Abstract
Objective
To determine the activation status and cytokine profiles of CD4+ T cells, CD8+ T cells, and CD19+ B cells from patients with early-stage Parkinson disease (PD) compared with healthy controls (HCs).
Methods
Peripheral blood samples from 41 patients with early-stage PD and 40 HCs were evaluated. Peripheral blood mononuclear cells were analyzed by flow cytometry for surface markers and intracellular cytokine production. Correlations of immunologic changes and clinical parameters were analyzed.
Results
Adaptive immunity plays a role in the pathogenesis of PD, yet the contribution of T cells and B cells, especially cytokine production by these cells, is poorly understood. We demonstrate that naive CD4+ and naive CD8+ T cells are significantly decreased in patients with PD, whereas central memory CD4+ T cells are significantly increased in patients with PD. Furthermore, IL-17–producing CD4+ Th17 cells, IL-4–producing CD4+ Th2 cells, and IFN-γ–producing CD8+ T cells are significantly increased in patients with PD. Regarding B cells, we observed a decrease in naive B cells and an increase in nonswitched memory and double-negative B cells. As well, TNF-α–producing CD19+ B cells were significantly increased in patients with PD. Notably, some of the changes observed in CD4+ T cells and B cells were associated with clinical motor disease severity.
Conclusions
These findings suggest that alterations in the adaptive immune system may promote clinical disease in PD by skewing to a more proinflammatory state in the early-stage PD patient cohort. Our study may shed light on potential immunotherapies targeting dysregulated CD4+ T cells, CD8+ T cells, and CD19+ B cells in patients with PD.
Parkinson disease (PD) is the second most common neurodegenerative disease after Alzheimer disease (AD) and is the most common movement disorder.1 Currently, approximately 2% of the population older than 60 years is affected.1 PD is clinically characterized by tremor, rigidity, and bradykinesia, along with nonmotor symptoms including mood disorders, constipation, and cognitive changes.1,2 The neuropathologic hallmarks of PD are the accumulation and aggregation of misfolded α-synuclein (α-Syn) forming Lewy bodies and Lewy neurites and the loss of dopaminergic (DA) neurons in the substantia nigra of the midbrain.1 The mechanisms underlying progressive degeneration in PD are not entirely clear, but several lines of evidence suggest that immune system dysregulation may play an important role.3,4
Much attention has been focused on the neuroinflammatory features of PD; cells of the innate immune system, both endogenous microglia and infiltrating monocytes/macrophages from the periphery, are relevant to the neuroinflammation observed in PD.5 A recent study demonstrated increased classical monocytes and TLR-positive monocytes in PD and that these are elevated in patients with PD thought to be at risk of faster cognitive decline.6 We have recently described evidence for inflammatory activation of monocytes in early PD, especially in female patients.7 Dysregulation of the immune system in conjunction with genetic mutations and environmental factors are contributing pathogenic factors in the initiation of PD.1 Genome-wide association studies have shown associations between PD and mutations/variants of α-Syn, leucine rich repeat kinase 2, major histocompatibility complex (MHC) Class II, and PINK1.8,9 Cytokine dysregulation, including high levels of the cytokines interleukin (IL)-6 and interferon (IFN)-γ, is associated with degeneration of DA neurons in patients with PD and animal models of PD.5
Studies indicate a role for the adaptive immune system in PD.10 Two major classes of lymphocytes, T cells and B cells, are responsible for induction of adaptive immunity, which confers antigen specificity and immunologic memory.5 Patients with PD have elevated numbers of CD4+ and CD8+ T cells in the ventral midbrain when compared with controls.11 T cells can generate an autoimmune response to posttranslationally modified α-Syn and other proteins in patients with PD, implicating autoimmune reactive T cells in PD.10,12 A recent study demonstrated that α-Syn–specific T-cell reactivity is associated with preclinical and early PD, and proinflammatory CD4+ T cells are most abundant shortly after diagnosis of motor symptoms.13
CD4+ T cells display considerable heterogeneity in their ability to differentiate into functional subsets (Th1, Th2, Th17, Tregs, and others), producing different cytokines and expressing distinct transcription factors.14 In general, CD4+ Th1 and Th17 cells are considered proinflammatory and pathogenic, whereas CD4+ Th2 and Tregs are anti-inflammatory. The literature regarding CD4+ T-cell subsets in PD is complex, with conflicting findings.15 Several groups have found increases in IL-17A–producing Th17 cells in peripheral blood from patients with PD, whereas others have not.16,17 Similarly, some groups have detected increased numbers of IFN-γ–producing Th1 cells in patients with PD, whereas others report no change.16 Discrepancies have also been noted in numbers of IL-4/IL-5–producing Th2 cells as well as Foxp3+ Treg cells,18,19 and conflicting results exist regarding cytokine production by CD8+ T cells in patients with PD.12 The disparities in published studies of T cells may be due, in part, to the small numbers of patients in many of the studies, heterogeneity of patients, and the use of different stimuli and methodologies for cytokine assessment.
Little is known about the role of B cells in PD. They are the only cell type capable of secreting antibodies, but they also perform other critical immune functions such as generating immunologic memory, antigen presentation, and regulatory cytokine production.20 B cells have important roles in the pathogenesis of neurologic diseases, including multiple sclerosis (MS).21 In MS, B cells contribute to both promotion of neuroinflammatory responses and neuronal injury, as well as resolution of neuroinflammation.22 These divergent functions are predicated on cytokine profiles. B cells have not been detected in the human PD brain, although they have been observed in some animal models of the disease.23 In the periphery, there are decreased numbers of CD19+ B cells in patients with PD compared with healthy controls (HCs).24,25
We hypothesize that aberrant responses of cells of the peripheral adaptive immune system (both T cells and B cells) occur early in PD and may contribute to neuroinflammation and disease progression. To test this hypothesis, we examined surface markers and intracellular cytokine production in CD4+ T cells, CD8+ T cells, and CD19+ B cells in patients with early-stage PD compared with HCs matched for age and sex.
