Abstract
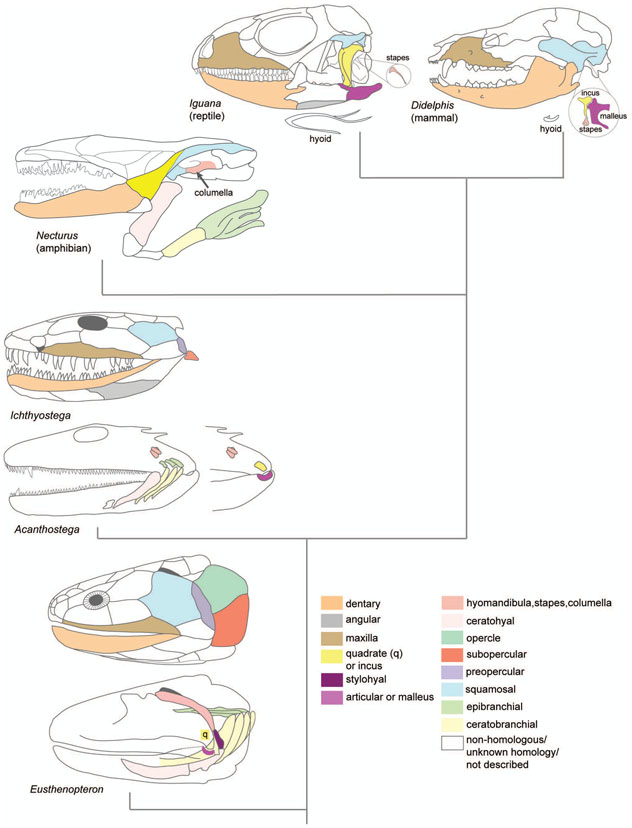
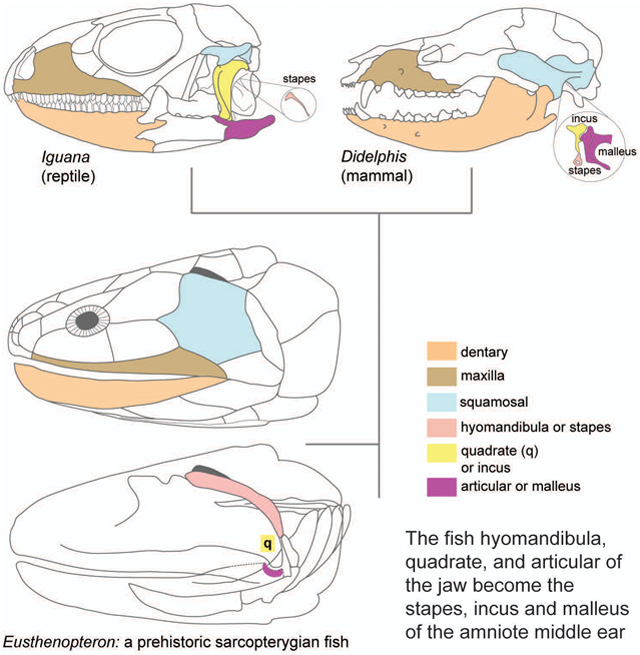
The evolution of the jaw represents a key innovation driving the diversification of vertebrate body plans and behavior. The pharyngeal apparatus originated as gill bars separated by slits in chordate ancestors to vertebrates. Later, with the acquisition of neural crest, pharyngeal arches gave rise branchial basket cartilages in jawless vertebrates (agnathans), and later bone and cartilage of the jaw, jaw support, and gills of jawed vertebrates (gnathostomes). Major events in the evolution of jaw structure from agnathans to gnathostomes include axial regionalization of pharyngeal elements and formation of a jaw joint. Hox genes specify the anterior-posterior identity of arches, and edn1, dlx, hand2, Jag1b-Notch2 signalling, and Nr2f factors specify dorsal-ventral identity. The formation of a jaw joint, an important step in the transition from an un-jointed pharynx in agnathans to a hinged jaw in gnathostomes involves interaction between nkx3.2, hand2, and barx1 factors. Major events in jaw patterning between fishes and reptiles include changes to elements of the second pharyngeal arch, including a loss of opercular and branchiostegal ray bones and transformation of the hyomandibula into the stapes. Further changes occurred between reptiles and mammals, including the transformation of the articular and quadrate elements of the jaw joint into the malleus and incus of the middle ear. Fossils of transitional jaw phenotypes can be analyzed from a developmental perspective, and there exists potential to use genetic manipulation techniques in extant taxa to test hypotheses about the evolution of jaw patterning in ancient vertebrates.
Graphical Abstract
Introduction
A true head represents one of the most sophisticated anatomical structures in vertebrates and is arguably one of the most significant drivers of vertebrate success (Northcutt, 2005; Wilkie & Morriss-Kay, 2001). The evolution of a protective cranium may have also contributed to the emergence of a complex brain and senses. The same cells that create facial structures also secrete factors that promote the growth of the midbrain and forebrain, and likely contributed to the overall increase in brain size (Le Douarin, Brito, & Creuzet, 2007). The evolution of a complex brain and senses, along with pharyngeal cartilages, allowed for the evolution of active predation and selective, intermittent feeding. A shift away from filter feeding associated with the evolution of a jaw allowed early vertebrates to diversify their behaviors, further driving evolution of the brain and sense organs, and eventually a separation of respiration from feeding. In vertebrates, the cranium evolved from intramembranous bony plates (dermatocranium) and inner cartilaginous (neurocranium) elements (Donoghue, Sansom, & Downs, 2006). The jaw evolved from repeating pharyngeal segments first present in chordate ancestors as respiratory structures, later giving rise to cartilaginous branchial baskets of jawless fishes and the bones and cartilages of the facial, upper and lower jaw, jaw support, and posterior gill or throat structures (viscero- or splanchnocranium) of jawed vertebrates (Donoghue et al., 2006; Kardong, 2012).
This review will focus on development and evolution of the jaw apparatus in pre-vertebrates, early vertebrates, agnathans (jawless fish), and gnathostomes (jawed fish), with an emphasis on understanding the changes to the jaw in fishes leading to early tetrapods. The focus on fish is relevant to evolutionary biologists who want to chart the macroevolution of pharyngeal systems in vertebrates from a developmental perspective. A detailed description of zebrafish pharyngeal development is included, as this species has been studied more extensively using molecular analysis than any other osteichthyan fish. Zebrafish studies provide a comprehensive framework to understand how gene expression produces distinct pharyngeal elements, and how changes to gene expression can alter developmental trajectories to produce variations in form. This review is also relevant to developmental biologists who are interested in the origin of the modern vertebrate jaw skeleton, including steps between lampreys and modern gnathostomes, and between fishes and tetrapods, and who may be able to offer hypotheses to explain the evolution of phenotypes in ancient taxa. Ancient taxa can only be directly studied based on their fossil record, however studying the development of extant species alongside fossil evidence can offer insights about key anatomical and molecular events that underlie the evolution of the pharyngeal system. This is not an exhaustive review of all ancient and extant fish lineages, many of which have fascinating individual jaw evolution stories, such as the ancient shark Helicoprion (Ramsay et al., 2015). There is also limited description of non-jaw cranial bone evolution, and is somewhat uncoupled from pharyngeal evolution. In this review, the focus is on elements that 1) persist within lineages or have homologs across evolutionary time, 2) are present in extant taxa, and 3) are of interest to developmental biologists who study pharyngeal patterning in models such as amphioxus, urochordates, lampreys, sharks, gars, lobe-finned sarcopterygian fishes, and zebrafish. In this review “jaw” will refer to the skeletal structures arising from the anterior first and second pharyngeal arches (including upper maxilla and lower mandibular jaw and jaw support skeleton). Gill and throat structures, arising from posterior pharyngeal arches will also be described. For a comprehensive review of the development of other arch derivatives such as vasculature, muscle patterning, and innervation, references are provided at the end of this paper (see Further Reading).
EARLY PHARYNGEAL ARCH STRUCTURE AND ROLE OF CRANIAL NEURAL CREST
In the early vertebrate embryo, the head forms from the anterior expansion and regionalization of the neural tube into a brain, which is covered in a layer of mesoderm and ectoderm. At the anterior region of the head, a prominence forms, called the frontonasal prominence, which will give rise to the forehead, nasal region, and primary palate (S. F. Gilbert & Barresi, 2016). The pharyngeal arches appear as a cranial to caudal series of repeating swellings and clefts on the lateral and ventral region of the face, caudal to the frontonasal prominence. Each pharyngeal arch has a mesoderm core, and while the outer half of the arch is covered in ectoderm, the inner half is covered in endoderm which lines the oral cavity and pharynx of the embryo (Shone & Graham, 2014). The formation of arches requires a process of segmentation of embryonic tissue. Separation of segments is driven by the endoderm, which evaginates laterally to the ectoderm surface, forming a pouch, on which either side forms the arch (Graham & Richardson, 2012). Paired box genes Pax1 and Pax9, expressed in the endoderm of the pharyngeal pouches, are factors associated with pouch formation and segmentation of the pharynx (Liu, Wang, Li, Huang, & Wang, 2013; Müller et al., 1996; Ogasawara, Shigetani, Hirano, Satoh, & Kuratani, 2000). In jawed vertebrates, typically the first and second pharyngeal arches are referred to as the mandibular and hyoid arches respectively, and the gill-bearing or throat structures are referred to as branchial arches (Kardong, 2012). In some references, “branchial arch” is used to refer to anterior pharyngeal segments (Mallatt, 1996).
The mesenchyme within the pharyngeal arches derives from a combination of cranial mesoderm and a special population of cells known as cranial neural crest cells. Sometimes referred to as the “fourth germ layer” (Hall, 2000), neural crest cells arise from a population of multipotent cells that delaminate from the neural plate at the junction with the ectoderm (the neural-ectoderm border). Neural crest is considered a vertebrate synapomorphy, meaning it evolved within the vertebrate clade, and is considered a critical innovation in the evolution of the head, peripheral nervous system, and sensory organs (Sauka-Spengler & Bronner-Fraser, 2008). The multipotent trunk neural crest cells undergo an epithelial to mesenchymal transition and then migrate from the neural-ectoderm junction throughout the body to give rise to cardiac cells, enteric nervous system cells, adrenal cells, neurons, glia, and melanocytes. Cranial neural crest cells migrate from the dorsal neural tube anteriorly and ventrally through the head of the embryo to populate the pharyngeal arches where these cells mingle with existing cranial mesoderm and differentiate into the craniofacial nerves and mesenchymal derivatives of skeletal cells, neurons, glia, and tendon (Calloni, Le Douarin, & Dupin, 2009; Grenier, Teillet, Grifone, Kelly, & Duprez, 2009). Cranial neural crest cells are not required for arch formation, demonstrated by neural crest ablation experiments in chick which do not impair arch and pouch formation, and based on the evidence that chordates lacking neural crest still produce pharyngeal structures (Escriva, Holland, Gronemeyer, Laudet, & Holland, 2002; Veitch, Begbie, Schilling, Smith, & Graham, 1999). Rather, specification of the arches is first determined by endoderm (G. Couly, Creuzet, Bennaceur, Vincent, & Le Douarin, 2002; Piotrowski & Nüsslein-Volhard, 2000).
In vertebrates, neural crest cell specifier genes, such as FoxD3, Slug/Snail, and Twist, Pax3/7, and SoxE factors (Figure 1) are associated with differentiation and are expressed in pre-migratory and migratory neural crest cells (Meulemans & Bronner-Fraser, 2004; Weider & Wegner, 2017). Some key factors associated with neural crest cell migration into the arches includes attractive/repulsive cues of Eph receptor/Ephrin ligand and Neuropilin (Nrp)/Semaphorin (Sema) ligand interactions between neural crest cells and their migratory environment (Davy, Aubin, & Soriano, 2004; Osborne, Begbie, Chilton, Schmidt, & Eickholt, 2005; Smith, Robinson, Patel, & Wilkinson, 1997; Yu & Moens, 2005). The pharyngeal mesoderm and endoderm also express guidance cues such as Twist and Tbx1, to ensure the proper migration of cells into arches (Soo et al., 2002; Vitelli, Morishima, Taddei, Lindsay, & Baldini, 2002). Following migration into the arches, neural crest cells differentiate into skeletogenic chondrocytes and osteoblasts which proliferate and secrete matrix to generate cartilage and bone, under regulatory factors such as bone morphogenic proteins (BMPs), SoxE factors, Pax genes, and Twist (Bhatt, Diaz, & Trainor, 2013; Meulemans & Bronner-Fraser, 2004; Monsoro-Burq, 2015). Most of the head skeleton in vertebrates is derived from cranial neural crest cells, generating bones of the cranium vault and face, and the lower jaw and gill elements. Other portions of the cranium, including elements mostly posterior to the frontal bone arise from the cranial paraxial head mesoderm (G. F. Couly, Coltey, & Le Douarin, 1992; Le Douarin, Ziller, & Couly, 1993; Yoshida, Vivatbutsiri, Morriss-Kay, Saga, & Iseki, 2008). Together, cranial neural crest cells and head paraxial mesoderm contribute to the complexity of the vertebrate head and face.
Figure 1:
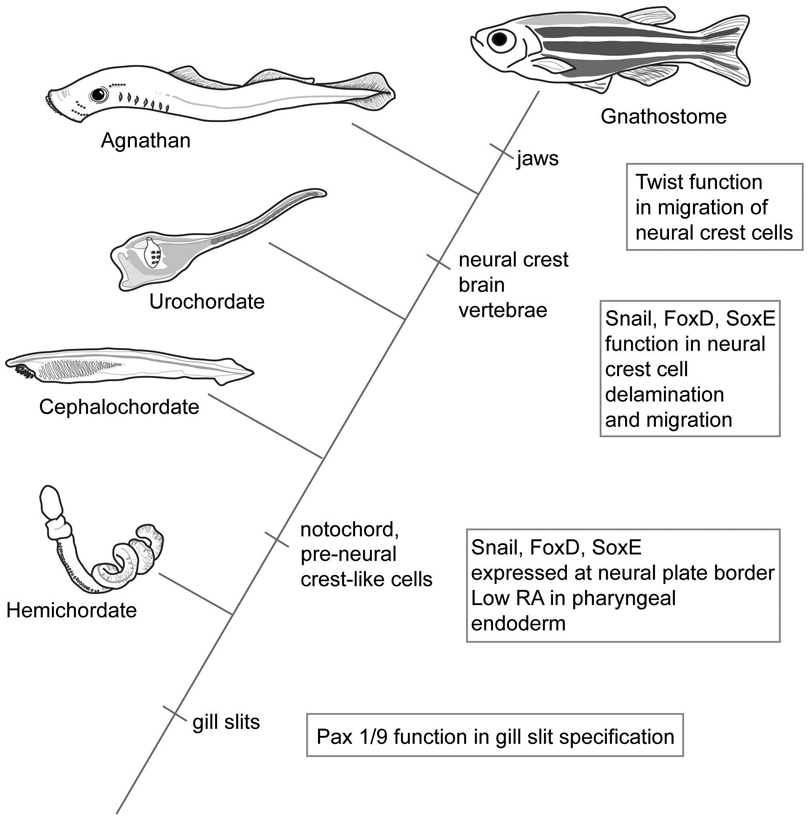
Evolution of gills slits and neural crest in hemichordates and chordates. Gill slits first appear in hemichordates, associated with the expression of Pax1/9 homologs. Snail, FoxD, and SoxE factors are expressed at the neural plate border in pre-vertebrate chordates, at locations where neural crest later evolves in vertebrates. In vertebrates, these factors function in neural crest cell delamination and migration. Low retinoic acid (RA) in pharyngeal endoderm specifies the anterior pharynx in pre-vertebrate chordates. Twist is associated with neural crest cell migration in gnathostomes, but does not function in this process in agnathans. Hemichordate redrawn from an image by C.B. Cameron, used with permission.
HEMICHORDATE AND CHORDATE ANCESTORS TO VERTEBRATES
The vertebrate pharyngeal apparatus has deep evolutionary origins, the product of the evolution of segmented, repeating gill-slit structures present in deuterostome ancestors of hemichordates and chordates (Gillis, Fritzenwanker, & Lowe, 2012). From stem groups, deuterostomes evolved into the Ambulacraria, which includes the phyla Echinodermata (e.g. sea urchins, sea stars) and Hemichordata (enteropneusta acorn worms and pterobranchia), and Chordata, which includes the subphyla Vertebrata, Urochordata (larvaceans, thaliaceans, ascidians), and Cephalocordata (amphioxus).
Hemichordates
Hemichordates are the only extant deuterostome out-group to chordates with gill slits whereas Echinodermata, which lack gill slits, are thought to have lost their ancestral gill structures secondarily (Gillis et al., 2012; Shu, Morris, Han, Zhang, & Liu, 2004). Enteropneusta hemichordates are burrowing or suspension feeders, ingesting water and organic matter into ciliated tracks in the mouth, expelling water through many repeating lateral pharyngeal slits in the pharynx wall into branchial pouches which exit the sides of the body through branchial pores (Kardong, 2012). Molecular analysis shows a conservation of expression of key transcription factors associated with formation of pharyngeal slits and branchial pores between hemichordates and vertebrates, confirming that the gill slit program was established prior to the chordate lineage, and that vertebrate pharyngeal complexity builds upon this basic plan (Gillis, Fritzenwanker, et al., 2012). For example, hemichordates (also urochordates and cephalochordates) express Pax1/9 homologs in the developing gill epithelia, indicating a conservation of the Pax1/9-mediated gill slit program (Figure 1) (Ogasawara, Wada, Peters, & Satoh, 1999; Simakov et al., 2015).
Cephalochordates
Modern amphioxus is considered to resemble an early chordate body plan, and the pharyngeal anatomy and patterns of gene expression in amphioxus gives clues as to its homology with modern vertebrates. Amphioxus is a burrowing, suspension-feeding organism with a muscular ciliated pharynx allowing passage of water and food to pharyngeal slits supported by fibrous connective tissue pharyngeal bars (Kardong, 2012). The pharyngeal slits and bars are enclosed by an atrium, and as food particles travel from the pharynx to the endostyle and then to the gut, and water is transported into the atrium cavity and out the posterior atriopore (Kardong, 2012). The gill structure of amphioxus is of a segmented repeating pattern of individual slits and bars. These pharyngeal structures are derived from mesoderm of the anterior somites as amphioxus lack neural crest (Koop et al., 2014a). Homologs of some vertebrate neural crest cell genes are expressed in regions near the neural plate border where in vertebrates the definitive neural crest originates, including AmphiSnail, a homolog of the vertebrate Snail, a specifier of neural crest cells and their sub lineages (Langeland, Tomsa, Jackman, & Kimmel, 1998). AmphiSnail-expressing cells do not migrate from the neural-ectoderm border, indicating that cells may have subsequently acquired migratory potential in ancestors to vertebrates. Other vertebrate neural crest specifier genes have homologs in amphioxus, including AmphiSoxE and AmphiFoxD expressed at the neural plate or neural plate border (Tai et al., 2016; J.-K. Yu, Holland, & Holland, 2002). Although amphioxus lack definitive neural crest, it is thought that early vertebrates acquired novel regulatory elements to utilize these factors to specify neural crest cell migration (Yu et al., 2002; Yu, Meulemans, McKeown, & Bronner-Fraser, 2008). In amphioxus, retinoic acid (RA) is produced in the middle third of the endoderm establishing the posterior limit of the pharynx (Escriva et al., 2002). AmphiHox1, also expressed in the middle third of the endoderm, mediates the activity of RA posterior to the pharynx to repress expression of Pax1/9, allowing normal patterning of the foregut/midgut into non-pharyngeal endoderm (Schubert, 2004). As in vertebrates, a low level of RA in the pharyngeal region is required for pharyngeal segmentation as low levels allow for the expression of Tbx1/10 and Pax1/9 genes, indicating that mechanisms of anterior positioning of the pharynx predated neural crest in chordates (Escriva et al., 2002; Holland & Holland, 1996; Koop et al., 2014b).
Urochordates
Among chordates, urochordates (also known as tunicates) are the closest living sister taxa to vertebrates (Delsuc, Brinkmann, Chourrout, & Philippe, 2006), and so their anatomy and development has been closely studied to understand the origin of vertebrate traits such as neural crest. As in cephalochordates, in urochordates, neural crest-like cells have been identified originating at the neural plate border. Whereas cephalochordates have non-migratory neural crest-like cells, urochordate neural crest-like cells can migrate short distances into the trunk to give rise to pigment cells (Jeffery, 2006; Jeffery et al., 2008a). This evidence points to the production of pigment as an original function of neural crest, and that these cells later acquired more diverse developmental fates in vertebrates (Jeffery, 2006). Experiments using the urochordate Ciona intestinalis have shown that expression of vertebrate neural crest specifiers including Twist, FoxDb, and Snail associated with the neural plate border is evidence of a rudimentary neural crest cell-forming population (Corbo, Erives, Di Gregorio, Chang, & Levine, 1997; Jeffery et al., 2008b). The question remains as to how these non-migratory or limited-migration neural crest-like cells in cephalochordates and urochordates acquired their migratory and mesenchymal fates in vertebrates. Analysis of factors associated with vertebrate neural crest migration and differentiation has led to the testing of function of these factors in urochordates. In vertebrates, only the cephalic (cranial and heart) neural-crest derived mesenchyme expresses Twist, and it is associated with migration and survival of neural crest cells in the pharyngeal arches and heart (Soo et al., 2002; Vincentz et al., 2008). Mis-expression of Ciona Twist in neural crest-like cells can induce them to become migrating mesenchymal cells similar to the neural crest-derived mesenchyme in vertebrates (Abitua, Wagner, Navarrete, & Levine, 2012). While neural crest-like cells are present in cephalochordates and urochordates and both groups possess factors in the neural crest gene regulatory network within their genomes, it is believed that the acquisition of ectomesenchymal determinants such as Twist led to the evolution of neural crest-derived head structures in vertebrates (Figure 1)(Abitua et al., 2012).
STEM-GROUP VERTEBRATES, EXTINCT AND EXTANT AGNATHAN VERTEBRATES
Haikouella and Haikouichthys
Based on fossil evidence of specimens such as Haikouella and Haikouichthys from the early Cambrian era, early vertebrates likely resembled modern amphioxus but with an endoskeleton composed of vertebral elements surrounding a notochord and a muscular pharynx with springy cartilaginous pharyngeal bars covered by gills (Holland & Chen, 2001; Mallatt & Chen, 2003; Shu et al., 2003). Along with changes to the pharynx a pump-like feeding strategy emerged, replacing the ciliary mechanism in pre-vertebrate chordates, which would have enhanced feeding and increased respiration capacity in early vertebrates. In Haikouella, debatably a stem group vertebrate, we see the emergence of a true head with upper lips, a buccal cavity, pharyngeal denticle or tooth, pharyngeal or visceral skeleton with possible gill filaments, sensory organs such as eyes, and a brain (Holland & Chen, 2001; Mallatt & Chen, 2003). Haikouichthys, a true vertebrate and stem craniate or sister to craniates, had nine visceral clefts resembling pharyngeal pouches, a likely cartilaginous visceral arch skeleton, eyes, possible nasal sacs and otic capsules, and a possibly cartilaginous cranium (Shu et al., 1999, 2003). The presence of definitive neural crest cell-derived structures forming a visceral skeleton supporting a muscular pump pharynx and possible early cranium along with the vertebrate characteristics of sensory placodes supports a shift towards predatory feeding, and the emergence of the head as a neomorphic unit, as theorized by Gans and Northcutt (Northcutt, 2005).
