense signals in the deconvoluted ESI-HRMS of trastuzumab conjugates (Tables 1, S2, Figures S6, S7) corresponded to “half antibody”. Signals corresponding to “full antibody” were weak, which is a common feature of such immunoconjugates as the smaller fragments are always detected with greater relative intensity. Additionally, all deconvoluted “half antibody” and “full antibody” signals were significantly broader than that observed for similarly prepared and characterized alkyne–trastuzumab immunoconjugates.34,42 We attribute this peak broadening to sar–dtm–trastuzumab and dfo–dtm–trastuzumab readily complexing trace metal ions: we have previously observed trace metal binding to chelator conjugates in our mass spectrometric analyses.43,44
Table 1. ESI-MS Data for Chelator–Trastuzumab Conjugates.
| half antibody conjugatea | m/z (observed) | m/z (calculated) |
|---|---|---|
| sar–dtm–trastuzumab | ||
| HL + 1 sar | 73125 | 73116 |
| HL + 2 sar | 73647 | 73639 |
| dfo–dtm–trastuzumab | ||
| HL + 1 dfo | ||
| HL + 2 dfo | 74015 | 74017 |
Abbreviation: HL = heavy chain + light chain (i.e., half antibody).
The combination of SDS-PAGE and ESI-HRMS data indicates that the new immunoconjugates consist of a heterogeneous mixture, comprised of predominantly “full antibody” and “half antibody” constructs (with low amounts of conjugate in which some heavy and light chains remain “unbridged”), and contain two–four chelators per antibody. This heterogeneity, and in particular the presence of both “full antibody” and “half antibody” constructs, is unlikely to significantly modify the immunoconjugates’ affinity for the target HER2 receptor or their biodistribution in vivo: we have previously shown that mixtures containing similar “disulfide-scrambled” trastuzumab species exhibit comparable antigen-binding and Fc-binding properties to native trastuzumab.42
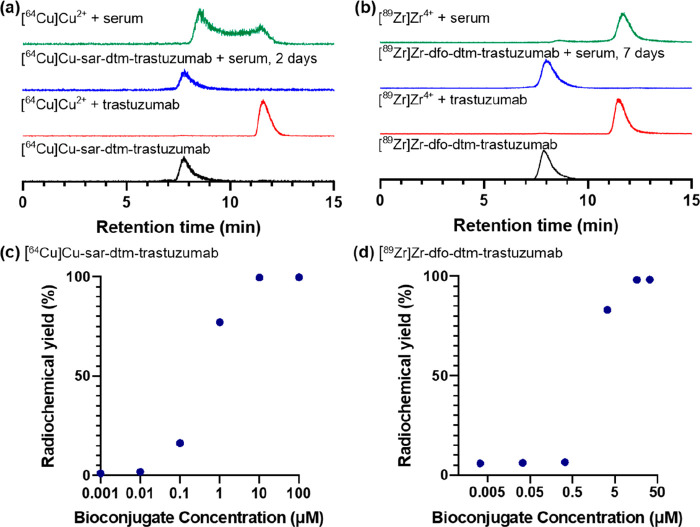
The immunoconjugate sar–dtm–trastuzumab was radiolabeled with [64Cu]Cu2+ at ambient temperature and pH 7 in 5 min, by addition of a solution of [64Cu]Cu2+ (5 MBq, 30 μL, 0.1 M NH4OAc) to the immunoconjugate (9.43 μL, 100 μg, 0.1 M NH4OAc), and analyzed by size exclusion (SE) HPLC. In SE-HPLC, a PBS mobile phase containing ethylenediamine tetraacetate (EDTA) was used, and under these conditions, [64Cu]Cu-sar–dtm–trastuzumab eluted at 7.8 min (Figure 2a, bottom trace) and unreacted [64Cu]Cu2+ eluted at 11.6 min as [64Cu][Cu(EDTA)]2–. This reaction provided [64Cu]Cu-sar–dtm–trastuzumab in near quantitative radiochemical yield (>98%). Similarly, dfo–dtm–trastuzumab (10 μL, 72.5 μg, 0.1 M NH4OAc) was radiolabeled with [89Zr]Zr4+ (0.1 MBq, 5 μL, 0.2 M HEPES) at room temperature and pH 7 in 10 min, to yield [89Zr]Zr-dfo–dtm–trastuzumab in 99% radiochemical yield (Figure 2b, bottom trace). In contrast, when a sample of unmodified trastuzumab was reacted with either [64Cu]Cu2+ or [89Zr]Zr4+, <2% of radioactivity was associated with the antibody, with the majority of unreacted radiometal eluting at 11.6 min (Figure 2a,b).
Figure 2.