Methods
Subjects
Subjects with early-stage idiopathic PD (diagnosed ≤2 years and on treatment ≤1 year) and HCs were recruited from the University of Alabama at Birmingham (UAB) Movement Disorders Clinic. Inclusion criteria for PD included diagnosis of PD by a fellowship-trained movement disorders specialist using UK Brain Bank criteria (at least 2 of the following: resting tremor, bradykinesia, and rigidity); male or female aged 30 years or older at the time of PD diagnosis; no more than 2 years since the initial diagnosis of PD and no more than 1 year on treatment for PD; and Hoehn and Yahr stage I or II.26 Exclusion criteria included diagnosis of an atypical PD syndrome due to drugs, metabolic disorders, encephalitis, or degenerative diseases other than PD; a clinical diagnosis of dementia as determined by the investigator; use of investigational drugs or devices within 60 days prior; known severe anemia (hematocrit <30); or a history of clinically significant autoimmune or inflammatory disorder. Inclusion criteria for HCs were age older than 30 years; no current diagnosis of PD or other neurodegenerative disorder; no history of PD in first-degree blood relatives; and a lack of positive responses on more than 3 items on a PD Screening Questionnaire.27 Exclusion criteria for HCs were known severe anemia (hematocrit <30); history of clinically significant autoimmune or inflammatory disorder; or use of immunomodulatory or anti-inflammatory drugs. The studies were conducted in accordance with recognized ethical guidelines and were approved by the Institutional Review Board of UAB (IRB Protocol #IRB-161128007). A total of 86 subjects, patients with PD (41) and HCs (45), were enrolled. Standard protocol approvals, registrations, and patient consents were used.
For both patients with PD and HCs, a clinical data set was collected including sex, age, clinical diagnosis, demographics, family history of disease, vital signs, Hamilton Anxiety Scale, REM Behavior Disorder Questionnaire, Movement Disorder Society Parkinson's Disease Rating Scale (MDS-UPDRS), Hoehn and Yahr score, Schwab & England Activities of Daily Living Scale, Montreal Cognitive Assessment (MoCA), Epworth Sleep Scale (ESS), Parkinson's Disease Biomarker's Program Behavioral History, and Parkinson's Disease Questionnaire (PDQ-39).
Sample Processing
Up to 50 mL of venous blood was collected from each participant in ethylenediaminetetraacetic acid tubes, and peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll-Paque density-gradient centrifugation, as described,13 and then divided into aliquots for T-cell and B-cell immunophenotyping. All samples were processed within 2 hours of receipt.
Flow Cytometry for Subsets and Intracellular Cytokine Expression in CD4+ T Cells, CD8+ T Cells, and B Cells
PBMCs (1 × 106) were blocked with Human TruStain FcX (BioLegend, San Diego, CA) before labeling with fluorochrome-conjugated monoclonal antibodies to T-cell and B-cell surface markers and then were fixed with 2% paraformaldehyde. The Aqua Live/Dead Kit was used to assess cell viability (Thermo Fisher Scientific). Antibodies used for CD4+ T-cell, CD8+ T-cell, and B-cell surface markers were from BioLegend except where noted otherwise: anti-CD45 Pacific Blue (clone HI30); anti-CD3 Brilliant Violet 605/Brilliant Violet 650 (clone OKT3); anti-CD4 PE (clone OKT4); anti-CD4 eFluor 450 (clone OKT4, eBioscience); anti-CD8α FITC (clone HIT8a); anti-CD25 PE/PerCP-Cyanine5.5 (clone M-A251); anti-CCR7 PE-Cy7 (clone G043H7); anti-CD45RA APC (clone HI100); anti-CD19 Brilliant Violet 650 (clone HIB19); anti-IgD FITC (clone IA6-2); anti-CD27 Brilliant Violet 421 (clone O323); anti-CD38 PerCP-Cyanine5.5 (clone HB-7); and anti-CXCR3 PE-Cy7 (clone G025H7). Antibodies used for intracellular cytokine expression in CD4+ T cells, CD8+ T cells, and B cells: anti-IFN-γ PE-Cy7 (clone B27, BD Bioscience); anti-IL-4 PE (clone 8D4-8); anti-IL-17A PerCP-Cyanine5.5 (clone eBio64DEC17, eBioscience); anti-Foxp3 Alexa Fluor 647 (clone 150D); anti-TNF-α PE (clone MAb11, BD Bioscience, 5 μL/test); anti-GM-CSF Brilliant Violet 421 (clone BVD2-21C11, BD Bioscience, 2 μL/test); anti-IL-10 (clone JES3-19F1, BD Bioscience, 2 μL/test); and anti- IL-6 (clone MQ2-13A5, BD Bioscience, 1 μL/test). All antibodies were diluted to 1:200 except where noted otherwise, and the incubation time on ice was 30–45 minutes.
PBMCs (1 × 106) from HCs and patients with PD were stimulated with PMA (50 ng/mL) and ionomycin (750 ng/mL) with GolgiStop for 4 hours. CD4+ T cells were examined for expression levels of IFN-γ (Th1 cells), IL-4 (Th2 cells), IL-17A (Th17 cells), and Foxp3 (Treg cells), and CD8+ T-cell production of IFN-γ and B-cell production of TNF-α, GM-CSF, IL-6, and IL-10 were analyzed by intracellular staining.28 Flow cytometry was performed on an LSRII flow cytometer (BD Biosciences), and data were analyzed using FlowJo software (Tree Star, Inc, Ashland, OR), as previously described.28
Data Analysis
Lymphocytes were gated using forward scatter and side scatter, and single cells were identified using forward-scatter area vs width. T cells were identified as CD45+CD3+ and then subdivided into CD4+ and CD8+ T cells. CD4+ T-cell and CD8+ T-cell subsets were identified as CD45RA+CCR7+–naive T cells, CCR7+CD45RA− central memory T cells (TCM), CCR7−CD45RA− effector memory T cells (TEM), and CCR7−CD45RA+ terminally differentiated memory T cells (TD) based on CCR7 and CD45RA expression. B-cell subsets were identified as CD27−IgD+–naive B cells, CD27+IgD− switched memory (SM) B cells, CD27+IgD+ nonswitched memory (NSM) B cells, and CD27−IgD− double-negative (DN) B cells based on IgD and CD27 expression. Statistical analyses were performed using descriptive statistics and Student t test distribution and 1-sided 2-sample Mann-Whitney rank-sum test comparing percentages of T-cell subsets (as a percentage of CD45+CD3+ lymphocytes) and B-cell subsets (CD19+CD3− lymphocytes) between patients with PD and HCs. One-way analysis of variance was used to compare differences between more than 2 samples. Frequencies of cell subsets are shown as mean ± SD.
