There is limited information on how hydrostatic pressure affects the fish heart’s response to other environmental challenges. Exposure to 80 bar of pressure increased the lumpfish’s activity and heart rate and eliminated their heart rate response to moderate hypoxia. However, it did not affect how heart rate changes with temperature.
Keywords: Biologgers, heart rate, hydrostatic pressure, hypoxia, lumpfish, temperature
Abstract
Studies on the effects of environmental changes with increasing depth (e.g. temperature and oxygen level) on fish physiology rarely consider how hydrostatic pressure might influence the observed responses. In this study, lumpfish (Cyclopterus lumpus, 200–400 g), which can exhibit vertical migrations of over 100 m daily and can be found at depths of 500 m or more, were implanted with Star-Oddi micro-HRT loggers. Then, their heart rate (fH) was measured in a pressure chamber when exposed to the following: (i) increasing pressure (up to 80 bar; 800 m in depth) at 10°C or (ii) increasing temperature (12–20°C), decreasing temperature (12 to 4°C) or decreasing oxygen levels (101–55% air saturation at 12°C) in the absence or presence of 80 bar of pressure. Additionally, we determined their fH response to chasing and to increasing temperature (to 22°C) at atmospheric pressure. Pressure-induced increases in fH (e.g. from 48 to 61 bpm at 12°C) were associated with hyperactivity. The magnitude of the rise in fH with temperature was greater in pressure-exposed vs. control fish (i.e. by ~30 bpm vs. 45 bpm between 5°C and 20°C). However, the relative increase (i.e. slope of the relationship) was not different between groups. In contrast, 80 bar of pressure eliminated the small (5 bpm) increase in fH when control fish were exposed to hypoxia. Exhaustive exercise and increasing temperature to 22°C resulted in a maximum fH of 77 and 81 bpm, respectively. Our research shows that pressure influences the fH response to environmental challenges and provides the first evidence that lumpfish have a limited capacity to increase fH.
Introduction
During vertical migrations to deeper waters, animals experience large changes in environmental conditions such as increases in hydrostatic pressure and reductions in temperature, oxygen (hypoxia) and light (Gross and Jaenicke, 1994; Andrzejaczek et al., 2019). However, due to the technical difficulties and high costs of gaining biological information while animals are under pressure (Guerrero et al., 2000; Shillito et al., 2014), there is still very little known about the physiological responses of fish to changing environmental conditions at depth (Andrzejaczek et al., 2019).
The physiological capacity of animals to cope with changes in their environment can inform biologists and managers of a species’ likelihood to be a ‘winner’ or ‘loser’ in the face of ocean warming and the expansion of oxygen minimum zones (Somero, 2010; Cooke et al., 2013). For example, changes in water temperature and oxygen levels have implications for future shifts in the bathymetric distribution of fish (Morris et al., 2015; Andrzejaczek et al., 2019). Specifically, it is primarily the capacity of the cardiovascular system that limits the depth range of ecologically and economically important species such as tuna and billfishes (Brill et al., 1998). Further, heart rate (fH), cardiac output and the scope available for fH may well determine the survival of fishes when exposed to abiotic and biotic challenges (Eliason et al., 2013; Farrell and Smith, 2017). Thus, it is critical that we learn more about the effects of these interacting variables on fish cardiovascular function.
Early research on the effects of hydrostatic pressure examined the tolerance and behavioural responses of a narrow range of fish species to acute increases in pressure (Brauer et al., 1974; Macdonald et al., 1987). Since then, the field has focused on the effects of acute and chronic pressure increases on the metabolic response of various fish species (e.g. see Sébert and Barthélémy, 1985a, b; Sébert and Macdonald, 1993; Speers-Roesch et al., 2004; Vettier et al., 2005, 2006). However, to our knowledge, no studies have measured the physiological response of fish to hypoxia in combination with pressure and very few studies have examined the combined effects of temperature and pressure (Sébert et al., 1995a, b; Scaion et al., 2008a, b). Further, even less is known about its effects on the cardiovascular system, and the published information is quite variable. For example, some studies indicate that pressure causes tachycardia in fish (Naroska, 1968 (c.f. Sébert and Macdonald, 1993); Sébert and Barthélémy, 1985b), while others report bradycardia (Belaud et al., 1976; Pennec et al., 1988), and the mechanisms mediating these effects are not yet known.
The common lumpfish (Cyclopterus lumpus) is an ecologically important marine species that is widely distributed on both sides of the Atlantic Ocean (Simpson et al., 2016; Powell et al., 2017). Further, it is a commercially important species due to the demand for their role as a substitute for sturgeon caviar, and their use as a cleaner fish in the Atlantic salmon (Salmo salar) aquaculture industry (Imsland et al., 2014; Powell et al., 2018). However, due to overfishing/harvesting, lumpfish have been designated as ‘Threatened’ by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC, 2017). Information on the physiological limits of this species will be important for its future conservation, and its proper management in the roe fishery and in the aquaculture industry. However, there is a limited understanding of the basic physiology of lumpfish and their tolerance to different environmental conditions (Ern et al., 2016; Jørgensen et al., 2017; Hvas et al., 2018; Hvas and Oppedal, 2019).
As mature adults, lumpfish migrate into shallow coastal waters in the spring and summer to reproduce. However, once the young become juveniles, they migrate out to the open ocean (Davenport, 1985). Most pelagic trawl records and video images suggest that lumpfish reside in the upper 60 m of the ocean, but they have also been found at depths up to 1000 m (Blacker, 1983; Rosen and Holst, 2013; Rosen et al., 2013). In addition, Kennedy et al. (2016) tagged Icelandic lumpfish with biologgers that recorded depth and temperature and they reported that the maximum recorded and extrapolated depth (based on temperature data) were 309 and 498 m, respectively, and that this species regularly engaged in daily vertical migrations of greater than 100 m.