(a) SE-HPLC radiochromatograms of [64Cu]Cu-sar–dtm–trastuzumab (black), [64Cu]Cu2+ with unmodified trastuzumab (red), [64Cu]Cu-sar–dtm–trastuzumab incubated in serum for 2 days (blue), and [64Cu]Cu2+ incubated in serum (green). (b) SEC-HPLC radiochromatograms of [89Zr]Zr-dfo–dtm–trastuzumab (black), [89Zr]Zr4+ with unmodified trastuzumab (red), [89Zr]Zr-dfo–dtm–trastuzumab incubated in serum for 7 days (blue), and [89Zr]Zr4+ incubated in serum (green). (c) Radiochemical yields for the reaction of [64Cu]Cu2+ with sar–dtm–trastuzumab at different concentrations (100 μM, 10 μM, 1 μM, 100 nM, 10 nM, and 1 nM) of immunoconjugate at ambient temperature for 5 min. (d) Radiochemical yields for the reaction of [89Zr]Zr4+ with dfo–dtm–trastuzumab at different concentrations (33 μM, 16.7 μM, 3.3 μM, 333 nM, 33 nM, and 3 nM) of immunoconjugate at ambient temperature for 10 min.
[64Cu]Cu-sar–dtm–trastuzumab and [89Zr]Zr-dfo–dtm–trastuzumab were additionally analyzed by SDS-PAGE with bright field imaging and autoradiography: a radioactivity signal from radiolabeled immunoconjugates was coincident with stained protein bands (Figure 1, lanes F and I). For [64Cu]Cu-sar–dtm–trastuzumab, 41.1% of 64Cu signal was associated with “full antibody”, 33.4% was associated with “half antibody”, 14.5% was associated with single heavy chain fragments, and 10.6% was associated with single light chain fragments. For [89Zr]Zr-dfo–dtm–trastuzumab, 54.4% of 89Zr signal was associated with “full antibody”, 34.9% was associated with “half antibody”, 7.7% was associated with single heavy chain fragments, and 3.0% was associated with single light chain fragments. The comparatively lower amount of radiolabeled single heavy chain and single light chain fragments for [89Zr]Zr-dfo–dtm–trastuzumab relative to [64Cu]Cu-sar–dtm–trastuzumab suggested that dithiol rebridging was more efficient for dfo–dbm than sar–dbm.
To assess the serum stability of [64Cu]Cu-sar–dtm–trastuzumab and [89Zr]Zr-dfo–dtm–trastuzumab ex vivo, the radiolabeled immunoconjugates were incubated in human serum at 37 °C. [64Cu]Cu-sar–dtm–trastuzumab and [89Zr]Zr-dfo–dtm–trastuzumab both exhibited high stability in the presence of serum proteins: SE-HPLC radiochromatographic analysis indicated that >99% of [64Cu]Cu-sar–dtm–trastuzumab remained intact after 2 days incubation in serum and >99% of [89Zr]Zr-dfo–dtm–trastuzumab remained intact after 7 days incubation in serum (Figure 2 a,b, S8, S9). In contrast, when [64Cu]Cu2+ was incubated in serum, SE-HPLC analysis showed that the majority of [64Cu]Cu2+ was complexed by a serum protein (retention time of 8.5 min) or low molecular weight species (retention time of 11.6 min). When [89Zr]Zr4+ was incubated in serum, the majority of radioactivity was associated with low molecular weight species (retention time of 11.8 min).
For preparations of radiopharmaceuticals based on chelator–protein bioconjugates, it is important that the amount of the precursor bioconjugate is relatively low. Typically, unlabeled immunoconjugate is not separated from the radiolabeled conjugate prior to in vivo administration, and high concentrations of unlabeled bioconjugate lead to in vivo receptor “blocking”, compromising PET image contrast. Furthermore, the ability to radiolabel in near quantitative radiochemical yields obviates purification steps. To assess the ability of sar–dtm–trastuzumab or dfo–dtm–trastuzumab to complex [64Cu]Cu2+ or [89Zr]Zr4+ respectively, solutions containing radiometal were added to increasingly dilute solutions of immunoconjugate (Figure 2c,d). Near quantitative radiochemical yields (>99%) of [64Cu]Cu-sar–dtm–trastuzumab were achieved at immunoconjugate concentrations as low as 10 μM, pH 7 and ambient temperature, with only 5 min reaction time. At a lower sar–dtm–trastuzumab concentration of 1 μM, a high radiochemical yield of 77% was still obtained. Near quantitative radiochemical yields (>99%) of [89Zr]Zr-dfo–dtm–trastuzumab were achieved at 16 μM of dfo–dtm–trastuzumab at pH 7, ambient temperature and only 10 min reaction time. At a lower concentration of 3 μM, a relatively high radiochemical yield of 83% was still observed.
In Vitro and in Vivo Characterization of [64Cu]Cu-sar–dtm–Trastuzumab
The binding of [64Cu]Cu-sar–dtm–trastuzumab to its target receptor, HER2, was evaluated in HER2-positive human ovarian cancer cells (SKOV3 cells). [64Cu]Cu-sar–dtm–trastuzumab retained specificity for HER2 receptor: the radiotracer showed uptake in SKOV3 cells, with uptake inhibited by increasing concentrations of unmodified trastuzumab (Figure S10).