Correlation analyses were performed using Spearman rank-order correlations to accommodate skewness in the variables. All significance levels are corrected using the Benjamini-Hochberg procedure for multiple testing, with overall Type I error controlled at p = 0.05. Statistical analysis was performed using JMP 14 (SAS Institute Inc) and graphs built with GraphPad Prism 8.0. p Values were ranked from the lowest to the highest, and then the critical p value (p. adj) was calculated as p = either the raw p value times m/i or the adjusted p value for the next higher raw p value, where i = the individual p value's rank, and m = total number of tests.
Data Availability
Anonymized data not published within this article will be made available on request from any qualified investigator.
Results
General Information on Clinical Cohort
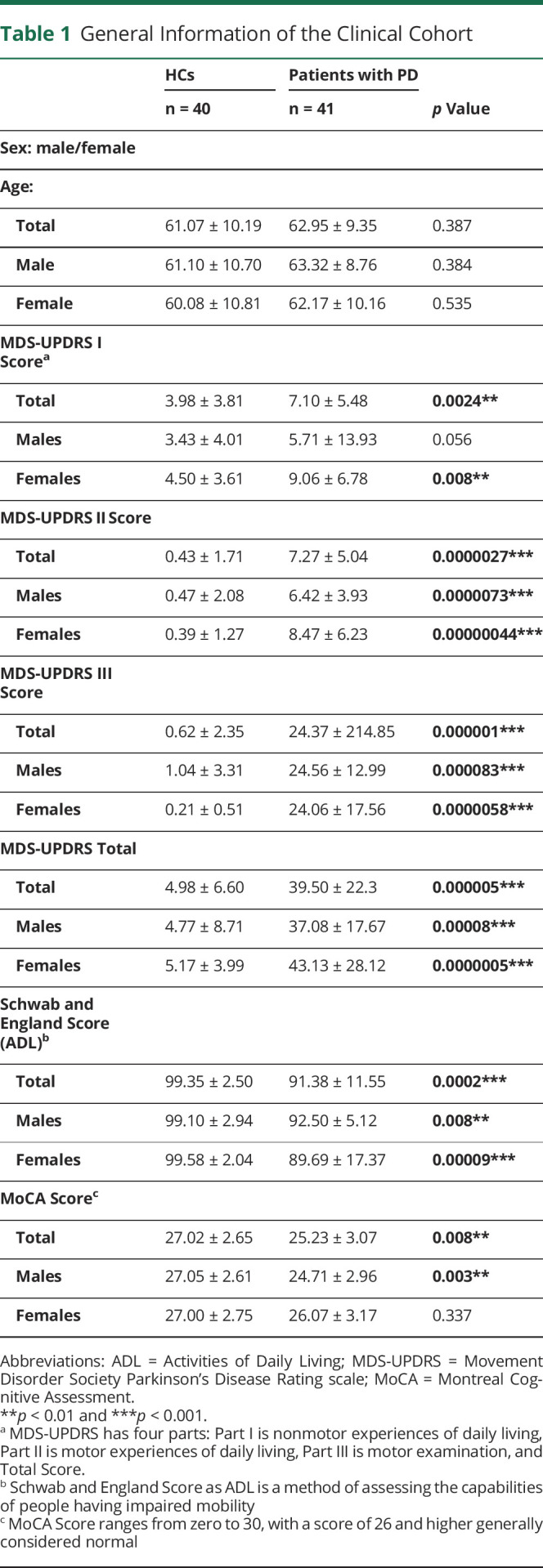
We collected clinical data and peripheral blood samples from patients with PD (n = 41) and HCs (n = 45) who signed patient consent forms as per the IRB. No PD cases were younger than 37 years, as such, 5 HCs younger than 37 years were excluded in our data analysis (eFigure 1A, links.lww.com/NXI/A506). We report on the 81 subjects included in this study: 40 HCs and 41 patients with PD. Analyses of samples were performed blinded to case or control status. There were 18 men (age: 39–80 years) and 22 women (age: 38–76 years) in the HC group, and 25 men (age: 40–77 years) and 16 women (age: 40–76 years) in the PD group. There were no significant differences in the ages of male and female groups between patients with PD and HCs (eFigure 1B, links.lww.com/NXI/A506).
We collected a clinical data set including MDS-UPDRS scores, the Schwab and England Scale of Independence, and MoCA scores for patients with PD and HCs as described in Table 1. There was no significant difference in ESS, Hamilton Anxiety scale, REM Behavior Disorder Questionnaire, Parkinson's Disease Biomarker's Program Behavioral History, and PDQ-39 between patients with PD and HCs. We focused our analysis of the correlation of adaptive immune cell populations and PD disease severity on MDS-UPDRS, MoCA, and Schwab and England scores.
Table 1.
