Abstract
Background:
Differential expression of chemokines/chemokine receptors in colorectal cancer (CRC) may enable molecular characterization of patients’ tumors for predicting clinical outcome.
Objective:
To evaluate the prognostic ability of these molecules in a CRC cohort and the CRC TCGA-dataset.
Methods:
Chemokine (CXCL-12α, CXCL-12β, IL-17A, CXCL-8, GM-CSF) and chemokine receptor (CXCR-4, CXCR-7) transcripts were analyzed by RT-qPCR in 76 CRC specimens (normal: 27, tumor: 49; clinical cohort). RNA-Seq data was analyzed from the TCGA-dataset (n=375). Transcript levels were correlated with outcome; analyses: univariate, multivariable, Kaplan-Meier.
Results:
In the clinical cohort, chemokine/chemokine receptor levels were elevated 3–10-fold in CRC specimens (P≤0.004) and were higher in patients who developed metastasis (P=0.03-<0.0001). CXCR-4, CXCR-7, CXCL-12α, CXCL-8, IL-17 and GM-CSF levels predicted metastasis (P≤0.0421) and/or overall survival (OS; P≤0.0373). The CXCR-4+CXCR-7+CXCL-12 marker (CXCR-4/7+CXCL-12(α/β) signature) stratified patients into risk for metastasis (P=0.0014; OR, 2.72) and OS (P=0.0442; OR, 2.7); sensitivity: 86.67%, specificity: 97.06%. In the TCGA-dataset, the CXCR-4/7+CXCL-12 signature predicted metastasis (P=0.011; OR, 2.72) and OS (P=0.0006; OR: 4.04). In both datasets, the signature was an independent predictor of clinical outcome.
Conclusions:
Results of 451 specimens from both cohorts reveal that the CXCR-4/7+CXCL-12 signature potentially predicts outcome in CRC patients and may allow earlier intervention.
Keywords: CXCL-12, CXCR-7, CXCR-4, CXCL-8, Colorectal Cancer
Introduction
Although overall incidence rates are declining, colorectal cancer (CRC) remains the third most common cancer in the United States, as well as the second leading cause of cancer-related deaths. CRC is also the most common malignancy of the gastrointestinal tract [1]. Improved public education and screening has allowed for increased overall 5-year survival (64.9%), but survival with metastatic disease remains dismal (13.9%) [2]. Locally advanced tumors present a prognostic quandary – patients with high T-stage and negative lymph node status (Stage II) can have significantly worse clinical outcomes than those with low T-stage (T1) and positive lymph nodes (Stage III) [3]. Molecular characterization of patients’ CRC may allow the identification of markers that distinguish between indolent and malignant CRC tumor types to accurately predict metastatic potential and inform the identification of therapeutic targets [4].
Several chemokines and chemokine receptors have been shown to promote CRC metastasis. C-X-C Motif Chemokine Ligand 8 (CXCL-8, also known as Interleukin-8) is a multifunctional glutamic acid-leucine-arginine positive, or ELR positive CXC chemokine classically associated with neutrophil migration that promotes tumor growth, motility, invasion, and angiogenesis [5]. CXCL-8 also regulates the expression of chemokine receptors such as the G-protein coupled receptor CXCR-7 [6]. CXCR-7 and another G-protein coupled receptor, CXCR-4, share a common ligand, C-X-C Motif Chemokine Ligand 12 (CXCL-12, also known as Stromal Derived Factor-1) [7–9]. The CXCR-7/CXCL-12 and CXCR-4/CXCL-12 signaling pathways have been shown to mediate tumor progression through pro-angiogenic and metastatic effects [10–14]. Six variant isoforms of CXCL-12 have been identified, of which α and β isoforms are known to promote tumor metastasis and angiogenesis [8, 15–17]. IL-17 is a family of pro-inflammatory cytokines that has been implicated in the pathogenesis of several inflammatory and autoimmune diseases, and more recently, colorectal cancer [18–22]. GM-CSF is a hematopoietic growth factor that stimulates production of granulocytes and monocytes (macrophages and dendritic cells). GM-CSF levels have been reported to be elevated in intestinal inflammation and several cancers including CRC, where it promotes tumor progression and angiogenesis [23–25].
Some studies have reported the prognostic potential of individual chemokines and chemokine receptors in predicting clinical outcome in CRC patients. High CXCL-8 expression has been shown to correlate with nodal disease, metastasis, and poor overall and disease-specific survival [26–28]. CXCL-12 and CXCR-4 have been shown to predict poor clinical outcome and resistance to radiotherapy [29–36]. Expression of CXCR-7 has been shown to correlate with both nodal disease and metastasis [37]. While the association between IL-17 and clinical prognosis has not been widely studied, there seems to be a consensus that higher expression of IL-17 is indicative of unfavorable prognosis. IL-17 levels have been shown to correlate with nodal disease, high TNM-staging and decreased OS [21, 38, 39]. However, one study has reported an association between higher IL-17 levels and prolonged OS [40].
We measured the expression of chemokines (CXCL-8, CXCL-12α, CXCL-12β, IL-17A and GM-CSF), and chemokine receptors (CXCR-4 and CXCR-7) in normal and tumor specimens from CRC patients and correlated the levels with clinical outcome. For validation, we used The Cancer Genome Atlas (TCGA) CRC dataset consisting of RNA-Seq and associated clinical data on 375 CRC specimens. We hypothesized that since these chemokines and receptors function in the same biochemical pathways, a combination of these molecules into a chemokine/chemokine receptor signature may better predict prognosis than individual molecules.
Materials and Methods
Patients and Tissue Specimens:
Normal colorectal (n=27) and primary tumor (n=49) specimens were collected from patients undergoing partial colectomy at the Medical College of Georgia, Augusta University (MCG-AU). All specimens were obtained based on their availability for research purpose through the Bio-Repository Alliance of Georgia for Oncology (BRAG-ONC) under a protocol approved by the AU institutional review board. The CRC TCGA-dataset was accessed through the UCSC Xena Browser [41]. The TCGA-dataset contains 375 patients on whom chemokine/chemokine receptor data were available, along with demographic and pathologic parameters and clinical outcome in terms of metastasis or survival. The dataset did not contain information regarding time to metastasis, and metastasis data was missing on 72 patients. Patient characteristics for both datasets are shown in Table 1.
Table 1: Patient characteristics.