Lamprey pharyngeal anatomy
Modern agnathan lineages comprise the Petromyzontidae (lampreys) and Myxinoidea (hagfishes) orders. Molecular analysis indicates they are a monophyletic group known as the cyclostomes (“round” and “mouth”) (Mallatt & Sullivan, 1998; Takezaki, Figueroa, Zaleska-Rutczynska, & Klein, 2003). Although modern lampreys and hagfishes have very derived pharyngeal structures associated with their rasping feeding strategies, they provide a useful model out-group to explore the origins of pharyngeal patterning in gnathostomes. Lampreys express factors associated with pharyngeal arch patterning including Pax1/9 and Tbx1/10 consistent with other ancestral chordates (Ogasawara et al., 2000; Tiecke et al., 2007), but as vertebrates, also have true cranial neural crest migrating into arches (Horigome et al., 1999). Studies of lamprey larvae indicate that some differences in neural crest-cell migration compared to gnathostomes. Cranial neural crest cell migration occurs in distinct streams as in gnathostomes, although lamprey neural crest cells exhibit more extensive migration and mixing to populate arches, compared to the more restrictive pattern observed in other vertebrates (McCauley & Bronner-Fraser, 2003). Specification and migration of early neural crest in lampreys involves factors such as Snail, FoxD3, and SoxE genes, although Twist is not expressed in pre-migratory or early migratory neural crest cells as it is in gnathostomes (Figure 1) (Sauka-Spengler, Meulemans, Jones, & Bronner-Fraser, 2007; York, Yuan, Zehnder, & McCauley, 2017). It is intriguing that lampreys do not express Twist in pre-migratory or early migrating neural crest cells, although they do express Twist in post-migratory crest cells within the arches (Sauka-Spengler et al., 2007). This indicates that Twist is not essential for early stages of neural crest cell migration in lamprey. Other differences in expression of neural crest development genes between lampreys and gnathostomes (i.e. Cadherins) suggests that the gnathostome gene regulatory network is a very derived state compared to what may be a more ancestral condition in lampreys (York et al., 2017).
During development, lamprey form eight pouches and nine pharyngeal arches (Richardson, Admiraal, & Wright, 2010). The first arch is the “mandibular” arch, the second has a portion of a gill hemibranch and so is technically a branchial arch although is also referred to as the “hyoid” arch as it is often considered to be homologous to the vertebrate second pharyngeal (hyoid) arch (Richardson et al., 2010). Within arches, neural crest-derived mesenchyme differentiates into cartilaginous structures. Compared to earlier chordates, there is specialization of the anterior first and second pharyngeal arch structures. The first pharyngeal arch is associated with formation of the curtain-like velum, rather than the mandibular cartilage of jawed vertebrates (Richardson et al., 2010). The velum separates the specialized respiratory branchial tube from the pharynx (Cohn, 2002; Richardson et al., 2010). The upper lip is likely to be formed in part by the dorsal aspect of the mandibular arch (Kuratani, Adachi, Wada, Oisi, & Sugahara, 2013). The second pharyngeal arch has no cartilage but contains a gill septum, and posterior third through ninth pharyngeal arches bear repeating cartilage bars and there are gill openings in between the third through eighth arches (Richardson et al., 2010). In agnathans, the pharyngeal structures do not form a joint with the cranium (paleostylic condition, see Kardong, 2012 for a phylogenetic tree showing the evolution of jaw articulations in vertebrates), a trait that evolved later in gnathostomes (Kardong, 2012).
Anterior-posterior pharyngeal patterning in lamprey
Among various species of gnathostomes studied, including zebrafish, Nile tilapia, sharks, Xenopus, chicks, and mice, the anterior-posterior identity of pharyngeal arch structures is associated with collinear expression of Hox genes (see more detailed discussion later; (Hunt et al., 1991; Hunter & Prince, 2002; S. C. Kuratani & Wall, 1992; Le Pabic, Scemama, & Stellwag, 2010; Oulion et al., 2011; Ozeki, Kurihara, Tonami, Watatani, & Kurihara, 2004). Vertebrates lack Hox expression in the first pharyngeal arch, however, in general the second through fourth arches express Hox2, Hox2/3 (arch 3), Hox2/3/4 (arch 4), paralog groups respectively, and posterior arches express various combinations of posteriorly-expressed Hox homologs (reviewed by Minoux & Rijli, 2010). The spatially restricted pattern of expression of each Hox gene is required for the correct regional specification of arch structures. Although lampreys are vertebrates and have a neural crest cell-derived cartilaginous pharyngeal skeleton, they lack a first pharyngeal arch-derived mandibular cartilage. The molecular and developmental basis for this is debated, and may be explained by the expression of a Hox6 homolog in the first arch or gill slit (Cohn, 2002). In lampreys, Cohn and colleagues (2002) report paralogous group HoxL6 is expressed in the first arch, and in amphioxus, AmphiHox6 is expressed in endoderm associated with the first gill slit (Cohn, 2002). However, a study by Takio and colleagues (2004) shows that the first arch in lampreys is also Hox-negative and second and third arches express LjHox2 and LjHox3d homologs, as in gnathostomes, and LjHox6w is expressed in posterior eighth endodermal pouch (Takio et al., 2004). This suggests the absence of mandibular jaws in lampreys is not due to the presence of Hox expression in the first arch. The discrepancy between the two studies may be due to the species used for analysis, although recent studies favor the interpretation of Takio and colleagues (2004) that a Hox-negative first arch is a general vertebrate trait (Cerny et al., 2010).
Dorsal-ventral pharyngeal patterning in lamprey
In zebrafish, dorsal-ventral patterning is controlled by ventral expression of edn1, hand2, dlx, jag1b, notch2, and Nr2f factors (see more detailed discussion later; Alexander et al., 2011; Barske et al., 2016, 2018; C. T. Miller, Schilling, Lee, Parker, & Kimmel, 2000; Craig T. Miller, Yelon, Stainier, & Kimmel, 2003; Talbot, Johnson, & Kimmel, 2010; Zuniga, Stellabotte, & Crump, 2010). Mice also require Edn1, Hand2, and Dlx factors for correct dorsal-ventral jaw patterning, and Jagged1-Notch2 signalling is involved in patterning of the mouse stapes and incus (homologous to the hyomandibula and palatoquadrate, respectively) (Beverdam et al., 2002; Depew, Lufkin, & Rubenstein, 2002; Ozeki et al., 2004, 2004; Teng et al., 2017)
Analysis of chordate phylogeny suggests that Edn1 did not exist in pre-vertebrate chordates, but emerged early in the vertebrate lineage prior to lampreys (Martinez-Morales, Henrich, Ramialison, Wittbrodt, & Martinez-Morales, 2007). Lampreys express homologs of vertebrate Edn1, Ednr, Dlx, and Hand2, although their expression patterns are not entirely homologous to jawed vertebrates (Kuraku, Takio, Sugahara, Takechi, & Kuratani, 2010). Edn1 is expressed in ectoderm and mesoderm in zebrafish (Miller, Schilling, Lee, Parker, & Kimmel, 2000; Zuniga, Stellabotte, & Crump, 2010). LjEdn-A is expressed in perioral surface ectoderm and both LjEdn-A and E homologs are expressed in upper lip ectoderm, and in pharyngeal arch mesenchyme, and LjEdn-C is expressed in lower lip ectoderm (Kuraku et al., 2010). Expression of LjEdnrα is present in the mesenchyme and ectoderm associated with lips and pharyngeal arches, and in migrating neural crest cells (Kuraku et al., 2010). As in gnathostomes, LjHandA is expressed ventrally in the oropharynx (Cerny et al., 2010; Kuraku et al., 2010). LjDlx homologs are variously expressed in the lip ectoderm, gill bars, and mesenchyme along the anterior-posterior axis of pharyngeal arch formation (Cerny et al., 2010; Kuraku et al., 2010). Kuraku and colleagues (2010) and others suggest there is no dorsal-ventral regionalization of Dlx expression as in gnathostomes (Kuraku et al., 2010; Neidert, Virupannavar, Hooker, & Langeland, 2001), although Cerny and colleagues (2010) indicate there is evidence of nesting of Dlx markers associated with expression of ventral specifiers Hand2 and MsxB (Cerny et al., 2010). The conservation of expression of these factors in lamprey as well as jawed vertebrates indicates that gene pathways associated with arch patterning predated the emergence of the jaw.
Hagfishes
Hagfishes have developmental similarities to other agnathans and characteristics conserved among all vertebrates. Hagfish embryos show evidence of migratory neural crest cells that express homologs of the vertebrate neural crest markers Pax6, Pax3/7, SoxEa and Sox9 (Ota, Kuraku, & Kuratani, 2007). Embryos express a Pax1/9 homolog in the pharyngeal pouches and a Tbx1/10 homolog is expressed in the arch mesenchyme (Oisi, Ota, Kuraku, Fujimoto, & Kuratani, 2013). As in lampreys, the mandibular arch forms velum structures and the mesoderm of the second, third, and fourth pharyngeal arches resemble these arches in lamprey (Oisi, Fujimoto, Ota, & Kuratani, 2015). In the hagfish, the pharynx is more caudal than in lamprey, and these differences, as well as unique features of the nasopharyngeal arrangement that is different to lampreys and other vertebrates indicates that hagfishes have acquired specific modifications to feeding and respiration unique to their order.
Ostracoderms and Galeaspids
Following the split of agnathan orders from the vertebrate lineage approximately 500 million years ago (mya) (Smith et al., 2013), other extinct forms of agnathans can be found in the fossil record that show the appearance of anatomical traits that foreshadow the emergence of gnathostomes. In the Late Cambrian, ancient ostracoderm (“bony” and “skin”) and galeaspid (“helmet shield”) fish debut, featuring a mineralized exoskeleton and cartilaginous endoskeleton. The cartilaginous endoskeleton in ostracoderms and galeaspids is consistent with the lamprey skeleton, however the ability to make dermal bone was an innovation in these fishes. Ostracoderms reveal the first evidence of cellular bone (Donoghue et al., 2006). Ostracoderms and galeaspids had the ability to generate a superficial layer of mineral on endoskeletal elements, although ossification where cartilage is replaced by bone, is believed to have evolved later (Donoghue et al., 2006; Nian-Zhong, Donoghue, Smith, & Sansom, 2005). Considerable debate exists about the origin of each mineralized tissue, bone, cartilage, dentin, and enamel in vertebrates, although paleontological analyses suggest these tissues may have evolved independently (Donoghue et al., 2006).
Changes to the anatomical arrangement of head structures in galeaspids compared to the cyclostomes suggests a new developmental landscape that allowed the anterior migration of neural crest cells into the jaw-forming region of the head (Shigetani et al., 2002). Cyclostomes have a single median nasohypophyseal placode that gives rise to a single nostril leading to a nasohypophyseal organ, which is a portal system associated with the hypothalamus (Gai, Donoghue, Zhu, Janvier, & Stampanoni, 2011; Kuratani, Nobusada, Horigome, & Shigetani, 2001; Uchida, Murakami, Kuraku, Hirano, & Kuratani, 2003). In the galeaspid Shuyu (430 mya), paired nasal sacs and changes to the patterning of the hypophyseal duct suggest these organs arose from separate placodes (Gai et al., 2011). Separation of these placodes suggests that changes to the non-skeletal face anatomy may have led to new migration patterns and apposition of neural crest cells within the head, leading to formation of skeletal tissue in novel regions. This is evident in ectomesenchymal trabeculae separating nasal sacs from the hypophysis, presaging a protective skeletal shelf to support the telencephalon in later jawed placoderms (Dupret, Sanchez, Goujet, Tafforeau, & Ahlberg, 2014).
Evolution of bone
Although cartilage is an ancient bilaterian trait, detectable in invertebrate protostomes, gene duplication events and acquisition of new regulatory sequences underlie further evolution of cartilage and mineralization of cartilage in vertebrates (Jandzik et al., 2014). Duplication and diversification of an ancestral fibrillar cartilage gene for type 2 collagen (Col2α1), a major constituent of the vertebrate skeleton, occurred at the chordate-vertebrate transition (Zhang & Cohn, 2006). In vertebrates, this Col2α1 gene is activated by the transcription factor Sox9 of the SoxE gene family, facilitated by SoxD, FGF and BMP signaling (Bi, Deng, Zhang, Behringer, & de Crombrugghe, 1999; Kumar, Ray, & Chapman, 2012; Lefebvre, Li, & de Crombrugghe, 1998). This core regulatory mechanism appears to also be present in amphioxus, although amphioxus does not have a neural crest-derived cartilaginous head skeleton. It has been demonstrated that SoxE acquired new cis-regulatory elements in vertebrates, and this lead to SoxE expression in neural crest cells, leading to the differentiation of cellular cartilage from neural crest (Jandzik et al., 2014; Tarazona, Slota, Lopez, Zhang, & Cohn, 2016).
The duplication of the ancestral bilaterian osteonectin (SPARC) gene into the osteonectin-like SPARC-like 1 (SPARCL1) gene in jawless agnathans coincides with the evolution of a mineralized exoskeleton (Kawasaki, Buchanan, & Weiss, 2007). This is followed by tandem duplication of SPARCL1 into a family of secretory calcium-binding phosphoprotein (SCPP) genes in vertebrates, which is believed to have lead to tissue mineralization (Kawasaki et al., 2007). SCPPs control calcium-phosphate levels in the extracellular environment, and crystallization of these deposits led to creation of mineralized matrix of the skeleton (Kawasaki & Weiss, 2003). It is hypothesized that duplications and diversifications of SCPPs and their regulatory sequences led to the formation of novel functions in developmentally similar tissues, leading to enamel, dentine, and bone (Donoghue et al., 2006; Kawasaki & Weiss, 2003). The evolution of genetic pathways to allow for formation of a mineralized endoskeleton, as seen in later vertebrates, was critical to the evolution of the vertebrate predatory feeding apparatus.
DEVELOPMENT AND ANATOMY OF THE JAW: AN EXAMPLE FROM ZEBRAFISH
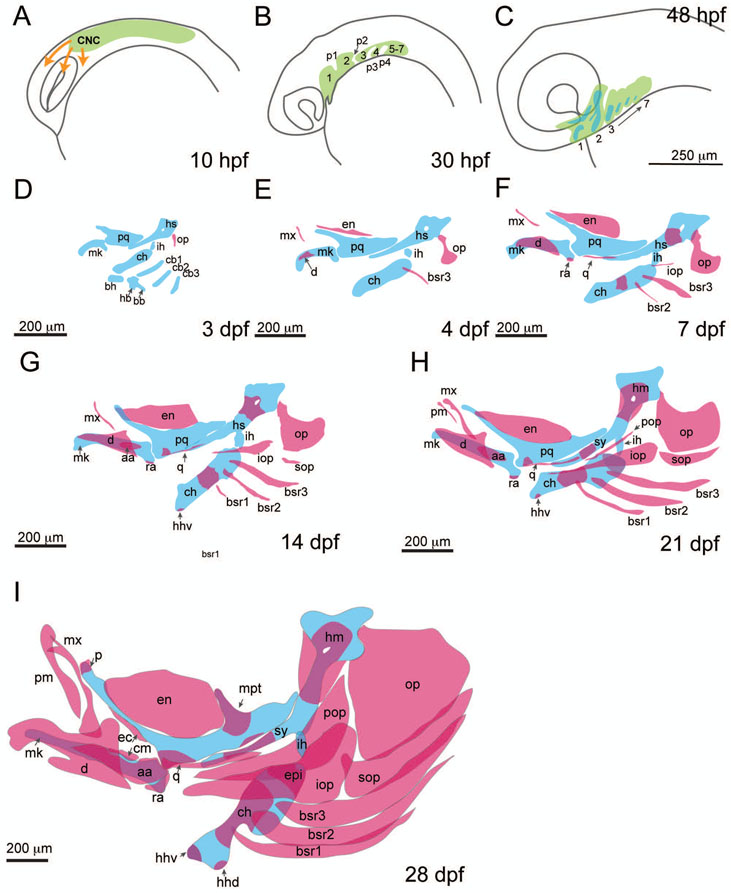
To establish the basic steps involved in fish jaw development, this section will outline the formation of the zebrafish pharyngeal skeleton from specification of the arches to emergence of skeletal elements and their patterning (Figures 2 and 3). This will cover zebrafish embryonic stages 0-3 days post-fertilization (dpf), through larval stages 4 dpf up to 28 dpf (juvenile stage begins 45 dpf, and adult 90 dpf) (Singleman & Holtzman, 2014). The zebrafish jaw is representative of the gnathostome fish template and can be used to understand both how the osteichthyan plan arose, and how it was modified over the course of evolution. Zebrafish jaw morphology builds on the pharyngeal structures of jawless vertebrates and early gnathostomes, where elements are added and specialized in their shape and function. As discussed later, this basic osteichthyan plan is modified in lobe finned sarcopterygians and tetrapodomorphs, the ancestors of terrestrial vertebrates, where structures were removed, reduced, or modified. The following pages will describe the development of the pharyngeal arches, and arch-derived structures, as well as the pouches. Descriptions are based on anatomy described in the FishFace Atlas of zebrafish craniofacial development (https://www.facebase.org/fishface/home/) and other descriptions with some additional information based on cellular studies (Cubbage & Mabee, 1996; Eames et al., 2013). Following the description of the development and anatomy of pharyngeal elements, a subsequent section will outline the molecular mechanisms currently known to pattern these elements in zebrafish.
Figure 2:
Stages of neural crest migration, pharyngeal arch patterning, and formation of the pharyngeal skeleton in zebrafish. A) Cranial neural crest cell migration begins at approximately 10hpf. B) By 30hpf the first through seventh pharyngeal arches (1-7) and first four pouches (p1-p4) are formed. Primordia for the posterior arches and pouches are present but arches and pouches are indistinct. C) By 48 hpf the precursors for skeletal elements (blue) can be detected within the arch mesenchyme (green). D) At 3dpf, cartilaginous elements of the first through fifth pharyngeal arches are present. Ventral midline elements are present, as is the opercle. E and F) Cartilaginous elements enlarge and more dermal bones form. The opercle becomes a fan-shape and by 6 or 7 dpf ossification of the hyosymplectic and ceratohyal begins. G-I) 14dpf-28dpf cartilaginous elements increase in size and cartilaginous elements undergo ossification. Dermal bones form and expand to encase cartilaginous elements. Cartilage is shown in blue, bone is shown in red. Anguloarticular (aa), branchiostegal ray (bsr), ceratobranchial (cb1-3), ceratohyal (ch), coronomeckelian (cm), cranial neural crest cells (CNC), dentary (d), dorsal hypohyal (hhd), ventral hypohyal (hhv), ectopterygoid (ec), entopterygoid (en), epihyal (epi), hyomandibula (hm), hyosymplectic (hs), interhyal (ih), interopercle (iop), maxilla (mx), Meckel’s cartilage (mk), metapterygoid (mpt), opercle (op), palatine (p), palatoquadrate (pq), premaxilla (pm), preopercle (pop), quadrate (q), retroarticular (ra), subopercle (sop), symplectic (sy).
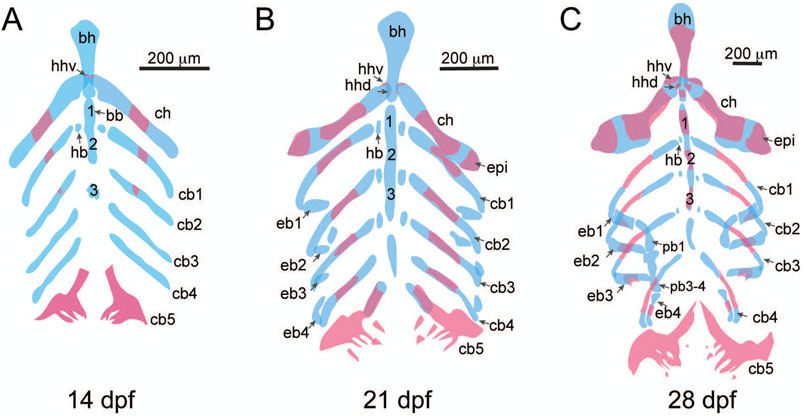
Figure 3:
Structures of the branchial skeleton. A) 14 dpf, ventral view of basibranchials (bb, 1-3), basihyal (bh), ceratobranchials (cb1-5), ceratohyal (ch), dorsal hypohyal (hhd), hypobranchials (hb), and ventral hypohyal (hhv). B) 21 dpf, ventral view, epibranchials (ep1-4) are present. C) 28 dpf, epibranchials have increased in size and are mineralizing, and pharyngobranchials (pb1-4) are present.
10-36hpf
At approximately 10 hours post-fertilization (hpf), cranial neural crest cells begin migrating from the anterior neural plate junction posterior to the eye, migrating rostrally throughout the head (Figure 2A). By 24hpf, neural crest cells are located in pharyngeal arches 1 and 2. The first pharyngeal arch is positioned immediately ventral and posterior to the eye, and surrounds the oral ectoderm of the mouth. The first pharyngeal pouch separates the dorsal halves of the first arch from the second. The second arch is located caudal or posterior to the pouch. Following the second arch is the second pouch, and subsequently arches 3-6, separated by respective pouches (Figure 2B). As development proceeds, the arches expand dorsal-ventrally, and first pharyngeal arch expands anteriorly ventral to the eye.
42-72hpf
As early as 2 dpf, the cells within the pharyngeal arch mesenchyme begin to condense and differentiate into chondrocytes and osteoblasts of the cartilage and dermal skeleton (Figure 2C). The Meckel’s cartilage forms in the ventral portion of the first arch, ventral to the oral ectoderm. The palatoquadrate cartilage forms in the dorsal portion of the first pharyngeal arch, dorsal to the oral ectoderm. The hyomandibula and ceratohyal form in the dorsal and ventral portions of pharyngeal second arch, and the symplectic and interhyal cartilages are visible. On the third day, the hyomandibula and symplectic form the hyosymplectic, and an interhyal between the hyosymplectic and ceratohyal forms (Figure 2D)(Kimmel et al., 1998). From the outset, a patent joint is visible between the posterior Meckel’s and the anterior palatoquadrate, which forms the early lower jaw joint in fish and will be enveloped by bones later in development. The basihyal, basibranchials, hypobranchials, and ceratobranchials are present in arches 3-5. The opercle bone, as evidenced in the second arch by a few cells forming a stick-like structure, is present as early as 60hpf (DeLaurier et al., 2014). The early neurocranium is formed of paired trabeculae joined rostrally by the ethmoid plate, and paired parachordals posteriorly (not shown, see DeLaurier et al, 2012). The trabeculae of the neurocranium are evident as early as 36 hpf, with the anterior ethmoid plate filling in by 54 hpf. In fishes, the trabeculae and parachordals are divided by a median parasphenoid bone (Kardong, 2012). The trabeculae and ethmoid will become the palate, and the parachordals will grow together to form the basal plate separating the otic capsules (Kardong, 2012).
4-7dpf
At 4dpf, the cartilage elements are enlarged. Among first arch elements, the maxilla bone is associated with the palatoquadrate and the dentary is associated with the Meckel’s cartilage (Figure 2E). Within second arch elements, at 4dpf the entopterygoid appears on the dorsal margin of the palatoquadrate. At 4dpf, one branchiostegal ray (ray 3) is present articulating with the ceratohyal. The opercle has acquired a fan-like shape. By 7dpf the retroarticular is associated with the Meckel’s cartilage (Figure 2F). The dentary has expanded to cover more of the Meckel’s cartilage. In the second arch by 6 or 7dpf there is evidence of ossification of the ceratohyal and hyomandibula, starting mid-shaft in the ceratohyal and at the mid-region of the head of the hyomandibula, surrounding the nerve foramen. Initially the symplectic appears to be part of the hyomandibula, but later becomes a separate ossified element. By 6-7 dpf, the second branchiostegal ray appears (ray 2). The interopercle bone is present at the dorsal margin of the ceratohyal near the hyoid joint. The opercle continues to grow posteriorly and ventrally. The quadrate is associated with the palatoquadrate.
14dpf
Among first arch elements, the dentary covers most of the shaft of the Meckel’s cartilage, and the anguloarticular is visible on the lateral aspect of the Meckel’s cartilage (Figure 2G). Among elements of the second arch, an area of ossification is present on the ventral and anterior region of the palatoquadrate, associated with the anterior projection of the quadrate bone. There is formation of the ventral hypohyal at the ventral and medial region of the ceratohyal. The interopercle bone has grown anteriorly, and the first branchiostegal ray (ray 1) appears. The subopercle bone is present ventral to the opercle, which has grown ventrally and posteriorly. The basihyal, basibranchials, hypobranchials, and ceratobranchials continue to grow throughout the larval and juvenile stages, and the ceratobranchials are showing evidence of mineralization (Figure 3A).
21dpf
At this stage the premaxilla appears anterior to the maxilla (Figure 2H). The hyosymplectic begins to separate into the hyomandibular bone and the symplectic bone, with separate ossification centers in each region. The hyomandibular portion of the element gains additional bone formation on the anterior and posterior sides of the element. The preopercle bone forms ventral to the symplectic cartilage and dorsal to the interopercle bone. The subopercle is broader and blade-like, extending along the ventral margin of the opercle. At 21 dpf, the metapterygoid forms as a small projection of cartilage on the dorsal aspect of the palatoquadrate, along the posterior margin of the entopterygoid bone. The epibranchial elements are present as small cartilages (Figure 3B).