General Information of the Clinical Cohort
Decreased Naive CD4+ and CD8+ T cells and Increased Central Memory CD4+ T cells in Patients With PD
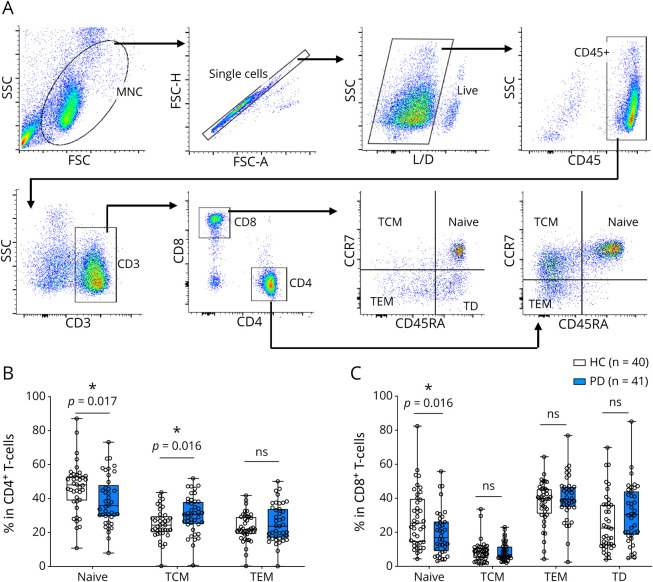
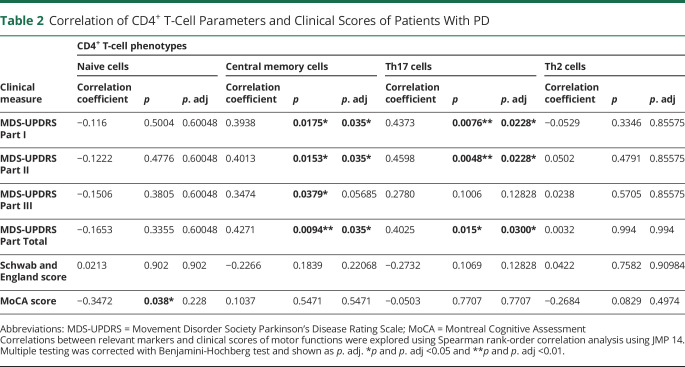
PBMCs from patients with PD (n = 41) and HCs (n = 40) were stained with the following surface markers: CD45, CD3, CD4, CD8, CCR7, and CD45RA. To determine subsets of CD4+ and CD8+ T cells, single, live CD45+CD3+CD4+ or CD45+CD3+CD8+ cells were gated and distinguished by the surface markers CCR7 and CD45RA (Figure 1A). Our data demonstrate a significant decrease of CCR7+CD45RA+–naive CD4+ T cells and an increase in CCR7+CD45RA− central memory (TCM) CD4+ T cells in patients with PD compared with HCs (Figure 1B). There were no differences in CCR7−CD45RA− effector memory (TEM) CD4+ T cells between patients with PD and HCs (Figure 1B). Importantly, we found that patients with higher CD4+ TCM cells show worsening of MDS-UPDRS scores, including Part I, Part II and total scores (Table 2).
Figure 1. CD4+ and CD8+ T-Cell Subsets in Healthy Controls and Patients With Parkinson Disease.
(A) Peripheral blood mononuclear cells were stained with surface markers for CD4+ and CD8+ T-cell subsets. (B) Frequencies of CD4+ T-cell subsets. (C) Frequencies of CD8+ T-cell subsets. *p < 0.05.
Table 2.
Correlation of CD4+ T-Cell Parameters and Clinical Scores of Patients With PD
Less is known about CD8+ T-cell subsets in patients with PD compared with CD4+ T cells. We examined CD8+ T-cell profiles, including CCR7+CD45RA+–naive, CCR7+CD45RA− TCM, CCR7−CD45RA− TEM, and CCR7−CD45RA+ terminally differentiated (TD) subsets (Figure 1A). Our results demonstrate a significant decrease of naive CD8+ T cells in patients with PD compared with HCs, with no significant differences observed in CD8+ TCM, TEM and TD cells (Figure 1C). No correlation was observed between decreased naive CD8+ T cells and clinical parameters in patients with PD (data not shown).
Increased IL-17A, IL-4–Producing CD4+ T cells, and IFN-γ–Producing CD8+ T cells in Patients With PD
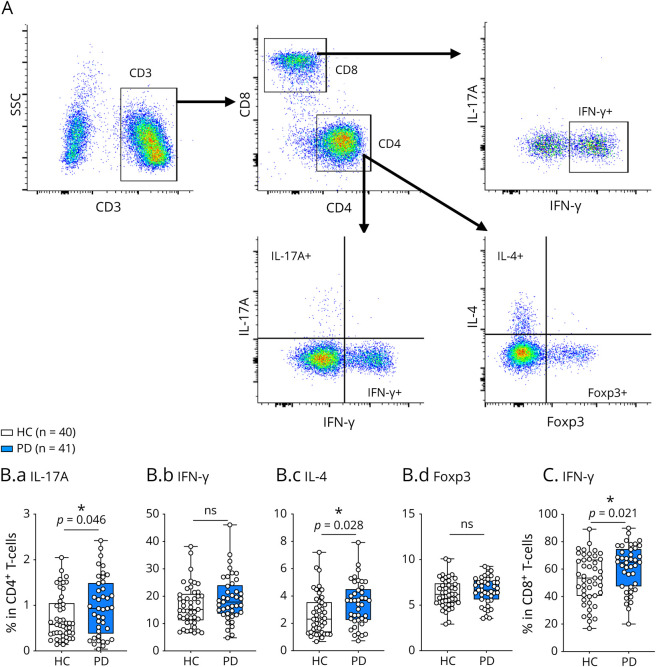
To investigate the differentiation of CD4+ T-cell subsets, we stimulated PBMCs with PMA and ionomycin in the presence of GolgiStop for 4 hours and determined cytokines (IFN-γ, IL-17A, and IL-4) or transcription factor (Foxp3) expression by intracellular staining (Figure 2A) compared with unstimulated cells. Cytokine expression in unstimulated cells was below detection by flow cytometry. Our results demonstrated significant increases in IL-17A+ Th17 and IL-4+ Th2 cells in patients with PD compared with HCs (Figure 2B), but no differences in IFN-γ+ Th1 and Foxp3+ Treg cells. Notably, PD patients with high IL-17A+ Th17 cells exhibit worsening MDS-UPDRS Part I, Part II, and total scores, indicated by correlation analysis (Table 2). No significant correlation was observed regarding IL-4+ Th2 cells and clinical parameters in patients with PD (data not shown).