We have previously assessed in vivo stability of radiometal–chelator complexes by quantifying the biodistribution of their IgG-based immunoconjugates in healthy mice over an extended period of time.3,19 In healthy mice, antibodies such as trastuzumab have long blood half-lives in the absence of diseased tissues/tumors that are positive for the target receptor. Here, in vivo studies in healthy animals aimed to assess the biodistribution and stability of [64Cu]Cu-sar–dtm–trastuzumab in vivo. [64Cu]Cu-sar–dtm–trastuzumab (1.0–2.5 MBq) was administered intravenously (via tail vein) to NOD scid gamma female mice. At 2 h, 1 day, and 2 days postinjection, PET/CT images were acquired, mice were culled, and organs and serum samples were collected for ex vivo analysis (Figures 3, S11).
Figure 3.
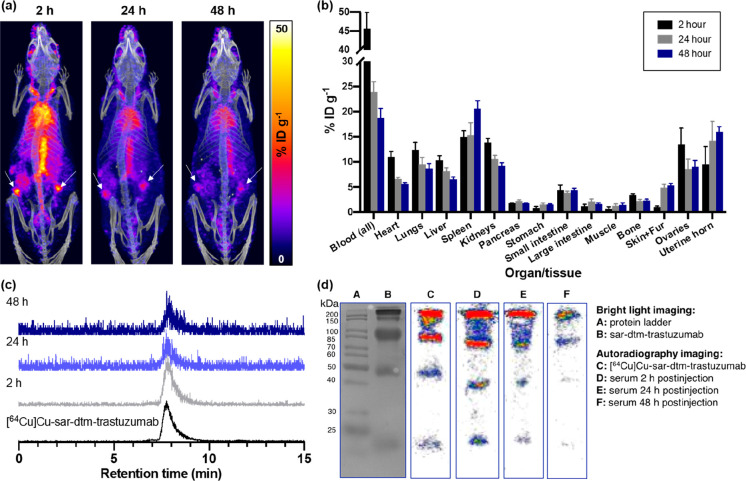
(a) PET/CT maximum intensity projections of a healthy female NOD scid gamma mouse administered [64Cu]Cu-sar–dtm–trastuzumab, at 2, 24, and 48 h postinjection. White arrows indicate ovaries that are positive for HER2. (b) Ex vivo biodistribution of [64Cu]Cu-sar–dtm–trastuzumab (n = 4 mice per time point). Error bars correspond to standard deviation. (c) SE-HPLC analysis of ex vivo serum samples shows a single radioactive signal in each chromatogram, with the retention time of each signal corresponding to [64Cu]Cu-sar–dtm–trastuzumab. (d) SDS-PAGE of ex vivo serum samples (bright light and autoradiography imaging) shows radioactive bands corresponding to [64Cu]Cu-sar–dtm–trastuzumab. The full gels are included in Figures S12, S13.
PET/CT imaging and ex vivo biodistribution (Figures 3a,b, S11) showed that significant 64Cu radioactivity remained in blood circulation over 1–2 days postinjection, although as typically observed, this activity decreased from 45.6 ± 4.3%ID (percentage injected dose) g–1 at 2 h postinjection to 18.7 ± 1.9%ID g–1 at 2 days postinjection. Importantly, 64Cu radioactivity in the liver did not increase from 2 h to 2 days postinjection. Prior studies3,45 have shown that loss of [64Cu]Cu from a bioconjugate (likely via transchelation to endogenous proteins including albumin in blood46 and superoxide dismutase47 and ceruloplasmin48 in the liver) results in increased accumulation of [64Cu]Cu in the liver over time. Our biodistribution data is consistent with prior reports that detail exceptional stability of [64Cu][Cu(sar)]2+ conjugates (both peptide and protein conjugates) in vivo.3,45
Radioactivity uptake and retention in healthy HER2-expressing tissue also indicated that [64Cu]Cu-sar–dtm–trastuzumab retains affinity for its target HER2 receptor (Figures 3a,b, S11). Notably, skin, uterine horn, and ovaries–healthy tissues that are known to express HER249,50 −showed either increased uptake or persistent retention of 64Cu radioactivity over the course of the study. For example, radioactivity uptake in skin increased from 1.0 ± 0.2%ID g–1 at 2 h postinjection to 4.9 ± 0.7%ID g–1 at 1 day postinjection (p = 3.1 × 10–5); at 2 days postinjection, skin samples showed retention of 64Cu radioactivity (5.3 ± 0.4%ID g–1).