28dpf
Dermal bones and cartilage ossifications continue to invest around cartilaginous elements, expanding in size and covering most of the surface of the jaws and gill structures (Figure 2I). Dentary, anguloarticular, retroarticular, and coronomeckelian (or Meckelian) bones surround the Meckel’s cartilage. The metapterygoid bone forms at the posterior portion of the palatoquadrate and the entopterygoid bone. The ectopterygoid forms as a blade-like bone at the anterior projection of the palatoquadrate, with the palatine bone forming at the anterior and dorsal tip of the palatoquadrate. The entopterygoid, metapterygoid, and palatine all contribute to elements of the upper palate or ventral structures surrounding the eye. The quadrate is associated with the ventral-anterior process of the palatoquadrate, and together the quadrate and the ossified portion of the palatoquadrate form the first jaw joint with the anguloarticular bone. This form of the jaw joint persists throughout life of the fish. At the posterior end of the ceratohyal the epihyal bone forms. All second arch dermal bones (opercular and branchiostegal ray series) are blade-like elements that overlap to provide a fan-like structure that during respiration to assist with the flow of water over the underlying gill elements. At this stage the epibranchials are more fully formed and the pharyngobranchials are present, and epibranchials are showing evidence of mineralization (Figure 3C).
MOLECULAR MECHANISMS OF PHARYNGEAL ARCH IDENTITY IN ZEBRAFISH
As zebrafish have been extensively studied to reveal cellular and molecular mechanisms that establish jaw development, the following section outlines key signaling pathways in zebrafish that establish axial identity and joint formation in the pharyngeal arches. Elements of these pathways underlie pharyngeal development in other vertebrates, such as mice, amphibians, lampreys, and other fish, indicating that variations in jaw patterning seen among ancient and modern taxa are the product of evolution of these conserved genetic networks.
Anterior-posterior identity: Hox genes, Retinoic Acid:
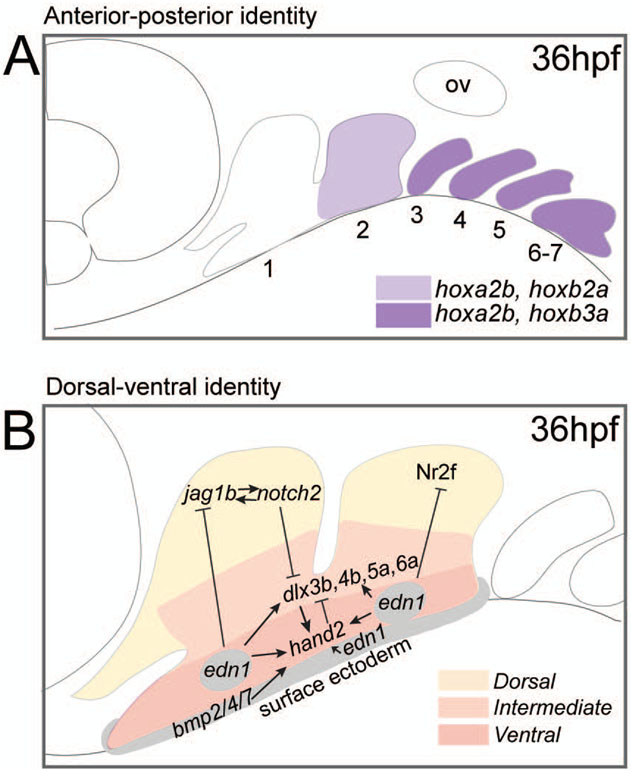
As in other vertebrates, the first arch in zebrafish is Hox-negative, and the arches posterior to the first arch express hox genes in a collinear manner (Figure 4A). Two members of the Hox paralog group 2, hoxa2b and hoxb2a are expressed in the second pharyngeal arch. Knockdown of these causes defects in second arch patterning, resulting in partial transformation into first arch structures (Hunter & Prince, 2002). Furthermore, ectopic mis-expression of hoxa2b and hoxb2a in the first arch causes transformations of first pharyngeal arch structures into second arch structures, also observed in mice and Xenopus (Gendron-Maguire, Mallo, Zhang, & Gridley, 1993; Hunter & Prince, 2002; Kitazawa et al., 2015; Ozeki et al., 2004; Pasqualetti, Ori, Nardi, & Rijli, 2000; Rijli et al., 1993). In zebrafish, other Hox paralog groups, such as Hox group 3 paralogs (hoxa3a, hoxb3a) and group 4 paralogs (hoxa4a, hoxb4a, and hoxd4a) are expressed in posterior gill arches and surrounding tissues (Hogan et al., 2004; Laue et al., 2008; Punnamoottil et al., 2008). As in lampreys and other chordates, retinoic acid is involved in anterior-posterior patterning of the arches of zebrafish, where low levels specify the anterior arches, and increasing levels posteriorly pattern the posterior arches (Begemann, Schilling, Rauch, Geisler, & Ingham, 2001; Escriva et al., 2002; Grandel et al., 2002; Ozeki et al., 2004; Yan, Jowett, & Postlethwait, 1998). Since the roles of Hox genes and retinoic acid are conserved in the lineages leading to gnathostomes (i.e. amphioxus and lamprey) and are also expressed in modern lineages (i.e. zebrafish), this indicates that these factors have retained their fundamental role in establishing anterior-posterior identity of pharyngeal arches throughout evolution (Begemann et al., 2001; Escriva et al., 2002; Grandel et al., 2002; Ozeki et al., 2004; Yan et al., 1998).
Figure 4:
Genetic mechanisms regulating anterior-posterior and dorsal-ventral patterning of the pharyngeal arches in zebrafish. A) Anterior-posterior patterning: expression of Hox paralogs in pharyngeal arches at approximately 36hpf. The first pharyngeal arch is hox-negative. The second pharyngeal arch expresses hoxa2b and hoxb2a, and third through seventh arches express hoxa2b and hoxb3a. Hoxa4a, hoxb4a, are hoxd4a are expressed in posterior gill arches, although the boundaries of expression in specific arches are not clear (not shown). B) Dorsal-ventral patterning: Jag1b-Notch2 signalling and Nr2f genes function in the dorsal domain, dlx3b, dlx4a, dlx5a, and dlx6a are expressed in the intermediate domain. Hand2 is expressed in the ventral domain. Bmp factors are expressed in the ventral epithelia. Edn1 is expressed in ventral pharyngeal arches and mesenchymal cores of arches. Factors function to activate or inhibit one another to specify boundaries of dorsal-ventral identity. Notch2 is expressed throughout the pharyngeal arches at 36 hpf, although Jag1b activates Notch2 in the dorsal domain. Adapted with copyright permission (Alexander et al., 2011; Barske et al., 2018; Hunter & Prince, 2002; Laue et al., 2008; Craig T. Miller et al., 2003; Punnamoottil et al., 2008; Talbot et al., 2010; Zuniga et al., 2011, 2010).
Dorsal-ventral identity
edn1
In zebrafish, the dorsal-ventral identity of pharyngeal arch structures is determined by edn1 expression in ectoderm, endoderm, and arch mesoderm, where Edn1 acts on post-migratory neural crest cells through Edn1 receptors (Miller et al., 2000; Miller, Yelon, Stainier, & Kimmel, 2003). In zebrafish, edn1 is expressed in the ventral region of the first and second pharyngeal arches and second and third pharyngeal pouches at 24hpf. At 30hpf, expression is located in the mesenchymal core of the first three arches and second through fourth pouches (Figure 4B) (Miller et al., 2000). Loss of edn1 in zebrafish (and mouse) causes loss or partial transformation of the ventral-most jaw and jaw support skeletal structures towards a dorsal-like identity (Miller et al., 2000; Ozeki, Kurihara, Tonami, Watatani, & Kurihara, 2004). Loss of edn1 causes reductions of ventral structures of first and second arches, including reductions of the Meckel’s cartilage, symplectic of the hyosymplectic, and ceratohyal cartilage, and a failure of formation of the first and second arch joints (Miller et al., 2000). In these mutants, first arch dermal bones may be malformed or fused, and second arch opercle bones may be absent or enlarged, and the branchiostegal ray may be absent (Kimmel, Ullmann, Walker, Miller, & Crump, 2003). Thus, the function of Edn1 is proposed to induce ventral and intermediate patterning by inducing patterning genes in these regions, and preventing dorsalization of the ventral arches by repressing expression of dorsal factors in intermediate and ventral domains.
dlx and hand2
Edn1 signalling induces the expression of dlx and hand2 in nested patterns of expression that specify dorsal and ventral identity of the jaw and jaw support skeleton (Figure 4B) (Miller et al., 2003; Talbot, Johnson, & Kimmel, 2010). In zebrafish, dlx2a is expressed throughout the pharyngeal arches as a broad post-migratory cranial neural crest cell marker (Miller et al., 2003). Knockdown of dlx2a causes defects to both dorsal and ventral domains of the pharyngeal arches (Talbot et al., 2010). dlx3b, dlx4b, dlx5a, and dlx6a are expressed in an intermediate domain within the pharyngeal arch primordia (Figure 4B), and dlx5a and dlx6a expression also extends ventrally (Talbot et al., 2010; Walker, Miller, Coffin Talbot, Stock, & Kimmel, 2006; Zuniga et al., 2010). dlx3b, dlx4b, and dlx5a have been shown to function redundantly to pattern elements within the intermediate domain (Talbot et al., 2010). Combined knockdown of these factors results in specific intermediate-domain patterning defects, including loss the symplectic and fusions between the first and second arch cartilages and second arch opercle and branchiostegal ray (Talbot et al., 2010). In dlx knockdowns, dorsal and ventral patterning is normal, indicating these factors are specific for patterning intermediate structures (Talbot et al., 2010). Hand2 is expressed in the ventral pharyngeal arch neural crest cells, adjacent to edn1 expression in pharyngeal mesoderm, endoderm, and ectoderm, and is positively regulated by edn1 and bmp (Alexander et al., 2011; Miller et al., 2003; Zuniga, Rippen, Alexander, Schilling, & Crump, 2011). Loss of hand2 causes shortening or loss of the Meckel’s cartilage and formation of an ectopic palatoquadrate-like structure in the midline (Miller et al., 2003; Talbot et al., 2010). Hand2 mutants also have a severe reduction of the ceratohyal and symplectic of the hyomandibula (Miller et al., 2003; Talbot et al., 2010). In hand2 mutants, dlx3b, dlx4b, and dlx5a are expanded ventrally, indicating a function of hand2 to restrict dlx factors to an intermediate location within the arches to impart an intermediate identity to the arches (Talbot et al., 2010).
Jagged1b-Notch2 and Nr2f
Dorsal pharyngeal arch patterning in zebrafish is determined by Jagged1b signalling through Notch2 (Zuniga et al., 2010). Notch2 represses dlx3b, dlx5a, and dlx6a expression to repress intermediate and ventral patterning, and positively regulates hey1 to promote dorsal identity (Figure 4B)(Zuniga et al., 2010). Jagged1b-Notch2 signalling in the dorsal arch is interpreted to inhibit cartilage formation, whereas ventral Edn1 is proposed to promote cartilage formation (Barske et al., 2016, 2018). During early development of the arches, ventral edn1 expression is induced by bmps, which also repress jag1b dorsally to ensure specification of the ventral and intermediate domains of the jaw (Alexander et al., 2011). Nuclear receptor 2f (Nr2f) genes are expressed in the dorsal domain of the arches and also function in limiting chondrogenesis of the upper jaw while repressing mandibular gene expression (Figure 4B)(Barske et al., 2018). It is proposed that edn1 represses ventral expression of Nr2f genes as edn1 mutants have expanded expression of Nr2f genes, and reduction of Nr2f can rescue the severe loss of ventral structures in edn1 mutants (Barske et al., 2018). Together, the Jagged1b-Notch2 and Nr2f signaling mechanisms, regulated by edn1, pattern dorsal identity of the arches, principally by Jagged1b-Notch2 repressing expression of ventral genes, and both factors functioning to repress formation of cartilage in the dorsal domain.
Patterning of the mandibular jaw joint
First arch joint formation: nkx3.2, hand2 and barx1
Specification of the jaw joint between the palatoquadrate and the Meckel’s cartilage is regulated by edn1, which induces nkx3.2 (formerly bapx1) expression in the jaw joint (Miller et al., 2003) Nkx3.2 in turn induces chd and gdf5, which are associated with articular joint formation in fish and tetrapods (Francis-West, Parish, Lee, & Archer, 1999; Miller et al., 2003; Storm & Kingsley, 1999). Loss of edn1 causes loss of nkx3.2, and knockdown of nkx3.2 induces fusions between dorsal and ventral elements, phenocopying joint defects when edn1 is reduced (Miller et al., 2003; Walker et al., 2006). Loss of hand2 causes expansion of the nkx3.2-expressing domain, as well as another joint marker trps1, suggesting that it normally functions to repress joint formation, potentially via repression of dlx3b, dlx4b, and dlx5a (Miller et al., 2003; Talbot et al., 2010). Together it is the positive and repressive function of edn1 and hand2 that position the first arch joint. nkx3.2-expressing cells also prefigure the midline basihyal cartilage, although expression in this region is not dependent on edn1 (Miller et al., 2003). Barx1, another regulator of joint formation, is expressed in pre-cartilage dorsal and ventral arch mesenchyme, but is not expressed in the intermediate domain, where first and second arch joints normally form (Nichols, Pan, Moens, & Kimmel, 2013). Loss of barx1 produces ectopic joints in sub-intermediate zones of the Meckel’s, ceratohyal, and ceratobranchial cartilages and abnormal proliferation and condensation of chondrocytes, suggesting that it functions to repress joint formation and promote cartilage formation (Nichols et al., 2013; Sperber & Dawid, 2008). The repressive function of barx1 on joint formation is due to repression of hand2 in the sub-intermediate zone, which in turn represses barx1 (Nichols et al., 2013). It is thought that this reciprocal repressive effect contributes to positioning the first arch jaw joint correctly in the intermediate zone, and promotes cartilage formation in the sub-intermediate zone (Nichols et al., 2013).
Evolution of the first jaw joint in vertebrates
While nkx3.2 is focally expressed in the first arch of gnathostomes and is associated with joint formation (Miller et al., 2003), in lamprey, LjBapxA/nkx3.2 expression is described in the trigeminal nerve ganglion and the mandibular arch ectoderm in by Kuraku and colleagues (2010)(Kuraku et al., 2010), and pharyngeal endoderm and ectoderm by Cerny and colleagues (2010)(Cerny et al., 2010) While barx1 is excluded from prospective joint-forming regions in the first arch of gnathostomes, Barx1 is expressed in all pharyngeal arches in lampreys, and contiguously throughout the first arch (Cerny et al., 2010; Nichols et al., 2013). Together this data indicates that acquisition of Edn1, ventral Hand2, and broadly expressed Dlx contributed to pharyngeal patterning in agnathan vertebrates, and the subsequent regionalization of Dlx led to dorsal-ventral patterning. Furthermore, the localization of bapx1/nkx3.2 to the intermediate region of the first arch, and exclusion of barx1 from this region may have led to the formation of the first arch jaw joint in gnathostomes (Cerny et al., 2010; Nichols et al., 2013).
Summary of dorsal-ventral, anterior-posterior, and joint patterning in gnathostome fish
Together a picture is emerging that early specification of ventral identity of the pharyngeal arches is determined by bmp induction of edn1 expression (in the pharyngeal endoderm and ectoderm), hand2 expression in the ventral arches, and restriction of jag1b to the dorsal arch (Alexander et al., 2011; Miller et al., 2003; Talbot et al., 2010; Zuniga et al., 2010). Subsequently, an intermediate domain emerges, induced by ventral expression of edn1, inducing expression of dlx genes, which are repressed by Jagged1b-Notch2 signalling (Alexander et al., 2011; Miller et al., 2000; Miller et al., 2003; Talbot et al., 2010; Zuniga et al., 2010). First arch jaw joint patterning requires balance between edn1 which induces nkx3.2, and activity of barx1 and hand2 to correctly position the joint, prevent ectopic joints, and promote cartilage formation (Miller et al., 2003; Nichols et al., 2013). Promotion of dermal bone formation in the dorsal portion of arches and inhibition of chondrogenesis is regulated by Jagged1b-Notch2 and Nr2f signaling, whereas ventral cartilage formation is positively induced by edn1 (Barske et al., 2016, 2018).
CARTILAGINOUS FISHES: EARLY GNATHOSTOMES, PLACODERMS, ACANTHODIANS, AND CHONDRICHTHYES
The following section examines the anatomy of ancient cartilaginous gnathostome fishes with an attempt to understand how changes to developmental programs could have contributed to evolution identity of pharyngeal elements and the emergence of a jaw joint. Anatomical studies are described in modern chondrichthyans, which offer a model for understanding the evolution of bone and cartilage.
Placoderm fishes
Approximately 444-416 million years ago, 30-70 million years after the appearance of ostracoderms, jawed vertebrate fishes arose in the Silurian (Anderson, Friedman, Brazeau, & Rayfield, 2011; Kardong, 2012). One group of the first gnathostomes were placoderm fishes, which lived from the mid-Silurian into the Devonian before becoming extinct and leaving no descendants (Kardong, 2012). These fish strongly resembled ostracoderms with a mineralized dermal skeleton, including a head shield comprised of large plates and a trunk covered in small plates, and a largely unmineralized endoskeleton. Unlike ostracoderms, placoderms are distinguished by well-developed jaws, sometimes with teeth, situating them as the first stem gnathostomes (Kardong, 2012). Placoderms likely had a bottom-feeding lifestyle similar to ostracoderms, although the presence of jaws indicates they were also predators, mostly notably in the Arthrodira Dunkleosteus, which had a substantial blade-like jaw formed from dermal bones (Hu, Lu, & Young, 2017). In Romundina placoderms, galeaspid features of separate nasal and hypophysial placodes and nasal capsules positioned between the eyes are present (Dupret et al., 2014), continuing the process of changes to the landscape in which ectomesenchyme could migrate and form new tissue interactions.
Analysis of the architecture of the jaw and cranium of Entelognathus and Romundina placoderms Figure 5, Table 1) shows that cranium anatomy resembles that of more ancient vertebrates such as galeaspid ostracoderms, whereas the jaw resembles that of derived gnathostomes, suggesting the beginnings of decoupling of each region in gnathostome evolution (Dupret et al., 2014; Zhu et al., 2013). In one study, the lower jaw of the Compagopiscis croucheri placoderm reveals jaws composed of cartilages and tooth structures homologous to crown gnathostomes. In this species, the lower jaw is specifically comprised of a first arch derived Meckel’s cartilage with evidence of surface ossifications (Rücklin et al., 2012). Descriptions of Arthrodira describe evidence of first arch structures homologous to osteichthyans, including the Meckel’s cartilage and the palatoquadrate, which may be partially or fully ossified (Hu et al., 2017; Young, 1986).
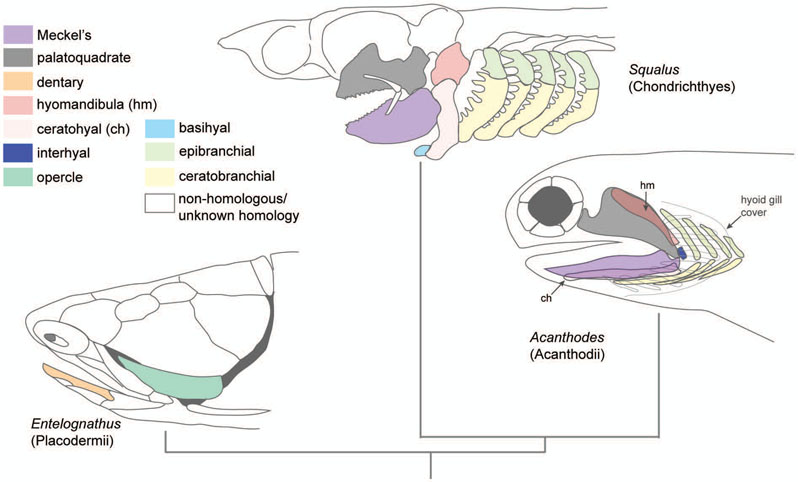
Figure 5:
Jaw patterning in cartilaginous fish classes Placodermii, Chondrichthyes, and Acanthodii. The fossil placoderm Entelognathus has a bony dermal head skeleton with evidence of an opercle and dentary. Other elements are also present such as maxillary bones, but not shown. Modern Squalus (dogfish shark) features cartilaginous elements only. Acanthodes has a cartilaginous endoskeleton with overlying dermal bone elements, including a hyoid gill covered in bony branchiostegal rays (grey outlines). The hyomandibula is beneath the palatoquadrate and the ceratohyal is beneath the Meckel’s cartilage. Note the presence of an interhyal and symmetry between epibranchial and ceratobranchial cartilages. Adapted with copyright permission (Janvier, 1996; Kardong, 2012; Zhu et al., 2013).
Table 1:
Features and structures of cartilaginous fish jaw skeletons. Blank spaces indicate that homologous element has not been reported. Elements that may be lost secondarily are indicated as “absent”.
| Cartilaginous fishes | ||||
|---|---|---|---|---|
| Placodermii (Carr et al., 2009; Hu et al., 2017; Rücklin et al., 2012; Young, 1986; Zhu et al., 2013) |
Acanthodii (Brazeau, 2009; Brazeau & de Winter, 2015; Friedman & Brazeau, 2010; Gardiner, 1984) |
Chondrichthyes (Gillis, Modrell, et al., 2012; Kent & Carr, 2001; Patterson, 1982) |
||
| Feature/structure | Element | |||
| Mineralization of cartilage | Present | Perichondral | Prismatic | |
| Dermal bone | Present | Present | Absent | |
| First pharyngeal arch | Anguloarticular or angular | Absent | ||
| Meckelian | Absent | |||
| Dentary | Present | Absent | ||
| Ectopterygoid | Absent | |||
| Entopterygoid | Absent | |||
| Maxilla | Present | Absent | ||
| Meckel’s cartilage | Present | Present | Present | |
| Metapterygoid | Absent | |||
| Palatoquadrate | Present | Present | Present | |
| Palatine | Absent | |||
| Premaxilla | Present | Absent | ||
| Quadrate | Absent | |||
| Retroarticular | Absent | |||
| Second pharyngeal arch | Branchiostegal ray | Hyoid gill cover | Absent | |
| Ceratohyal | Present | Present | Present | |
| Epihyal | Present | Absent | ||
| Hyomandibula | Present | Present | Present | |
| Symplectic | Absent | |||
| Interhyal | Present | Limited evidence | Absent | |
| Interopercle | Absent | |||
| Opercle | Present | Hyoid gill cover | Absent | |
| Opercular cartilage | Present | Absent | ||
| Preopercle | Absent | |||
| Subopercle | Absent | |||
| Ventral and branchial arch structures | Basibranchial | Present | Present | Present |
| Basihyal | Present | Present | Present | |
| Ceratobranchials | Present | Present | ||
| Epibranchials | Present | Present | ||
| Hypohyals | Present | Absent | ||
| Hypobranchials | Present | Present | ||
| Urohyal | Present | Absent | ||
Second arch cartilages are also present in placoderms including the basihyal, ceratohyal, hyomandibula, epihyal, interhyals, urohyal, paired basibranchials, and opercular elements, which may also be ossified by perichondral ossification (Carr, Johanson, & Ritchie, 2009; Hu et al., 2017; Young, 1986). Mouth roof structures of the primary palate, including the neurocranium, parasphenoid, and ethmoid plate are present in placoderms (Carr et al., 2009; Young, 1986). Other osteichthyans-like dermal bones have been observed in Entelognathus including the dentary associated with the mandible, a maxilla and premaxilla associated with the snout, a palatoquadrate, and opercle bones and opercular cartilages of the lateral face (Zhu et al., 2013). Analysis of the position and shape of many of these bones suggests they are the ancient homologs of these structures in osteichthyans (Zhu et al., 2013). In placoderms, the palatoquadrate of the mandibular arch forms an attachment to the neurocranium, the first true attachment of the cranium with the jaw, forming an autostylic joint. In this form, the second arch hyoid elements are not attached to the cranium and do not suspend the jaw (Carr et al., 2009). In the placoderms, the palatoquadrate is partially supported by the external dermal skeleton, and the opercle bone (referred to as the submarginal plate in some sources) is supported by the hyomandibula that remains connected to the neurocranium (Carr et al., 2009).