Patient characteristics in the clinical specimen cohort and in the available TCGA-dataset. Follow-up information was not available for time to metastasis in the TCGA-dataset. Note: In the TCGA-dataset, metastasis data is same as M-stage (metastasis at the time of diagnosis), as the follow-up data are not available to determine whether any M-stage negative patients developed metastasis later.
| Parameter | Clinical cohort | TCGA-dataset |
|---|---|---|
| Specimens | Normal = 27; Tumor = 49 | Tumor = 375 |
| Gender | Male: 25; Female: 24 | Male: 207; Female: 168 |
| Age (years) | Median: 64; Mean: 62.3 ± 12.58 | Median: 66; Mean: 64.44 ± 13.09 |
| T-stage | T1: 4; T2: 11; T3: 28; T4: 6 | T1: 11; T2: 57; T3: 256; T4: 49 Missing: 2 |
| N-stage | (−): 25; (+): 24 | (−): 204; (+): 167 Missing:4 |
| LVI | (−): 34; (+): 15 | (−): 227; (+): 102 Missing: 46 |
| PNI | (−): 39; (+): 10 | (−): 168; (+): 58 Missing: 149 |
| Metastasis | (−): 34; (+): 15 | (−): 252; (+): 51 Missing: 72 |
| Metastasis (months) | 26.97 ± 35.82 | |
| Overall survival | (−): 42; (+): 7 | (−): 280; (+): 86 Missing: 9 |
| Follow up months (overall survival) | 36.3 ± 36.76 | 30.78 ± 26.6 |
Note: (−) indicates negative or no event; (+) means positive or event, i.e. death or metastasis as indicated.
Reverse transcription quantitative PCR (RT-qPCR):
RNA was extracted from approximately 30–50 mg of tissue using the RNeasy kit (Qiagen, Germantown, MD). RNA was reverse transcribed (RT; iScript; Bio-Rad Laboratories, Hercules, CA) and subjected to quantitative PCR using gene-specific primers (Table 2). Normalized transcript levels for each gene were calculated as (100/2ΔCq); ΔCq = Cq(transcript) – Cq(β actin) [42–44]. Each specimen was analyzed at least in duplicate in each assay. The average normalized value from each specimen was used when calculating the data for each marker in the specimen cohort.
Table 2:
RT-qPCR primer sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| CXCR-4 | 5’TCATCAAGCAAGGGTGTGAG3’ | 5’TGGCTCCAAGGAAAGCATAG3’ |
| CXCR-7 | 5’CAGCTTCATCAATCGCAACTA3’ | 5’AGCTTGGTGAGCCCTGTTT3’ |
| CXCL-12α | 5’AAGCACAACAGCCAAAAAGG3’ | 5’TGCCCTTTCATCTCTCACAA3’ |
| CXCL-12β | 5’CGCCTTTCCCAGGTGCTAAC3’ | 5’TGGTCTGCTTAGGGGATTTGG3’ |
| CXCL-8 | 5’TTTGCCAAGGAGTGCTAAAGA3’ | 5’GATAAATTTGGGGTGGAAAGG3’ |
| IL-17A | 5’GCCCAAATTCTGAGGACAAG3’ | 5’GGAGATTCCAAGGTGAGGTG3’ |
| GM-CSF | 5’ AGCCTCACCAAGCTCAAGG3’ | 5’ AATCTGGGTTGCACAGGAAG3’ |
Statistical Analysis:
Differences in biomarker levels between normal colorectal tissues and CRC were compared using the Mann-Whitney test, as the data showed non-normal distribution by the Shapiro-Wilk test; P-values were two-tailed. Similarly, differences in the levels of biomarkers between CRC with respect to lymph node (LN) status, T-stage, metastasis, lymphovascular invasion (LVI) and perineural invasion (PNI) were also compared using the Mann-Whitney test. All reported P-values in this study are two-tailed. The levels of the combined biomarker signature CXCR-4+CXCR-7+CXCL-12 (CXCR-4/7+CXCL-12 signature) was calculated as follows: [1-intercept + (α1 × (CXCR-4)1) + (α2 × (CXCR-7)1) + (α3 × (CXCL-12)1]; α1, α2 and α3: CXCR-4, CXCR-7 and CXCL-12 coefficients, respectively; (CXCR-4)1, (CXCR-7)1 and (CXCL-12)1: the levels of respective markers in specimen # 1 [44, 45]. The intercept and coefficients for each marker were computed by simultaneously analyzing the three markers in the logistic regression model (Table 3). Logistic regression single-parameter model (univariate analysis) was used to determine the association of clinical parameters and the marker levels/marker signature with metastasis and overall survival (OS). Based on Youden’s index from the ROC curve, optimal cut-off values were calculated to compute sensitivity and specificity. Cox-proportional hazards model (multivariable analysis) was used to determine which clinical demographic and pathologic parameters and/or biomarkers were significant in predicting metastasis and/or OS. It has been reported that for the multivariable logistic regression to be reliable, at least 10 events per variable (EPV) are required in a cohort of 1000 patients [46]. In the study cohort of 49 patients, 15 patients developed metastasis and the Cox multivariable regression analysis had 7 independent variables. This results in an EPV of 43 in a cohort of 1000. Similarly, in this cohort 7 patients died during follow-up. This results in an EPV of 20. In the TCGA cohort of 375 patients, 86 patients died during follow-up, resulting in an EPV of 33. Stratified Kaplan-Meier plots were prepared for markers that significantly predicted metastasis in both univariate and multivariable analyses.
Table 3:
Intercepts and coefficients for CXCR-4/7+CXCL-12 signature (CXCR-4/7+CXCL-12β in clinical cohort). Logistic regression analysis was used to calculate intercepts and coefficients (α) for the calculation of the signature. For both cohorts, the signature was calculated using metastasis and overall survival as a categorical variable.
| Metastasis | |||
|---|---|---|---|
| Clinical cohort | TCGA-dataset | ||
| Intercept | Coefficient α | Intercept | Coefficient α |
| 4.663 | CXCR-4: −2.9178 CXCR-7: −1.3486 CXCL-12β: 4.7398 |
3.5927 | CXCR-4: 0.0522 CXCR-7: −0.5131 CXCL-12: 0.2282 |
| Overall Survival | |||
| Clinical cohort | TCGA-dataset | ||
| Intercept | Coefficient α | Intercept | Coefficient α |
| 2.3296 | CXCR-4: 0.02996 CXCR-7: −0.5862 CXCL-12β: 0.5028 |
0.3529 | CXCR-4: 0.1022 CXCR-7: 0.1708 CXCL-12: −0.18 |
Results
Differential expression of chemokines in normal and CRC tissues.
The transcript levels of CXCR-4, CXCR-7, CXCL-12α, CXCL-12β, IL-17A, CXCL-8 and GM-CSF were measured in 76 colorectal tissue specimens. We initially evaluated the differential expression of these potential markers in matched normal and cancer specimens (n=17). The approach of matching normal and tumor specimens from the same patient was adopted to reduce/negate variations in biomarker levels due to comorbid conditions, treatment history, or genetic makeup of the subjects. Median expression of all markers was increased 9- to 22-fold in tumor specimens compared to the matched normal colon specimens; the difference was statistically significant for all markers (Figure 1A). We then added additional unmatched normal colon and tumor specimens to our cohort and analyzed the differential expression of the markers in this expanded cohort. Median expression of all markers except for CXCL-12α was increased 4- to 16-fold in tumor specimens as compared to normal colon tissues. The differences in the levels between tumor and normal specimens were statistically significant for all markers except CXCL-12α (Figure 1B).
Figure 1: Differential expression of markers in clinical specimens.