Serum samples were obtained from mice administered [64Cu]Cu-sar–dtm–trastuzumab at 2 h, 1 day, and 2 days postinjection and analyzed by SE-HPLC and SDS-PAGE. In each SE-HPLC chromatogram, only a single signal was observed, with a retention time matching that of [64Cu]Cu-sar–dtm–trastuzumab (Figure 3c). Serum samples were also subjected to SDS-PAGE separation, followed by autoradiography imaging, which revealed radioactive bands (Figure 3d, lanes D–F) that corresponded to denatured fragments of both unlabeled sar–dtm–trastuzumab (Figure 3d, lane B) and [64Cu]Cu-sar–dtm–trastuzumab (Figure 3d, lane C). Significantly, neither SE-HPLC nor SDS-PAGE analyses revealed any radiolabeled species with molecular weights corresponding to serum albumin (with a molecular weight of 66.5 kDa), which is known to (i) react with maleimide derivatives released in vivo as a result of intramolecular retro-Michael reactions of bioconjugates, resulting in transfer of maleimide groups and their cargo from bioconjugates to albumin,21−24 and additionally, (ii) compete with [64Cu]Cu-radiolabeled chelator conjugates of peptides and proteins for [64Cu]Cu2+ binding, resulting in release of [64Cu]Cu from chelator conjugates.46 In short, our experiments evidence the high stability of [64Cu]Cu-sar–dtm–trastuzumab in vivo.
Concluding Remarks: Usability of Chelators Containing Dibromomaleimide Groups for Site-Specific Radiolabeling of an IgG Antibody
The majority of chelator–antibody conjugates are prepared from chelators containing reactive electrophilic groups that react with solvent accessible primary amines of lysine side chains. While these conjugation protocols are very simple to implement (even for researchers inexperienced in protein chemistry), such methods mostly lead to heterogeneous mixtures of chelator–protein conjugates that can exhibit suboptimal pharmacokinetics and decreased affinity for target receptors.6−8 Immunoconjugates containing conventional maleimide groups can enable site-specific attachment to reduced thiol groups of disulfide bonds of antibody derivatives, but the resulting conjugates undergo retro-Michael reactions in the biological milieu, resulting in loss of radioactive cargo.21−24 Many new chemical innovations in site-specific modification of antibodies have been adapted to stably incorporate chelators into antibodies and their derivatives;9−14 however, these methods involve enzymatic reactions, engineering of specific peptide sequences into the antibody and/or multistep procedures, and are therefore not simple to implement and tend to require extensive optimization for them to work reliably.
Dibromomaleimide chemistry is superbly suited to simple, stable, and site-specific incorporation of radionuclides into antibodies for receptor-targeted molecular imaging. We have prepared dibromomaleimide derivatives of (i) a dfo chelator, commonly used for [89Zr]Zr4+ radiolabeling of antibodies, and (ii) a sarcophagine chelator, which is considered state-of-the-art for [64Cu]Cu2+ radiolabeling of proteins and peptides, and successfully incorporated both new bifunctional chelators into the HER2-targeted IgG antibody, trastuzumab. These are the first examples of radiometal-labeled chelator–antibody conjugates that have been prepared using dibromomaleimide technology, enabling site-specific attachment of two different chelators to the IgG trastuzumab antibody. Importantly, the methods we describe are simple to implement and produce chelator–antibody conjugates that can be readily radiolabeled with the PET radiometals [89Zr]Zr4+ and [64Cu]Cu2+ in near-quantitative radiochemical yields at ambient temperature. We have evidenced the high stability of [64Cu]Cu-sar–dtm–trastuzumab both in serum and in vivo: it is stable to both demetalation of [64Cu]Cu, consistent with the known high stability of [64Cu][Cu(sar)]2+ complexes, and transfer of the [64Cu][Cu(sar)]2+ to endogenous thiols, consistent with the reported stability of dithiomaleamic acid groups. Our future efforts will compare the biological properties of these site-specifically radiolabeled immunoconjugates with radiolabeled immunoconjugates modified using either conventional maleimide conjugation chemistry or non-site-specific (stochastic) conjugation chemistry.
Acknowledgments
This research was supported by a Cancer Research UK Career Establishment Award (C63178/A24959), King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1), the NPIF EPSRC Doctoral King’s College London 2017 (EP/R512552/1), the EPSRC programme for Next Generation Molecular Imaging and Therapy with Radionuclides (EP/S019901/1, “MITHRAS”), the Wellcome Multiuser Equipment Radioanalytical Facility funded by Wellcome Trust (212885/Z/18/Z), the Centre for Medical Engineering funded by the Wellcome Trust and the Engineering and Physical Sciences Research Council (WT088641/Z/09/Z), and EPSRC grant EP/R034621/1. The King’s College London Centre for Biomolecular Spectroscopy is funded by Wellcome Trust (202762/Z/16/Z) and the British Heart Foundation (IG/16/2/32273). N.J.L. is grateful for a Royal Society Wolfson Research Merit award.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.bioconjchem.0c00710.
Experimental descriptions, full SDS-PAGE data, ESI-MS, NMR, HPLC chromatograms, PET/CT image analysis, cell binding study data (PDF)
The authors declare the following competing financial interest(s): We declare that V.C. and J.R.B. are Directors of the UCL spin-off ThioLogics.