Acanthodians
Another group of early gnathostomes, the Acanthodians, existed contemporaneously with placoderms during the Silurian and may have even arisen in the Late Ordovician, preceding the appearance of placoderms. Whereas placoderms abruptly disappeared at the end of the Devonian, Acanthodians persisted into the Permian (Brazeau & de Winter, 2015). The placement of acanthodians in gnathostome phylogeny and relationship to modern gnathostomes is controversial, as analysis of fossil remains suggest that they possessed characteristics of both osteichthyans and chondrichthyans (Davis, Finarelli, & Coates, 2012). Increasing evidence indicates that they are most likely stem chondrichthyans (Burrow & Rudkin, 2014; Coates et al., 2018). Whether they are stem chondrichthyans or osteichthyes, they may be oldest jawed fishes possessing skeletal traits and jaw structures of crown gnathostomes (Davis et al., 2012). There is no evidence that acanthodians had a significantly mineralized endoskeleton (Brazeau, 2009), and if they represent a stem chondrichthyan, loss of perichondral ossification may have emerged in the acanthodian ancestor to cartilaginous fishes. Analysis of fossil features of the early Devonian acanthodian Ptomacanthus anglicus (418-412 mya) and later Acanthodes bronni (100my later) remains featured osteichthyans-like fins, dermal cranial bone, a cranium comprised of neurocranium, and visceral skeletal elements including palatoquadrates, hyomandibula, Meckel’s cartilage, ceratobranchials, ceratohyal, basihyal, and hypohyal (Figure 5, Table 1) (Brazeau, 2009; Brazeau & de Winter, 2015; Friedman & Brazeau, 2010). In Acanthodes, their hyomandibula perichondrally ossifies in two segments, there is no nerve foramen in the hyomandibula, and there is limited evidence of examples with an interhyal (Gardiner, 1984). Although they did not have an opercle bone, they had a hyoid gill cover covered in dermal branchiostegal rays (Janvier, 1996). Acanthodians had symmetrical upper and lower pharyngeal elements, implying that in acanthodians, as in lampreys, the Dlx code to specify dorsal-ventral identity may not have been fully employed yet (Koentges & Matsuoka, 2002; Kuraku et al., 2010). However, unlike lampreys, which have a non-jointed branchial “basket” pharyngeal skeleton without separated upper and lower segments, acanthodians have jointed upper and lower elements. This suggests the ability to make jaw joints evolved in the ancestor to acanthodians, which may have involved the evolution of localization of nkx3.2 to the joint-forming region or restriction of barx1 from the future joint-forming region.
Chondrichthyes
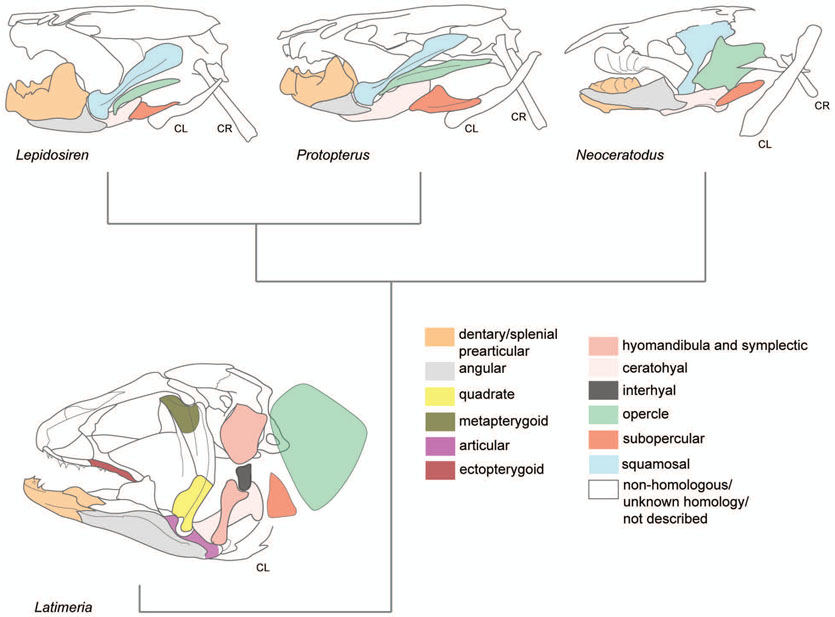
Chondrichthyes (“cartilaginous fishes”) arose during the Devonian (416-359 mya)(Sallan & Coates, 2010), or possibly earlier, and co-existed with placoderms and ostracoderms. Whereas placoderms and ostracoderms disappeared abruptly at the end of the Devonian, and Acanthodians disappeared in the Permian, the branch of chondrichthyes that gave rise to elasmobranches (sharks and skates) and holocephalans (chimaeras) expanded and diversified during the Carboniferous and hundreds of species exist today (Kardong, 2012). Modern chondrichthyans lack the ability to undergo ossification of cartilage, although since they arose from placoderm or acanthodian ancestors with ossified cartilage, it is hypothesized that they lost this trait secondarily. Analysis of the genome of the elephant shark Callorhinchus milii demonstrates that sharks have the ancestral genes Sparc and Sparcl1, but do not have other SCPP family genes (SPP1, MEPE, IBSP, DMP1 and DSPP) that arose due to a subsequent tandem duplication of Sparcl1 (Venkatesh et al., 2014). These genes are present in osteichthyans and tetrapods, and are responsible for ossification of collagen. Analysis of other chondrichthyan genomes as well as the sea lamprey genome shows the absence of these SCPP genes, and the presence of spp1 in zebrafish and medaka osteichthyans indicates that tandem duplication of Sparcl1 may have occurred after the split of osteichthyes from the chondrichthyan lineage (Venkatesh et al., 2014). It is inferred that the absence of cartilage ossification in chondrichthyans may be associated with the absence of these ossification genes. What remains difficult to explain by this hypothesis is the evidence of ossification in placoderm ancestors to chondrichthyans. An alternative explanation may be that the tandem duplication of Sparcl1 occurred before the common ancestor to chondrichthyes and osteichthyes, and chondrichthyans lost elements of this gene cluster, whereas osteichthyans retained duplicates. Chondrichthyans do retain the ability to ossify some parts of their body as evident by their mineralized teeth, fin spines, and placoid body scales (all body parts that do not require chondral ossification), but do not have other dermal bone characteristics such as a bony dermatocranium. The reason for this is unclear, but suggests that the underlying genetic program for making teeth, spines, and mineral scales may involve different gene pathways than ossification of cartilage and dermal bone formation processes, and that the latter programs have undergone evolutionary changes in chondrichthyans. Sharks do have a surface mineralization of cartilage in the form of a prismatic block covering, and histological analyses indicate this is a unique feature of chondrichthyans that has no evolutionary relationship with cartilage ossification (Dean, Mull, Gorb, & Summers, 2009). This characteristic is evident among the earliest elasmobranch fossils dating to early Devonian, approximately 400 mya, as Doliodus problematicus had prismatic calcified cartilage of the neurocranium and visceral skeleton, as well as teeth (Miller, Cloutier, & Turner, 2003).
When examining a modern chondrichthyan, compared to osteichthyans, the absence of dermal bone and perichondral ossification of the cranium, jaws, and gill structures is strikingly evident. In many ways, chondrichthyan head skeletons resemble osteichthyans if all mineralized skeletal elements were removed, and if osteichthyans “grew” their larval cartilages to adult forms without these elements becoming encapsulated by bone (Figure 5, Table 1). Modern chondrichthyans entirely lack any dermatocranium present in osteichthyans, which forms as dermal bone, so with no cartilaginous precursor there is no portion of this structure that could be retained in chondrichthyans. Instead, to protect their brains and delicate sensory systems, chondrichthyans adapted their neurocranium to become a braincase known as the chondrocranium. As with the neurocranium in osteichthyans, the chondrocranium in chondrichthyans forms the primary palate of the mouth. However, whereas the bony dermatocranium forms a dorsal cover to the brain in osteichthyans, in chondrichthyans the brain, eyes, and olfactory apparatus are housed in the neurocranium-chondrocranium (Kardong, 2012).
The basic visceral skeleton of chondrichthyans resembles that of other gnathostomes. Chondrichthyans have a first arch skeleton comprised of Meckel’s cartilage and palatoquadrate (Figure 5, Table 1). Unlike other gnathostomes, both elements have teeth, where the Meckel’s cartilage forms the lower jaw, and the palatoquadrate forms the upper jaw, articulating to create a biting mouth. Each element is attached to the hyoid skeleton, which in turn is attached to the chondrocranium. Among sharks that feed by attacking prey with an open mouth, the Meckel’s and palatoquadrate can be extended at once from the hyoid, creating an agile, articulated swinging bite used to attack prey (McNeil, Lowry, Larson, & Griffing, 2016). When not attacking, the Meckel’s and palatoquadrate can retract beneath the chondrocranium to restore the streamlined, hydrodynamic profile of elasmobranchs. The second arch skeleton is comprised of the hyomandibula (referred to as epihyal in sharks), a symplectic that is a hypertrophied interhyal, and ceratohyal cartilage (Gillis, Modrell, & Baker, 2012; Patterson, 1982). Chondrichthyans have various configurations of the ventral pharyngeal and gill arch skeleton, but a representative Squalus acanthias (dogfish shark) possesses a single median basihyal, paired hypobranchials and ceratobranchials, and two basibranchials (Kent & Carr, 2001). The median basihyal articulates with the hyoid and first gill arch (Wischnitzer & Wischnitzer, 2006). In chondrichthyans, there is an absence of opercle bone elements. Because of the lack of the opercular gill flap, the branchial gills are exposed in chondrichthyans, opening to the sides of the head. The joint between the posterior Meckel’s and anterior palatoquadrate forms the primary jaw joint, and remains the embryonic, functional jaw joint in all living, non-mammals.
In early (and most modern) chondrichthyans the pharyngeal apparatus was attached to the cranium by two independent articulations of the palatoquadrate and hyosymplectic (amphistylic). In modern sharks, the swinging Meckel’s-palatoquadrate jaw unit is suspended in part by the ceratohyal, and not the hyomandibula. In the process of becoming a jaw-supporting structure, the ceratohyal has moved anteriorly in elasmobranchs, reducing the size of the first gill slit (Kardong, 2012). In some benthic species, the first gill slit has become modified to become the spiracle, which is a tube or pouch opening to the surface, lined with sensory cilia and a cupula similar to lateral line sensory organs in fishes (Barry & Boord, 1984; Barry, Hall, & Bennett, 1988). Flexion of the hyomandibula to protrude the jaw causes changes in shape to the inner spiracle, resulting in changes in mechanoreception (Barry et al., 1988). The spiracle can also supply oxygenated water to the gills when mouth breathing is obstructed (Graham et al., 2014). In fast-swimming species that breathe entirely through their mouths, the first gill slit has been reduced or disappeared entirely (Tomita, Toda, Ueda, Uchida, & Nakaya, 2012). In manta rays, the spiracle and oral valves both function in buccal-pumping respiration in utero, but the spiracle closes shortly after hatching and does not function in respiration in adult rays (Tomita et al., 2012). In either case, modifications to the jaw anatomy of sharks are associated with changes to gill slit architecture and acquisition of new functions in the form of the spiracle.
EVOLUTION OF JAWS IN OSTEICHTHYES
The following section examines the anatomy of ancient and modern osteichthyan fishes with an attempt to understand how changes to jaw patterning facilitated their diversification.
The superclass of osteichthyes, or “bony fishes” arose from a placoderm ancestor in the Ordovician or Silurian, splitting off from chondrichthyes around 427mya (Broughton, Betancur-R, Li, Arratia, & Ortí, 2013; Lu, Giles, Friedman, den Blaauwen, & Zhu, 2016). As with chondrichthyans, osteichthyans co-existed with ostracoderms, placoderms, chondrichthyans, and acanthodians during the Silurian and Devonian. Following the Devonian extinction, recovery of placoderms and acanthodians was limited, whereas chondrichthyans and other osteichthyans underwent rapid diversification and expansion, becoming the predominant vertebrate species on the planet (Sallan & Coates, 2010). Osteichthyes are divided between the classes of actinopterygians (ray-finned fishes) and sarcopterygians (lobe-finned fishes), groups that diverged from one another during the Silurian (Broughton et al., 2013; Lu et al., 2016). Actinopterygians gave rise to many branches of ray-finned fishes and most modern fishes, including the staggeringly vast teleost infraclass. The sarcopterygian lineage, represented by far fewer groups, gave rise to land-dwelling tetrapods in the Late Devonian. Today, sarcopterygian fish species represent less than 0.02% of vertebrates and actinopterygian fishes represent approximately 44% all vertebrates (Hinchliff et al., 2015). Among actinopterygians over 99% are teleosts, with teleosts representing 43.9% of all vertebrate species (Hinchliff et al., 2015).
Evolution of pharyngeal arches and origins of jaws in ray-finned Actinopterygians
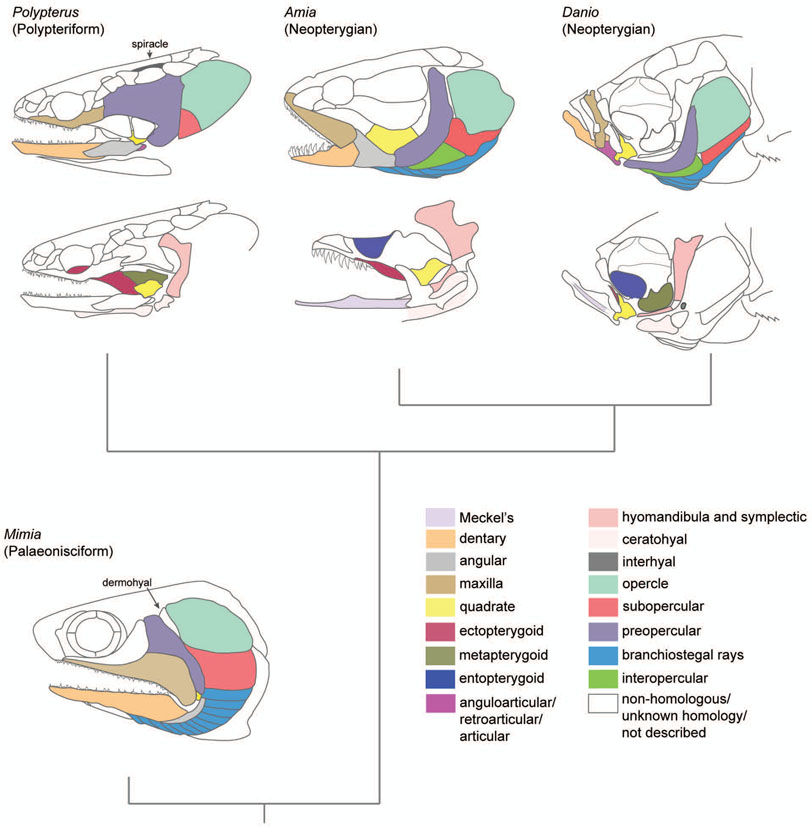
Palaeonisciform actinopterygians
The middle to Late Devonian and Carboniferous saw the emergence of palaeonisciform actinopterygians. These early actinopterygians had dermal head skeletons, with an ossified endoskeleton. Over time, the dermal head skeletons become reduced in actinopterygians, while the endoskeleton became increasingly ossified, although the endoskeleton in some taxa remain primarily cartilaginous. The early palaeonisciform Cheirolepis genus, the earliest definitive actinopterygian, had a large mouth, a hyomandibula, and pre-opercle, subopercle, and opercle bones covering gills, branchiostegal rays, and a maxilla and premaxilla (Arratia Fuentes, Wilson, Cloutier, & Schultze, 2004; Giles et al., 2015; Janvier, 1996). In Mimia, a true actinopterygian of the Late Devonian, ossification of cartilage is evident (Janvier, 1996). The palatoquadrate is attached to the neurocranium at two locations, and the hyomandibula is attached to the palatoquadrate and cranium. Mimia features an ecto- and entopterygoid, dentary, tooth plates on elements, a dermohyal, and there is an interhyal between the ceratohyal and hyomandibula, and the ceratohyal joins a hypohyal to meet in the ventral midline at the basibranchial (Figure 6, Table 2) (Janvier, 1996). In early actinopterygians the “suspension jaw” appears, with the hyomandibula, palatoquadrate, and preopercle forming articulations with each other and the cranium, enabling mouth opening by raising the neurocranium and lowering the mandible (Janvier, 1996). In actinopterygians there appears a distinct opercular process on the posterior hyomandibula for articulation of the opercle (Gardiner, 1984). In modern osteichthyans the jaw-cranium joint is hyostylic, where the lower jaw loses its direct attachment to the cranium, but instead attaches to the hyomandibula, which in turn is attached to the chondrocranium (Kardong, 2012).
Figure 6:
Jaw patterning in actinopterygian fishes. For Polypterus, Amia, and Danio, dermatocranial bones are illustrated at the top of figure and viscerocranial elements below. For Mimia, only dermatocranial elements are shown. The fossil palaeonisciform Mimia has both cartilage ossification and dermal bone element representing basic features of actinopterygians. Polypterus, an extant form of polypteriform actinopterygian has transformed the first gill slit into a spiracle for respiration. The ceratohyal is found in two parts, and anterior and posterior element. Amia, representative of an early neopterygian actinopterygian has a sturdy dermal skeleton overlying a hyomandibula with a separate symplectic element and interopercle bone. Danio, a representative neopterygian teleost has a substantially reduced maxilla and dentary compared to earlier forms of actinopterygians, adapted for protrusion of the maxilla during suction feeding. Adapted with copyright permission (Gardiner, 1984; Jarvik, 1980; Jollie, 1984a).
Table 2:
Features and structures of actinopterygian jaw skeletons. Blank spaces indicate that homologous element has not been reported. Elements that may be lost secondarily are indicated as “absent”.
| Actinopterygians | |||||
|---|---|---|---|---|---|
| Palaeonisciform (Mimia and Cheirolepis) (Arratia Fuentes et al., 2004; Gardiner, 1984; Giles et al., 2015; Janvier, 1996) |
Polypterus (Jollie, 1984a; Pehrson, 1947) |
Amia calva (Jarvik, 1980) |
Teleost (Danio) (Cubbage & Mabee, 1996; Eames et al., 2013) |
||
| Feature/structure | Element | ||||
| Mineralization of cartilage | Present | Present | Present | ||
| Dermal bone | Present | Present | Present | Present | |
| First pharyngeal arch | Anguloarticular or angular | Angular | Angular | Present | |
| Meckelian | Mento-meckelian | Mento-meckelian | Coronomeckelian or Mentomeckelian | ||
| Dentary | Present | Dentosplenial | Dentary/infradentary | Present | |
| Ectopterygoid | Present | Present | Present | Present | |
| Entopterygoid | Present | Present | Present | Present | |
| Maxilla | Present | Present | Present | Present | |
| Meckel’s cartilage | Present | Present | Present | ||
| Metapterygoid | Present | Present | Present | ||
| Palatoquadrate | Present | Present | Present | Present | |
| Palatine | Present | Dermopalatine | Present | ||
| Premaxilla | Present | Present | Present | Present | |
| Quadrate | Present | Present | Present | Present | |
| Retroarticular | “Articulary region” in Cheirolepis | Articular process not separate bone | Retroarticular process | Present | |
| Second pharyngeal arch | Branchiostegal ray | Present | Absent | Present | Present |
| Ceratohyal | Present | Anterior and posterior elements | Present | Present | |
| Epihyal | Present | ||||
| Hyomandibula | Present | Present | Present | Present | |
| Symplectic | None | Present | Present | ||
| Interhyal | Present | None | Present | ||
| Interopercle | Present | Present | |||
| Opercle | Present | Present | Present | Present | |
| Opercular cartilage | Present | ||||
| Preopercle | Present | Present | Present | Present | |
| Subopercle | Present | Present | Present | Present | |
| Ventral and branchial arch structures | Basibranchial | Present | Present | Present | Present |
| Basihyal | Present | ||||
| Ceratobranchials | Present | Present | Present | Present | |
| Epibranchials | Present | Present | Present | ||
| Hypohyals | Present | Present | Present | Present | |
| Hypobranchials | Present | Present | Present | Present | |
| Urohyal | Present | Present | |||
Although jaw patterning and function in actinopterygians is generally defined by characteristics of neopterygii, two early lineages of palaeonisciform actinopterygians have jaw adaptations convergent with earlier and later forms, offering insights into what may have been elements of a basal actinopterygian jaw pattern. One group of palaeonisciform actinopterygians are the acipenseriformes, which include modern sturgeon and paddlefishes. Like sharks, acipenseriformes also have a reduced first gill slit that functions as a spiracle (Mims & Shelton, 2015). These fishes have dermal bone elements consistent with all actinopterygians, including opercular bones, although their endoskeletal jaw elements remain mostly cartilaginous (Carroll & Wainwright, 2003). Like chondrichthyans, these cartilaginous jaw elements are articulated from a mobile hyoid arch, and neither the palatoquadrate nor the Meckel’s cartilage is attached to the neurocranium (Carroll & Wainwright, 2003). This anatomical arrangement, studied in detail in sturgeon, allows them to feed in a manner similar to sharks, protruding their jaws away from the neurocranium (Carroll & Wainwright, 2003) it is believed that this feeding mechanism is unique to acipenseriformes among actinopterygians, and is an example of convergent evolution with sharks (Carroll & Wainwright, 2003).
Polypteriform actinopterygians
An extant group of actinopterygians are the polypteriformes (polypterus, or bichirs), and are considered by many to be the definitive representative of a actinopterygian “bauplan” (Bartsch, Gemballa, & Piotrowski, 1997; Kralovic, Horáček, & Cerny, 2010). Polypteriformes are bimodal respirators, using internal gills in water and paired dorsal spiracles to inhale air from the water surface (Graham et al., 2014). Air that is inhaled through spiracles, or less predominantly, by surface gulping by the mouth, enters paired lungs, and is expired through the opercular opening. As in other taxa with spiracles, the polypteriform spiracle is a transformation of the first gill slit (Graham et al., 2014). This highly modified pharyngeal slit structure, transposed from the lateral and ventral side of the head to the top of the head gives polypteriforms an advantage living in shallow and stagnant waters, conditions where water-only respirators could not survive. Polypterus can swim with their heads underwater allowing them to feed and hide, and in hypoxic conditions where air-breathing is a necessity (Graham et al., 2014). Although the polypterus lineage does not give rise to sarcopterygians, sarcopterygian stem tetrapod fossils also have spiracles. This indicates that the ability to modify the first gill arch evolved convergently in other osteichthyans taxa to achieve surface air breathing, which would have been an advantage navigating the biodiverse shallows of low-oxygenated Late Devonian shores (McGhee, 2012). Polypterus have a concave joint between the articular and quadrate, and a retroarticular process (Jollie, 1984b; Pehrson, 1947), similar to that of neopterygians, although relatively smaller and more shallow (Figure 6, Table 2). A separate retroarticular bone seems absent in Polypterus, and the one-piece articular is considered primitive. Subsequent lineages of osteichthyans including the ancestors of tetrapods also lack a separate retroarticular bone and their jaw joint is between the articular and quadrate bones. In Polypterus, the angular bone is braced on their pectoral girdle. Polypterus have a Meckel’s cartilage, ecto- and entopterygoid, an opercle with a process, preopercle, subopercle, and small opercular cartilage, which may be associated with an articular facet for the opercle bone, or lines the dorsal margin of the opercle, and they lack branchiostegal rays (Gardiner, 1984; Jollie, 1984a). The ceratohyal is found in two parts, an anterior and posterior ceratohyal, although some sources indicate the posterior ceratohyal is actually an interhyal (Jollie, 1984a; Kent & Carr, 2001; Pehrson, 1947). Sources indicate that Polypterus lacks a symplectic and interhyal (Jollie, 1984a; Pehrson, 1947). Like Mimia, polypterus possess a dermohyal bone element fused with their hyomandibula (Gardiner, 1984; Jollie, 1984a) Polypterus possesses a single basibranchial and a urohyal, a toothed premaxilla overlaid by a maxilla, and a toothed pterygoid on the roof of the mouth (Gardiner, 1984; Jollie, 1984a).