Given that lumpfish can be found at a variety of depths and that data on their cardiovascular physiology and environmental tolerances are lacking, we set out to determine how hydrostatic pressure influences their capacity to deal with other abiotic factors. Star-Oddi micro-HRT loggers have been shown to provide reliable measurements of fH in other fish species (Brijs et al., 2019; Zrini and Gamperl, 2021) and would allow us to collect measurements of fH in a sealed pressure chamber (IPOCAMP, formally named ‘Incubateur Pressurisé pour l’Observation et la Culture d’Animaux Marins Profonds’). Considering the lack of information on the effects of pressure on the cardiovascular system of fish, we used these two unique pieces of equipment to examine: (i) the fH response of lumpfish to increasing hydrostatic pressure up to 80 bar (800 m in depth); (ii) the effect of prior exposure to 80 bar on the post-chase fH of lumpfish; and (iii) the fH response to decreasing temperature (12 to 5°C), increasing temperature (12 to 20°C) or decreasing oxygen levels (101–55% air saturation at 12°C) in the absence and presence of 80 bar of pressure. The experiment investigating the effect of increased pressure and temperature (which would be unlikely to occur unless fish were in the proximity of hydrothermal vents) was performed to more fully understand how temperature and pressure interact in the control of fH in fishes. In addition, we measured the lumpfish’s fH response during an acute warming protocol (increase by 2°C h−1 up to 22°C) at atmospheric pressure. This latter experiment was performed to determine if the limited maximum fH observed for this species in the IPOCAMP was similar to that measured by a standard warming protocol.
Materials and methods
Animal husbandry
All work described was approved by the Institutional Animal Care Committee of Memorial University (Protocol #17-95-KG) and followed the standards and guidelines outlined by the Canadian Council on AnimalCare.
The lumpfish used in these studies were reared at the Ocean Science Centre (Memorial University; Newfoundland, Canada) at 6°C. The lumpfish were then transferred to one of two 0.5-m3 tanks on 23 April 2018 (n = 56), 23 July 2018 (n = 22) and 16 January 2019 (n = 20) with densities never exceeding 16.8 kg m−3. Tanks were supplied with seawater at ~7.5°C and a 14-hour light:10-hour dark photoperiod. The temperature in these tanks was raised at a rate of 0.5°C per day to 10 or 12°C, this difference in temperature due to requirements of concurring projects in our multi-use facility. Lumpfish were held at these temperatures for a minimum of 14 days before use in experiments.
Logger implantation
The Star-Oddi (Gardabaer, Iceland; see https://www.star-oddi.com) micro-HRT logger (25.4 mm in length, 8.3 mm in diameter, 3.3 g in air) records and stores fH, electrocardiograms (ECGs) and temperature. These loggers were programmed using the company’s Mercury software prior to implantation.
To prepare the loggers for implantation, 3-O silk suture was tied around the body of the logger and the loggers and surgical equipment were cleaned and sterilized in 70% ethanol. The lumpfish were anaesthetized in seawater containing 0.15 g l−1 of tricaine methanesulfonate (MS-222; Skar et al., 2017). After losing equilibrium, the fish were moved onto a wetted surgical sponge where their gills were irrigated with flowing and aerated ~10°C seawater containing 0.075 g l−1 of MS-222. A 1.5- to 2.0-cm mid-ventral incision was made in the fish’s body wall beginning immediately posterior to the sucker. The logger was then inserted (blunt end first) in a posterior direction and pulled anteriorly to within 0.5 cm of the pericardium using the anchoring suture. A cutting-edge needle was used to pass the suture through the skin to secure the logger to the body wall at the anterior of the incision. Finally, the incision was closed with continuous stitches. One or two suture knots were also attached to the dorsal muscle to allow for the identification of fish in their holdingtank.
IPOCAMP set-up
Experiments #1 and #2 used IPOCAMP chambers (Autoclave, France; 19 l vessel, 60 cm high by 20 cm in diameter) (Fig. S1). The temperature of the chambers was controlled by a heater/chiller that regulated the temperature of both the in-flowing water and of the glycol jacket that surrounded each chamber. The water flowing into the IPOCAMPs came from a 50-l reservoir in which the oxygen level was controlled by a fibre-optic oxygen probe connected to a Witrox 1 oxygen system equipped with WitroxCTRL software and solenoid valves (Loligo Systems, Denmark). This system regulated the reservoir’s water oxygen content within relatively narrow limits (±2% air saturation) by bubbling air or nitrogen into the reservoir when water oxygen levels reached the lower and upper set points, respectively. These set points were determined by monitoring the oxygen content in the water leaving the chamber, as recorded by a Fibox 3 LCD oxygen metre (PreSens, Germany). A PT1000 probe was used to measure temperature in the reservoir (±0.15°C), and a pipe inspection camera was inserted into one of the view ports in the lid of the IPOCAMP to record the behaviour and activity of the fish during all experiments. Fibre-optic light sources and red filters were inserted into the other two view ports to provide adequate light and to maintain photoperiod.
Experiment #1: heart rate response to hydrostatic pressure and the fish’s maximum post-exercise heart rate
After implantation, lumpfish (n = 14, 0♀: 0♂: 3 immature: 11 not measured, 237.8 ± 5.3 g, 18.1 ± 0.3 cm, mean ± S.E.M.) were returned to their tank for recovery. At 48 hours post-surgery, two fish were transferred to a container with mesh sides (38.7 cm long × 24.8 cm wide × 29.2 cm deep) that was floating in the tank to be fasted for ~66 hours before being transferred to the IPOCAMP. This was necessary as water supplying the IPOCAMP passed through a fine filter that was easily clogged by faecal matter. Then fish, two at a time, were placed on the platforms of an insert that was lowered into the IPOCAMP (Fig. S1) and acclimated to the chamber overnight at 10°C and at 0 bar of pressure.
Immediately following surgery, the pre-programmed micro-HRT loggers saved ECGs and recorded fH (at 100 Hz for 6 seconds) and temperature every 4 hours during the recovery period. On the morning following acclimation to the IPOCAMP, the loggers began to save ECGs and record fH (100 Hz for 6 seconds) and temperature every 2 minutes. Then, the lumpfish were exposed to 0 pressure (n = 6; for use as time-matched controls) or to increasing levels of hydrostatic pressure (n = 8). Hydrostatic pressure was initially increased to 20 bar over 2 minutes, then held at this pressure for 8 minutes. Thereafter, pressure was increased to 35, 50, 65 and finally to 80 bar using the same protocol. The lumpfish were then decompressed in the opposite sequence (see Fig. 1 for a schematic representation of this experiment).
Figure 1.