Supplementary Material
References
- Bensch F.; van der Veen E. L.; Lub-De Hooge M. N.; Jorritsma-Smit A.; Boellaard R.; Kok I. C.; Oosting S. F.; Schröder C. P.; Hiltermann T. J. N.; van der Wekken A. J.; et al. (2018) 89Zr-Atezolizumab Imaging as a Non-Invasive Approach to Assess Clinical Response to PD-L1 Blockade in Cancer. Nat. Med. 24, 1852–1858. 10.1038/s41591-018-0255-8. [DOI] [PubMed] [Google Scholar]
- Jackson J. A.; Hungnes I. N.; Ma M. T.; Rivas C. (2020) Bioconjugates of Chelators with Peptides and Proteins in Nuclear Medicine: Historical Importance, Current Innovations, and Future Challenges. Bioconjugate Chem. 31, 483–491. 10.1021/acs.bioconjchem.0c00015. [DOI] [PubMed] [Google Scholar]
- Cooper M. S.; Ma M. T.; Sunassee K.; Shaw K. P.; Williams J. D.; Paul R. L.; Donnelly P. S.; Blower P. J. (2012) Comparison of 64Cu-Complexing Bifunctional Chelators for Radioimmunoconjugation: Labeling Efficiency, Specific Activity, and in Vitro/in Vivo Stability. Bioconjugate Chem. 23, 1029–1039. 10.1021/bc300037w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosjan M. J. W. D.; Perk L. R.; Visser G. W. M.; Budde M.; Jurek P.; Kiefer G. E.; Van Dongen G. A. M. S. (2010) Conjugation and Radiolabeling of Monoclonal Antibodies with Zirconium-89 for PET Imaging Using the Bifunctional Chelate p-Isothiocyanatobenzyl-Desferrioxamine. Nat. Protoc. 5, 739–743. 10.1038/nprot.2010.13. [DOI] [PubMed] [Google Scholar]
- Morais M.; Ma M. T. (2018) Site-Specific Chelator-Antibody Conjugation for PET and SPECT Imaging with Radiometals. Drug Discovery Today: Technol. 30, 91–104. 10.1016/j.ddtec.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell C. A.; Mundo E. E.; Zhang C.; Bumbaca D.; Valle N. R.; Kozak K. R.; Fourie A.; Chuh J.; Koppada N.; Saad O.; et al. (2011) Impact of Drug Conjugation on Pharmacokinetics and Tissue Distribution of Anti-STEAP1 Antibody-Drug Conjugates in Rats. Bioconjugate Chem. 22, 1994–2004. 10.1021/bc200212a. [DOI] [PubMed] [Google Scholar]
- Giersing B. K.; Rae M. T.; CarballidoBrea M.; Williamson R. A.; Blower P. J. (2001) Synthesis and Characterization of 111In-DTPA-N-TIMP-2: A Radiopharmaceutical for Imaging Matrix Metalloproteinase Expression. Bioconjugate Chem. 12, 964–971. 10.1021/bc010028f. [DOI] [PubMed] [Google Scholar]
- Junutula J. R.; Raab H.; Clark S.; Bhakta S.; Leipold D. D.; Weir S.; Chen Y.; Simpson M.; Tsai S. P.; Dennis M. S.; et al. (2008) Site-Specific Conjugation of a Cytotoxic Drug to an Antibody Improves the Therapeutic Index. Nat. Biotechnol. 26, 925–932. 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- Zeglis B. M.; Davis C. B.; Aggeler R.; Kang H. C.; Chen A.; Agnew B. J.; Lewis J. S. (2013) Enzyme-Mediated Methodology for the Site-Specific Radiolabeling of Antibodies Based on Catalyst-Free Click Chemistry. Bioconjugate Chem. 24, 1057–1067. 10.1021/bc400122c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook B. E.; Adumeau P.; Membreno R.; Carnazza K. E.; Brand C.; Reiner T.; Agnew B. J.; Lewis J. S.; Zeglis B. M. (2016) Pretargeted PET Imaging Using a Site-Specifically Labeled Immunoconjugate. Bioconjugate Chem. 27, 1789–1795. 10.1021/acs.bioconjchem.6b00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeglis B. M.; Davis C. B.; Abdel-Atti D.; Carlin S. D.; Chen A.; Aggeler R.; Agnew B. J.; Lewis J. S. (2014) Chemoenzymatic Strategy for the Synthesis of Site-Specifically Labeled Immunoconjugates for Multimodal PET and Optical Imaging. Bioconjugate Chem. 25, 2123–2128. 10.1021/bc500499h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeger S.; Zimmermann K.; Blanc A.; Grünberg J.; Honer M.; Hunziker P.; Struthers H.; Schibli R. (2010) Site-Specific and Stoichiometric Modification of Antibodies by Bacterial Transglutaminase. Angew. Chem., Int. Ed. 49, 9995–9997. 10.1002/anie.201004243. [DOI] [PubMed] [Google Scholar]