Neopterygian actinopterygians: lepisosteiformes and Amia calva
Palaeonisciform actinopterygian lineages gave rise to neopterygians in the Late Carboniferous. Early branches that are extant today include gars (lepisosteiformes) and bowfins (Amia calva, or amiiformes), although the neopterygians are overwhelmingly dominated by the teleosts. Neopterygians are distinguished from earlier forms of actinopterygians by their reduced dermal bones and scales, which presumably increased agility, and their enhanced jaw mobility (Kardong, 2012). Although they do not have spiracles, they utilize bimodal respiration of water and air via gills and mouth, or mouth alone (Burggren et al., 2016; Hedrick & Jones, 1999). A key feature of neopterygian jaws is the wide range of rotation of the Meckel’s-palatoquadrate joint, flaring of hyoid, and changes to jaw musculature, which allows a wide gape and the ability to catch prey in a rapid striking motion. Gars have elongated upper and lower jaws, and a rostrally-positioned jaw joint. The dentary (a dermal bone) is very long, and the underlying Meckel’s can be one third to half of its length (Kammerer, Grande, & Westneat, 2006). To catch prey, gars open their mouths by elevation of the maxilla and depression of the mandible, the head is rotated laterally, and jaws are closed around prey by lowering the upper jaw and adducting the mandible (Kammerer et al., 2006). Examination of juvenile gar lower jaw joints reveals a synovial-like architecture including joint cavities covered in articular chondrocytes and an external, fibrous synovial membrane encasing the cavity (Askary et al., 2017). These anatomical features, along with molecular similarities indicate these joints bear homologies to synovial joints in mammals, indicating synovial joint evolution is an early osteichthyans trait (Askary et al., 2016). Like gars, Amia calva retains a substantial dermal cranium (Figure 6, Table 2), with a sturdy maxilla and premaxilla, which along with jaws performs rhythmic chewing of prey (Gintof, Konow, Ross, & Sanford, 2010). Amia also has a synovial-like jaw joint between the quadrate and articular bones, although it is not known if this joint bears the same histological and molecular characteristics of the gar or mammals. Amia has the addition of a interopercle bone, between the opercle and subopercle, likely a modified branchiostegal ray, and eleven branchiostegal rays (Gardiner, 1984). In neopterygians including Amia, the symplectic forms as an antero-ventral extension of the hyomandibula, ossifies independently of the hyomandibula, and becomes part of the quadrate (Gardiner, 1984).
Neopterygian actinopterygians: Teleosts
Teleosts emerged in the Carboniferous from a basal neopterygian approximately 284 mya (Broughton et al., 2013). The teleost lineage is distinguished by a third round of whole genome duplication (3R WGD) which occurred in the ancestor of teleosts 284-328 mya (Amores et al., 1998; Broughton et al., 2013; Ozeki et al., 2004). This duplication and the following diversification of paralogous genes is believed to underlie the vast number of teleost lineages and their remarkable range of physiological adaptations (Ozeki et al., 2004). Compared to Amia and gars, teleosts have reduced dermal bone of dermatocranium, with a loss of some elements and a substantial thinning of others. Teleosts are dominated by acanthomorphs, or spiny-fin-rayed fishes, which features over 21,000 species including such familiar taxa as tuna, flatfishes, trigger fishes, snappers, sticklebacks, wrasses, clownfishes, zebrafishes, and seahorses (Hinchliff et al., 2015; Wainwright & Longo, 2017). Acanthomorphs have the basic actinopterygian cranium and jaw morphology described earlier, with some notable modifications such as secondary metamorphosis of the head skeleton in the order pleuronectiformes (e.g. flounder) to migrate one eye to the contralateral side of the head associated with a change in lifestyle from pelagic swimmer to a shallow burrowing fish. In the last 100 million years, from the Late Cretaceous onwards, teleosts have taken the actinopterygian protrusion jaw mechanism to the extreme with their powerful and rapid suction feeding adaptation (Bellwood, Goatley, Bellwood, Delbarre, & Friedman, 2015). Teleost suction feeders project their upper jaw anteriorly, caused by lowering of the mandible and an anterior and ventral rotation of the mandibular joints of the premaxilla and maxilla, resulting in their mouths and pharynx creating a large tubular hollow (Motta, 1984). Expansion of the pharynx is assisted by the depression of the hyoid skeleton, expansion of the cheek bones (quadrate, hyomandibula, metapterygoid, entopterygoid, symplectic) (Figure 6, Table 2), and expulsion of water through the opercular flap (Wainwright, McGee, Longo, & Hernandez, 2015). This rapid expansion and intake of water creates a low-pressure environment in the mouth, causing nearby water to rush in, carrying potential prey with it. This can extend the mouth up to 20% the length of the body (Bellwood et al., 2015), allowing the predator to reach prey from a further distance, and much more surreptitiously, than fishes that rely on a biting catch to capture prey. The majority of acanthomorphs posses not only an upper and lower set of jaws, but also possess a set of toothed gill elements forming the pharyngeal jaws. These additional jaws allow fish to further manipulate and process food taken into the pharynx, and represent another feeding adaptation among these fish (Wainwright, 2005; Wainwright & Longo, 2017).
Examination of zebrafish and stickleback lower jaw joints reveals a mammalian-like synovial architecture as in gars, including joint-lining cells, a fibrous capsule surrounding the joint, and expression of orthologous joint markers such as prg4, encoding the lubricin proteoglycan gene (Askary et al., 2016). Zebrafish jaw joints have expression of other markers associated with synovial joints, including elevated expression of hyaluronan synthase and reduced type II and X Collagen and other extracellular matrix proteins in articular compared to non-articular chondrocytes (Askary et al., 2016). Evidence of synovial-like jaw joints in gars and zebrafish indicates this trait may have evolved in vertebrate lineages prior to tetrapods (Askary et al., 2016).
EVOLUTION OF JAWS IN LOBE-FINNED SARCOPTERYGIANS
The following section examines the anatomy of ancient and modern sarcopterygian fishes with a focus on changes to the second pharyngeal elements and evolution of the squamosal bone in Latimeria and rhipidistian lungfishes. In these taxa, second arch elements are highly modified or lost associated with adaptations to feeding, and in rhipidistians, a trend towards air breathing.
Sarcopterygian fishes appear in the early Devonian, include two main clades, the Actinistia clade, which includes coelacanths, and the Rhipidistia clade, which includes dipnoi (lungfishes) and tetrapods (Kardong, 2012). Early sarcopterygians were believed to employ a mode of prey capture where mouth gape was achieved by flexion of the anterior neurocrania at the juncture of an intracranial joint in the neurocranium (Friedman & Brazeau, 2010). Derived features of sarcopterygians include a sarcopterygian-specific urohyal (present in ancient actinistians, dipnoans, and Latimeria), a broad joint between the hyomandibula and neurocranium, and an articulation between the last two gill arches (Friedman & Brazeau, 2010; Gardiner, 1984; Janvier, 1996).
Actinistia
Analysis of fossils and the extant coelacanth Latimeria, the only living branch of Actinistia, shows this intracranial joint, and separation of the neurocrania into an anterior ethmosphenoid portion (lateral ethmoids, parasphenoid, basisphenoid) and a posterior otoccipital portion (housing the otic and occipital regions of braincase) (Dutel, Herbin, Clément, & Herrel, 2015; Dutel, Herrel, Clément, & Herbin, 2013). This intracranial joint configuration, along with the basicranial muscle, has been shown to generate a high biting force in Latimeria that would have made it possible to consume a range of different sized and hard prey items. Among ancestral taxa, variations in this structure are believed to underlie variations in feeding behavior among sarcopterygians (Dutel, Herbin, et al., 2015).
Other features of Latimeria show features common to osteichthyans previously discussed. The suspensorium is comprised of a palatoquadrate complex (pterygoid, quadrate, metapterygoid elements) and hyoid complex (hyomandibula, interhyal, symplectic, and ceratohyal) which connects the mandible to the posterior neurocranium (amphistyly)(Figure 7, Table 3)(Dutel et al., 2013; Dutel, Herrel, Clément, & Herbin, 2015; Kardong, 2012). The palatoquadrate complex is attached to the neurocranium and palate by muscle attachments (Dutel et al., 2013). The symplectic cartilage of the hyoid arch forms independently of the short hyomandibula and articulates with the retroarticular on the lower jaw, and the interhyal and ceratohyal, separate from the joint between the quadrate and lower jaw (Dutel et al., 2013; Dutel, Herrel, et al., 2015; Gardiner, 1984). The ceratohyal forms a joint with the symplectic, interhyal, and basibranchial, and the mandibula is comprised of a dentary, angular, and articular where the quadrate forms a cartilage-covered socket-like joint with the articular, as seen in actinopterygians previously discussed (Dutel et al., 2013). There is no maxilla, and the premaxilla is small (Janvier, 1996). The opercle bone is large compared to other osteichthyans, lined on the ventro-posterior margin by a opercular cartilage, and there is an absence of branchiostegal rays (Gardiner, 1984; Janvier, 1996).
Figure 7:
Jaw patterning in sarcopterygian fishes. In Latimeria, the hyomandibula and symplectic are separate elements. In Lepidosiren, Protopterus, Neoceratodus (Rhipdistians), the hyomandibula and symplectic are absent. The opercle is a plate-like structure in Neoceratodus, but a blade-like structure in Lepidosiren and Protopterus. Rhipdistians have a novel element, the squamosal forming the adult jaw joint. This arises near the larval quadrate from a separate primordium. In Rhipdistians cranial ribs (CR) articulate with the neurocranium and assist with respiration. CL = clavicle. Adapted with copyright permission (Criswell, 2015; Dutel, Herrel, et al., 2015).
Table 3:
Features and structures of sarcopterygian jaw skeletons. Blank spaces indicate that homologous element has not been reported. Elements that may be lost secondarily are indicated as “absent”.
| Sarcopterygians | |||||
|---|---|---|---|---|---|
|
Latimeria (Dutel, Herrel, et al., 2015; Gardiner, 1984; Janvier, 1996) |
Lepidosiren (Bartsch, 1994; Criswell, 2015; Gardiner, 1984; Machado et al., 2010) |
Protopterus (Criswell, 2015; Gardiner, 1984; Machado et al., 2010) |
Neoceratodus (Bartsch, 1994; Criswell, 2015; Gardiner, 1984; Kemp, 1999, 2013; Machado et al., 2010) |
||
| Feature/structure | Element | ||||
| Mineralization of cartilage | Present | Present | Present | Present | |
| Dermal bone | Present | Present | Present | Present | |
| First pharyngeal arch | Anguloarticular or angular | Angular | Angular | Angular | Angular |
| Meckelian | |||||
| Dentary | Present | Termed prearticular | Termed prearticular | Termed prearticular | |
| Ectopterygoid | Present | ||||
| Entopterygoid | Derived tooth-like structure | ||||
| Maxilla | Absent | Fused with premaxilla | |||
| Meckel’s cartilage | Present | Present | Present | ||
| Metapterygoid | Present | ||||
| Palatoquadrate | Present | Present | |||
| Palatine | Autopalatine | ||||
| Premaxilla | Present | Fused with maxilla | |||
| Quadrate | Present | In development, replaced by squamosal | |||
| Retroarticular | Present | ||||
| Squamosal | Present | Present | Present | ||
| Second pharyngeal arch | Branchiostegal ray | Absent | Modified or absent | Modified or absent | Modified or absent |
| Ceratohyal | Present | Present | Present | Present | |
| Epihyal | |||||
| Hyomandibula | Present | Absent | Absent | Reduced | |
| Symplectic | Present | ||||
| Interhyal | Present | ||||
| Interopercle | |||||
| Opercle bone | Present | Present | Present | Present | |
| Opercular cartilage | Present | Present | Present | ||
| Preopercle | Present | ||||
| Subopercle | Present | Present | Present | Present | |
| Ventral and branchial arch structures | Basibranchial | Present | Present | Present | |
| Basihyal | Present | Present | |||
| Ceratobranchials | Present | ||||
| Epibranchials | |||||
| Hypohyals | Present | ||||
| Hypobranchials | Reduced or absent | Reduced or absent | Reduced or absent | ||
| Urohyal | Present | ||||
Rhipidistians
Among rhipidistians, the intracranial joint was independently lost in lungfishes (dipnoi) and tetrapods, and in tetrapods it is thought that biting force was likely reduced compared stem sarcopterygians, and other feeding strategies were employed (Dutel, Herbin, et al., 2015). In both lungfishes and early tetrapods, air-breathing evolved independently by the middle Devonian, driven by aquatic hypoxia (low ocean oxygen levels) and possibly an increase in metabolic activity by these fishes (Clement & Long, 2010). In lungfishes, the mouth forms a buccal pump, which expands to take in surface air, then compresses to force air into a single or paired set of lungs, with excess air leaving through the mouth or nostrils (Kardong, 2012). All Devonian lungfishes were likely entirely dependent on gill-breathing, and buccal respiration likely arose later (Janvier, 1996). In studies of ancient Rhinodipterus and the extant African Protopterus lungfish, a buccal cavity is created through hyoid depression assisted by a mobile ceratohyal and pectoral girdle (Clement & Long, 2010). In Protopterus, the South American lungfish Lepidosiren paradoxa, and the Australian lungfish Neoceratodus forsteri, cranial ribs are attached to the neurocranium and assist with respiration (Clack, 2002; Clement & Long, 2010; Machado, Wellendorf, & Brito, 2010).
In general, modern lungfishes have an array of head and jaw adaptations that are specific and derived, and they are not basal tetrapods, however their early tetrapod jaw evolution may have involved similar mechanisms. Overall, extant lungfishes are reported to have more cartilaginous skeletons than actinopterygians, fewer dermal bones, and unusual fusions between elements. Dipnoans have fused premaxillary and maxillary bones, and both maxillary and mandible are characterized by large bones with tooth-like bony projections derived from the entopterygoids and prearticulars (Janvier, 1996). Dipnoans have reduced dentary bones and a single tooth plate at the median joint of the lower jaw (Gardiner, 1984). The lower jaw joint articulates with a squamosal bone that arises near the larval quadrate from a separate primordium (Bartsch, 1994). As in teleosts, they have an additional ossification called a basihyal anterior to the first basibranchial, which is also seen in some amphibians and reptiles (Gardiner, 1984). There are a series of submandibular bones not articulated with the ceratohyal, which are debated as either modified branchiostegal rays or a non-homologous gill-cover structures (Gardiner, 1984). In both actinistians and dipnoans, there is a single broad triangular basibranchial, and a reduction or loss of hypobranchials (Gardiner, 1984). In dipnoans, the upper jaw is composed of the upper tooth plate and pterygoid, the hyomandibula is small or absent, and the trabeculae and base of the neurocranium are not present (Bartsch, 1994; Criswell, 2015; Machado et al., 2010). All lungfishes have an autostylic jaw, where the palatoquadrate is attached to the cranium, and the hyomandibula is not involved in jaw suspension (Bartsch, 1994). This is also the case for amniotes.
Both Protopterus and Lepidosiren have similar morphologies, related to their recent common origin (Kemp, Cavin, & Guinot, 2017). Both represent departures from the osteichthyans plan, most notably by a reduction of the number of elements and a substantial loss or reduction of elements of the hyoid and posterior gill arch structures, including a loss of the hyomandibula, and no external gill structures in adulthood (Figure 7, Table 3) (Sanchez et al., 2001). In Protopterus and Lepidosiren the lower jaw is composed large prearticular elements with coronoid tooth-like processes, angular bones, and a Meckel’s cartilage (Criswell, 2015; Machado et al., 2010). The prearticular is articulated with the blade or paddle like squamosal to form the lower jaw joint. The slender stick-like operculum lies ventrally along the squamosal, and articulates along its anterior margin with the squamosal and prearticular (Criswell, 2015). The subopercle is ventral and posterior to the operculum, and articulates with a curved ceratohyal (Criswell, 2015). In Lepidosiren, there are opercular cartilages associated with the inner surfaces of the opercle and subopercle (Gardiner, 1984).
In Neoceratodus forsteri, an extant lungfish from Australia, the Meckel’s persists as a cartilaginous element throughout life, covered in angular and prearticular bones (Figure 7, Table 3). During development, the jaw joint is comprised of the Meckel’s articulating with the quadrate, although in adults it is formed of articular and squamosal bones (Criswell, 2015; Kemp, 2013). In this lungfish, elements such as the Meckel’s, basihyal, hypohyal, and basibranchials remain cartilaginous and only the curved or spatulate ceratohyal becomes partially ossified to contribute to the adult visceral skeleton (Kemp, 1999; Kemp, 2013). Opercle and preopercle bones are present, and the opercle is more plate-like and less blade-like compared to Protopterus and Lepidosiren (Criswell, 2015; Kemp, 1999). As in Lepidosiren, there are opercular cartilages associated with the opercle and subopercle (Gardiner, 1984). The hyomandibula is small and unossified, and has no role in the suspensory system, but functions in opercular opening (Bartsch, 1994). The presence of a hyomandibula, although small, is consistent with the general osteichthyan plan, and the position of Neoceratodus on an more ancient lineage of sarcopterygians (Kemp et al., 2017). Loss of the hyomandibula in Lepidosiren and Protopterus represents a derived feature shared by these sister lineages. The Neoceratodus is a facultative air breather, preferring to breath water, and so has functional branchial gills (Kind, Grigg, & Booth, 2002). It is possible that the retention of a hyomandibula in Neoceratodus may be to facilitate action of the hyoid during gill respiration.
ORIGINS OF JAWS IN TETRAPODOMORPHA: RHIPIDISTIAN “FISHAPODS” AND LABYRINTHODONTS
The following section examines the anatomy of the ancestors to tetrapods, the tetrapodomorphs, with a focus on how the pharyngeal skeleton, in particular how loss or modifications to the second pharyngeal arch and posterior arch skeletal elements, was associated with a shift towards a terrestrial lifestyle.
Rhipidistian Fishapods
Among rhipidistians, the tetrapodomorpha branch of lobe-finned fish split from dipnoans and underwent changes that represent transitions to living in shallow water, air breathing, and eventually giving rise to descendants that migrated onto land. These “fishapod” rhipidistian fishes are notable by their progressive loss of opercular and branchiostegal ray structures, as well as modifications to the hyomandibula away from being a jaw suspensory role towards a sound transduction function in the ear (Shubin, 2009). A well-known early fishapod is Eusthenopteron (osteolepiformes) from the Late Devonian. This fish lived entirely in water, showing no signs of living on land. Even though it lived in water, Eusthenopteron is considered a transitional form towards tetrapods, as it possesses tetrapod-like traits, particularly in changes to the limb skeleton associated with terrestrial life (Boisvert, 2005). The jaw of Eusthenopteron is very much like other rhipidistian fishes. There is an inter-cranial joint, and Eusthenopteron has a large squamosal, a quadratojugal, an opercle, subopercle, and bar-shaped preopercle of the dermal skeleton (Figure 8, Table 4) (Janvier, 1996). It also had a palatoquadrate articulated with the braincase (Janvier, 1996). This palatoquadrate resembles that of most osteichthyans, and has a broad entopterygoid bone and metapterygoid (epipterygoid in tetrapods) bone associated with it (Brazeau & Ahlberg, 2006). The hyomandibula is elongate and projects ventrally, and articulates with the braincase, palatoquadrate, opercle, and branchial elements (Downs, Daeschler, Jenkins, & Shubin, 2008; Janvier, 1996). The metapterygoid and entopterygoid of the palatoquadrate along with the hyomandibula create a narrow, slot-like spiracle, where the metapterygoid creates the lateral wall of a respiratory spiracle, and the entopterygoid associates with the hyomandibula (Brazeau & Ahlberg, 2006). In Eusthenopteron, there is a ceratohyal, urohyal, five branchial arches, two basibranchials, hypohyal, and bones of the lower jaw including a dentary and articular (Clack, 2002; Janvier, 1996; Jarvik, 1980). There is a “stylohyal” element between the hyomandibula and ceratohyal, indicated as a “symplectic” in Brazeau and Ahlberg 2006 (Brazeau & Ahlberg, 2006; Jarvik, 1980).
Figure 8:
Jaw patterning in rhipidistian fishapods and labyrinthodonts. In Eusthenopteron, a prehistoric sarcopterygian fish, the hyomandibula is a blade-like element adjacent to the spiracle. There is no symplectic. The ceratohyal is in two parts. In Ichthyostega and Acanthostega, the squamosal is a cranial vault bone, and the opercular series is much reduced, where the subopercle and preopercle bones are very small elements. Fossil evidence of the deeper elements of the jaw in Acanthostega reveals a small hyomandibula and evidence of gill structures. In the amphibian Necturus, the ceratohyal is present, as are ceratobranchials and epibranchials, associated with gill structures. A columella (hyomandibula, stapes) is present. In the modern Iguana, the hyomandibula has become a hearing organ, the stapes that connects the quadrate to the inner ear. The second arch-derived hyoid is a separate throat structure. In Eusthenopteron, Acanthostega, and reptiles, the jaw joint is between the articular and quadrate. In mammals such as Didelphis (possum) the lower jaw is exclusively formed from the dentary forming a joint with the squamosal. Besides the hyomandibula-derived stapes, the quadrate has become transformed into the incus bone, and the articular has become the malleus of the middle ear (Valdezate et al., 2015). The styloid process is a projection from the temporal bone and is derived from the second pharyngeal arch (Kent and Carr, 2001) (not shown). Adapted with copyright permission (Clack, 2002; Jarvik, 1980; Kardong, 2012; Porter & Witmer, 2015).
Table 4:
Features and structures of Rhipidistian fishapods, labyrinthodonts, amphibians, and amniote jaw skeletons. Blank spaces indicate that homologous element has not been reported. Elements that may be lost secondarily are indicated as “absent”.
| Fishapods, amphibians, and amniotes | ||||||
|---|---|---|---|---|---|---|
|
Eusthen opteron (Brazeau & Ahlberg, 2006; Clack, 2002; Jarvik, 1980) |
Ichthyostega and Acanthostega (Clack, 2002; Janvier, 1996; Porro et al., 2015) |
Amphibians Necturus and frogs (Kent and Carr, 2001; S.G. Gilbert, 1986) |
Reptile (Kardong, 2012; Kent & Carr, 2001) |
Mammal (Kardong, 2012; Kent & Carr, 2001; Presley & Steel, 1978) |
||
| Feature/ structure |
Element | |||||
| Mineralization of cartilage | Present? | Present | Present | Present | ||
| Dermal bone | Present | Present | Present | Present | Present | |
| First pharyngeal arch | Anguloarticular or angular | Angular | Angular | Angular | Angular | Absent |
| Meckelian | Present | |||||
| Dentary | Present | Present | Present | Present | Present | |
| Ectopterygoid | Present | Present | “Pterygoid” | Present | Present in development | |
| Entopterygoid | Present | Present | “Pterygoid” | |||
| Maxilla | Maxillary | Present | Present in frogs | Present | Present | |
| Meckel’s cartilage | Present | Present | Present | |||
| Metapterygoid | Present | “Pterygoid” | Epipterygoid | Alisphenoid portion of sphenoid bone | ||
| Palatoquadrate | Present | |||||
| Palatine | Present | Present | Present | Present | Present | |
| Premaxilla | Present | Present | Present | Present | Absent | |
| Quadrate | Present | Present | Present | Present | Incus | |
| Articular | Articular | Articular | Articular present in frogs Present in Necturus? (Articular cartilage?) | Articular | Malleus | |
| Squamosal | Present | Present | Present | Present | Temporal bone | |
| Second pharyngeal arch | Branchiostegal ray | Absent | Absent | Absent | Absent | Absent |
| Ceratohyal | Ceratohyal 1 and 2 | Present in Acanthostega | Present in Necturus, hyoid in frogs | Anterior horn of hyoid | Styloid process Anterior horn of hyoid | |
| Epihyal | ||||||
| Hyomandibula | Present | Stapes | Columella | Stapes | Stapes | |
| Symplectic | ‘symplectic’ or ‘stylohyal’ | Absent | Absent | Absent | Absent | |
| Interhyal | ||||||
| Interopercle | Absent | Absent | Absent | Absent | Absent | |
| Opercle bone | Present | Absent | Absent | Absent | Absent | |
| Opercular cartilage | Absent | Absent | Absent | |||
| Preopercle | Present | Present | Absent | Absent | Absent | |
| Subopercle | Present | Present | Absent | Absent | Absent | |
| Ventral and branchial arch structures | Basibranchial | Present | Present | Absent | ||
| Basihyal | Entoglossus | Body of hyoid | ||||
| Ceratobranchials | Present | Present in Acanthostega | Present in Necturus | Second horn of hyoid, Body of hyoid, Last horn of hyoid Cricoids and arytenoids | Second horn of hyoid, body of hyoid, thyroid cartilages, cricoids and arytenoids | |
| Epibranchials | Present | Present in Acnathostega | Present in Necturus | |||
| Hypohyals | Present | Present in Necturus Body of hyoid in frogs | ||||
| Hypobranchials | Present | |||||
| Urohyal | Present | Absent | Absent | |||
Panderichthys another Late Devonian fishapod, has traits similar to Eusthenopteron including similar palatoquadrate, hyomandibula, and urohyal endoskeletal elements, and an opercular series (Janvier, 1996). However, Panderichthys also shows the emergence of tetrapod-like characteristics among rhipidistians, including a flattened cranium with dorsally-positioned orbits and a jugal articulating with the quadratojugal (Janvier, 1996). Unlike Eusthenopteron, Panderichthys may have been a facultative air-breather, respirating through enlarged dorsal spiracles, which are associated with a reduced hyomandibula and opercular series. Transformations to the spiracular region, associated with changes to skeletal elements, is believed to represent the origin of the tetrapod middle ear (Brazeau & Ahlberg, 2006). Compared to Eusthenopteron, Panderichthys has a shallower palatoquadrate with a narrowed entopterygoid, reducing the amount of contact between the palatoquadrate and hyomandibula, creating a wider and straighter spiracle (Brazeau & Ahlberg, 2006). The hyomandibula has a dorsal portion with an opercular articulation, but it is missing the distal/ventral portion found in other osteichthyans. Together, the distal/ventral reduction of the hyomandibula and separation from the entopterygoid are believed to be transitions of the hyomandibula into a stapes found in tetrapods (Brazeau & Ahlberg, 2006). These features of the hyomandibula are also seen in another Devonian tetrapod morph, Gogonasus (Long, Young, Holland, Senden, & Fitzgerald, 2006).