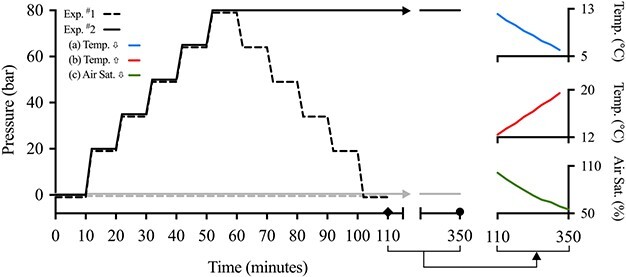
Protocols for compression and/or decompression in Experiment #1 (dotted lines) and #2 (solid lines). In Experiment #1, control lumpfish (grey lines) were held at 0 bar and the pressure-exposed fish (black lines) were compressed to 80 bar in a stepwise protocol and then decompressed to 0 bar using the same protocol (110 total minutes; diamond represents end of experiment). In Experiment #2, control fish were held at 0 bar and pressure-exposed fish were compressed to 80 bar using the stepwise protocol and held at pressure. After 1 hour, control and pressure-exposed fish were exposed to either: (a) decreasing temperature, (b) increasing temperature or (c) decreasing oxygen (340–350 total minutes; circle represents end of experiment).
Following the experimental protocol, most lumpfish were removed from the IPOCAMP and euthanized. However, four fish from each treatment were given 1 hour in the chamber for the pressure-exposed group to recover. Then, these fish were placed into a tote (75 cm long × 40 cm wide × 45 cm deep, 30 l) and continuously chased with a net for 1.5 minutes. Lumpfish were discouraged from attaching to the tote by constant poking/encouragement with the net. This allowed for an estimation of the fish’s maximum fH in response to exercise with and without prior exposure to pressure. However, no fish lost equilibrium during the chase or appeared to reach complete exhaustion, and thus their maximum post-chase fH may not have been reached.
Experiment #2: influence of hydrostatic pressure on heart rate and its response to changes in temperature and hypoxia
Lumpfish were recovered from surgery, fasted and transferred to the IPOCAMP chamber as described in Experiment #1, but at 12°C. The micro-HRT loggers were set to save ECGs and record fH (100 Hz for 6 seconds) and temperature every 4 hours on the day of being transferred to the IPOCAMP, every 2 minutes during the increase in pressure and when initially held at 80 bar (i.e. between 8:00 and 10:20 AM) and every 5 minutes for the rest of the duration of the experiment; the latter used to save logger memory and batterylife.
On the morning following acclimation to the IPOCAMP, lumpfish were exposed to increasing pressure to 80 bar and acclimated at this pressure for 1 hour, or maintained at atmospheric pressure (0 bar, control fish) for the same total duration (Fig. 1). The lumpfish were then exposed to one of the following treatments. In the first trial, lumpfish were exposed to decreasing temperature from 12°C to 5°C (n = 14, 7♀: 2♂: 3 immature: 2 not measured, 350.1 ± 12.4 g, 21.1 ± 0.2 cm). In the second trial, lumpfish (n = 15, 1♀: 0♂: 8 immature: 6 not measured, 404.9 ± 14.1 g, 21.2 ± 0.3 cm) were exposed to increasing temperature from 12 to 20°C (~2°C lower than the previously calculated critical thermal maximum or CTMAX for lumpfish; Ern et al., 2016). The rate of temperature change in both trials was ~ 2°C h−1. Finally, lumpfish (n = 16, 2♀: 6♂: 4 immature: 4 not measured, 435.8 ± 23.9 g, 21.9 ± 0.4 cm) were exposed to decreasing oxygen from 100% to 50% air saturation (~10% air saturation above their Pcrit calculated at 10°C; Ern et al., 2016). Temperature and oxygen were not brought close to the CTMAX or Pcrit of the lumpfish because they can attach to the platforms with their suckers even when unconscious, making loss of equilibrium difficult to determine (Ern et al., 2016). In addition, decompression and removal of fish from the pressure chamber takes a considerable amount of time. This posed ethical concerns for the welfare of thefish.
Experiment #3: the normobaric heart rate response to increased temperature
Lumpfish (n = 12, 544.5 ± 19.1 g, 23.7 ± 0.4 cm) were implanted with loggers, four at a time, then returned to their holding tank for recovery for 72 hours. After recovery, the fish were transferred into individual buckets (26.5 cm in diameter × 23.5 cm deep, 8 l) in a water bath with flowing seawater and sufficient aeration to maintain water oxygen levels near 100% saturation. The lumpfish were given 24 hours at 12°C to acclimate to the buckets, during which time, photoperiod was maintained at 14-hour light:10-hourdark.
On the morning following transfer, the pre-programmed micro-HRT loggers began saving ECGs and recording fH (100 Hz for 6 seconds) and temperature every 5 minutes at 8:00 AM. At 9:00 AM, water temperature was increased by 2°C h−1 to a maximum of 22°C (n = 8). Some lumpfish were also held at 12°C to serve as time-matched controls (n = 4).
Following all experiments, the fish were euthanized in 0.6 g l−1 MS-222 in order to perform post-mortem dissections and recover the data. Post-mortem dissections were conducted to record the distance from the front of the logger to the pericardium, the logger’s final position, to look for any signs of inflammation or infection and to determine sex based on the absence (i.e. immature fish) or presence of ovaries or testes.
Calculation of heart rate parameters
All reported measurements of fH were calculated manually using a previously described method (Zrini and Gamperl, 2021). Briefly, the average time between R wave peaks was measured (in seconds), and then 60 was divided by this number to obtain the fish’s fH in beats per minute (bpm). Quality index (QI) measurements for each ECG also were provided by the Mercury software (with QI0 indicating very good quality, QI1 and QI2 indicating decreasing quality and QI3 meaning that no R-R interval was detected). The absolute difference between fH values calculated by the on-board logger algorithm and those manually calculated ranged from 0 to 389 bpm (avg. absolute difference: QI0 = 1.4 bpm, QI1 = 9.1 bpm, QI2 = 15.9 bpm and QI3 = 21.9 bpm). When ECG artefacts made the PQRS complex unidentifiable, manual calculation was not possible and the data were not included. Percentage change in fH was calculated for each fish based on initial fH values in each experiment (e.g. as a percentage of 0 or 80 bar values prior to changes in temperature of oxygen level). Heart rate variability (HRV) was calculated as the standard deviation of time between successive R wave peaks (in ms). Heart rate scope (bpm) was determined in the pressure-exposed fish (Experiments #1 and #2) as the difference between mean fH at 0 bar and that measured once 80 bar was reached.