- Paterson B. M.; Alt K.; Jeffery C. M.; Price R. I.; Jagdale S.; Rigby S.; Williams C. C.; Peter K.; Hagemeyer C. E.; Donnelly P. S. (2014) Enzyme-Mediated Site-Specific Bioconjugation of Metal Complexes to Proteins: Sortase-Mediated Coupling of Copper-64 to a Single-Chain Antibody. Angew. Chem., Int. Ed. 53, 6115–6119. 10.1002/anie.201402613. [DOI] [PubMed] [Google Scholar]
- Ahn S. H.; Vaughn B. A.; Solis W. A.; Lupher M. L.; Hallam T. J.; Boros E. (2020) Site-Specific 89Zr- and 111In-Radiolabeling and in Vivo Evaluation of Glycan-free Antibodies by Azide-Alkyne Cycloaddition with a Non-natural Amino Acid. Bioconjugate Chem. 31, 1177–1187. 10.1021/acs.bioconjchem.0c00100. [DOI] [PubMed] [Google Scholar]
- Ilieva K. M.; Fazekas-Singer J.; Achkova D. Y.; Dodev T. S.; Mele S.; Crescioli S.; Bax H. J.; Cheung A.; Karagiannis P.; Correa I.; et al. (2017) Functionally Active Fc Mutant Antibodies Recognizing Cancer Antigens Generated Rapidly at High Yields. Front. Immunol. 8, 1112. 10.3389/fimmu.2017.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier D.; Sharma S. K.; Adumeau P.; Rodriguez C.; Fung K.; Zeglis B. M. (2019) The Impact of FcGRI Binding on Immuno-PET. J. Nucl. Med. 60, 1174–1182. 10.2967/jnumed.118.223636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.; DiLillo D. J.; Bournazos S.; Giddens J. P.; Ravetch J. V.; Wang L. X. (2017) Modulating IgG Effector Function by Fc Glycan Engineering. Proc. Natl. Acad. Sci. U. S. A. 114, 3485–3490. 10.1073/pnas.1702173114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinianow J. N.; Gill H. S.; Ogasawara A.; Flores J. E.; Vanderbilt A. N.; Luis E.; Vandlen R.; Darwish M.; Junutula J. R.; Williams S. P.; et al. (2010) Site-Specifically 89Zr-Labeled Monoclonal Antibodies for ImmunoPET. Nucl. Med. Biol. 37, 289–297. 10.1016/j.nucmedbio.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Ma M. T.; Meszaros L. K.; Paterson B. M.; Berry D. J.; Cooper M. S.; Ma Y.; Hider R. C.; Blower P. J. (2015) Tripodal Tris(hydroxypyridinone) Ligands for Immunoconjugate PET Imaging with 89Zr4+: Comparison with Desferrioxamine-B. Dalton Trans. 44, 4884–4900. 10.1039/C4DT02978J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Olafsen T.; Anderson A. L.; Wu A.; Raubitschek A. A.; Shively J. E. (2002) Reduction of Kidney Uptake in Radiometal Labeled Peptide Linkers Conjugated to Recombinant Antibody Fragments. Site-Specific Conjugation of DOTA-Peptides to a Cys-Diabody. Bioconjugate Chem. 13, 985–995. 10.1021/bc025565u. [DOI] [PubMed] [Google Scholar]
- Ravasco J. M. J. M.; Faustino H.; Trindade A.; Gois P. M. P. (2019) Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chem. - Eur. J. 25, 43–59. 10.1002/chem.201803174. [DOI] [PubMed] [Google Scholar]
- Baldwin A. D.; Kiick K. L. (2011) Tunable Degradation of Maleimide-Thiol Adducts in Reducing Environments. Bioconjugate Chem. 22, 1946–1953. 10.1021/bc200148v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie R. J.; Fleming R.; Bezabeh B.; Woods R.; Mao S.; Harper J.; Joseph A.; Wang Q.; Xu Z. Q.; Wu H.; et al. (2015) Stabilization of Cysteine-Linked Antibody Drug Conjugates with N-Aryl Maleimides. J. Controlled Release 220, 660–670. 10.1016/j.jconrel.2015.09.032. [DOI] [PubMed] [Google Scholar]
- Shen B. Q.; Xu K.; Liu L.; Raab H.; Bhakta S.; Kenrick M.; Parsons-Reponte K. L.; Tien J.; Yu S. F.; Mai E.; et al. (2012) Conjugation Site Modulates the in Vivo Stability and Therapeutic Activity of Antibody-Drug Conjugates. Nat. Biotechnol. 30, 184–189. 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- Badescu G.; Bryant P.; Bird M.; Henseleit K.; Swierkosz J.; Parekh V.; Tommasi R.; Pawlisz E.; Jurlewicz K.; Farys M.; et al. (2014) Bridging Disulfides for Stable and Defined Antibody Drug Conjugates. Bioconjugate Chem. 25, 1124–1136. 10.1021/bc500148x. [DOI] [PubMed] [Google Scholar]