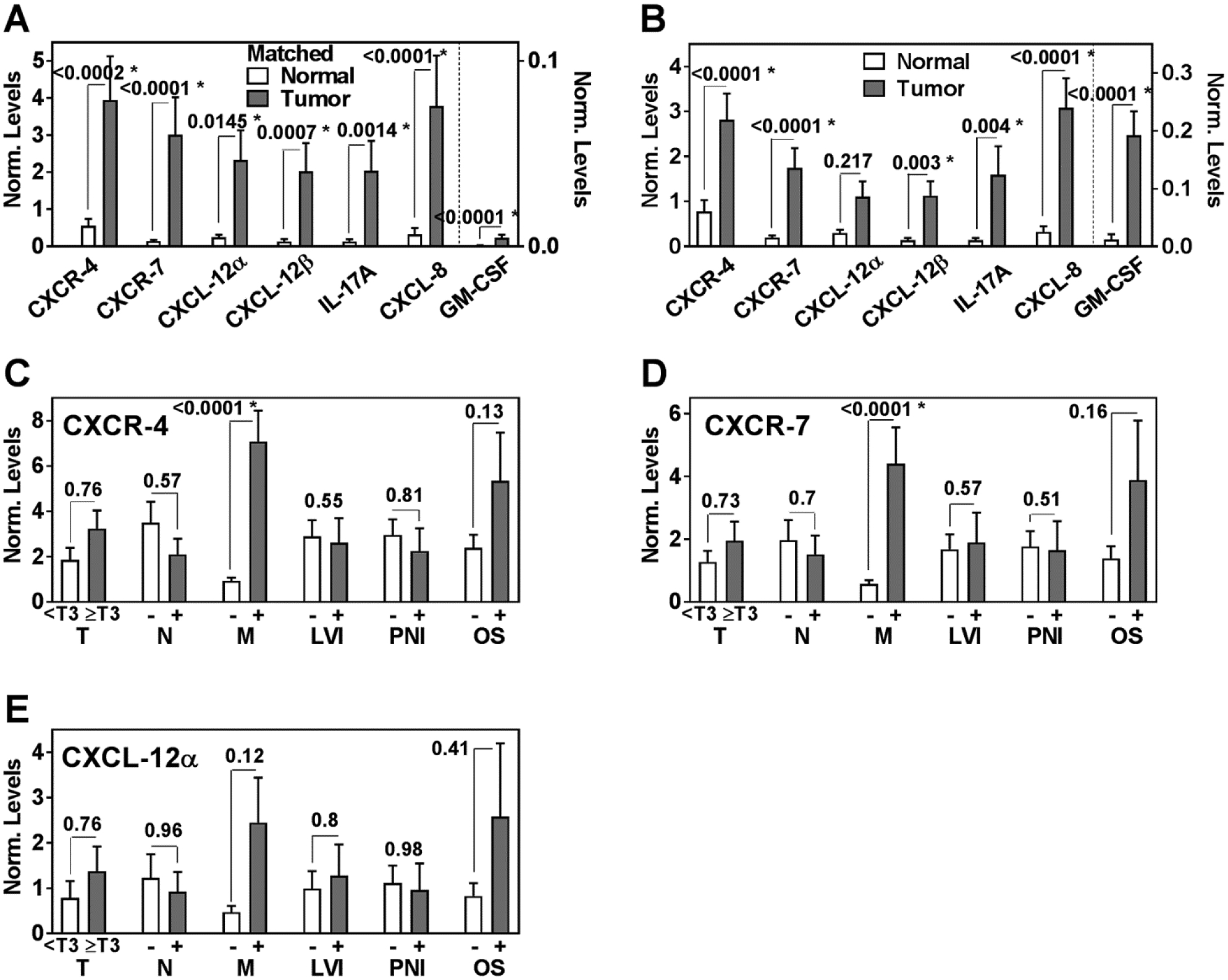
Mean ± SEM was plotted for each marker, statistical significance was analyzed by the Mann-Whitney test. A Comparison of marker levels between matched normal (17) and CRC (17) specimens. Note: GM-CSF values are plotted on the right axis. B Comparison of marker levels between normal (27) and CRC (49) specimens. Note: GM-CSF values are plotted on the right axis. C – E Differences in CXCR-4, CXCR-7 and CXCL-12α marker levels with respect to clinical parameters, i.e. T-stage, LN status, metastasis, LVI, PNI and OS. OS: (−) is indicative of survival and (+) is indicative of death.
Association of chemokine levels with prognosis
Of the 49 patients in the cohort, 10 had metastasis at the time of surgery and 5 developed metastasis during follow-up. Except for CXCL-12α, expression of all markers was significantly higher in tumors from patients who had metastasis as compared to those who did not (Figures 1, 2). Of the 49 patients in the clinical cohort, 7 patients died during the median follow-up of 32.5 months. The levels of all markers were also higher in patients who died during follow-up, but the increase was statistically significant only for CXCL-8 (Figures 1, 2).
Figure 2: Differential expression of markers in clinical specimens.
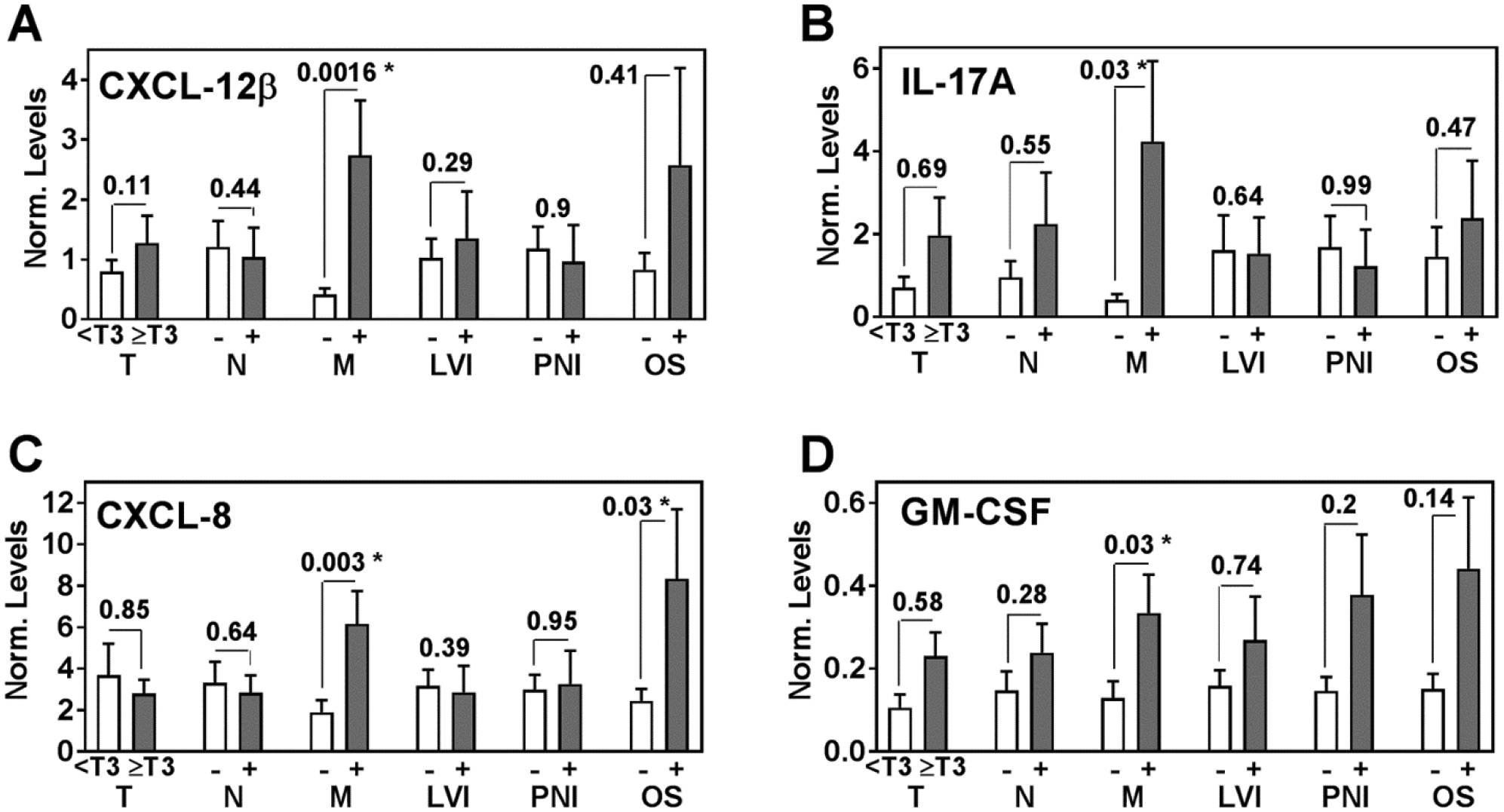
Mean ± SEM was plotted for each marker, statistical significance was analyzed by the Mann-Whitney test. A – D Differences in CXCL-12β, IL-17A, CXCL-8 and GM-CSF marker levels with respect to clinical parameters, i.e. T-stage, LN status, metastasis, LVI, PNI and OS. OS: (−) is indicative of survival and (+) is indicative of death.