Figure 2. Cytokine Production by CD4+ and CD8+ T Cells in HCs and Patients With PD.
(A) Gating strategy of T cells. (B). IFN-γ+Th1 (B.a), IL-4+Th2 (B.b), IL-17A + Th17 (B.c), and Foxp3+Treg (B.d) cells in HCs and patients with PD. (C) Frequency of IFN-γ+CD8+ T cells in HCs and patients with PD. *p < 0.05. HC = healthy control; PD = Parkinson disease.
To investigate cytokine production by CD8+ T cells, IFN-γ production was examined (Figure 2A). We found that IFN-γ+ CD8+ T cells were significantly increased in patients with PD compared with HCs (Figure 2C); however, no correlation was observed between increased IFN-γ+ CD8+ T cells and clinical parameters in patients with PD (data not shown).
Increased Proinflammatory Properties of B Cells in Patients With PD
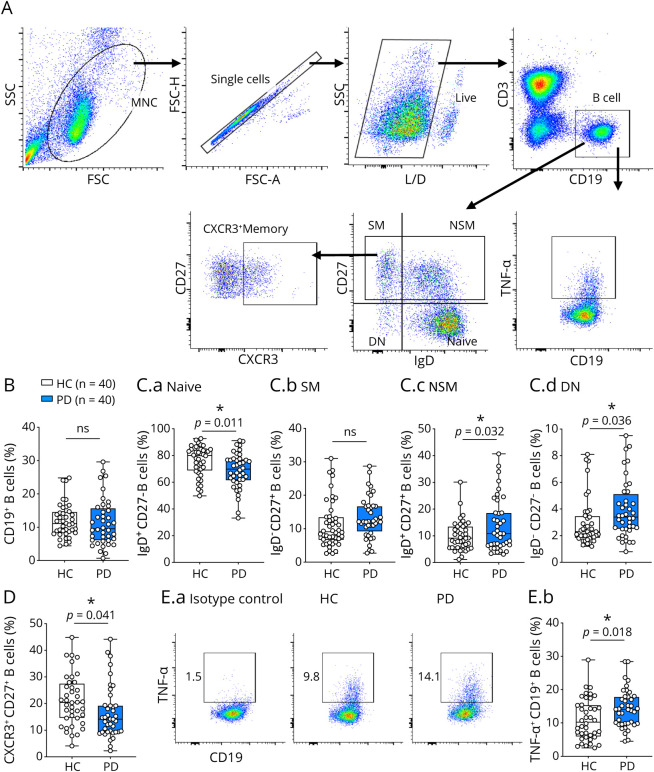
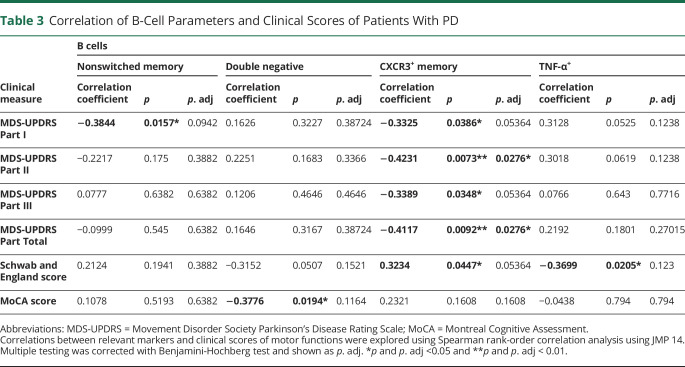
There is a paucity of information regarding B cells in the context of PD. As such, we examined the immune profiles of B cells in patients with early-stage PD and HCs. Because of an insufficient number of PBMCs from one of the patients with PD, we examined PBMC samples from 40 patients with PD and 40 HCs. All B cells were gated as CD3−CD19+, and different B-cell subsets were further determined using IgD and CD27; these include IgD+CD27−–naive B cells, IgD−CD27+ SM B cells, IgD+CD27+ NSM B cells, and IgD−CD27− DN B cells (Figure 3A). Our results indicated no significant difference of total CD19+ B cells between patients with PD and HCs (Figure 3B). A significant decrease in naive B cells, with a significant increase in both NSM B cells and DN B cells, was observed in patients with PD compared with HCs, with no difference in SM B cells (Figure 3C). No correlation was found between NSM or DN B cells and clinical outcomes (Table 3).
Figure 3. B-Cell Subsets and TNF-α Production in HCs and Patients With Parkinson Disease.
(A). Gating strategy of CD3−CD19+ B cells. (B). Total CD19+ B cells. (C). B-cell subsets. (D). CXCR3+CD27+ memory B cells. (E). TNF-⍺+CD19+ B cells in HCs and patients with Parkinson disease. *p < 0.05.
Table 3.
Correlation of B-Cell Parameters and Clinical Scores of Patients With PD
The chemokines CXCL9, CXCL10, and CXCL11 specifically bind to CXCR3, which leads to the infiltration of immune cells to inflammatory sites.29 We determined that CXCR3+CD27+ memory B cells were significantly decreased in patients with PD compared with HCs (Figure 3D). As shown in Table 3, patients with decreased CX3CR1+CD27+ memory B cells show increased disease severity (UPDRS Part II and total scores).