Lumpfish activity
Video was recorded during all experiments by connecting the pipe inspection camera in the view ports of the IPOCAMP’s lid to a laptop running VideoVelocity (CandyLabs, Vancouver, Canada). From these videos, the activity of all individuals was scored by assigning fish with a rank for each 10-minute period during exposure to pressure: 0 represented fish that were completely inactive; 1 represented fish that were mostly inactive but had some spontaneous activity; 2 represented fish that were mostly active, but had some periods of rest; and 3 represented fish that were active for the entire 10-minute period. Activity was ranked for all pressure steps in Experiments #1 and #2.
Statistical analyses
To assess the effects of surgical recovery on fH, linear mixed-effects (LME) models were used with photoperiod (day time between 6:00 AM and 7:59 PM and night time between 8:00 PM and 5:59 AM) and day (where N1 was the first night following surgery and D1–D5 represent the subsequent days) as the fixed-effects, an interaction term, and fish as a random factor.
The effects of pressure on fH, the percentage change in fH, HRV, activity rank and the percentage of good quality (i.e. QI0) ECGs were assessed using LME models with pressure step (0, 20, 35, 50, 65 and 80 bar, and either the reverse during decompression or 10-minute increments when pressure was held at 80 bar) and treatment (control fish at 0 bar or pressure-exposed fish) as the fixed effects, an interaction term and fish as a random factor. For all pressure-exposed fish in Experiments #1 and 2, the effects of sex (immature, female, male), temperature (10°C or 12°C) and activity rank at 0 bar on resting fH (at 0 bar) or fH scope (0 vs. 80 bar) were analysed by one-way ANOVAs and unpaired t-tests, while their relationship with weight (g), length (cm) and activity during pressure exposure was assessed using linear regressions. Lastly, the effect of resting fH on fH scope was determined using linear regression.
Linear regression analysis was also used to examine the relationships between fH and the percentage change in fH for each environmental parameter (decreased temperature, increased temperature, decreased oxygen and acute warming protocol on data up to 20.8°C), including determining whether the slopes and intercepts were different between treatments. Finally, an LME model with temperature/oxygen and treatment as fixed effects, an interaction term and fish as a random factor was used to assess changes in the percentage of QI0ECGs.
The LME models were performed in RStudio (v. 1.2.1335, RStudio Inc., Boston, MA, USA; http://www.rstudio.com) with the nlme package (Pinheiro et al., 2020), while the other analyses were performed using Prism 7 (GraphPad Software, Inc., San Diego, CA, USA). Assumptions of normality, homogeneity and independence were analysed by visual inspection of Q-Q plots and histograms of the residuals, residual-fit plots and residual lag plots, respectively, for data analysed in RStudio. The estimated marginal means, or emmeans, package (Singmann et al., 2019) was used to perform Bonferroni post-hoc tests on all LME models and Tukey’s multiple comparison tests were performed following one-way ANOVAs. The level of statistical significance was P < 0.05. All values presented in the text are means ± standard errors of the mean (S.E.M.).
Results
Heart rate recovery and diel patterns post-surgery
Following implantation of the micro-HRT loggers, the fH of lumpfish recovering in the holding tank at 10°C was recorded for 5 days (Fig. 2A). Average daily fH decreased significantly during the recovery period (P < 0.0001; Fig. 2B; Table S1), from 61.8 ± 0.9 and 59.1 ± 1.1 bpm (day-time and night-time values) on the first day to 54.4 ± 0.9 and 51.9 ± 1.0 bpm on the final day of recovery. There was also a significant effect of photoperiod (P = 0.0015). However, the diel variation was relatively small (2–4 bpm) and only significant on the first day post-surgery.
Figure 2.
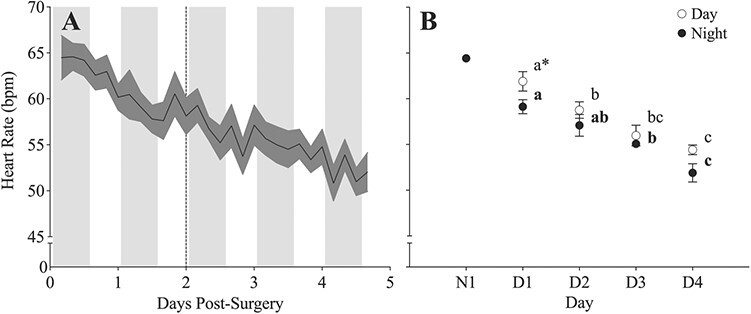
Moving average (A) and average (B) heart rate (fH, bpm) values in free-swimming lumpfish (n = 14) recorded every 4 hours for 5 days following the surgical implantation of the Star-Oddi micro-HRT logger. After 48 hours (dotted line), two lumpfish were transferred to containers inside the tank to be fasted for an additional ~66 hours. (A) Fish were on a 14-hour light:10-hour dark photoperiod (grey bars represent periods of darkness/night-time). (B) Open symbols represent day-time, while dark symbols represent night-time values. Dissimilar lower-case letters indicate a significant difference within a photoperiod group (for night-time values the letters are bolded), while an asterisk represents a significant difference between day-time and night-time values at each measurement point. The symbols represent means ± S.E.M. with each value representing the average of 3 data points per fish. Note: N1 was not included in the analysis.