The marker levels did not significantly associate with T-stage, N-stage, lymphovascular invasion (LVI) or PNI (Figures 1, 2). However, in univariate analysis all markers, except CXCL-12β, significantly associated with metastasis (Table 4). In multivariable analysis, all markers except IL-17A and GM-CSF significantly predicted metastasis (Table 4). Kaplan-Meier plots showed that higher levels of CXCR-4, CXCR-7 and CXCL-8 significantly stratified the patients into higher risk for metastasis (Figure 3A, 3B, 3C). Out of the 15 patients who were positive for metastasis, 10 had metastasis at the time of surgery. CXCR-4 and CXCR-7 levels were higher in 8 and 9 of these patients, respectively. Therefore, elevated CXCR-4 and CXCR-7 levels indicated 6 – 7-fold increased risk of having metastasis at the time of surgery; CXCR-4: χ2: 8.0; P=0.0047; RR, 5.8; 95% CI, 1.6–22.6; CXCR-7: χ2=6.2; P=0.013; RR, 7.3; 95% CI, 1.4–43.4.
Table 4: Relationship of clinical parameters and marker levels to metastasis and OS in the clinical cohort.
Univariate analysis (single parameter logistic regression) was performed to evaluate the ability of clinical parameters and marker levels to predict metastasis and OS. P values are two-tailed. Multivariable analysis was performed using the Cox-proportional hazards model. Parameters included in the multivariable analysis were age, gender, T-stage, LN, LVI, PNI, and each marker/signature. Results are based on Wald’s test.
| Univariate Analysis | ||||||
|---|---|---|---|---|---|---|
| Metastasis | Overall Survival | |||||
| Parameter | X2 | P Value | OR; 95% CI | X2 | P Value | OR; 95% CI |
| Age | 2.38 | 0.1230 | NS | 0.40 | 0.5262 | NS |
| Gender | 1.04 | 0.3084 | NS | 0.22 | 0.6420 | NS |
| T-stage | 3.58 | 0.0585 | NS | 0.20 | 0.6531 | NS |
| LN | 0.16 | 0.6858 | NS | 1.54 | 0.2146 | NS |
| LVI | 0.08 | 0.7838 | NS | 0.56 | 0.4525 | NS |
| PNI | 0.51 | 0.4731 | NS | 2.31 | 0.1282 | NS |
| CXCR-4 | 8.40 | 0.0038 | 3.23; 1.45–7.14 | 2.79 | 0.0951 | NS |
| CXCR-7 | 8.34 | 0.0039 | 3.23; 1.47–7.14 | 3.30 | 0.0694 | NS |
| CXCL-12α | 4.13 | 0.0421 | 1.52; 1.01–2.22 | 2.70 | 0.1004 | NS |
| CXCL-12β | 3.47 | 0.0624 | NS | 2.24 | 0.1344 | NS |
| CXCL-8 | 4.98 | 0.0257 | 1.22; 1.02–1.43 | 4.34 | 0.0373 | 1.16; 1.01–1.35 |
| IL-17A | 4.43 | 0.0353 | 1.54; 1.03–2.33 | 0.25 | 0.6170 | NS |
| GM-CSF | 4.20 | 0.0405 | 11.11; 1.11–100 | 4.63 | 0.0314 | 12.5; 1.25–100 |
| CXCR-4/7+CXCL-12β signature | 10.16 | 0.0014 | 2.7; 1.47–5.03 | 4.05 | 0.0442 | 2.7; 1.03–7.14 |
| Multivariable Analysis | ||||||
| Metastasis | Overall Survival | |||||
| Parameter | x2 | P value | RR; 95% CI | X2 | P Value | RR; 95% CI |
| CXCR-4 | 12.47 | 0.0004 | 1.24; 1.11–1.41 | NS | ||
| CXCR-7 | 7.31 | 0.007 | 8.95; 1.81–49.06 | NS | ||
| CXCL-12α | 3.88 | 0.049 | 1.17; 0.99–30 | NS | ||
| CXCL-12β | 5.38 | 0.0204 | 1.24; 1.03–1.5 | NS | ||
| CXCL-8 | 3.89 | 0.049 | 1.04; 1–1.08 | 7.66 | 0.0056 | 1.12; 1.04–1.23 |
| CXCR-4/7+CXCL-12β signature |
12.89 | 0.0003 | 1.1; 1.05–1.17 | 5.06 | 0.0245 | 4.29; 1.29–18.12 |
NS – not significant. Note: only significant parameters are shown.
Figure 3: Kaplan-Meier plots for time to metastasis based on marker levels in the clinical cohort.