To determine the cytokine profiles of B cells, we examined the proinflammatory cytokines TNF-α, GM-CSF, and IL-6 and anti-inflammatory cytokine IL-10 in PMA/ionomycin-stimulated CD19+ B cells. Importantly, we found a significant increase in the frequency of TNF-α+CD19+ B cells in patients with PD compared with HCs (Figure 3E); however, no correlation with clinical score was observed (Table 3). The expression levels of GM-CSF, IL-6, and IL-10 were undetectable by flow cytometry analysis after PMA/ionomycin stimulation. However, preliminary analysis in a small subset of patients of mRNA levels indicated a trend toward increased IL-6 mRNA and decreased IL-10 mRNA levels in unstimulated B cells purified from patients with PD (n = 5) compared with HCs (n = 5) (data not shown).
Discussion
This study demonstrates that subsets and cytokine expression profiles of peripheral adaptive immune cells in patients with early-stage PD show distinct differences compared with HCs matched for age and sex. These peripheral abnormalities may drive PD disease initiation and progression through enhanced infiltration of immune cells and enhanced neuroinflammation in the CNS.
Although there have been numerous studies examining cytokine production by CD4+ T cells in PD, the results are conflicting.19,30 Chen et al.,18 reported an increase of Th1 and Th17 cells and a decrease of Th2 and Treg cells in the peripheral blood of patients with PD. Two other reports also demonstrated increased frequencies of Th17 cells in PD patients.17,30 However, another study demonstrated an increase in Th1 cells and TNF-α–producing CD4+ T cells and a corresponding reduction in Th2 and Th17 cells in patients with PD.16 Our findings demonstrate an increase in proinflammatory CD4+ Th17 cells and anti-inflammatory CD4+ Th2 cells in patients with early-stage PD, with no significant changes in Th1 and Treg cells. Patients with higher CD4+ Th17 cells exhibit worsening MDS-UPDRS scores. As noted, there were correlations between Th17 cells and the subscales I and II of the MDS-UPDRS, which measure nonmotor symptoms, but not with subscale III, which measures motor symptoms.1 It may be that nonmotor symptoms are more prominent and progressive in early stages of PD disease than motor symptoms.1,2 IL-17–producing Th17 cells have pathogenic functions in a number of autoimmune and neurologic diseases. A recent study demonstrated that a key function of IL-17 was to mobilize myeloid cells that prime pathogenic T cells in peripheral immune organs, which then traffic to the CNS and cause autoimmune disease.31 IL-17 also causes neuronal damage,32 and this finding was recently confirmed using a human autologous iPSC-based model.17 Collectively, these findings implicate IL-17 in promoting inflammatory responses in the CNS as well as mediating neuronal damage, actions relevant to PD pathogenesis. IL-4–producing Th2 cells, on the other hand, have predominantly anti-inflammatory functions in the CNS and also exert protective effects on neurons. T cell–derived IL-4 mediates neuroprotection in CNS injury models,33 and intranasal administration of IL-4 improves clinical symptoms of EAE and promotes neuronal recovery.34 Several studies have reported decreased numbers of Th2 cells in patients with PD compared with HCs.16,18 However, recent studies demonstrate that α-Syn peptides can induce IL-4/IL-5–producing Th2 cells in patients with PD, which are significantly increased compared with HCs.12,13 The precise role of IL-4 in PD is unclear, especially given that IL-4 can exert both pro- and anti-inflammatory properties, depending on disease context and the microenvironment.35 Future studies will address the functionality of IL-4 in patients with early-stage PD. Taken together, our data suggest that an imbalance of CD4+ T-cell subsets may be linked to PD pathobiology and disease severity.
CD8+ T cells play important roles in the pathogenesis of numerous CNS disorders. IFN-γ is a representative cytokine of CD8+ T cells and contributes to their cytotoxic properties. CD8+ T cells from patients with PD recognize α-Syn peptides and produce IFN-γ, which potentially contributes to pathogenic immune responses in PD.12,13 Our results demonstrate an increase of IFN-γ–producing CD8+ T cells in response to PMA/ionomycin in patients with PD. A recent study demonstrated that CD8+ T cells from patients with AD and patients with mild cognitive impairment, in response to the same stimuli, secreted higher levels of IFN-γ compared with controls.36 Dopamine neurons can express MHC Class I in response to IFN-γ, which then renders them susceptible to cell death by cytotoxic CD8+ T cells.37 Thus, the increase in IFN-γ–producing cytotoxic CD8+ T cells may contribute to both neuroinflammation and neuronal damage in patients with PD.
We present novel findings on B-cell subsets and cytokine profiles in patients with PD. We observed a significant decrease in naive B cells, accompanied by a significant increase in NSM and DN B cells in patients with PD. NSM B cells are involved in early inflammatory responses.38 DN B cells are detected at low levels in peripheral blood of heathy individuals39 and are expanded in elderly patients40 and patients with AD.41 The DN B-cell subset is also implicated in inflammatory responses. At present, the function of these subsets in patients with PD is not known and deserves further investigation. We also noted a decrease in circulating CXCR3+ memory B cells in patients with PD. This phenotype of B cells has enhanced chemotaxis in response to various chemokines. Whether this decrease in circulating CXCR3+ memory B cells in patients with PD reflects enhanced migration to secondary lymphoid organs is an intriguing possibility.