Experiment #1: the heart rate response to hydrostatic pressure and the fish’s maximum post-exercise heart rate
Heart rate, the percentage change in fH, activity, HRV and percentage of QI0 ECGs remained constant in the control fish (Fig. 3). At 10°C, hydrostatic pressure had a significant effect on fH and the percentage change in fH (P = 0.0025; P = 0.0012; Table S2). Heart rate began to increase between 35 and 50 bar, and while further increases were limited, fH reached 61.5 ± 1.7 bpm (129.1 ± 3.8% of initial values) by 80 bar as compared to 48.1 ± 1.4 bpm in the control fish at the same time point (Fig. 3A). The lumpfish’s fH remained elevated during decompression. Following removal from the IPOCAMP, maximum post-exercise fH was 73.2 ± 1.4 and 76.8 ± 1.2 bpm (in control and pressure-exposed fish, respectively), suggesting that the pressure-induced increase in fH was only ~47% of the fish’s scope for increases in fH (Fig. 3A). Pressure significantly increased the activity of the fish exposed to 80 bar (P = 0.0006; Fig. 3E). At 0 bar, pressure-exposed fish had an average activity rank of 0.63 ± 0.18 and fish were either not moving (rank = 0) or swimming very little (rank = 1). Initial increases in activity began immediately upon compression, and activity rank peaked at 50 bar at 2.38 ± 0.18. Activity gradually decreased to normal levels during decompression.
Figure 3.
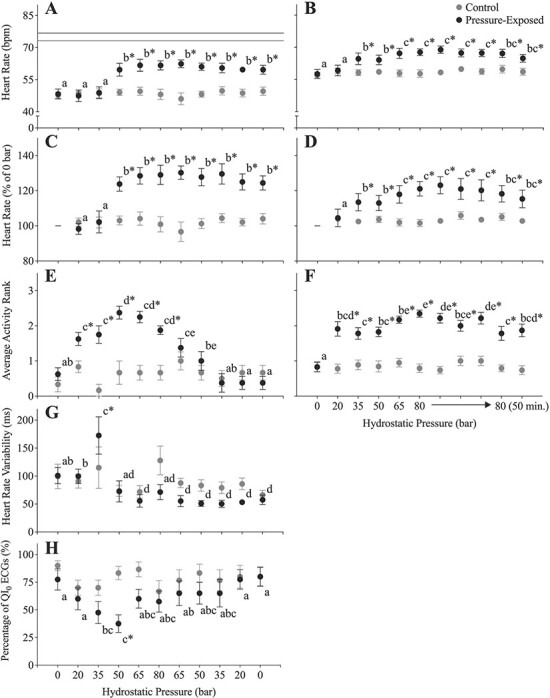
Lumpfish were held in the IPOCAMP at atmospheric pressure (0 bar, grey symbols) or exposed to hydrostatic pressure in a step-wise protocol (black symbols). Pressure was initially increased to 20 bar over 2 minutes, then held for 8 minutes. Pressure was then increased in a similar manner to 35, 50, 65 and 80 bar, followed by decompression in the opposite sequence (A, C, E, G, H; controls and 8 pressure exposed) or held at 80 bar for 1 hour (B, D, F; 19 controls and 23 pressure exposed). Heart rate (fH in bpm; A and B), the percentage change in fH (as a % of resting values at 0 bar; C and D) and HRV (in ms; G) were manually calculated from ECGs and the percentage of QI0 ECGs (H) were provided by the Star-Oddi Mercury software. The average activity rank (E and F) was determined from video recordings; where 0 represents fish that were completely inactive, 1 represents fish that were mostly inactive but had some spontaneous activity, 2 represents fish that were mostly active but had some periods of inactivity and 3 represents fish that were active for the entire 10-minute period. Maximum fH post-chase following removal from the IPOCAMP is represented as a grey (control; n = 4) or line in panel A. Dissimilar lower-case letters indicate a significant difference within the pressure-exposed group (no differences existed in the control group), while an asterisk indicates a significant difference (P < 0.05) between the pressure-exposed and control groups at a particular pressure step. Data were recorded every 2 minutes and the symbols represent means ± S.E.M (5 per fish).
Overall, treatment did not significantly affect HRV or the quality of ECGs (P = 0.2932 and P = 0.0519, respectively; Table S2). However, there was a significant interaction between treatment and ‘pressure step’. In the pressure-exposed group, HRV decreased from 100.9 ± 14.5 ms at the beginning to 71.4 ± 13.5 ms at 80 bar and to 57.6 ± 8.5 ms after decompression (Fig. 3G). Interestingly, HRV spiked to ~173 ms at 35 bar, this value significantly higher than measured in the control group at this pressure. The percentage of QI0 ECGs fell from 0 to 50 bar, and then gradually returned to initial values; the values at 35 and 50 bar were significantly different from that of the control group at these pressures (Fig. 3H). Interestingly, 35–50 bar of pressure corresponded to the beginning of fH increases, and the relationship between the percentage of QI0 values and pressure was a mirror image of that for activity.
Experiment #2: influence of hydrostatic pressure on the heart rate response to changes in temperature and hypoxia
At 12°C, the effects of hydrostatic pressure were similar to those at 10°C. However, fH began to increase at 35 bar instead of 50 bar (Fig. 3B). Heart rate was significantly elevated by hydrostatic pressure (P = 0.0053) throughout the period of exposure, and this was also reflected in values of fH when expressed as a percentage of initial values (P < 0.0001 for both parameters; Table S3). Heart rate did not change over the experiment in control fish. When pressure reached 80 bar, fH was 67.7 ± 1.6 bpm compared to 57.7 ± 1.7 bpm in control fish at the same sampling point (i.e. 121.1 ± 4.1% of initial values) and fH remained elevated above control values after 1 hour of acclimation to 80 bar. Similar to Experiment #1, the lumpfish were significantly more active when exposed to increased pressure (P < 0.0001). Activity peaked at 2.35 ± 0.12 at 80 bar and remained elevated for the 50 minutes of exposure to 80 bar of pressure.
When data from fish in Experiments #1 and #2 were combined, weight and length had no effect on resting fH (i.e. values at 0 bar), but sex significantly affected this parameter (P = 0.015), with females having a 14.6- and 14.1-bpm higher fH than immature or male fish, respectively (P = 0.014 and P = 0.092; Fig. S2; Table S4). Neither weight, length, sex, temperature nor activity affected the fH scope between 0 and 80 bar (P > 0.05; Fig. S2; Table S4). However, this fH scope was negatively correlated with resting fH, with fish with high fH values at 0 bar having a smaller increase in fH, or a decrease in fH, in response to pressure-exposure (P < 0.0001; Fig. S2; Table S4).