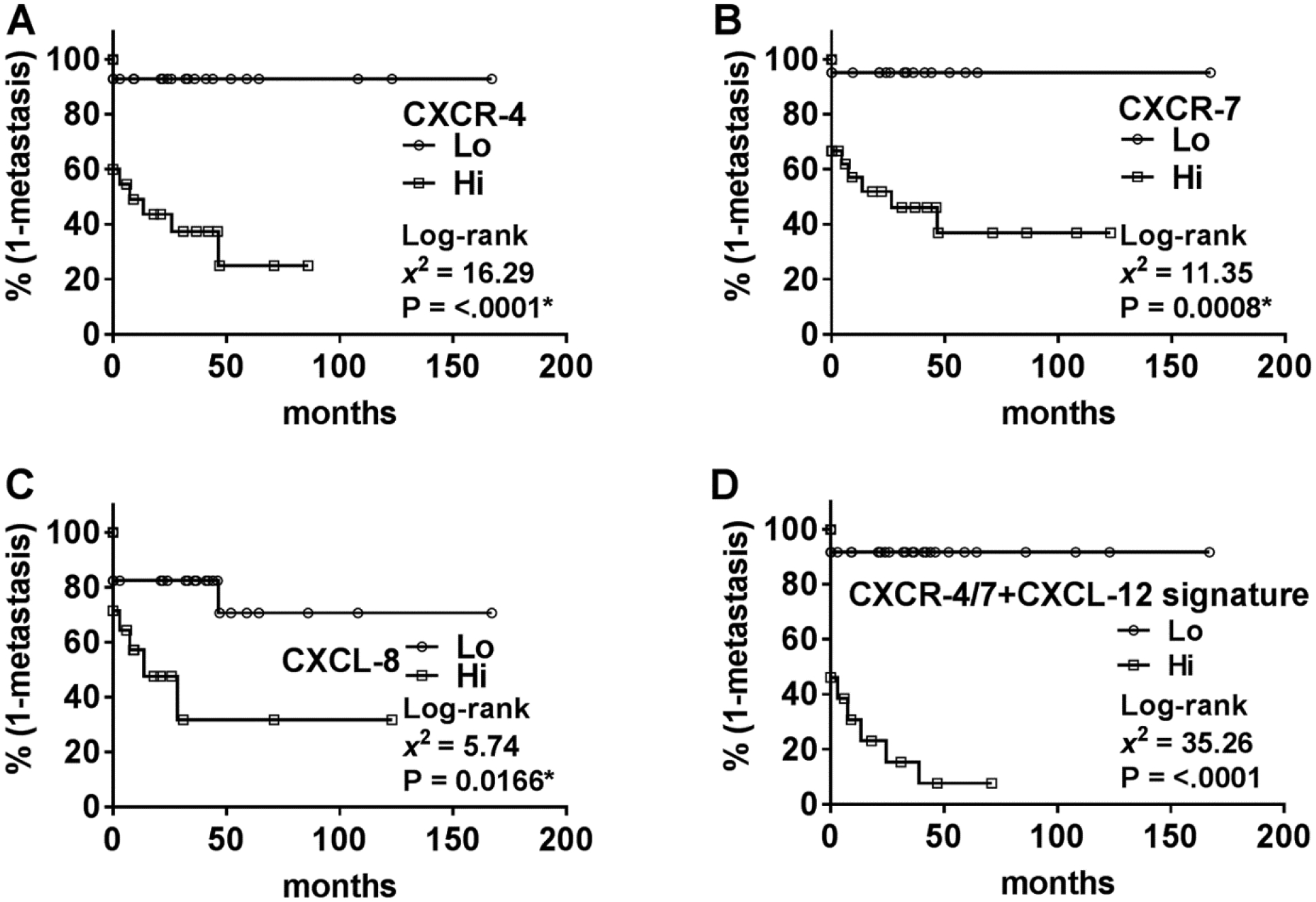
A-D Data on marker levels in the clinical specimen cohort were stratified as high and low expression based on Youden’s index from the ROC curve (generated from the univariate logistic regression analysis). Stratified data were then used to generate Kaplan-Meier plots with respect to metastasis.
In univariate analysis, higher levels of CXCL-8 and GM-CSF significantly correlated with increased risk of death (i.e., decreased OS; Table 4). In multivariable analysis CXCL-8 levels significantly predicted OS (Table 4).
Association of chemokine levels with prognosis in the TCGA CRC dataset.
We next analyzed whether the observed correlation between differential expression of chemokines or chemokine receptors and clinical outcome could be validated in the available CRC TCGA-dataset of 375 patients (Table 1). In this TCGA-dataset, follow-up data are not available regarding metastasis. Therefore, M-stage data was used to determine whether any marker detected synchronous metastasis at the time of diagnosis. In univariate analysis, none of the markers correlated with metastasis or OS, except IL-17A, which showed a significant but negative correlation with both; IL-17A levels were lower in tumors of those patients who had metastasis or died during follow-up (Table 5). In Kaplan-Meier analysis, lower IL-17A levels significantly stratified patients into higher risk of death (Figure 4A). In multivariable analysis, no markers significantly associated with OS (Table 5).
Table 5: Relationship of clinical parameters and marker levels to metastasis and OS in the TCGA-dataset.
Univariate analysis (single parameter logistic regression) was performed to evaluate the ability of clinical parameters and marker levels to predict metastasis and OS. P values are two-tailed. Multivariable analysis was performed using the Cox-proportional hazards model. Parameters included in analysis were age, gender, T-stage, LN, LVI, PNI, and each marker. Results are based on Wald’s test; only significant parameters are shown.
| Univariate Analysis | ||||||
|---|---|---|---|---|---|---|
| Metastasis | Overall Survival | |||||
| Parameter | X2 | P Value | OR; 95% CI | X2 | P Value | OR; 95% CI |
| Age | 4.28 | 0.0386 | 1.02; 1–1.05 | 13.46 | 0.0002 | 1.04; 1.02–1.06 |
| Gender | 0.17 | 0.6815 | NS | 0.44 | 0.506 | NS |
| T-stage | 27.51 | <.0001 | 1.18; 1.1–1.23 | 10.84 | 0.001 | 1.08; 1.03–1.11 |
| LN | 35.06 | <.0001 | 14.73; 6.05–35.91 | 12.31 | 0.0004 | 2.45; 1.49–4.05 |
| LVI | 23.05 | <.0001 | 5.56; 2.76–11.21 | 2.61 | 0.106 | NS |
| PNI | 11.77 | 0.0006 | 4.18; 1.85–9.46 | 0.61 | 0.4354 | NS |
| CXCR-4 | 0.13 | 0.7186 | NS | 0.28 | 0.5998 | NS |
| CXCR-7 | 3.26 | 0.0711 | NS | 0.37 | 0.5435 | NS |
| CXCL-12 | 0.15 | 0.7003 | NS | 0.36 | 0.5513 | NS |
| CXCL-8 | 0.28 | 0.5978 | NS | 2.11 | 0.1461 | NS |
| IL-17A | 6.11 | 0.0135 | 0.76; 0.62–0.95 | 4.1 | 0.0429 | 0.85; 0.73–1 |
| GM-CSF | 0.03 | 0.8614 | NS | 0.6 | 0.4376 | NS |
| CXCR-4/7+CXCL-12 signature | 6.46 | 0.011 | 2.7; 1.26–5.88 | 11.74 | 0.0006 | 4.04; 1.82–8.97 |
| Multivariable Analysis | ||||||
| Overall Survival | ||||||
| Parameter | x2 | P value | RR; 95% CI | |||
| Age | 5.37 | 0.0204 | 1.03; 1.01–1.06 | |||
| LN | 14.04 | 0.0002 | 4.81; 2.18–11.38 | |||
| CXCR-4/7+CXCL-12 signature | 7.56 | 0.006 | 3.18; 1.31–6.96 | |||
Note: TCGA-dataset does not distinguish between CXCL-12 isoforms. The dataset also does not have follow-up information regarding metastasis. Therefore, M-stage data (i.e., synchronous metastasis) data was used to evaluate the association of clinical parameters with metastasis in univariate analysis. However, Cox-proportional hazard analysis could not be performed for metastasis.