- Walsh S. J.; Omarjee S.; Galloway W. R. J. D.; Kwan T. T. L.; Sore H. F.; Parker J. S.; Hyvönen M.; Carroll J. S.; Spring D. R. (2019) A General Approach for the Site-Selective Modification of Native Proteins, Enabling the Generation of Stable and Functional Antibody-Drug Conjugates. Chem. Sci. 10, 694–700. 10.1039/C8SC04645J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell A. J.; Walsh S. J.; Robertson N. S.; Sore H. F.; Spring D. R. (2020) Efficient and Selective Antibody Modification with Functionalised Divinyltriazines. Org. Biomol. Chem. 18, 4739–4743. 10.1039/D0OB01002B. [DOI] [PubMed] [Google Scholar]
- Martínez-Sáez N.; Sun S.; Oldrini D.; Sormanni P.; Boutureira O.; Carboni F.; Compañón I.; Deery M. J.; Vendruscolo M.; Corzana F.; et al. (2017) Oxetane Grafts Installed Site-Selectively on Native Disulfides to Enhance Protein Stability and Activity In Vivo. Angew. Chem., Int. Ed. 56, 14963–14967. 10.1002/anie.201708847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahou C.; Richards D. A.; Maruani A.; Love E. A.; Javaid F.; Caddick S.; Baker J. R.; Chudasama V. (2018) Highly Homogeneous Antibody Modification through Optimisation of the Synthesis and Conjugation of Functionalised Dibromopyridazinediones. Org. Biomol. Chem. 16, 1359–1366. 10.1039/C7OB03138F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruani A.; Szijj P. A.; Bahou C.; Nogueira J. C. F.; Caddick S.; Baker J. R.; Chudasama V. (2020) A Plug-and-Play Approach for the de Novo Generation of Dually Functionalized Bispecifics. Bioconjugate Chem. 31, 520–529. 10.1021/acs.bioconjchem.0c00002. [DOI] [PubMed] [Google Scholar]
- Schumacher F. F.; Nunes J. P. M.; Maruani A.; Chudasama V.; Smith M. E. B.; Chester K. A.; Baker J. R.; Caddick S. (2014) Next Generation Maleimides Enable the Controlled Assembly of Antibody-Drug Conjugates via Native Disulfide Bond Bridging. Org. Biomol. Chem. 12, 7261–7269. 10.1039/C4OB01550A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda L.; Maruani A.; Schumacher F. F.; Miranda E.; Chudasama V.; Chester K. A.; Baker J. R.; Smith M. E. B.; Caddick S. (2013) Acid-Cleavable Thiomaleamic Acid Linker for Homogeneous Antibody-Drug Conjugation. Chem. Commun. 49, 8187–8189. 10.1039/c3cc45220d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes J. P. M.; Morais M.; Vassileva V.; Robinson E.; Rajkumar V. S.; Smith M. E. B.; Pedley R. B.; Caddick S.; Baker J. R.; Chudasama V. (2015) Functional Native Disulfide Bridging Enables Delivery of a Potent, Stable and Targeted Antibody-Drug Conjugate (ADC). Chem. Commun. 51, 10624–10627. 10.1039/C5CC03557K. [DOI] [PubMed] [Google Scholar]
- Morais M.; Nunes J. P. M.; Karu K.; Forte N.; Benni I.; Smith M. E. B.; Caddick S.; Chudasama V.; Baker J. R. (2017) Optimisation of the Dibromomaleimide (DBM) Platform for Native Antibody Conjugation by Accelerated Post-Conjugation Hydrolysis. Org. Biomol. Chem. 15, 2947–2952. 10.1039/C7OB00220C. [DOI] [PubMed] [Google Scholar]
- Forte N.; Livanos M.; Miranda E.; Morais M.; Yang X.; Rajkumar V. S.; Chester K. A.; Chudasama V.; Baker J. R. (2018) Tuning the Hydrolytic Stability of Next Generation Maleimide Cross-Linkers Enables Access to Albumin-Antibody Fragment Conjugates and Tri-ScFvs. Bioconjugate Chem. 29, 486–492. 10.1021/acs.bioconjchem.7b00795. [DOI] [PubMed] [Google Scholar]
- Pham T. T.; Lu Z.; Davis C.; Li C.; Sun F.; Maher J.; Yan R. (2020) Iodine-124 Based Dual Positron Emission Tomography and Fluorescent Labeling Reagents for in Vivo Cell Tracking. Bioconjugate Chem. 31, 1107–1116. 10.1021/acs.bioconjchem.9b00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M. T.; Karas J. A.; White J. M.; Scanlon D.; Donnelly P. S. (2009) A New Bifunctional Chelator for Copper Radiopharmaceuticals: A Cage Amine Ligand with a Carboxylate Functional Group for Conjugation to Peptides. Chem. Commun. 3237–3239. 10.1039/b903426a. [DOI] [PubMed] [Google Scholar]