B cells produce cytokines that regulate innate and adaptive immune responses.20 Our findings demonstrate a significant increase in TNF-α–producing B cells in patients with PD compared with controls. There has been heightened interest in TNF in the context of PD.42 The level of TNF-α in CSF is a candidate risk biomarker for the detection of PD at the prodromal stage,43 but the source(s) of TNF-α is unknown. The association of increased production of TNF-α in B cells and disease status of patients with PD will need to be examined in a larger PD cohort and in a longitudinal manner. A recent study reported that patients with inflammatory bowel disease (IBD) using anti-TNF medications had a nearly 80% reduction in the onset of PD.44 Furthermore, inhibition of soluble TNF signaling with a dominant-negative TNF inhibitor led to an ∼50% rescue of DA neurons in several PD preclinical models.45 These findings suggest that TNF-α may play a key role in the pathophysiology of PD. We will continue to explore the capacity of B cells from patients with PD to produce other proinflammatory mediators and determine whether these cells have pathogenic properties. The findings of B-cell abnormalities in patients with early-stage PD are novel, and further examination of B cells may provide relevant biomarkers in patients with PD. Validation of the importance of TNF-α–producing B cells in PD may suggest anti-TNF medications as possible therapy. TNF blockers have been successfully used for patients with IBD and rheumatoid arthritis for many years,46 and there are intriguing data from both the PD and AD literature suggesting that this strategy may be relevant for neurodegenerative diseases.42 However, it should be noted that TNF inhibition had a detrimental effect in patients with multiple sclerosis, promoting demyelination and disease severity.47,48
We acknowledge that there are limitations to this study. First, we were not able to follow our patients with PD in a longitudinal manner to assess immune phenotypes or disease progression in individual patients over time. A recent case study of an individual with blood samples before clinical diagnosis and shortly after diagnosis of motor PD indicated that elevated α-Syn–reactive T cells were features of premotor and early-motor PD.13 Future longitudinal analysis of PD patient cohorts may determine whether dysregulation of peripheral adaptive immune cells predicts a more rapid progression of clinical symptoms. Second, it is difficult to determine when the clinical symptoms of PD begin. We have used the window of diagnosis within 2 years and no more than 1 year of treatment as a practical definition of early PD, but the disease process likely begins substantially earlier than this.1 Third, the patients with PD in this study were diagnosed, and the control cohort was selected purely using clinical criteria. There is the possibility that some of the subjects may eventually prove to have some other disease than PD; based on prior clinical studies, this is usually in the range of 10% of the sample.49 We will continue to explore cytokine profiles in adaptive immune cells of patients with PD in a longitudinal manner to determine whether certain immune cell subsets can be used as potential biomarkers to guide the development of therapeutic strategies.
Acknowledgment
The authors thank Jenna Smith, RN, Candace Cromer, and Courtney Blair for assistance with study coordination and sample collection and the study participants who made this work possible. The Comprehensive Flow Cytometry Core at the University of Alabama at Birmingham is supported by NIH Grants P30AR048311 and P30AI27667.
Glossary
- AD
Alzheimer disease
- DA
dopaminergic
- DN
double negative
- ESS
Epworth Sleep Scale
- HC
healthy control
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- IRB
Institutional Review Board
- MDS-UPDRS
Movement Disorder Society Parkinson's Disease Rating Scale
- MHC
major histocompatibility complex
- MoCA
Montreal Cognitive Assessment
- MS
multiple sclerosis
- NSM
nonswitched memory
- PBMC
peripheral blood mononuclear cell
- PD
Parkinson disease
- SM
switched memory
- TD
terminally differentiated
- α-Syn
α-synuclein
Appendix. Authors
Contributor Information
Zhaoqi Yan, Email: zhaoqi.yan@gladstone.ucsf.edu.
Wei Yang, Email: weiyang@uab.edu.
Hairong Wei, Email: hairongwei@gmail.com.
Marissa N. Dean, Email: mndean@uabmc.edu.
David G. Standaert, Email: dstandaert@uab.edu.
Gary R. Cutter, Email: cutterg@uab.edu.
Etty N. Benveniste, Email: tika@uab.edu.
Study Funding
The Michael J. Fox Foundation Grant (to E.N.B.), NIH Grants P50NS108675 (to D.G.S. and E.N.B.) and R01NS057563 (to E.N.B.), and National Multiple Sclerosis Society Grant RG-1606-24794 (to H.Q.).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386(9996):896-912. [DOI] [PubMed] [Google Scholar]
- 2.Przedborski S. The two-century journey of Parkinson disease research. Nat Rev Neurosci. 2017;18(4):251-259. [DOI] [PubMed] [Google Scholar]
- 3.Marogianni C, Sokratous M, Dardiotis E, et al. Neurodegeneration and inflammation-an interesting interplay in Parkinson's Disease. Int J Mol Sci. 2020;21(22):8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Aguzzi A. Immunotherapy for neurodegeneration? Science. 2019;364(6436):130-131. [DOI] [PubMed] [Google Scholar]
- 5.Schonhoff AM, Williams GP, Wallen ZD, et al. Innate and adaptive immune responses in Parkinson's disease. Prog Brain Res. 2020;252:169-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wijeyekoon RS, Kronenberg-Versteeg D, Scott KM, et al. Peripheral innate immune and bacterial signals relate to clinical heterogeneity in Parkinson's disease. Brain Behav Immun. 2020;87:473-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlisle SM, Qin H, Hendrickson RC, et al. Sex-based differences in the activation of peripheral blood monocytes in early Parkinson disease. NPJ Parkinsons Dis. 2021;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sliter DA, Martinez J, Hao L, et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 2018;561(7722):1258-1262. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Hollenbach JA, Norman PJ, Creary LE, et al. A specific amino acid motif of HLA-DRB1 mediates risk and interacts with smoking history in Parkinson's disease. Proc Natl Acad Sci USA. 2019;116(15):7419-7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garretti F, Agalliu D, Lindestam Arlehamn CS, et al. Autoimmunity in Parkinson's Disease: the role of alpha-Synuclein-specific T cells. Front Immunol. 2019;10:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brochard V, Combadiere B, Prigent A, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119(1):182-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulzer D, Alcalay RN, Garretti F, et al. T cells from patients with Parkinson's disease recognize α-synuclein peptides. Nature. 2017;546(7660):656-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindestam Arlehamn CS, Dhanwani R, Pham J, et al. alpha-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson's disease. Nat Commun. 2020;11(1):1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16(3):149-163. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Chen S, Liu J. The role of T cells in the pathogenesis of Parkinson's disease. Prog Neurobiol. 2018;169:1-23. [DOI] [PubMed] [Google Scholar]