NS – not significant.
Figure 4: Kaplan-Meier plots for overall survival based on marker levels in the TCGA cohort.
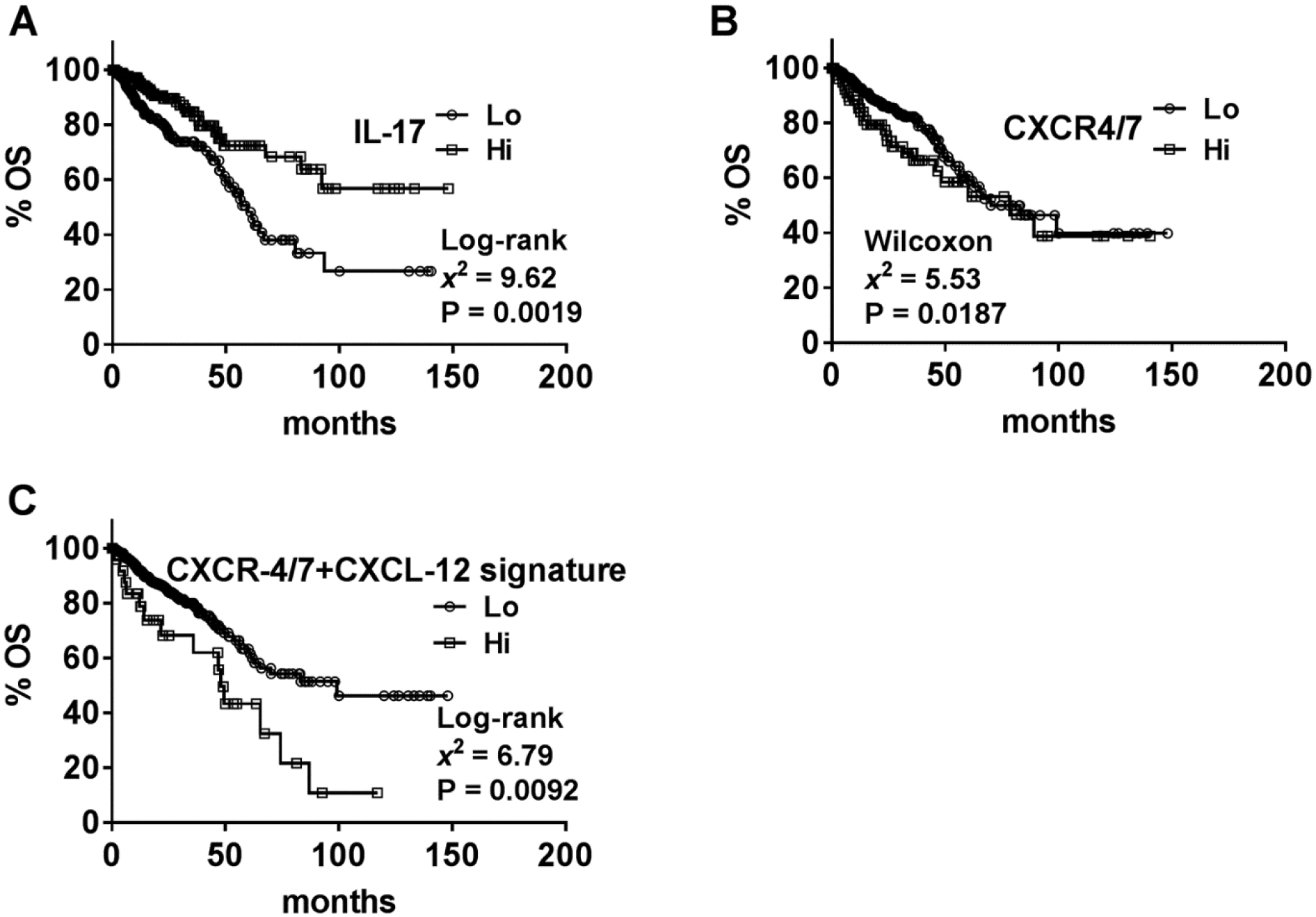
A-C Data on marker levels in the TCGA cohort were stratified as high and low expression based on Youden’s index from the ROC curve (generated from the univariate logistic regression analysis). Stratified data were then used to generate Kaplan-Meier plots with respect to OS. Note: B Results of the Wilcoxon test are shown for CXCR4/7 since the Log-rank test was not significant.
Association of a novel chemokine/chemokine receptor signature to predict prognosis in the TCGA-dataset and clinical cohort.
In the TCGA-dataset, all marker levels, except IL-17A, failed to correlate with metastasis or OS. Therefore, we analyzed whether a combination of markers, instead of individual marker levels, could successfully predict clinical outcome. We found that a combined CXCR-4 and CXCR-7 marker significantly predicted metastasis in univariate analysis (χ2: 4.35; P=0.0371). While the combined CXCR-4 and CXCR-7 marker failed to predict OS in univariate analysis, in Kaplan-Meier analysis this marker significantly stratified patients for risk of death according to the Wilcoxon test (Figure 4B). Upon addition of CXCL-12 into the signature, the signature was now able to predict both metastasis and OS. This CXCR-4/7+CXCL-12 signature significantly predicted metastasis and/or OS in univariate and multivariable analyses (Table 5). The CXCR-4/7+CXCL-12 signature also risk-stratified patients in regards to OS in Kaplan-Meier analysis (Figure 4C). We then sought to validate this finding in our clinical cohort. Since the TCGA-dataset does not differentiate between the isoforms of CXCL-12, we tested the CXCR-4/7+CXCL-12 signature in our clinical cohort with both CXCL-12α and CXCL-12β. The CXCR-4/7+CXCL-12β signature significantly predicted metastasis and OS in univariate and multivariable analyses (Table 4). Efficacy analyses showed that the CXCR-4/7+CXCL-12β signature had 86.67% sensitivity and 97.06% specificity to predict metastasis. The signature had relatively high sensitivity (85.71%) but lower specificity (59.52%) to predict OS. In the clinical cohort, the CXCR-4/7+CXCL-12β signature significantly stratified patients for risk of metastasis (Figure 3D). Eight out of ten patients who had metastasis at the time of surgery also had elevated CXCR-4/7+CXCL-12β signature, which correlated to a 10-fold increased risk of synchronous metastasis; χ2: 16.28; P<.0001; RR, 10; 95% CI, 2.75–38.14. The combination of CXCR-4/7 with CXCL-12α (CXCR-4/7+CXCL-12α signature) could also significantly predict metastasis in univariate (χ2: 5.03; P=0.0249) and multivariable (χ2: 14.14; P=0.0002) analyses.