- Ma M. T.; Cooper M. S.; Paul R. L.; Shaw K. P.; Karas J. A.; Scanlon D.; White J. M.; Blower P. J.; Donnelly P. S. (2011) Macrobicyclic Cage Amine Ligands for Copper Radiopharmaceuticals: A Single Bivalent Cage Amine Containing Two Lys3-Bombesin Targeting Peptides. Inorg. Chem. 50, 6701–6710. 10.1021/ic200681s. [DOI] [PubMed] [Google Scholar]
- Paterson B. M.; Buncic G.; McInnes L. E.; Roselt P.; Cullinane C.; Binns D. S.; Jeffery C. M.; Price R. I.; Hicks R. J.; Donnelly P. S. (2015) Bifunctional 64Cu-Labelled Macrobicyclic Cage Amine Isothiocyanates for Immuno-Positron Emission Tomography. Dalton Trans. 44, 4901–4909. 10.1039/C4DT02983F. [DOI] [PubMed] [Google Scholar]
- Donnelly P. S.; Harrowfield J. M.; Skelton B. W.; White A. H. (2000) Carboxymethylation of Cage Amines: Control of Alkylation by Metal Ion Coordination. Inorg. Chem. 39, 5817–5830. 10.1021/ic000346a. [DOI] [PubMed] [Google Scholar]
- Morais M., Forte N., Chudasama V., and Baker J. R. (2019) Application of Next-Generation Maleimides (NGMs) to Site-Selective Antibody Conjugation. In Bioconjugation. Methods in Molecular Biology (Mass S., and Devoogdt N., Eds.) pp 15–24, Vol. 2033, 10.1007/978-1-4939-9654-4_2. [DOI] [PubMed] [Google Scholar]
- Bahou C.; Love E. A.; Leonard S.; Spears R. J.; Maruani A.; Armour K.; Baker J. R.; Chudasama V. (2019) Disulfide Modified IgG1: An Investigation of Biophysical Profile and Clinically Relevant Fc Interactions. Bioconjugate Chem. 30, 1048–1054. 10.1021/acs.bioconjchem.9b00174. [DOI] [PubMed] [Google Scholar]
- Imberti C.; Terry S. Y. A.; Cullinane C.; Clarke F.; Cornish G. H.; Ramakrishnan N. K.; Roselt P.; Cope A. P.; Hicks R. J.; Blower P. J.; et al. (2017) Enhancing PET Signal at Target Tissue in Vivo: Dendritic and Multimeric Tris(Hydroxypyridinone) Conjugates for Molecular Imaging ofnvβ3 Integrin Expression with Gallium-68. Bioconjugate Chem. 28, 481–495. 10.1021/acs.bioconjchem.6b00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusnir R.; Cakebread A.; Cooper M. S.; Young J. D.; Blower P. J.; Ma M. T. (2019) The Effects of Trace Metal Impurities on Ga-68-radiolabelling with a Tris(3-hydroxy-1,6-dimethylpyridin-4-one) (THP) Chelator. RSC Adv. 9, 37214–37221. 10.1039/C9RA07723E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M.; Roselt P.; Denoyer D.; Cullinane C.; Binns D.; Noonan W.; Jeffery C. M.; Price R. I.; White J. M.; Hicks R. J.; et al. (2014) PET Imaging of Tumours with a 64Cu Labeled Macrobicyclic Cage Amine Ligand Tethered to Tyr3-Octreotate. Dalton Trans. 43, 1386–1396. 10.1039/C3DT52647J. [DOI] [PubMed] [Google Scholar]
- Cole W. C.; DeNardo S. J.; Meares C. F.; McCall M. J.; DeNardo G. L.; Epstein A. L.; O’Brien H. A.; Moi M. K. (1986) Serum Stability of 67Cu Chelates: Comparison with 111In and 57Co. Int. J. Radiat. Appl. Instrumentation. 13, 363–368. 10.1016/0883-2897(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Bass L. A.; Wang M.; Welch M. J.; Anderson C. J. (2000) In Vivo Transchelation of Copper-64 from TETA-Octreotide to Superoxide Dismutase in Rat Liver. Bioconjugate Chem. 11, 527–532. 10.1021/bc990167l. [DOI] [PubMed] [Google Scholar]
- Mirick G. R.; O’Donnell R. T.; Denardo S. J.; Shen S.; Meares C. F.; Denardo G. L. (1999) Transfer of Copper from a Chelated 67Cu-Antibody Conjugate to Ceruloplasmin in Lymphoma Patients. Nucl. Med. Biol. 26, 841–845. 10.1016/S0969-8051(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Press M.; Cordon-Cardo C.; Slamon D. (1990) Expression of the HER-2/Neu Proto-Oncogene in Normal Human Adult and Fetal Tissues. Oncogene 5, 953–962. [PubMed] [Google Scholar]
- Krähn G.; Leiter U.; Kaskel P.; Udart M.; Utikal J.; Bezold G.; Peter R. U. (2001) Coexpression Patterns of EGFR, HER2, HER3 and HER4 in Non-Melanoma Skin Cancer. Eur. J. Cancer 37, 251–259. 10.1016/S0959-8049(00)00364-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.