In control and pressure-exposed fish, fH fell with decreasing temperature (P < 0.0001 and P < 0.0001, respectively; Fig. 4A; Table S5). The fH of pressure-exposed fish was 7.0 bpm higher than that of control fish before the temperature began to decrease (i.e. at 80 bar), and the slopes of the relationship between fH and temperature were significantly different (P = 0.007; Table S5) as fH in both groups was 38–39 bpm at ~5.7°C. In contrast, the Q10 values, and the slopes of the relationships between relative fH (as a percentage of the initial value) and temperature, were not significantly different between the control and pressure-exposed groups (Fig. 4A; Table 1).
Figure 4.
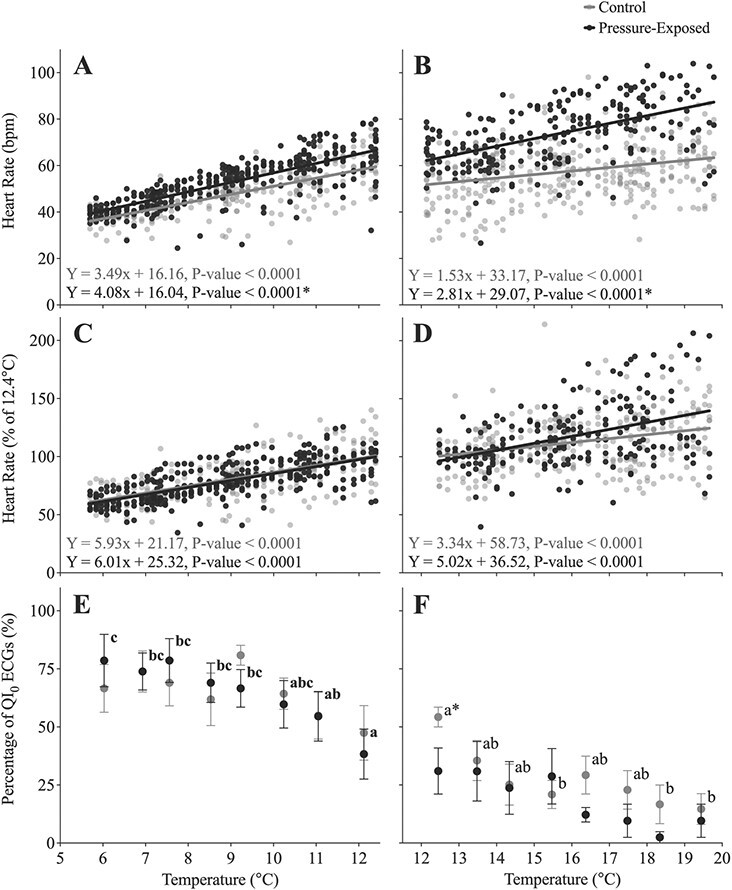
Lumpfish held at atmospheric pressure (grey symbols; 7 per experiment) or exposed to 80 bar of pressure (black symbols; 7 per experiment) were exposed to decreasing (at 2°C h−1; A, C, E) or increasing (at 2°C h−1; B, D, F) temperature in the IPOCAMP chamber. Heart rate (fH in bpm; A and B) and the percentage change in fH (as a % of resting values at 0 or 80 bar; C and D) were manually calculated from the ECGs. An asterisk indicates a significant difference between the slopes for relationships between control and pressure-exposed groups. The percentage of QI0 ECGs (E and F) was provided by the Star-Oddi Mercury software. In these panels, significant differences within the control (regular letters) or pressure-exposed groups (bold letters) are represented by dissimilar lower-case letters and an asterisk indicates a significant difference (P < 0.05) between the pressure-exposed and control groups at a particular temperature step. Data were recorded every 5 minutes, and the symbols represent means ± S.E.M (6 values per fish).
Table 1.
Summary of Q10 and average maximum values of fH recorded in lumpfish
| Experiment | Location | Treatment | Maximum fH (bpm) | Temperature (°C) | Q10 |
|---|---|---|---|---|---|
| #1 | Bucket | Chase – Control | 73 | 10 | - |
| Chase – Pressure | 77 | 10 | - | ||
| #2 | IPOCAMP | Control | - | 12.2–6.1 | 2.10 |
| Pressure | - | 12.0–5.9 | 2.07 | ||
| #2 | IPOCAMP | Control | 64 | 12.4–19.3 | 1.37 |
| Pressure | 83 | 12.5–19.6 | 1.39 | ||
| #3 | Bucket | Acute Warming | 81 | 12.3–20.8 | 1.67 |
Heart rate was 16.0 bpm higher in the pressure-exposed group before temperature was raised and increased in both control and pressure-exposed fish with temperature (P < 0.0001 and P < 0.0001, respectively; Fig. 4B). The slope of this relationship was significantly greater in the pressure-exposed group (P = 0.0078; Table S5). However, the Q10 values for fH (1.37 and 1.39) and the slopes of the relationships between temperature and relative fH (i.e. as a percentage of initial values) were again not significantly different (P = 0.1069). When the two temperature challenges are considered together, the fH of control and pressure-exposed lumpfish increased from 38 to 64 bpm and 39 to 83 bpm, respectively, from 5 to 20°C.
When held at atmospheric pressure in the IPOCAMP, the fH of lumpfish increased slightly with decreasing oxygen (P < 0.0001; Fig. 5A; Table S5), i.e. from 58.7 ± 3.2 bpm at 106% air sat. to 62.3 ± 2.9 bpm at 57% air sat. However, exposure to 80 bar of hydrostatic pressure eliminated the effect of decreasing oxygen level, i.e. fH remained unchanged from 103% to 57% air sat. (P = 0.7859). The same relationships were evident when fH data was calculated as a percentage of initial values (Fig. 5B; Table S5).
Figure 5.