Discussion
Molecular characterization of CRC for individualized prognostic evaluation is clinically relevant from the perspective of identifying metastasis or predicting metastatic potential, allowing for closer observation and/or individually tailored therapy. Although the expression of these chemokines and receptors has been shown to associate with advanced disease and worse prognosis, to our knowledge no group has examined quantifiable expression levels of these chemokines and associated receptors simultaneously using a chemokine/chemokine receptor signature to evaluate clinical outcome. Furthermore, these results were validated in an independent dataset - the CRC TCGA-dataset.
The seven chemokines and chemokine receptors that were included in our study are known to promote tumor growth, metastasis and/or angiogenesis [5–9,11–13]. However, the biomarker potential of these molecules has not yet been definitively determined. As oncology continues to move toward individualized therapies, the routine measurement of biomarker expression in resected specimens might allow identification of patients at higher risk for metastasis and disease progression. In turn this could open the door to tailored surveillance and treatments. In our cohort, we found an increased expression of all examined markers, i.e. CXCR-4, CXCR-7, CXCL-12α, CXCL-12β, IL-17A, CXCL-8 and GM-CSF in CRC tissues as compared to normal colon tissues; differences were statistically significant for all markers except CXCL-12α. CXCL-8 levels have been shown to associate with advanced stage, nodal disease, metastasis and increased risk of mortality.26−28 However, in our study the prognostic ability of CXCL-8 was not consistent among the two cohorts, i.e., the clinical cohort and the TCGA-dataset. For example, while in the clinical cohort CXCL-8 modestly associated with metastasis and OS, it did not predict metastasis or OS in the TCGA-dataset.
Another contrasting result was obtained regarding IL-17A. Similar to other published studies21,38,39, in our study a significant but modest association was observed between increased IL-17A levels and metastasis in the clinical cohort. However, IL-17A levels negatively correlated with metastasis and OS in the TCGA-dataset. This discrepancy in the results of our clinical cohort and the TCGA-dataset with regards to IL-17A may be because IL-17 is an inflammatory cytokine and not a tumor cell intrinsic cytokine. The discrepancy could also possibly be influenced by the lack of follow-up information for metastasis in the TCGA-dataset. Nevertheless, in multivariable analysis IL-17A was not a significant predictor of metastasis or OS in the clinical cohort, suggesting limited prognostic significance of IL-17A in CRC. Our study therefore reveals that simultaneous analyses of multiple markers in different datasets is necessary to identify candidate markers that could be useful in the clinical management of CRC patients.
The CXCR-4/CXCL-12 signaling pathway has previously been implicated in the development of metastasis and angiogenesis, and has been shown to associate with clinical outcome [7–11,13]. In our study, the combined CXCR-4 and CXCR-7 marker significantly predicted metastasis in the TCGA-dataset. Furthermore, this marker stratified patients for risk of death in Kaplan-Meier analysis with the Wilcoxon test statistic, suggesting that the combined CXCR-4 and CXCR-7 marker is an early predictor of OS in CRC. Our study also demonstrates that the CXCR-4/7+CXCL-12 signature has significantly higher potential as a prognostic marker. The CXCR-4/7+CXCL-12 signature was the only marker that was an independent predictor of both metastasis and OS in the clinical cohort and the TCGA-dataset. The signature also had good efficacy for predicting clinical outcome (86.7% sensitivity and 97.1% specificity for predicting metastasis). A limitation of our study is that cancer-specific survival was not available in both the clinical cohort and the TCGA-dataset and the latter also did not have follow-up information for metastasis. Therefore, although the CXCR-4/7+CXCL-12 signature was an independent predictor of OS and had high sensitivity (85.7%) to predict OS, the specificity was modest (59.5%). Nevertheless, this study including a total of 451 specimens shows that the CXCR-4/7+CXCL-12 signature may have potential for early detection of metastasis. This is further supported by our observation that the CXCR-4/7+CXCL-12β signature was also able to detect metastasis at the time of surgery in the clinical cohort. Therefore, elevated CXCR-4, CXCR-7 and CXCL-12 levels in a primary tumor specimen could be indicative of micrometastases. If further validated, this could allow individualized decisions regarding early adjuvant treatment for controlling disease progression.
Another limitation of the study is that, for certain markers, differences were observed in results between the clinical cohort and the TCGA-dataset; however, this may be explained by the limitations of the TCGA-dataset itself. The TCGA-dataset had a significantly smaller proportion of patients with positive M-stage (synchronous metastasis) and patients with missing M-stage data, but had a higher proportion of patients with T3 and T4 stage tumors which have a propensity to metastasize. It is plausible that some of these patients developed metastasis at a later date but were misclassified as false positives given that the M-stage was negative. Despite these limitations, the CXCR-4/7+CXCL-12 signature consistently showed independent prognostic significance with reasonable efficacy to predict clinical outcome in CRC patients. Further investigation into the clinical significance of this biomarker is warranted in order to define its relationship to and accuracy for predicting clinical outcome.
Supplementary Material
Funding:
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number 1R01CA227277-01A1 to V.B.L, 1F31 CA236437-01 to D.S.M)
Abbreviations:
- CRC
colorectal cancer
- CXCL-8
C-X-C Motif Chemokine Ligand 8
- CXCL-12
C-X-C Motif Chemokine Ligand 12
Footnotes
The authors declare no potential conflicts of interest.
References
- [1].U.S.C.S.W. Group. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-based Report. 2017. [cited 2018.