In Tiktaalik, a stem tetrapod, changes to the head skeleton suggest it is an intermediate form between Panderichthys and Acanthostega. Tiktaalik has a horizontally oriented and shallow entopterygoid, and a short and horizontal hyomandibula, as in Panderichthys (Downs et al., 2008). As in Panderichthys, the hyomandibula does not form an articulation with the palatoquadrate; however, unlike in Panderichthys, Tiktaalik had no opercle bone, and so the hyomandibula also has no opercular joint (Downs et al., 2008). As in other finned tetrapodomorphs, Tiktaalik has a branchial skeleton comprised of two basibranchials, a urohyal, hypohyals, ceratohyals, hypobranchials, and ceratobranchials (Downs et al., 2008). Like Panderichthys, Tiktaalik has a flattened head, and dorsal eyes, suggesting a lifestyle of navigating and predating in shallow waters (Shubin, 2009). It is proposed by Downs and colleagues (2008), that with the transition to air breathing, there was less need for a coordinated suspensorium involving the hyomandibula to aide in gill respiration (Downs et al., 2008). In tetrapodomorpha the shift from coordinated gill respiration, and perhaps as a consequence, the loss of selective pressure on maintaining joints between the hyomandibula and other elements meant it could change shape and orientation, eventually becoming co-opted into a hearing organ in tetrapods.
Labyrinthodonts
The tetrapod clade of rhipidistian fishes first made forays onto land in the Late Devonian or Carboniferous, about 335 mya (Broughton et al., 2013). Early tetrapods are referred to as labyrinthodonts, and it is from within the labyrinthodont clade that modern amphibians arose (Kardong, 2012). Labyrinthodonts date from the Late Devonian, and include Acanthostega and Ichthyostega. Although aquatic, tetrapods had limbs and girdles, rather than lobefins, which could support their trunks in shallow water (Callier, Clack, & Ahlberg, 2009). The jaw and pharynx of true tetrapods are distinguished by the complete absence of opercle bones and the branchiostegal rays. Nasals, frontal, jugals, prefrontals and postfrontals, parietals and post-parietals form the front of upper face and cranium (Porro, Rayfield, & Clack, 2015; Schoch, 2006). Both Acanthostega and Ichthyostega have gills, indicating a primarily aquatic and not terrestrial existence, but various changes to the second pharyngeal hyoid arch are associated with a shift towards a lifestyle partially on land (Clack et al., 2003).
In Acanthostega, the hyomandibular retains the distal loss as in Panderichthys, is transversely orientated, and is transformed into a stapes of the ear, although it likely did not function in sound transduction, but may have had a function in supporting the spiracles (Brazeau & Ahlberg, 2006; Clack, 1989). Since the opercle is lost in Acanthostega, the hyomandibula does not have an opercular articulation as in Panderichthys (Brazeau & Ahlberg, 2006). The palatoquadrate morphology, including a narrowing of the entopterygoid is nearly identical to that in Panderichthys (Brazeau & Ahlberg, 2006). The lower jaw has a Meckel’s cartilage, dentary, splenial, postsplenial, angular, and surangular, and articular, coronoids, and prearticular, and features an upturned snout (Figure 8, Table 4) (Porro et al., 2015). Analysis of the functional mechanics of jaws suggests that Acanthostega fed more like a tetrapodomorph fish than fully terrestrial tetrapods (Neenan, Ruta, Clack, & Rayfield, 2014). In Ichthyostega the hyomandibula is transformed into a hearing organ. Described as thin and disc-like, it projects into the otic chamber of the ear, articulating with the sacculus of the inner ear (Clack et al., 2003). The stapes in Ichthyostega has two heads separated by a large foramen, where one head articulates with the basioccipital of the cranium, and another the fenestra ovalis associated with the sacculus (Clack et al., 2003). It is proposed that movement of the stapes would have created vibrations to the inner ear, via an air or water-filled spiracular tract (Clack et al., 2003). This ear is not considered a precursor to the typannic ear of temnospondyls, but represents a system for sound transduction in water in early tetrapods (Clack et al., 2003).
EVOLUTION OF JAWS IN AMNIOTES
As aquatic tetrapods made their way onto land and became obligate air-breathers, the gill skeletal elements were gradually reduced and slits closed. The aquatic respiration apparatus became repurposed into structures for airborne-hearing and throat structures. There are many steps in this process between aquatic amphibians and amniotes, and retention of some structures in certain taxa, including during development that may involve a gill apparatus in some amphibians that is later transformed or lost. This section of the review will highlight the major changes that have occurred to form the jaw, ear, and throat structures in amphibians and amniotes (reptiles, mammals, birds). Other reviews discuss changes in amphibians in detail, and also specifically the evolution of multiple structures of the middle ear (Clack, 2002; Thomson, 1966).
As an overview, in amniotes, the first pharyngeal arch structures contribute to the Meckel’s cartilage, maxilla and mandible of the jaw, malleus and incus of the ear, and wings of sphenoid (alisphenoid), the second arch contributes to Reichert’s cartilage, the stapes, styloid process of the temporal bone in mammals, and the lesser horn and upper body of the hyoid bone. The third arch (first branchial) also contributes to the greater horn and lower body of the hyoid, and the fourth arch to the thyroid and epiglottis cartilages in mammals (and a third hyoid element in reptiles). The sixth arch gives rise to the cuneiform, corniculate, cricoid and arytenoid cartilages of the throat. Arch seven has been lost entirely in amniotes (Kent & Carr, 2001). The hyoid and posterior cartilages are midline structures, forming rings or curved structures surrounding the trachea or larynx. The gill pouches give rise to cavities in the pharynx, and glands such as the thyroid, thymus, and parathyroid glands (S. F. Gilbert & Barresi, 2016). The following are a series of descriptions of the transformations that have occurred to pharyngeal arch element in amniotes, with reference to changes to developmental programs underlying these transformations.
Upper jaw and maxillary structures
Amphibians and reptiles retain the elongated maxillary bones of early tetrapods and a relatively small and posterior braincase. Mammals have a comparatively enlarged braincase and a smaller maxilla and the premaxilla may be fused with the maxilla (Atkins & Franz-Odendaal, 2015). Substantial changes are present in the amniotes associated with separation of the airway from the digestive system. Whereas fishes and amphibians have a primary palate to form the roof of the mouth, in amniotes a secondary palate develops to separate the oral and nasal cavities. In fishes and amphibians, this primary palate forms from the palatine, pterygoid, vomer, ethmoid, and parasphenoid (neurocranium-derived) (Kent & Carr, 2001). In amniotes the secondary palate forms from lateral swellings of the maxilla during development forming palatine shelves that fuse in the midline of the oral cavity (Li, Lan, & Jiang, 2017). Elements of the primary palate are substantially changed and have moved anterior to the incisive fossa in the roof of the mouth, the paired palatine and pterygoid (reptiles and birds only) and unpaired midline vomer are located at the posterior region of the palate, pushed posteriorly by the shelves of the maxilla. The ethmoid in mammals is located in the mid-facial orbital region. The parasphenoid is a midline palate element of reptiles, birds and some mammals (monotremes and marsupials), but is absent in placental mammals (Atkins & Franz-Odendaal, 2015).
Mandibular first arch and associated structures
Like fishes, the scaffold of the lower jaw in amphibians and amniotes develops as the Meckel’s cartilage and becomes enveloped by overlying dermal bones. The overlying mandibular bones of these tetrapods are similar to that in osteichthyans, in particular to rhipidistian fishes. Amphibian mandibles include a dentary, articular, and quadrate (Kent & Carr, 2001). Amniote mandibles always include a dentary, and different amniotes may have combinations of angular, surangular, splenial, coronoids, prearticulars and articular bones (Figure 8). Reptiles retain a splenial, and coronoid, although these are absent in birds. In mammals, only the dentary remains, having gradually overtaken the majority of the jaw with evolution of the synapsids, expanding dorsally to form a ramus, which articulates with the squamosal of the temporal bone (Figure 8, Table 4) (Kent & Carr, 2001). In amphibians, reptiles and birds, the jaw suspension is metautostylic, where attachment of the jaw to the braincase is through one articulation though the quadrate, whereas in mammals the attachment of the jaw to the squamosal is considered craniostylic (Kardong, 2012). In osteichthyans, the palatoquadrate forms the entopterygoid (epipterygoid), metapterygoid and quadrate. Reptiles and birds retain the quadrate for articulation of the cranium with lower jaw, and pterygoid structures for portions of the base of the cranium and palate. In mammals the epipterygoid becomes the alisphenoid and the quadrate and articular become reduced and internalized into the ear as the incus and malleus, respectively (Kent & Carr, 2001). Although the ear bones are completely separated from the jaw in extant mammals, fossil evidence suggests there were transitional forms of partial ear bone attachment (of the malleus) to a persistent Meckel’s in early mammals, which served as a supporting arrangement for the middle ear structures (Weil, 2011). The first gill slit or pouch, reduced to a spiracle in early tetrapods, becomes the cavity of the middle ear and Eustachian tube in amniotes, connecting the pharyngeal cavity (as in fishes) to the inner ear (S. F. Gilbert & Barresi, 2016).
Hyoid second arch and associated structures
As previously described in early tetrapods, the hyomandibula becomes a true stapes of the middle ear in mammals and the columella (“little column”) in amphibians, reptiles, and birds (Kent & Carr, 2001). Intriguingly, the same Jagged1-Notch2 signalling mechanism for correct patterning of the zebrafish dorsal hyoid arch skeleton is required for mouse stapes development, as loss of either factor in neural crest cells causes the stapes to be smaller and lacking a fully patent foramen (Teng et al., 2017). Once released from a jaw suspension role, the hyomandibula became disassociated with the quadrate, becoming enveloped by the first pharyngeal pouch, and attaching to the precursor of the typannic membrane of the middle ear (Kent & Carr, 2001). While suspended in the middle ear, a portion of the stapes is embedded in the fenestris ovalis of the inner ear and transmits and amplifies the sound vibrations received at the tympanic membrane, via the malleus and incus (Kardong, 2012). In amphibians, such as the Necturus, a ceratohyal persists in the pharynx, and articulates with hypohyals (S. G. Gilbert, 1986), while in frogs the ceratohyal is transformed into the hyoid (Kent & Carr, 2001) (Figure 8). In amniotes, the hyomandibula and ceratohyal contribute to the styloid process, a projection from the temporal bone, and the ceratohyal contributes to the lesser horn of the hyoid of the throat (Kitazawa et al., 2015). The stylohyoid muscle attaches the styloid process to the hyoid bone, and functions in elevating the hyoid in swallowing (Kent & Carr, 2001). In amniotes, the basihyal, a midline cartilage in fishes and Necturus, becomes the midline body of the hyoid (Kent & Carr, 2001). The second pharyngeal pouch creates a wall surrounding of the tonsils, and a pocket will bud off from the second pouch and migrate posteriorly to become the thyroid gland (S. F. Gilbert & Barresi, 2016).
Third through sixth pharyngeal arch
In amphibians such as Necturus, the third through sixth pharyngeal arches are comprised of ceratobranchials and epibranchials functioning in gill respiration (S. G. Gilbert, 1986). In frogs, the third and fourth arches are associated with the hyoid, and the fifth and sixth arches are associated with laryngeal cartilages (Kardong, 2012; Kent & Carr, 2001). In amniotes, the third pharyngeal arch cartilages contribute to the second horn of the hyoid in reptiles and mammals (Kent & Carr, 2001). The third pouch creates the thymus which functions in differentiation of T lymphocytes, and one pair of the parathyroid glands, which secrete endocrine factors (S. F. Gilbert & Barresi, 2016). In mice, loss of Hoxa3 and Pax1 causes thymus and parathyroid gland defects (Su, Ellis, Napier, Lee, & Manley, 2001). Since Pax1 is associated with gill slit formation in hemichordates and amphioxus, this indicates that there may be conserved aspects of development of gill slits in hemichordates and amphioxus, and formation of pouch glands in mice (Escriva et al., 2002; Ogasawara et al., 1999; Su et al., 2001). The fourth branchial arch cartilages form the last horn of the hyoid in reptiles, and the thyroid cartilages in mammals and the sixth arch gives rise to the cricoid and arytenoid cartilages associated with laryngeal vocalization (Kent & Carr, 2001). The fourth pharyngeal pouch creates another pair of parathyroid glands (S. F. Gilbert & Barresi, 2016).
Conclusion
The major events associated with jaw evolution include acquisition of pharyngeal slits, formation of joints between the upper and lower segments of the pharyngeal arches, reduction and loss of posterior second arch dermal bones, morphogenesis of the first pouch to become a spiracle or Eustachian tube. Evolution of the jaw into an ear apparatus involved loss of the symplectic and reduction and dissociation of the hyomandibula from a role in jaw support in fishes to a role in hearing in reptiles, and a transition from a quadrate-articular jaw joint in reptiles to a dentary-squamosal joint in mammals. From agnathans to amniotes, there exists deep conservation of gene regulatory pathways that establish pharyngeal arch development among vertebrates, including expression of Pax genes that create gill slits, Hox genes that segment pharyngeal arches, and the Edn1/Hand2/Dlx and Jagged1-Notch2 pathways which give jaw elements a dorsal-ventral identity. Although the molecular mechanisms to establish the axial position of elements are increasingly understood, a new and exciting phase of jaw research aims to understand how changes to gene regulatory networks could produce the major events in morphogenesis over evolutionary time. For example, it is not known how jaw joints were acquired in gnathostomes from agnathan ancestors, as the earliest gnathostome fossils have a patent joint that is homologous to modern jaw joints and there exist no transitional forms in the fossil record. If ancient agnathan ancestors to gnathostomes looked anything like extant agnathans, they may have had a branchial basket formed of continuous cartilage that became segmented into dorsal and ventral elements. As previously mentioned, new modes of expression of factors such as nkx3.2, hand2, and barx1 may have led to the exclusion of cartilage formation within ancient agnathan gill bars, leading to the formation of a flexible junction between cartilage elements (Nichols et al., 2013). Understanding these genetic mechanisms in gnathostomes, and how joints are lost in mutants, sets the stage for potential future studies to ectopically generate jaw joints in agnathans.
Although this review has focused on the changes in structure of elements over time, some elements have remained remarkably unchanged since the early Devonian, and seem to be present in nearly all extant gnathostome taxa. The dentary, Meckel’s cartilage, hyomandibula, palatoquadrate-derived elements, and articular appear early in the evolutionary record, and are retained in most taxa. Their persistence indicates their importance in jaw development, suggesting the developmental pathways leading to their formation have been largely unperturbed since ancient times. Other jaw elements have been gained over time, such as the squamosal and angular, whereas other elements were gained and subsequently lost, such as the branchiostegal rays and the interopercle. Understanding how cellular populations contribute to the formation of these elements, and what genes are expressed associated with their formation or loss could explain how jaw elements appeared, changed, and disappeared over time and between lineages. The process by which the hyomandibula became dissociated from the jaw and became the columella/stapes is well documented in the fossil record. It remains an intriguing developmental question, which could be studied in reptiles and mammals, as to how the quadrate and articular element dissociated from the jaw joint in reptiles to become the incus and malleus of the middle ear of mammals.
Among ancient and extant taxa, extreme differences in shape and relative size of some elements suggest they possess more developmental plasticity and therefore are more prone to evolution than others. For example, the opercle has tremendous shape variation even among teleost fishes (Kimmel, Small, & Knope, 2017), and is variably reduced or lost in ancient fish lineages. As previously revealed with studies of mef2ca and indian hedgehog (ihh) mutant zebrafish, changes to expression of genes associated with opercle patterning can resulting in extreme phenotypic outcomes which evolution can act upon (DeLaurier et al., 2014; Huycke, Eames, & Kimmel, 2012; Nichols et al., 2016). The symplectic seems to be rather developmentally sensitive, as many zebrafish mutants are prone to loss or reductions of the symplectic (e.g. Furin and Edn1 pathways, wnt5b, itga8) (Sisson, Dale, Mui, Topczewska, & Topczewski, 2015; Talbot et al., 2016; Walker et al., 2006). The developmental fragility of the symplectic may underlie the independent loss of this element in ancient fish lineages, and may have promoted the dissociation of the hyomandibula from the second arch jaw skeleton to become a middle ear element in labyrinthodonts. Evolution of jaw musculature and endoderm, not discussed in this paper likely also had important influences on skeletal patterning. For example, fras1 zebrafish mutants have defects in first pharyngeal pouch formation that leads to fusions of skeletal elements (Talbot et al., 2012). Given the prevalence of Eustachian tube and hearing defects in humans, understanding how interactions between pharyngeal endoderm and jaw skeletal elements evolved could provide important insights into mechanisms underlying human ear disorders.
Acknowledgments
I would like to acknowledge Susan Chapman, Alexandra Roach, and Jared Talbot for helpful feedback on drafts of this manuscript and Allen Dennis for informative discussions about geological events in the Devonian era. This work was supported by a Developmental Research Program (DRP) award to A.D. though SCINBRE (NIGMS/NIH) grant P20GM103499.
Footnotes
No conflicts of interest
Contributor Information
April DeLaurier, University of South Carolina Aiken, Biology and Geology, South Carolina, United States..
John Gerhart, University of California, Berkeley, Molecular and Cell Biolofy, Berkeley, California..