- 16.Kustrimovic N, Comi C, Magistrelli L, et al. Parkinson's disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J Neuroinflamm. 2018;15(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommer A, Maxreiter F, Krach F, et al. Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson's Disease. Cell Stem Cell. 2018;23(1):123-131 e6. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Qi B, Xu W, et al. Clinical correlation of peripheral CD4+cell subsets, their imbalance and Parkinson's disease. Mol Med Rep. 2015;12(4):6105-6111. [DOI] [PubMed] [Google Scholar]
- 19.Saunders JA, Estes KA, Kosloski LM, et al. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson's disease. J Neuroimmune Pharmacol. 2012;7(4):927-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cyster JG, Allen CDC. B cell responses: cell interaction dynamics and decisions. Cell. 2019;177(3):524-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabatino JJ Jr, Probstel AK, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20(12):728-745. [DOI] [PubMed] [Google Scholar]
- 22.Li R, Patterson KR, Bar-Or A. Reassessing B cell contributions in multiple sclerosis. Nat Immunol. 2018;19(7):696-707. [DOI] [PubMed] [Google Scholar]
- 23.Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67(12):1149-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens CH, Rowe D, Morel-Kopp MC, et al. Reduced T helper and B lymphocytes in Parkinson's disease. J Neuroimmunol. 2012;252(1-2):95-99. [DOI] [PubMed] [Google Scholar]
- 25.Jiang S, Gao H, Luo Q, et al. The correlation of lymphocyte subsets, natural killer cell, and Parkinson's disease: a meta-analysis. Neurol Sci. 2017;38(8):1373-1380. [DOI] [PubMed] [Google Scholar]
- 26.Rocca WA, Maraganore DM, McDonnell SK, et al. Validation of a telephone questionnaire for Parkinson's disease. J Clin Epidemiol. 1998;51(6):517-523. [DOI] [PubMed] [Google Scholar]
- 27.Tanner C, Gilley D, Goetz C. A brief screening questionnaire for parkinsonism. Ann Neurol. 1990;28:267-268. [Google Scholar]
- 28.Yan Z, Yang W, Parkitny L, et al. Deficiency of Socs3 leads to brain-targeted EAE via enhanced neutrophil activation and ROS production. JCI Insight. 2019;5(9):e126520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorensen TL, Trebst C, Kivisakk P, et al. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127(1-2):59-68. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Liu Y, Niu Y, et al. Increased abundance of myeloid-derived suppressor cells and Th17 cells in peripheral blood of newly-diagnosed Parkinson's disease patients. Neurosci Lett. 2017;648:21-25. [DOI] [PubMed] [Google Scholar]
- 31.McGinley AM, Sutton CE, Edwards SC, et al. Interleukin-17A serves a priming role in autoimmunity by recruiting IL-1beta-producing myeloid cells that promote pathogenic T cells. Immunity. 2020;50(2):892-906. [DOI] [PubMed] [Google Scholar]
- 32.Siffrin V, Radbruch H, Glumm R, et al. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33(3):424-436. [DOI] [PubMed] [Google Scholar]
- 33.Walsh JT, Hendrix S, Boato F, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125(2):699-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogelaar CF, Mandal S, Lerch S, et al. Fast direct neuronal signaling via the IL-4 receptor as therapeutic target in neuroinflammation. Sci Transl Med. 2018;10(430):e2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gadani SP, Cronk JC, Norris GT, et al. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189(9):4213-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gate D, Saligrama N, Leventhal O, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer's disease. Nature. 2020;577(7790):399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cebrian C, Zucca FA, Mauri P, et al. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun. 2014;5:3633-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seifert M, Przekopowitz M, Taudien S, et al. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci USA. 2015;112(6):E546-E555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei C, Anolik J, Cappione A, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178(10):6624-6633. [DOI] [PubMed] [Google Scholar]
- 40.Colonna-Romano G, Bulati M, Aquino A, et al. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev. 2009;130(10):681-690. [DOI] [PubMed] [Google Scholar]
- 41.Bulati M, Buffa S, Martorana A, et al. Double negative (IgG+IgD-CD27-) B cells are increased in a cohort of moderate-severe Alzheimer's disease patients and show a pro-inflammatory trafficking receptor phenotype. J Alzheimers Dis. 2015;44(4):1241-1251. [DOI] [PubMed] [Google Scholar]
- 42.Clark IA, Vissel B. Therapeutic implications of how TNF links apolipoprotein E, phosphorylated tau, alpha-synuclein, amyloid-beta and insulin resistance in neurodegenerative diseases. Br J Pharmacol. 2018;175(20):3859-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majbour NK, Aasly JO, Hustad E, et al. CSF total and oligomeric alpha-Synuclein along with TNF-alpha as risk biomarkers for Parkinson's disease: a study in LRRK2 mutation carriers. Transl Neurodegener. 2020;9(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peter I, Dubinsky M, Bressman S, et al. Anti-tumor necrosis factor therapy and incidence of Parkinson Disease among patients with Inflammatory Bowel Disease. JAMA Neurol. 2018;75(8):939-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harms AS, Barnum CJ, Ruhn KA, et al. Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson's Disease. Mol Ther. 2011;19(1):46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Haens GR. Top-down therapy for IBD: rationale and requisite evidence. Nat Rev Gastroenterol Hepatol. 2010;7(2):86-92. [DOI] [PubMed] [Google Scholar]
- 47.Gregory AP, Dendrou CA, Attfield KE, et al. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature. 2012;488(7412):508-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The Lenercept Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53(3):457-465. [PubMed] [Google Scholar]
- 49.Jankovic J, Rajput AH, McDermott MP, et al. The evolution of diagnosis in early Parkinson disease. Arch Neurol. 2000;57(3):369-372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available on request from any qualified investigator.