- [2].Surveillance Epidemiology and Results End (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2016 Sub (1973–2014), Journal [Google Scholar]
- [3].Kim MJ, et al. , Survival paradox between stage IIB/C (T4N0) and stage IIIA (T1–2N1) colon cancer, Ann Surg Oncol 22 (2015), 505–12. [DOI] [PubMed] [Google Scholar]
- [4].Inamura K, Colorectal Cancers: An Update on Their Molecular Pathology, Cancers (Basel) 10 (2018), [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xie K, Interleukin-8 and human cancer biology, Cytokine Growth Factor Rev 12 (2001), 375–91. [DOI] [PubMed] [Google Scholar]
- [6].Singh RK and Lokeshwar BL, The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth, Cancer Res 71 (2011), 3268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vandercappellen J, Van Damme J, and Struyf S, The role of CXC chemokines and their receptors in cancer, Cancer Lett 267 (2008), 226–44. [DOI] [PubMed] [Google Scholar]
- [8].Sun X, et al. , CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression, Cancer Metastasis Rev 29 (2010), 709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maksym RB, et al. , The role of stromal-derived factor-1--CXCR7 axis in development and cancer, Eur J Pharmacol 625 (2009), 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu D, et al. , Drug Design Targeting the CXCR4/CXCR7/CXCL12 Pathway, Curr Top Med Chem 16 (2016), 1441–51. [DOI] [PubMed] [Google Scholar]
- [11].Rupertus K, et al. , Interaction of the chemokines I-TAC (CXCL11) and SDF-1 (CXCL12) in the regulation of tumor angiogenesis of colorectal cancer, Clin Exp Metastasis 31 (2014), 447–59. [DOI] [PubMed] [Google Scholar]
- [12].Hu TH, et al. , SDF-1/CXCR4 promotes epithelial-mesenchymal transition and progression of colorectal cancer by activation of the Wnt/beta-catenin signaling pathway, Cancer Lett 354 (2014), 417–26. [DOI] [PubMed] [Google Scholar]
- [13].Benedicto A, Romayor I, and Arteta B, CXCR4 receptor blockage reduces the contribution of tumor and stromal cells to the metastatic growth in the liver, Oncol Rep 39 (2018), 2022–2030. [DOI] [PubMed] [Google Scholar]
- [14].Guo F, et al. , CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks, Oncogene 35 (2016), 816–26. [DOI] [PubMed] [Google Scholar]
- [15].Teicher BA and Fricker SP, CXCL12 (SDF-1)/CXCR4 pathway in cancer, Clin Cancer Res 16 (2010), 2927–31. [DOI] [PubMed] [Google Scholar]
- [16].Kollmar O, et al. , Stromal cell-derived factor-1 promotes cell migration and tumor growth of colorectal metastasis, Neoplasia 9 (2007), 862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Altenburg JD, et al. , A naturally occurring splice variant of CXCL12/stromal cell-derived factor 1 is a potent human immunodeficiency virus type 1 inhibitor with weak chemotaxis and cell survival activities, J Virol 81 (2007), 8140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Onishi RM and Gaffen SL, Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease, Immunology 129 (2010), 311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zepp JA, et al. , IL-17A-Induced PLET1 Expression Contributes to Tissue Repair and Colon Tumorigenesis, J Immunol 199 (2017), 3849–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wu D, et al. , Interleukin-17: a promoter in colorectal cancer progression, Clin Dev Immunol 2013 (2013), 436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen ZY, et al. , A prognostic classifier consisting of 17 circulating cytokines is a novel predictor of overall survival for metastatic colorectal cancer patients, Int J Cancer 136 (2015), 584–92. [DOI] [PubMed] [Google Scholar]
- [22].Hurtado CG, et al. , Roles for Interleukin 17 and Adaptive Immunity in Pathogenesis of Colorectal Cancer, Gastroenterology 155 (2018), 1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aliper AM, et al. , A role for G-CSF and GM-CSF in nonmyeloid cancers, Cancer Med 3 (2014), 737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Geremia A and Arancibia-Carcamo CV, Innate Lymphoid Cells in Intestinal Inflammation, Front Immunol 8 (2017), 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen Y, et al. , An epithelial-to-mesenchymal transition-inducing potential of granulocyte macrophage colony-stimulating factor in colon cancer, Sci Rep 7 (2017), 8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xia W, et al. , Prognostic value, clinicopathologic features and diagnostic accuracy of interleukin-8 in colorectal cancer: a meta-analysis, PLoS One 10 (2015), e0123484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xiao YC, et al. , CXCL8, overexpressed in colorectal cancer, enhances the resistance of colorectal cancer cells to anoikis, Cancer Lett 361 (2015), 22–32. [DOI] [PubMed] [Google Scholar]
- [28].Cheng XS, et al. , CCL20 and CXCL8 synergize to promote progression and poor survival outcome in patients with colorectal cancer by collaborative induction of the epithelial-mesenchymal transition, Cancer Lett 348 (2014), 77–87. [DOI] [PubMed] [Google Scholar]
- [29].Yoshitake N, et al. , Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer, Br J Cancer 98 (2008), 1682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang SC, et al. , Nuclear expression of CXCR4 is associated with advanced colorectal cancer, Int J Colorectal Dis 25 (2010), 1185–91. [DOI] [PubMed] [Google Scholar]
- [31].Akishima-Fukasawa Y, et al. , Prognostic significance of CXCL12 expression in patients with colorectal carcinoma, Am J Clin Pathol 132 (2009), 202–10; quiz 307. [DOI] [PubMed] [Google Scholar]
- [32].Choi YJ, et al. , The prognostic role of serum C-X-C chemokine receptor type 4 in patients with metastatic or recurrent colorectal cancer, Onco Targets Ther 9 (2016), 3307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li YP, et al. , Role of CXCR4 and SDF1 as prognostic factors for survival and the association with clinicopathology in colorectal cancer: A systematic meta-analysis, Tumour Biol 39 (2017), 1010428317706206. [DOI] [PubMed] [Google Scholar]
- [34].Lv S, et al. , The association of CXCR4 expression with prognosis and clinicopathological indicators in colorectal carcinoma patients: a meta-analysis, Histopathology 64 (2014), 701–12. [DOI] [PubMed] [Google Scholar]
- [35].Wang D, et al. , Activation of CXCL12/CXCR4 renders colorectal cancer cells less sensitive to radiotherapy via up-regulating the expression of survivin, Exp Biol Med (Maywood) 242 (2017), 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhao H, et al. , CXCR4 over-expression and survival in cancer: a system review and meta-analysis, Oncotarget 6 (2015), 5022–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yang D, et al. , Expression of chemokine receptor CXCR7 in colorectal carcinoma and its prognostic significance, Int J Clin Exp Pathol 8 (2015), 13051–8. [PMC free article] [PubMed] [Google Scholar]
- [38].Liu J, et al. , IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma, Biochem Biophys Res Commun 407 (2011), 348–54. [DOI] [PubMed] [Google Scholar]
- [39].Tosolini M, et al. , Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer, Cancer Res 71 (2011), 1263–71. [DOI] [PubMed] [Google Scholar]
- [40].Lin Y, et al. , Interleukin-17 is a favorable prognostic marker for colorectal cancer, Clin Transl Oncol 17 (2015), 50–6. [DOI] [PubMed] [Google Scholar]
- [41].K. A Goldman Mary C B, Brooks Angela N, Zhu Jingchun, Haussler David., The UCSC Xena Platform for cancer genomics data visualization and interpretation., bioRxiv. (2018), [Google Scholar]
- [42].Kramer MW, et al. , Association of hyaluronic acid family members (HAS1, HAS2, and HYAL-1) with bladder cancer diagnosis and prognosis, Cancer 117 (2011), 1197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chi A, et al. , Molecular characterization of kidney cancer: association of hyaluronic acid family with histological subtypes and metastasis, Cancer 118 (2012), 2394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gahan JC, et al. , Chemokine and chemokine receptor expression in kidney tumors: molecular profiling of histological subtypes and association with metastasis, J Urol 187 (2012), 827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lokeshwar SD, et al. , Molecular Characterization of Renal Cell Carcinoma: A Potential Three-MicroRNA Prognostic Signature, Cancer Epidemiol Biomarkers Prev 27 (2018), 464–472. [DOI] [PubMed] [Google Scholar]
- [46].van Domburg R, et al. , Tools and techniques--statistics: how many variables are allowed in the logistic and Cox regression models?, EuroIntervention 9 (2014), 1472–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