References
- Abitua PB, Wagner E, Navarrete IA, & Levine M (2012). Identification of a rudimentary neural crest in a non-vertebrate chordate. Nature, 492(7427), 104. 10.1038/nature11589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C, Zuniga E, Blitz IL, Wada N, Le Pabic P, Javidan Y, … Schilling TF (2011). Combinatorial roles for BMPs and Endothelin 1 in patterning the dorsal-ventral axis of the craniofacial skeleton. Development (Cambridge, England), 138(23), 5135–5146. 10.1242/dev.067801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, … Postlethwait JH (1998). Zebrafish hox clusters and vertebrate genome evolution. Science (New York, N.Y.), 282(5394), 1711–1714. [DOI] [PubMed] [Google Scholar]
- Anderson PSL, Friedman M, Brazeau MD, & Rayfield EJ (2011). Initial radiation of jaws demonstrated stability despite faunal and environmental change. Nature, 476(7359), 206–209. 10.1038/nature10207 [DOI] [PubMed] [Google Scholar]
- Anthwal N, Urban DJ, Luo ZX, Sears KE, & Tucker AS (2017). Meckel’s cartilage breakdown offers clues to mammalian middle ear evolution. Nature Ecology & Evolution, 1(4), 93. 10.1038/s41559-017-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arratia Fuentes G, Wilson MVH, Cloutier R, & Schultze H-P (Eds.). (2004). Recent advances in the origin an early radiation of vertebrates: honoring Hans-Peter Schultze. München: F. Pfeil. [Google Scholar]
- Askary A, Smeeton J, Paul S, Schindler S, Braasch I, Ellis NA, … Crump JG (2016). Ancient origin of lubricated joints in bony vertebrates. eLife, 5. 10.7554/eLife.16415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askary A, Xu P, Barske L, Bay M, Bump P, Balczerski B, … Crump JG (2017). Genome-wide analysis of facial skeletal regionalization in zebrafish. Development (Cambridge, England), 144(16), 2994–3005. 10.1242/dev.151712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins JB, & Franz-Odendaal TA (2015). The evolutionary and morphological history of the parasphenoid bone in vertebrates. Acta Zoologica, 97(2), 255–263. 10.1111/azo.12131 [DOI] [Google Scholar]
- Barry MA, & Boord RL (1984). The spiracular organ of sharks and skates: anatomical evidence indicating a mechanoreceptive role. Science (New York, N.Y.), 226(4677), 990–992. [DOI] [PubMed] [Google Scholar]
- Barry MA, Hall DH, & Bennett MV (1988). The elasmobranch spiracular organ. I. Morphological studies. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 163(1), 85–92. [DOI] [PubMed] [Google Scholar]
- Barske L, Askary A, Zuniga E, Balczerski B, Bump P, Nichols JT, & Crump JG (2016). Competition between Jagged-Notch and Endothelin1 Signaling Selectively Restricts Cartilage Formation in the Zebrafish Upper Face. PLoS Genetics, 12(4), e1005967. 10.1371/journal.pgen.1005967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barske L, Rataud P, Behizad K, Del Rio L, Cox SG, & Crump JG (2018). Essential Role of Nr2f Nuclear Receptors in Patterning the Vertebrate Upper Jaw. Developmental Cell, 44(3), 337–347.e5. 10.1016/j.devcel.2017.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch P (1994). Development of the cranium of Neoceratodus forsteri, with a discussion of the suspensorium and the opercular apparatus in Dipnoi. Zoomorphology, 114(1), 1–31. 10.1007/BF00574911 [DOI] [Google Scholar]
- Bartsch P, Gemballa S, & Piotrowski T (1997). The Embryonic and Larval Development of Polypterus senegalus Cuvier, 1829: its Staging with Reference to External and Skeletal Features, Behaviour and Locomotory Habits. Acta Zoologica, 78(4), 309–328. [Google Scholar]
- Begemann G, Schilling TF, Rauch GJ, Geisler R, & Ingham PW (2001). The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development (Cambridge, England), 128(16), 3081–3094. [DOI] [PubMed] [Google Scholar]
- Bellwood DR, Goatley CHR, Bellwood O, Delbarre DJ, & Friedman M (2015). The Rise of Jaw Protrusion in Spiny-Rayed Fishes Closes the Gap on Elusive Prey. Current Biology: CB, 25(20), 2696–2700. 10.1016/j.cub.2015.08.058 [DOI] [PubMed] [Google Scholar]
- Beverdam A, Merlo GR, Paleari L, Mantero S, Genova F, Barbieri O, … Levi G (2002). Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis (New York, N.Y.: 2000), 34(4), 221–227. 10.1002/gene.10156 [DOI] [PubMed] [Google Scholar]
- Bhatt S, Diaz R, & Trainor PA (2013). Signals and switches in Mammalian neural crest cell differentiation. Cold Spring Harbor Perspectives in Biology, 5(2). 10.1101/cshperspect.a008326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, & de Crombrugghe B (1999). Sox9 is required for cartilage formation. Nature Genetics, 22(1), 85–89. 10.1038/8792 [DOI] [PubMed] [Google Scholar]
- Boisvert CA (2005). The pelvic fin and girdle of Panderichthys and the origin of tetrapod locomotion. Nature, 438(7071), 1145–1147. 10.1038/nature04119 [DOI] [PubMed] [Google Scholar]
- Brazeau MD (2009). The braincase and jaws of a Devonian “acanthodian” and modern gnathostome origins. Nature, 457(7227), 305–308. 10.1038/nature07436 [DOI] [PubMed] [Google Scholar]
- Brazeau MD, & Ahlberg PE (2006). Tetrapod-like middle ear architecture in a Devonian fish. Nature, 439(7074), 318–321. 10.1038/nature04196 [DOI] [PubMed] [Google Scholar]
- Brazeau MD, & de Winter V (2015). The hyoid arch and braincase anatomy of Acanthodes support chondrichthyan affinity of “acanthodians.” Proceedings. Biological Sciences, 282(1821), 20152210. 10.1098/rspb.2015.2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton RE, Betancur-R R, Li C, Arratia G, & Ortí G (2013). Multi-locus phylogenetic analysis reveals the pattern and tempo of bony fish evolution. PLOS Currents Tree of Life. 10.1371/currents.tol.2ca8041495ffafd0c92756e75247483e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren WW, Bautista GM, Coop SC, Couturier GM, Delgadillo SP, García RM, & González CAA (2016). Developmental cardiorespiratory physiology of the air-breathing tropical gar, Atractosteus tropicus. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 311(4), R689–R701. 10.1152/ajpregu.00022.2016 [DOI] [PubMed] [Google Scholar]
- Burrow CJ, & Rudkin D (2014). Oldest near-complete acanthodian: the first vertebrate from the Silurian Bertie Formation Konservat-Lagerstätte, Ontario. PloS One, 9(8), e104171. 10.1371/journal.pone.0104171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callier V, Clack JA, & Ahlberg PE (2009). Contrasting developmental trajectories in the earliest known tetrapod forelimbs. Science (New York, N.Y.), 324(5925), 364–367. 10.1126/science.1167542 [DOI] [PubMed] [Google Scholar]
- Calloni GW, Le Douarin NM, & Dupin E (2009). High frequency of cephalic neural crest cells shows coexistence of neurogenic, melanogenic, and osteogenic differentiation capacities. Proceedings of the National Academy of Sciences of the United States of America, 106(22), 8947–8952. 10.1073/pnas.0903780106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RK, Johanson Z, & Ritchie A (2009). The phyllolepid placoderm Cowralepis mclachlani: insights into the evolution of feeding mechanisms in jawed vertebrates. Journal of Morphology, 270(7), 775–804. 10.1002/jmor.10719 [DOI] [PubMed] [Google Scholar]
- Carroll AM, & Wainwright PC (2003). Functional morphology of prey capture in the sturgeon, Scaphirhynchus albus. Journal of Morphology, 256(3), 270–284. 10.1002/jmor.10095 [DOI] [PubMed] [Google Scholar]
- Cerny R, Cattell M, Sauka-Spengler T, Bronner-Fraser M, Yu F, & Medeiros DM (2010). Evidence for the prepattern/cooption model of vertebrate jaw evolution. Proceedings of the National Academy of Sciences of the United States of America, 107(40), 17262–17267. 10.1073/pnas.1009304107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack JA (1989). Discovery of the earliest-known tetrapod stapes. Nature, 342(6248), 425–427. 10.1038/342425a0 [DOI] [PubMed] [Google Scholar]
- Clack JA (2002). Gaining Ground: The Origin and Evolution of Tetrapods. Indiana University Press. [Google Scholar]
- Clack JA, Ahlberg PE, Finney SM, Dominguez Alonso P, Robinson J, & Ketcham RA (2003). A uniquely specialized ear in a very early tetrapod. Nature, 425(6953), 65–69. 10.1038/nature01904 [DOI] [PubMed] [Google Scholar]
- Clement AM, & Long JA (2010). Air-breathing adaptation in a marine Devonian lungfish. Biology Letters, 6(4), 509–512. 10.1098/rsbl.2009.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates MI, Finarelli JA, Sansom IJ, Andreev PS, Criswell KE, Tietjen K, … La Riviere PJ (2018). An early chondrichthyan and the evolutionary assembly of a shark body plan. Proceedings. Biological Sciences, 285(1870). 10.1098/rspb.2017.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn MJ (2002). Evolutionary biology: lamprey Hox genes and the origin of jaws. Nature, 416(6879), 386–387. 10.1038/416386a [DOI] [PubMed] [Google Scholar]
- Corbo JC, Erives A, Di Gregorio A, Chang A, & Levine M (1997). Dorsoventral patterning of the vertebrate neural tube is conserved in a protochordate. Development (Cambridge, England), 124(12), 2335–2344. [DOI] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, & Le Douarin NM (2002). Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development (Cambridge, England), 129(4), 1061–1073. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, & Le Douarin NM (1992). The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development (Cambridge, England), 114(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Criswell KE (2015). The comparative osteology and phylogenetic relationships of African and South American lungfishes (Sarcopterygii: Dipnoi). Zoological Journal of the Linnean Society, 174(4), 801–858. 10.1111/zoj.12255 [DOI] [Google Scholar]
- Cubbage CC, & Mabee PM (1996). Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, Cyprinidae). Journal of Morphology, 229(2), 121–160. [DOI] [PubMed] [Google Scholar]
- Davis SP, Finarelli JA, & Coates MI (2012). Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes. Nature, 486(7402), 247–250. 10.1038/nature11080 [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, & Soriano P (2004). Ephrin-B1 forward and reverse signaling are required during mouse development. Genes & Development, 18(5), 572–583. 10.1101/gad.1171704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean MN, Mull CG, Gorb SN, & Summers AP (2009). Ontogeny of the tessellated skeleton: insight from the skeletal growth of the round stingray Urobatis halleri. Journal of Anatomy, 215(3), 227–239. 10.1111/j.1469-7580.2009.01116.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLaurier A, Huycke TR, Nichols JT, Swartz ME, Larsen A, Walker C, … Kimmel CB (2014). Role of mef2ca in developmental buffering of the zebrafish larval hyoid dermal skeleton. Developmental Biology, 385(2), 189–199. 10.1016/j.ydbio.2013.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, & Philippe H (2006). Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature, 439(7079), 965. 10.1038/nature04336 [DOI] [PubMed] [Google Scholar]
- Depew MJ, Lufkin T, & Rubenstein JLR (2002). Specification of jaw subdivisions by Dlx genes. Science (New York, N.Y.), 298(5592), 381–385. 10.1126/science.1075703 [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Sansom IJ, & Downs JP (2006). Early evolution of vertebrate skeletal tissues and cellular interactions, and the canalization of skeletal development. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 306(3), 278–294. 10.1002/jez.b.21090 [DOI] [PubMed] [Google Scholar]
- Downs JP, Daeschler EB, Jenkins FA, & Shubin NH (2008). The cranial endoskeleton of Tiktaalik roseae. Nature, 455(7215), 925–929. 10.1038/nature07189 [DOI] [PubMed] [Google Scholar]
- Dupret V, Sanchez S, Goujet D, Tafforeau P, & Ahlberg PE (2014). A primitive placoderm sheds light on the origin of the jawed vertebrate face. Nature, 507(7493), 500–503. 10.1038/nature12980 [DOI] [PubMed] [Google Scholar]
- Dutel H, Herbin M, Clément G, & Herrel A (2015). Bite force in the extant coelacanth Latimeria: the role of the intracranial joint and the basicranial muscle. Current Biology: CB, 25(9), 1228–1233. 10.1016/j.cub.2015.02.076 [DOI] [PubMed] [Google Scholar]
- Dutel H, Herrel A, Clément G, & Herbin M (2013). A reevaluation of the anatomy of the jaw-closing system in the extant coelacanth Latimeria chalumnae. Die Naturwissenschaften, 100(11), 1007–1022. 10.1007/s00114-013-1104-8 [DOI] [PubMed] [Google Scholar]
- Dutel H, Herrel A, Clément G, & Herbin M (2015). Redescription of the hyoid apparatus and associated musculature in the extant coelacanth Latimeria chalumnae: functional implications for feeding. Anatomical Record (Hoboken, N.J.: 2007), 298(3), 579–601. 10.1002/ar.23103 [DOI] [PubMed] [Google Scholar]
- Eames BF, DeLaurier A, Ullmann B, Huycke TR, Nichols JT, Dowd J, … Kimmel CB (2013). FishFace: interactive atlas of zebrafish craniofacial development at cellular resolution. BMC Developmental Biology, 13, 23. 10.1186/1471-213X-13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriva H, Holland ND, Gronemeyer H, Laudet V, & Holland LZ (2002). The retinoic acid signaling pathway regulates anterior/posterior patterning in the nerve cord and pharynx of amphioxus, a chordate lacking neural crest. Development (Cambridge, England), 129(12), 2905–2916. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Parish J, Lee K, & Archer CW (1999). BMP/GDF-signalling interactions during synovial joint development. Cell and Tissue Research, 296(1), 111–119. [DOI] [PubMed] [Google Scholar]
- Friedman M, & Brazeau MD (2010). A Reappraisal of the Origin and Basal Radiation of the Osteichthyes. Journal of Vertebrate Paleontology, 30(1), 36–56. 10.1080/02724630903409071 [DOI] [Google Scholar]
- Gai Z, Donoghue PCJ, Zhu M, Janvier P, & Stampanoni M (2011). Fossil jawless fish from China foreshadows early jawed vertebrate anatomy. Nature, 476(7360), 324–327. 10.1038/nature10276 [DOI] [PubMed] [Google Scholar]
- Gardiner BG (1984). Devonian Palaeoniscid Fishes: New Specimens of Mimia and Moythomasia from the Upper Devonian of Western Australia. University of California Press. [Google Scholar]
- Gendron-Maguire M, Mallo M, Zhang M, & Gridley T (1993). Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell, 75(7), 1317–1331. [DOI] [PubMed] [Google Scholar]
- Gilbert SF, & Barresi MJF (2016). Developmental Biology. Sinauer. [Google Scholar]
- Gilbert SG (1986). Pictoral anatomy of the necturus. Seattle: University of Washington Press. [Google Scholar]
- Giles S, Coates MI, Garwood RJ, Brazeau MD, Atwood R, Johanson Z, & Friedman M (2015). Endoskeletal structure in Cheirolepis (Osteichthyes, Actinopterygii), An early ray-finned fish. Palaeontology, 58(5), 849–870. 10.1111/pala.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JA, Fritzenwanker JH, & Lowe CJ (2012). A stem-deuterostome origin of the vertebrate pharyngeal transcriptional network. Proc. R. Soc. B, 279(1727), 237–246. 10.1098/rspb.2011.0599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JA, Modrell MS, & Baker CVH (2012). A timeline of pharyngeal endoskeletal condensation and differentiation in the shark,Scyliorhinus canicula, and the paddlefish,Polyodon spathula. Zeitschrift Fur Angewandte Ichthyologie = Journal of Applied Ichthyology, 28(3), 341–345. 10.1111/j.1439-0426.2012.01976.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gintof C, Konow N, Ross CF, & Sanford CPJ (2010). Rhythmic chewing with oral jaws in teleost fishes: a comparison with amniotes. The Journal of Experimental Biology, 213(11), 1868–1875. 10.1242/jeb.041012 [DOI] [PubMed] [Google Scholar]
- Graham A, & Richardson J (2012). Developmental and evolutionary origins of the pharyngeal apparatus. EvoDevo, 3, 24. 10.1186/2041-9139-3-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JB, Wegner NC, Miller LA, Jew CJ, Lai NC, Berquist RM, … Long JA (2014). Spiracular air breathing in polypterid fishes and its implications for aerial respiration in stem tetrapods. Nature Communications, 5, 3022. 10.1038/ncomms4022 [DOI] [PubMed] [Google Scholar]
- Grandel H, Lun K, Rauch G-J, Rhinn M, Piotrowski T, Houart C, … Brand M (2002). Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development (Cambridge, England), 129(12), 2851–2865. [DOI] [PubMed] [Google Scholar]
- Grenier J, Teillet M-A, Grifone R, Kelly RG, & Duprez D (2009). Relationship between neural crest cells and cranial mesoderm during head muscle development. PloS One, 4(2), e4381. 10.1371/journal.pone.0004381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK (2000). The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evolution & Development, 2(1), 3–5. [DOI] [PubMed] [Google Scholar]
- Hedrick MS, & Jones DR (1999). Control of gill ventilation and air-breathing in the bowfin amia calva. Journal of Experimental Biology, 202(1), 87–94. [DOI] [PubMed] [Google Scholar]
- Hinchliff CE, Smith SA, Allman JF, Burleigh JG, Chaudhary R, Coghill LM, … Cranston KA (2015). Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Proceedings of the National Academy of Sciences, 112(41), 12764–12769. 10.1073/pnas.1423041112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BM, Hunter MP, Oates AC, Crowhurst MO, Hall NE, Heath JK, … Lieschke GJ (2004). Zebrafish gcm2 is required for gill filament budding from pharyngeal ectoderm. Developmental Biology, 276(2), 508–522. 10.1016/j.ydbio.2004.09.018 [DOI] [PubMed] [Google Scholar]
- Holland LZ, & Holland ND (1996). Expression of AmphiHox-1 and AmphiPax-1 in amphioxus embryos treated with retinoic acid: insights into evolution and patterning of the chordate nerve cord and pharynx. Development (Cambridge, England), 122(6), 1829–1838. [DOI] [PubMed] [Google Scholar]
- Holland ND, & Chen J (2001). Origin and early evolution of the vertebrates: New insights from advances in molecular biology, anatomy, and palaeontology. BioEssays, 23(2), 142–151. [DOI] [PubMed] [Google Scholar]
- Horigome N, Myojin M, Ueki T, Hirano S, Aizawa S, & Kuratani S (1999). Development of Cephalic Neural Crest Cells in Embryos of Lampetra japonica, with Special Reference to the Evolution of the Jaw. Developmental Biology, 207(2), 287–308. 10.1006/dbio.1998.9175 [DOI] [PubMed] [Google Scholar]
- Hu Y, Lu J, & Young GC (2017). New findings in a 400 million-year-old Devonian placoderm shed light on jaw structure and function in basal gnathostomes. Scientific Reports, 7(1), 7813. 10.1038/s41598-017-07674-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P, Gulisano M, Cook M, Sham MH, Faiella A, Wilkinson D, … Krumlauf R (1991). A distinct Hox code for the branchial region of the vertebrate head. Nature, 353(6347), 861–864. 10.1038/353861a0 [DOI] [PubMed] [Google Scholar]
- Hunter MP, & Prince VE (2002). Zebrafish hox paralogue group 2 genes function redundantly as selector genes to pattern the second pharyngeal arch. Developmental Biology, 247(2), 367–389. [DOI] [PubMed] [Google Scholar]
- Huycke TR, Eames BF, & Kimmel CB (2012). Hedgehog-dependent proliferation drives modular growth during morphogenesis of a dermal bone. Development (Cambridge, England), 139(13), 2371–2380. 10.1242/dev.079806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandzik D, Garnett AT, Square TA, Cattell MV, Yu J-K, & Medeiros DM (2014). Evolution of the new vertebrate head by co-option of an ancient chordate skeletal tissue. Nature, 518(7540), 534. 10.1038/nature14000 [DOI] [PubMed] [Google Scholar]
- Janvier P (1996). Early Vertebrates. New York: Oxford University Press. [Google Scholar]
- Jarvik E (1980). Basic Structure and Evolution of Vertebrates (Vol. 1). Academic Press. [Google Scholar]
- Jeffery WR (2006). Ascidian neural crest-like cells: phylogenetic distribution, relationship to larval complexity, and pigment cell fate. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 306(5), 470–480. 10.1002/jez.b.21109 [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Chiba T, Krajka FR, Deyts C, Satoh N, & Joly J-S (2008a). Trunk lateral cells are neural crest-like cells in the ascidian Ciona intestinalis: insights into the ancestry and evolution of the neural crest. Developmental Biology, 324(1), 152–160. 10.1016/j.ydbio.2008.08.022 [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Chiba T, Krajka FR, Deyts C, Satoh N, & Joly J-S (2008b). Trunk lateral cells are neural crest-like cells in the ascidian Ciona intestinalis: insights into the ancestry and evolution of the neural crest. Developmental Biology, 324(1), 152–160. 10.1016/j.ydbio.2008.08.022 [DOI] [PubMed] [Google Scholar]
- Jollie M (1984a). Development of the head and pectoral skeleton of Amia with a note on the scales. Gegenbaurs Morphologisches Jahrbuch, 130(2), 315–351. [PubMed] [Google Scholar]
- Jollie M (1984b). Development of the head and pectoral skeleton of Polypterus with a note on scales (Pisces: Actinopterygii). Journal of Zoology, 204(4), 469–507. 10.1111/j.1469-7998.1984.tb02382.x [DOI] [Google Scholar]
- Kammerer CF, Grande L, & Westneat MW (2006). Comparative and developmental functional morphology of the jaws of living and fossil gars (Actinopterygii: Lepisosteidae). Journal of Morphology, 267(9), 1017–1031. 10.1002/jmor.10293 [DOI] [PubMed] [Google Scholar]
- Kardong KV (2012). Vertebrates: Comparative anatomy, function, evolution (6th edition). New York: McGraw-Hill. [Google Scholar]
- Kawasaki K, Buchanan AV, & Weiss KM (2007). Gene duplication and the evolution of vertebrate skeletal mineralization. Cells, Tissues, Organs, 186(1), 7–24. 10.1159/000102678 [DOI] [PubMed] [Google Scholar]
- Kawasaki K, & Weiss KM (2003). Mineralized tissue and vertebrate evolution: the secretory calcium-binding phosphoprotein gene cluster. Proceedings of the National Academy of Sciences of the United States of America, 100(7), 4060–4065. 10.1073/pnas.0638023100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A (1999). Ontogeny of the skull of the Australian lungfish Neoceratodus forsteri (Osteichthyes: Dipnoi). Journal of Zoology, 248(1), 97–137. 10.1111/j.1469-7998.1999.tb01027.x [DOI] [Google Scholar]
- Kemp A (2013). Cartilage, bone, and intermandibular connective tissue in the Australian lungfish, Neoceratodus forsteri (Osteichthyes: Dipnoi). Journal of Morphology, 274(10), 1085–1089. 10.1002/jmor.20164 [DOI] [PubMed] [Google Scholar]
- Kemp A, Cavin L, & Guinot G (2017). Evolutionary history of lungfishes with a new phylogeny of post-Devonian genera. Palaeogeography, Palaeoclimatology, Palaeoecology, 471, 209–219. 10.1016/j.palaeo.2016.12.051 [DOI] [Google Scholar]
- Kent GC, & Carr RK (2001). Comparative anatomy of the vertebrates (9th ed.). New York: McGraw-Hill. [Google Scholar]
- Kimmel CB, Miller CT, Kruze G, Ullmann B, BreMiller RA, Larison KD, & Snyder HC (1998). The shaping of pharyngeal cartilages during early development of the zebrafish. Developmental Biology, 203(2), 245–263. 10.1006/dbio.1998.9016 [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Small CM, & Knope ML (2017). A rich diversity of opercle bone shape among teleost fishes. PloS One, 12(12), e0188888. 10.1371/journal.pone.0188888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ullmann B, Walker M, Miller CT, & Crump JG (2003). Endothelin 1-mediated regulation of pharyngeal bone development in zebrafish. Development (Cambridge, England), 130(7), 1339–1351. [DOI] [PubMed] [Google Scholar]
- Kind PK, Grigg GC, & Booth DT (2002). Physiological responses to prolonged aquatic hypoxia in the Queensland lungfish Neoceratodus forsteri. Respiratory Physiology & Neurobiology, 132(2), 179–190. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Fujisawa K, Narboux-Nême N, Arima Y, Kawamura Y, Inoue T, … Kurihara H (2015). Distinct effects of Hoxa2 overexpression in cranial neural crest populations reveal that the mammalian hyomandibular-ceratohyal boundary maps within the styloid process. Developmental Biology, 402(2), 162–174. 10.1016/j.ydbio.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Koentges G, & Matsuoka T (2002). Evolution. Jaws of the fates. Science (New York, N.Y.), 298(5592), 371–373. 10.1126/science.1077706 [DOI] [PubMed] [Google Scholar]
- Koop D, Chen J, Theodosiou M, Carvalho JE, Alvarez S, de Lera AR, … Schubert M (2014a). Roles of retinoic acid and Tbx1/10 in pharyngeal segmentation: amphioxus and the ancestral chordate condition. EvoDevo, 5(1), 36. 10.1186/2041-9139-5-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop D, Chen J, Theodosiou M, Carvalho JE, Alvarez S, de Lera AR, … Schubert M (2014b). Roles of retinoic acid and Tbx1/10 in pharyngeal segmentation: amphioxus and the ancestral chordate condition. EvoDevo, 5(1), 36. 10.1186/2041-9139-5-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralovic M, Horáček I, & Cerny R (2010). Mouth development in the Senegal bichir Polypterus senegalus does not involve the oropharyngeal membrane: possible implications for the ecto-endoderm boundary and tooth initiation. Journal of Applied Ichthyology, 26(2), 179–182. 10.1111/j.1439-0426.2010.01400.x [DOI] [Google Scholar]
- Kumar M, Ray P, & Chapman SC (2012). Fibroblast growth factor and bone morphogenetic protein signaling are required for specifying prechondrogenic identity in neural crest-derived mesenchyme and initiating the chondrogenic program. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 241(6), 1091–1103. 10.1002/dvdy.23768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraku S, Takio Y, Sugahara F, Takechi M, & Kuratani S (2010). Evolution of oropharyngeal patterning mechanisms involving Dlx and endothelins in vertebrates. Developmental Biology, 341(1), 315–323. 10.1016/j.ydbio.2010.02.013 [DOI] [PubMed] [Google Scholar]
- Kuratani S, Adachi N, Wada N, Oisi Y, & Sugahara F (2013). Developmental and evolutionary significance of the mandibular arch and prechordal/premandibular cranium in vertebrates: revising the heterotopy scenario of gnathostome jaw evolution. Journal of Anatomy, 222(1), 41–55. 10.1111/j.1469-7580.2012.01505.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani SC, & Wall NA (1992). Expression of Hox 2.1 protein in restricted populations of neural crest cells and pharyngeal ectoderm. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 195(1), 15–28. 10.1002/aja.1001950103 [DOI] [PubMed] [Google Scholar]