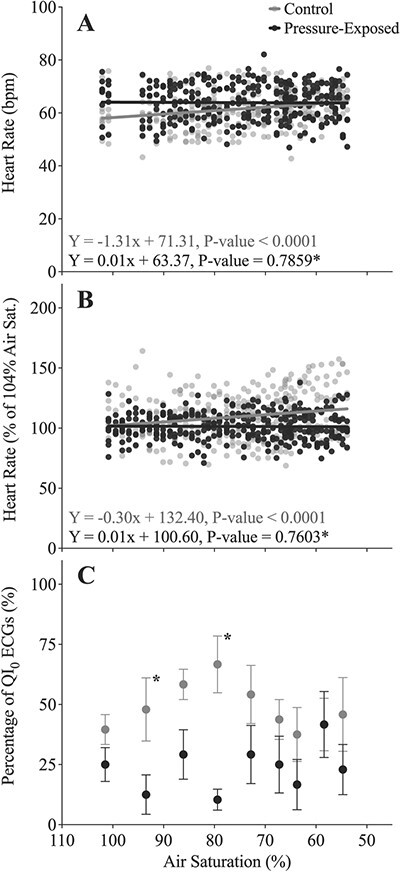
(A) Heart rate (fH, bpm) and (B) the percentage change in fH (as a % of initial values at 0 or 80 bar) in lumpfish exposed to decreasing oxygen levels (air saturation, %) in the IPOCAMP chamber over 3–4 hours. Prior to the decrease in oxygen levels, lumpfish were held at atmospheric pressure (black circles; n = 8) or exposed to 80 bar of pressure (grey circles; n = 8). An asterisk represents a significant difference in the slopes of the relationships between control and pressure-exposed lumpfish. (C) The percentage of QI0 ECGs were provided by the Star-Oddi Mercury software, and in this panel, an asterisk represents a significant difference between pressure-exposed and control groups at a particular oxygen level. Within each treatment group, there were no differences in the quality of ECGs. Data were recorded every 5 minutes, and the symbols represent means ± S.E.M (6 values per fish).
Hydrostatic pressure had a significant effect on the percentage of QI0 ECGs during the decreasing oxygen experiment (P = 0.0292; Table S6), but not in the decreasing or increasing temperature experiments (P = 0.9784 and P = 0.1939, respectively). On average, QI0 ECG values were ~25% fewer in pressure-exposed fish compared to control fish in the hypoxia experiment (48.4% vs. 23.6%). Conversely, decreasing or increasing temperature, but not decreasing oxygen (P < 0.0001, P < 0.0001 and P = 0.2698, respectively; Fig. 4E and F; Fig. 5C) strongly affected the quality of the ECGs. Overall, the percentage of QI0 ECGs fell from 72.6% at 6.0°C to 12.3% at 19.4°C.
Experiment #3: the normobaric heart rate response to increased temperature
When lumpfish underwent an acute warming protocol under normobaric conditions, fH was 52.4 ± 2.5 bpm at 12.3°C, peaked at 81.0 ± 3.6 bpm at 20.8°C (P < 0.0001) and then declined to 71.7 ± 3.6 bpm by 22.1°C (a scope of ~29 bpm between 12.3°C and 20.8°C; Q10 = 1.67) (Fig. 6A; Table 1; Table S5). This value for maximum fH was ~17 bpm higher than the corresponding value reached in fish held at atmospheric pressure in the IPOCAMP (~64 bpm at 19.6°C), but comparable to the maximum fH recorded for lumpfish at 80 bar (~83 bpm at 19.6°C). Increasing temperature also resulted in a significant decrease in the percentage of QI0 ECGs; this value being significantly lower than the time-matched control group at temperatures >15°C (P = 0.0041; Fig. 6C; Table S6). Overall, the percentage of QI0 values decreased from 45.8% at 12.3°C to 1.8% at 21.6°C (compared to 41.7% and 67.9% in the time-matched controls).
Figure 6.
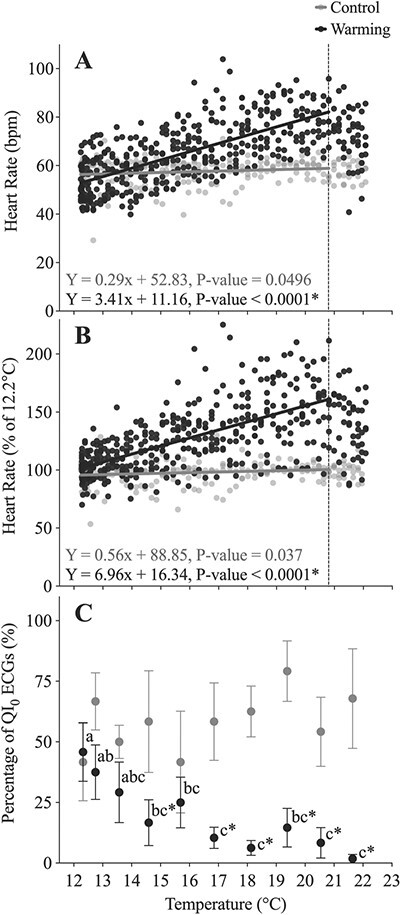
(A) Heart rate (fH, bpm) and (B) the percentage change in fH (as a % of initial values at 12.2°C) in lumpfish during an acute warming protocol (black symbols; n = 12) in a water table, where temperature was increased at 2°C h−1 vs. when fish were held at a constant temperature of 12°C (grey symbols; n = 4). An asterisk indicates a significant difference (P < 0.05) in the slopes between control fish and those exposed to increasing temperature. (C) The percentage of QI0 ECGs was provided by the Star-Oddi Mercury software. In this panel, significant differences within the warming group (there were no differences in the control group) are represented by dissimilar lower-case letters, and an asterisk represents a difference between the warming and control group at a particular temperature. Data were recorded every 5 minutes, and the symbols represent means ± S.E.M (6 per fish). The dotted line indicates the temperature of 20.8°C. Beyond this temperature the fH of the lumpfish began to decrease, and thus, these data were not included in the linear regression.
Discussion
Post-surgical recovery and diel patterns in heart rate
While not a primary goal of this research, the fH of lumpfish was recorded following the implantation of micro-HRT loggers to monitor recovery. This is because netting, handling, anaesthesia and surgery induce a physiological stress response in fish leading to increased fH (Altimiras and Larsen, 2000; Hill and Forster, 2004; Rothwell et al., 2005; Grӓns et al., 2014). Initially, the day-time fH of lumpfish was 62 bpm, but this value was ~60 bpm after 48 hours of recovery and 54 bpm by 5 days (at 10°C). Given the limited maximum fH in this species (~80 bpm, see below), it might be expected that they would have a low resting fH so that they would have a scope for fH comparable to other teleost species (1.8- to 2.6-fold increase in fH during CTMAX tests; Gollock et al., 2006; Clark et al., 2008; Vornanen et al., 2014; Penney et al., 2014; Motyka et al., 2017). These data may indicate that the lumpfish had not fully recovered from surgery and that this explains their high resting fH. This would agree with recent research on Atlantic salmon showing that while fH decreases most during the first week post-implantation it continues to fall for 2–3 weeks post-surgery (Hvas et al., 2020; Zrini and Gamperl, 2021). However, it is also possible given the low aerobic scope and Ucrit of this species (Hvas et al., 2018) that a large scope for fH is not required.