- Kuratani S, Nobusada Y, Horigome N, & Shigetani Y (2001). Embryology of the lamprey and evolution of the vertebrate jaw: insights from molecular and developmental perspectives. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 356(1414), 1615–1632. 10.1098/rstb.2001.0976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeland JA, Tomsa JM, Jackman WR, & Kimmel CB (1998). An amphioxus snail gene: expression in paraxial mesoderm and neural plate suggests a conserved role in patterning the chordate embryo. Development Genes and Evolution, 208(10), 569–577. [DOI] [PubMed] [Google Scholar]
- Laue K, Daujat S, Crump JG, Plaster N, Roehl HH, Kimmel CB, … Hammerschmidt M (2008). The multidomain protein Brpf1 binds histones and is required for Hox gene expression and segmental identity. Development, 135(11), 1935–1946. 10.1242/dev.017160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Brito JM, & Creuzet S (2007). Role of the neural crest in face and brain development. Brain Research Reviews, 55(2), 237–247. 10.1016/j.brainresrev.2007.06.023 [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Ziller C, & Couly GF (1993). Patterning of neural crest derivatives in the avian embryo: in vivo and in vitro studies. Developmental Biology, 159(1), 24–49. 10.1006/dbio.1993.1219 [DOI] [PubMed] [Google Scholar]
- Le Pabic P, Scemama J-L, & Stellwag EJ (2010). Role of Hox PG2 genes in Nile tilapia pharyngeal arch specification: implications for gnathostome pharyngeal arch evolution. Evolution & Development, 12(1), 45–60. 10.1111/j.1525-142X.2009.00390.x [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Li P, & de Crombrugghe B (1998). A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. The EMBO Journal, 17(19), 5718–5733. 10.1093/emboj/17.19.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Lan Y, & Jiang R (2017). Molecular and Cellular Mechanisms of Palate Development. Journal of Dental Research, 96(11), 1184–1191. 10.1177/0022034517703580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang H, Li G, Huang H-Z, & Wang Y-Q (2013). The function of DrPax1b gene in the embryonic development of zebrafish. Genes & Genetic Systems, 88(4), 261–269. [DOI] [PubMed] [Google Scholar]
- Long JA, Young GC, Holland T, Senden TJ, & Fitzgerald EMG (2006). An exceptional Devonian fish from Australia sheds light on tetrapod origins. Nature, 444(7116), 199–202. 10.1038/nature05243 [DOI] [PubMed] [Google Scholar]
- Lu J, Giles S, Friedman M, den Blaauwen JL, & Zhu M (2016). The Oldest Actinopterygian Highlights the Cryptic Early History of the Hyperdiverse Ray-Finned Fishes. Current Biology: CB, 26(12), 1602–1608. 10.1016/j.cub.2016.04.045 [DOI] [PubMed] [Google Scholar]
- Machado LP, Wellendorf H, & Brito PM (2010). On the type material of Lepidosiren paradoxa Fitzinger, 1837 (Sarcopterygii, Dipnoi). Comptes Rendus Biologies, 333(1), 56–60. 10.1016/j.crvi.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Mallatt J (1996). Ventilation and the origin of jawed vertebrates: a new mouth. Zoological Journal of the Linnean Society, 117(4), 329–404. 10.1111/j.1096-3642.1996.tb01658.x [DOI] [Google Scholar]
- Mallatt J, & Chen J (2003). Fossil sister group of craniates: predicted and found. Journal of Morphology, 258(1), 1–31. 10.1002/jmor.10081 [DOI] [PubMed] [Google Scholar]
- Mallatt J, & Sullivan J (1998). 28S and 18S rDNA sequences support the monophyly of lampreys and hagfishes. Molecular Biology and Evolution, 15(12), 1706–1718. 10.1093/oxfordjournals.molbev.a025897 [DOI] [PubMed] [Google Scholar]
- Martinez-Morales J-R, Henrich T, Ramialison M, Wittbrodt J, & Martinez-Morales J-R (2007). New genes in the evolution of the neural crest differentiation program. Genome Biology, 8(3), R36. 10.1186/gb-2007-8-3-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley DW, & Bronner-Fraser M (2003). Neural crest contributions to the lamprey head. Development (Cambridge, England), 130(11), 2317–2327. [DOI] [PubMed] [Google Scholar]
- McGhee GR (2012). Extinction: Late Devonian Mass Extinction. eLS. 10.1002/9780470015902.a0001653.pub3 [DOI] [Google Scholar]
- McNeil B, Lowry D, Larson S, & Griffing D (2016). Feeding Behavior of Subadult Sixgill Sharks (Hexanchus griseus) at a Bait Station. PLoS ONE, 11(5). 10.1371/journal.pone.0156730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans D, & Bronner-Fraser M (2004). Gene-regulatory interactions in neural crest evolution and development. Developmental Cell, 7(3), 291–299. 10.1016/j.devcel.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Miller CT, Schilling TF, Lee K, Parker J, & Kimmel CB (2000). sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development. Development (Cambridge, England), 127(17), 3815–3828. [DOI] [PubMed] [Google Scholar]
- Miller CT, Yelon D, Stainier DYR, & Kimmel CB (2003). Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development (Cambridge, England), 130(7), 1353–1365. [DOI] [PubMed] [Google Scholar]
- Miller RF, Cloutier R, & Turner S (2003). The oldest articulated chondrichthyan from the Early Devonian period. Nature, 425(6957), 501–504. 10.1038/nature02001 [DOI] [PubMed] [Google Scholar]
- Mims SD, & Shelton WL (2015). Paddlefish Aquaculture. John Wiley & Sons. [Google Scholar]
- Minoux M, & Rijli FM (2010). Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development (Cambridge, England), 137(16), 2605–2621. 10.1242/dev.040048 [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq AH (2015). PAX transcription factors in neural crest development. Seminars in Cell & Developmental Biology, 44, 87–96. 10.1016/j.semcdb.2015.09.015 [DOI] [PubMed] [Google Scholar]
- Motta PJ (1984). Mechanics and Functions of Jaw Protrusion in Teleost Fishes: A Review. Copeia, 1984(1), 1–18. 10.2307/1445030 [DOI] [Google Scholar]
- Müller TS, Ebensperger C, Neubüser A, Koseki H, Balling R, Christ B, & Wilting J (1996). Expression of avian Pax1 and Pax9 is intrinsically regulated in the pharyngeal endoderm, but depends on environmental influences in the paraxial mesoderm. Developmental Biology, 178(2), 403–417. 10.1006/dbio.1996.0227 [DOI] [PubMed] [Google Scholar]
- Neenan JM, Ruta M, Clack JA, & Rayfield EJ (2014). Feeding biomechanics in Acanthostega and across the fish-tetrapod transition. Proceedings. Biological Sciences, 281(1781), 20132689. 10.1098/rspb.2013.2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidert AH, Virupannavar V, Hooker GW, & Langeland JA (2001). Lamprey Dlx genes and early vertebrate evolution. Proceedings of the National Academy of Sciences of the United States of America, 98(4), 1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nian-Zhong W, Donoghue PCJ, Smith MM, & Sansom IJ (2005). Histology of the galeaspid dermoskeleton and endoskeleton, and the origin and early evolution of the vertebrate cranial endoskeleton. JOURNAL OF VERTEBRATE PALEONTOLOGY, 25(4), 745–756. [Google Scholar]
- Nichols JT, Blanco-Sánchez B, Brooks EP, Parthasarathy R, Dowd J, Subramanian A, … Kimmel CB (2016). Ligament versus bone cell identity in the zebrafish hyoid skeleton is regulated by mef2ca. Development (Cambridge, England), 143(23), 4430–4440. 10.1242/dev.141036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JT, Pan L, Moens CB, & Kimmel CB (2013). barx1 represses joints and promotes cartilage in the craniofacial skeleton. Development (Cambridge, England), 140(13), 2765–2775. 10.1242/dev.090639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt R (2005). The new head hypothesis revisited. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 304(4), 274–297. 10.1002/jez.b.21063 [DOI] [PubMed] [Google Scholar]
- Ogasawara M, Shigetani Y, Hirano S, Satoh N, & Kuratani S (2000). Pax1/Pax9-Related genes in an agnathan vertebrate, Lampetra japonica: expression pattern of LjPax9 implies sequential evolutionary events toward the gnathostome body plan. Developmental Biology, 223(2), 399–410. 10.1006/dbio.2000.9756 [DOI] [PubMed] [Google Scholar]
- Ogasawara M, Wada H, Peters H, & Satoh N (1999). Developmental expression of Pax1/9 genes in urochordate and hemichordate gills: insight into function and evolution of the pharyngeal epithelium. Development (Cambridge, England), 126(11), 2539–2550. [DOI] [PubMed] [Google Scholar]
- Oisi Y, Fujimoto S, Ota KG, & Kuratani S (2015). On the peculiar morphology and development of the hypoglossal, glossopharyngeal and vagus nerves and hypobranchial muscles in the hagfish. Zoological Letters, 1, 6. 10.1186/s40851-014-0005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oisi Y, Ota KG, Kuraku S, Fujimoto S, & Kuratani S (2013). Craniofacial development of hagfishes and the evolution of vertebrates. Nature, 493(7431), 175–180. 10.1038/nature11794 [DOI] [PubMed] [Google Scholar]
- Osborne NJ, Begbie J, Chilton JK, Schmidt H, & Eickholt BJ (2005). Semaphorin/neuropilin signaling influences the positioning of migratory neural crest cells within the hindbrain region of the chick. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 232(4), 939–949. 10.1002/dvdy.20258 [DOI] [PubMed] [Google Scholar]
- Ota KG, Kuraku S, & Kuratani S (2007). Hagfish embryology with reference to the evolution of the neural crest. Nature, 446(7136), 672. 10.1038/nature05633 [DOI] [PubMed] [Google Scholar]
- Oulion S, Borday-Birraux V, Debiais-Thibaud M, Mazan S, Laurenti P, & Casane D (2011). Evolution of repeated structures along the body axis of jawed vertebrates, insights from the Scyliorhinus canicula Hox code. Evolution & Development, 13(3), 247–259. 10.1111/j.1525-142X.2011.00477.x [DOI] [PubMed] [Google Scholar]
- Ozeki H, Kurihara Y, Tonami K, Watatani S, & Kurihara H (2004). Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mechanisms of Development, 121(4), 387–395. 10.1016/j.mod.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Nardi I, & Rijli FM (2000). Ectopic Hoxa2 induction after neural crest migration results in homeosis of jaw elements in Xenopus. Development (Cambridge, England), 127(24), 5367–5378. [DOI] [PubMed] [Google Scholar]
- Patterson C (1982). Morphology and Interrelationships of Primitive Actinopterygian Fishes. American Zoologist, 22(2), 241–259. 10.1093/icb/22.2.241 [DOI] [Google Scholar]
- Pehrson T (1947). Some New Interpretations of the Skull in Polypterus. Acta Zoologica, 28, 399–455. [Google Scholar]
- Piotrowski T, & Nüsslein-Volhard C (2000). The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Developmental Biology, 225(2), 339–356. 10.1006/dbio.2000.9842 [DOI] [PubMed] [Google Scholar]
- Porro LB, Rayfield EJ, & Clack JA (2015). Descriptive anatomy and three-dimensional reconstruction of the skull of the early tetrapod Acanthostega gunnari Jarvik, 1952. PloS One, 10(3), e0118882. 10.1371/journal.pone.0118882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley R, & Steel FL (1978). The pterygoid and ectopterygoid in mammals. Anatomy and Embryology, 154(1), 95–110. [DOI] [PubMed] [Google Scholar]
- Punnamoottil B, Kikuta H, Pezeron G, Erceg J, Becker TS, & Rinkwitz S (2008). Enhancer detection in zebrafish permits the identification of neuronal subtypes that express Hox4 paralogs. Developmental Dynamics, 237(8), 2195–2208. 10.1002/dvdy.21618 [DOI] [PubMed] [Google Scholar]
- Ramsay JB, Wilga CD, Tapanila L, Pruitt J, Pradel A, Schlader R, & Didier DA (2015). Eating with a saw for a jaw: functional morphology of the jaws and tooth-whorl in Helicoprion davisii. Journal of Morphology, 276(1), 47–64. 10.1002/jmor.20319 [DOI] [PubMed] [Google Scholar]
- Richardson MK, Admiraal J, & Wright GM (2010). Developmental anatomy of lampreys. Biological Reviews, 85(1), 1–33. 10.1111/j.1469-185X.2009.00092.x [DOI] [PubMed] [Google Scholar]
- Rijli FM, Mark M, Lakkaraju S, Dierich A, Dollé P, & Chambon P (1993). A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell, 75(7), 1333–1349. [DOI] [PubMed] [Google Scholar]
- Rücklin M, Donoghue PCJ, Johanson Z, Trinajstic K, Marone F, & Stampanoni M (2012). Development of teeth and jaws in the earliest jawed vertebrates. Nature, 491(7426), 748–751. 10.1038/nature11555 [DOI] [PubMed] [Google Scholar]
- Sallan LC, & Coates MI (2010). End-Devonian extinction and a bottleneck in the early evolution of modern jawed vertebrates. Proceedings of the National Academy of Sciences of the United States of America, 107(22), 10131–10135. 10.1073/pnas.0914000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Soncini R, Wang T, Koldkjaer P, Taylor EW, & Glass ML (2001). The differential cardio-respiratory responses to ambient hypoxia and systemic hypoxaemia in the South American lungfish, Lepidosiren paradoxa. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 130(4), 677–687. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, & Bronner-Fraser M (2008). A gene regulatory network orchestrates neural crest formation. Nature Reviews. Molecular Cell Biology, 9(7), 557–568. 10.1038/nrm2428 [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Meulemans D, Jones M, & Bronner-Fraser M (2007). Ancient Evolutionary Origin of the Neural Crest Gene Regulatory Network. Developmental Cell, 13(3), 405–420. 10.1016/j.devcel.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Schoch RR (2006). Skull ontogeny: developmental patterns of fishes conserved across major tetrapod clades. Evolution & Development, 8(6), 524–536. 10.1111/j.1525-142X.2006.00125.x [DOI] [PubMed] [Google Scholar]
- Schubert M (2004). Retinoic acid signaling acts via Hox1 to establish the posterior limit of the pharynx in the chordate amphioxus. Development, 132(1), 61–73. 10.1242/dev.01554 [DOI] [PubMed] [Google Scholar]
- Shigetani Y, Sugahara F, Kawakami Y, Murakami Y, Hirano S, & Kuratani S (2002). Heterotopic Shift of Epithelial-Mesenchymal Interactions in Vertebrate Jaw Evolution. Science, 296(5571), 1316–1319. 10.1126/science.1068310 [DOI] [PubMed] [Google Scholar]
- Shone V, & Graham A (2014). Endodermal/ectodermal interfaces during pharyngeal segmentation in vertebrates. Journal of Anatomy, 225(5), 479–491. 10.1111/joa.12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu D-G, Luo H-L, Morris SC, Zhang X-L, Hu S-X, Chen L, … Chen L-Z (1999). Lower Cambrian vertebrates from south China. Nature, 402(6757), 42. 10.1038/46965 [DOI] [Google Scholar]
- Shu D-G, Morris SC, Han J, Zhang Z-F, & Liu J-N (2004). Ancestral echinoderms from the Chengjiang deposits of China. Nature, 430(6998), 422. 10.1038/nature02648 [DOI] [PubMed] [Google Scholar]
- Shu D-G, Morris SC, Han J, Zhang Z-F, Yasui K, Janvier P, … Liu H-Q (2003). Head and backbone of the Early Cambrian vertebrate Haikouichthys. Nature, 421(6922), 526–529. 10.1038/nature01264 [DOI] [PubMed] [Google Scholar]
- Shubin N (2009). Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body. Vintage Books. [Google Scholar]
- Simakov O, Kawashima T, Marlétaz F, Jenkins J, Koyanagi R, Mitros T, … Gerhart J (2015). Hemichordate genomes and deuterostome origins. Nature, 527(7579), 459–465. 10.1038/nature16150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleman C, & Holtzman NG (2014). Growth and Maturation in the Zebrafish, Danio Rerio: A Staging Tool for Teaching and Research. Zebrafish, 11(4), 396–406. 10.1089/zeb.2014.0976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson BE, Dale RM, Mui SR, Topczewska JM, & Topczewski J (2015). A role of glypican4 and wnt5b in chondrocyte stacking underlying craniofacial cartilage morphogenesis. Mechanisms of Development, 138 Pt 3, 279–290. 10.1016/j.mod.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Robinson V, Patel K, & Wilkinson DG (1997). The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Current Biology: CB, 7(8), 561–570. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Kuraku S, Holt C, Sauka-Spengler T, Jiang N, Campbell MS, … Li W (2013). Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nature Genetics, 45(4), 415. 10.1038/ng.2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo K, O’Rourke MP, Khoo P-L, Steiner KA, Wong N, Behringer RR, & Tam PPL (2002). Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Developmental Biology, 247(2), 251–270. [DOI] [PubMed] [Google Scholar]
- Sperber SM, & Dawid IB (2008). barx1 is necessary for ectomesenchyme proliferation and osteochondroprogenitor condensation in the zebrafish pharyngeal arches. Developmental Biology, 321(1), 101–110. 10.1016/j.ydbio.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, & Kingsley DM (1999). GDF5 coordinates bone and joint formation during digit development. Developmental Biology, 209(1), 11–27. 10.1006/dbio.1999.9241 [DOI] [PubMed] [Google Scholar]
- Su D, Ellis S, Napier A, Lee K, & Manley NR (2001). Hoxa3 and pax1 regulate epithelial cell death and proliferation during thymus and parathyroid organogenesis. Developmental Biology, 236(2), 316–329. 10.1006/dbio.2001.0342 [DOI] [PubMed] [Google Scholar]
- Tai A, Cheung M, Huang Y-H, Jauch R, Bronner ME, & Cheah KSE (2016). SOXE neofunctionalization and elaboration of the neural crest during chordate evolution. Scientific Reports, 6, 34964. 10.1038/srep34964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezaki N, Figueroa F, Zaleska-Rutczynska Z, & Klein J (2003). Molecular phylogeny of early vertebrates: monophyly of the agnathans as revealed by sequences of 35 genes. Molecular Biology and Evolution, 20(2), 287–292. 10.1093/molbev/msg040 [DOI] [PubMed] [Google Scholar]
- Takio Y, Pasqualetti M, Kuraku S, Hirano S, Rijli FM, & Kuratani S (2004). Evolutionary biology: lamprey Hox genes and the evolution of jaws. Nature, 429(6989), 1 p following 262. [DOI] [PubMed] [Google Scholar]
- Talbot JC, Johnson SL, & Kimmel CB (2010). hand2 and Dlx genes specify dorsal, intermediate and ventral domains within zebrafish pharyngeal arches. Development (Cambridge, England), 137(15), 2507–2517. 10.1242/dev.049700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JC, Nichols JT, Yan Y-L, Leonard IF, BreMiller RA, Amacher SL, … Kimmel CB (2016). Pharyngeal morphogenesis requires fras1-itga8-dependent epithelial-mesenchymal interaction. Developmental Biology, 416(1), 136–148. 10.1016/j.ydbio.2016.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JC, Walker MB, Carney TJ, Huycke TR, Yan Y-L, BreMiller RA, … Kimmel CB (2012). fras1 shapes endodermal pouch 1 and stabilizes zebrafish pharyngeal skeletal development. Development (Cambridge, England), 139(15), 2804–2813. 10.1242/dev.074906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazona OA, Slota LA, Lopez DH, Zhang G, & Cohn MJ (2016). The genetic program for cartilage development has deep homology within Bilateria. Nature, 533(7601), 86–89. 10.1038/nature17398 [DOI] [PubMed] [Google Scholar]
- Teng CS, Yen H-Y, Barske L, Smith B, Llamas J, Segil N, … Crump JG (2017). Requirement for Jagged1-Notch2 signaling in patterning the bones of the mouse and human middle ear. Scientific Reports, 7(1), 2497. 10.1038/s41598-017-02574-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson KS (1966). The evolution of the tetrapod middle ear in the rhipidistian-amphibian transition. American Zoologist, 6(3), 379–397. [DOI] [PubMed] [Google Scholar]
- Tiecke E, Matsuura M, Kokubo N, Kuraku S, Kusakabe R, Kuratani S, & Tanaka M (2007). Identification and developmental expression of two Tbx1/10-related genes in the agnathan Lethenteron japonicum. Development Genes and Evolution, 217(10), 691–697. 10.1007/s00427-007-0181-0 [DOI] [PubMed] [Google Scholar]
- Tomita T, Toda M, Ueda K, Uchida S, & Nakaya K (2012). Live-bearing manta ray: how the embryo acquires oxygen without placenta and umbilical cord. Biology Letters, 8(5), 721–724. 10.1098/rsbl.2012.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Murakami Y, Kuraku S, Hirano S, & Kuratani S (2003). Development of the adenohypophysis in the lamprey: evolution of epigenetic patterning programs in organogenesis. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 300(1), 32–47. [DOI] [PubMed] [Google Scholar]
- Veitch E, Begbie J, Schilling TF, Smith MM, & Graham A (1999). Pharyngeal arch patterning in the absence of neural crest. Current Biology: CB, 9(24), 1481–1484. [DOI] [PubMed] [Google Scholar]
- Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, … Warren WC (2014). Elephant shark genome provides unique insights into gnathostome evolution. Nature, 505(7482), 174–179. 10.1038/nature12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincentz JW, Barnes RM, Rodgers R, Firulli BA, Conway SJ, & Firulli AB (2008). An absence of Twist1 results in aberrant cardiac neural crest morphogenesis. Developmental Biology, 320(1), 131–139. 10.1016/j.ydbio.2008.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitelli F, Morishima M, Taddei I, Lindsay EA, & Baldini A (2002). Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Human Molecular Genetics, 11(8), 915–922. [DOI] [PubMed] [Google Scholar]
- Wainwright PC (2005). Functional Morphology of the Pharyngeal Jaw Apparatus. In Fish Physiology (Vol. 23, pp. 77–101). Academic Press. Retrieved from http://www.sciencedirect.com/science/article/pii/S1546509805230030 [Google Scholar]
- Wainwright PC, & Longo SJ (2017). Functional Innovations and the Conquest of the Oceans by Acanthomorph Fishes. Current Biology: CB, 27(11), R550–R557. 10.1016/j.cub.2017.03.044 [DOI] [PubMed] [Google Scholar]
- Wainwright PC, McGee MD, Longo SJ, & Hernandez LP (2015). Origins, Innovations, and Diversification of Suction Feeding in Vertebrates. Integrative and Comparative Biology, 55(1), 134–145. 10.1093/icb/icv026 [DOI] [PubMed] [Google Scholar]
- Walker MB, Miller CT, Coffin Talbot J, Stock DW, & Kimmel CB (2006). Zebrafish furin mutants reveal intricacies in regulating Endothelin1 signaling in craniofacial patterning. Developmental Biology, 295(1), 194–205. 10.1016/j.ydbio.2006.03.028 [DOI] [PubMed] [Google Scholar]
- Weider M, & Wegner M (2017). SoxE factors: Transcriptional regulators of neural differentiation and nervous system development. Seminars in Cell & Developmental Biology, 63, 35–42. 10.1016/j.semcdb.2016.08.013 [DOI] [PubMed] [Google Scholar]
- Weil A (2011). Mammalian evolution: A jaw-dropping ear. Nature, 472(7342), 174–176. 10.1038/472174a [DOI] [PubMed] [Google Scholar]
- Wilkie AO, & Morriss-Kay GM (2001). Genetics of craniofacial development and malformation. Nature Reviews. Genetics, 2(6), 458–468. 10.1038/35076601 [DOI] [PubMed] [Google Scholar]
- Wischnitzer S, & Wischnitzer E (2006). Atlas and Dissection Guide for Comparative Anatomy. Macmillan. [Google Scholar]
- Yan YL, Jowett T, & Postlethwait JH (1998). Ectopic expression of hoxb2 after retinoic acid treatment or mRNA injection: disruption of hindbrain and craniofacial morphogenesis in zebrafish embryos. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 213(4), 370–385. [DOI] [PubMed] [Google Scholar]
- York JR, Yuan T, Zehnder K, & McCauley DW (2017). Lamprey neural crest migration is Snail-dependent and occurs without a differential shift in cadherin expression. Developmental Biology, 428(1), 176–187. 10.1016/j.ydbio.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, & Iseki S (2008). Cell lineage in mammalian craniofacial mesenchyme. Mechanisms of Development, 125(9–10), 797–808. 10.1016/j.mod.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Young GC (1986). The relationships of placoderm fishes. Zoological Journal of the Linnean Society, 88(1), 1–57. 10.1111/j.1096-3642.1986.tb00876.x [DOI] [Google Scholar]
- Yu H-H, & Moens CB (2005). Semaphorin signaling guides cranial neural crest cell migration in zebrafish. Developmental Biology, 280(2), 373–385. 10.1016/j.ydbio.2005.01.029 [DOI] [PubMed] [Google Scholar]
- Yu J-K, Holland ND, & Holland LZ (2002). An amphioxus winged helix/forkhead gene, AmphiFoxD: insights into vertebrate neural crest evolution. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 225(3), 289–297. 10.1002/dvdy.10173 [DOI] [PubMed] [Google Scholar]
- Yu J-K, Meulemans D, McKeown SJ, & Bronner-Fraser M (2008). Insights from the amphioxus genome on the origin of vertebrate neural crest. Genome Research, 18(7), 1127–1132. 10.1101/gr.076208.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, & Cohn MJ (2006). Hagfish and lancelet fibrillar collagens reveal that type II collagen-based cartilage evolved in stem vertebrates. Proceedings of the National Academy of Sciences, 103(45), 16829–16833. 10.1073/pnas.0605630103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Yu X, Ahlberg PE, Choo B, Lu J, Qiao T, … Zhu Y (2013). A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature, 502(7470), 188–193. 10.1038/nature12617 [DOI] [PubMed] [Google Scholar]
- Zuniga E, Rippen M, Alexander C, Schilling TF, & Crump JG (2011). Gremlin 2 regulates distinct roles of BMP and Endothelin 1 signaling in dorsoventral patterning of the facial skeleton. Development (Cambridge, England), 138(23), 5147–5156. 10.1242/dev.067785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga E, Stellabotte F, & Crump JG (2010). Jagged-Notch signaling ensures dorsal skeletal identity in the vertebrate face. Development (Cambridge, England), 137(11), 1843–1852. 10.1242/dev.049056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Clack JA (2002). Gaining Ground: The Origin and Evolution of Tetrapods. Indiana University Press. [Google Scholar]
- Eames BF, DeLaurier A, Ullmann B, Huycke TR, Nichols JT, Dowd J, … Kimmel CB (2013). FishFace: interactive atlas of zebrafish craniofacial development at cellular resolution. BMC Developmental Biology, 13, 23. 10.1186/1471-213X-13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisdal A, & Trainor PA (2014). Development and evolution of the pharyngeal apparatus. Wiley Interdisciplinary Reviews. Developmental Biology, 3(6), 403–418. 10.1002/wdev.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert FR, Singh AJ, Afoyalan O, Kioussi C, & Dietrich S (2018). To roll the eyes and snap a bite - function, development and evolution of craniofacial muscles. Seminars in Cell & Developmental Biology. 10.1016/j.semcdb.2017.12.013 [DOI] [PubMed] [Google Scholar]