Overall, there was a significant effect of photoperiod on the fH of lumpfish during the recovery period (Fig. 2). However, day-time values were only significantly different than night-time values on the first day post-surgery, and diel variations in fH were relatively small (2 to 4 bpm). Conversely, Atlantic salmon had average diel variations of 7 bpm (and up to 14 bpm) at similar temperatures and time points post-surgery that were synchronous with changes in swimming activity (Zrini and Gamperl, 2021). It is possible that the dampened diel variations in fH were related to low day-time activity of the lumpfish; however, this parameter was not monitored in this study. While limited evidence suggests that lumpfish behave diurnally (Imsland et al., 2015; Leclercq et al., 2018), further investigation is needed to understand diel patterns of behaviour and physiology in this species, especially given the flexibility of these characteristics.
Heart rate response to hydrostatic pressure
The primary goal of this research was to investigate the effect of hydrostatic pressure on the fH of lumpfish. In response to an acute exposure to 80 bar of pressure, the fH of 10°C-acclimated lumpfish increased by ~14 bpm (20–30%) above resting values (Fig. 3A and C). Further, this tachycardia was sustained during 1 hour at 80 bar (Fig. 3B and D) and only diminished slightly during decompression. Previous research on this topic is extremely limited, possibly due to technical limitations (Guerrero et al., 2000). However, our results are generally consistent with other studies that have measured the effect of hydrostatic pressure on fH in fishes at temperatures within the middle of a species’ thermal range. For example, Sébert and Barthélémy (1985b) reported that (i) exposure to ~101 bar of pressure increased the fH of freshwater eels acclimated to 15–20°C by ~30–80% and that tachycardia was sustained during the 1 hour of pressure exposure; and (ii) while fH did fall to some degree during decompression, it was still not back to pre-exposure levels by 1 hour. Also, Naroska (1968) showed that abrupt compression to ~50 bar produced a transient tachycardia in 5°C eel pout (Zoarces viviparous) and Belaud et al. (1976) found that pressure induced a tachycardia below the temperature of 24.5°C in eels (c.f. Sébert and Macdonald, 1993). Further, our results agree with studies on the effects of pressure on oxygen consumption (ṀO2) in a variety of teleost species (reviewed in Sebert and Macdonald, 1993). While the relationship between ṀO2 and fH can be influenced by changes in stroke volume (Farrell and Smith, 2017), this latter data is still highly relevant to our conclusions (Armstrong, 1986; Lucas, 1994); especially given the limited fH data on the following topics.
With regards to the pressure at which increases in fH begin in fishes, the data are difficult to compare as the maximum hydrostatic pressure the fish is exposed, the rate of compression and temperature all influence the fH response to increased pressure (Sébert and Macdonald, 1993). In this study, fH began to increase between 30 and 50 bar and this is consistent with Sébert and Barthélémy (1985b) where, after no change or a brief bradycardia in some eels, fH began to increase at 40 to 50 bar. In contrast, the pressure at which the ṀO2 of male and female eels began to increase was between 50 and 80 bar (Scaion et al., 2008a), and Speers-Roesch et al. (2004) demonstrated that pressures as low as 3 bar increased ṀO2 in the bloater (Coregonus hoyi). The latter data suggest that the normal depth range of a given species likely has a significant effect on the sensitivity of their responses to increasing pressure. In this study, the increase in fH induced by hydrostatic pressure was only ~47% of the available scope for fH. Again, this is consistent with Sébert and Barthélémy (1985b) who reported that while the maximum temperature-induced fH in eels is ~120 bpm, fH when exposed to ~101 bar did not exceed 60 bpm. These data indicate that fish at high pressure (at least those whose life history includes excursions to the applied pressures) still have a considerable scope available for increases in fH.
Many authors attribute the reported increases in ṀO2 to a simultaneous increase in motor activity during compression (Sébert and Barthélémy, 1985a; Simon et al., 1989; Sébert et al., 1997; Vettier et al., 2003), which Speers-Roesch et al. (2004) suggested was partially related to compression of the swim bladder in bloater, and thus a loss of buoyancy. Lumpfish do not possess a swim bladder (Powell et al., 2017) and were supported by a platform in the pressure chamber. Nonetheless, they became agitated and more active during compression and this hyperactivity was maintained for 1 hour at 80 bar (see Fig. 3E and F); a behavioural response that may simply be an attempt to escape this novel situation. Sébert and Barthélémy (1985b) found that increases in motor activity during compression in eels were associated with tachycardia. These data strongly suggest that increased activity was primarily responsible for the increase in fH with pressure exposure. However, it is also possible that the observed tachycardia was in part related to alterations in the neurohormonal control of fH. This conclusion is based on three lines of evidence. First, exposing isolated eel hearts (which are free of neurohormonal control) to increased pressure resulted in a pronounced bradycardia, not tachycardia (Pennec et al., 1988). Second, Belaud et al. (1976) and Sébert and Barthélémy (1985b) show that atropine, and adrenergic agonists and antagonists, markedly alter the magnitude of the tachycardic response when eels are exposed to increased hydrostatic pressure. Third, HRV was considerably lower in the pressure-exposed group near the end of the compression period at 80 bar and remained lower during decompression (Fig. 3G). The mechanisms involved in pressure-induced increases in fH require investigation but could be related to alterations in cholinergic or adrenergic tone, or receptor function/affinity associated with changes in pacemaker cell membrane fluidity (Sébert and Barthélémy, 1985b). Further, several studies have provided evidence that a pressure-induced decrease in membrane fluidity, or ‘rigidification’, results in ‘compression-induced histotoxic hypoxia’ in fish (Sébert and Barthélémy, 1985a; Sébert et al., 1987; Sébert et al., 1993). However, this latter hypothesis/phenomenon is controversial and is not supported by data on the ṀO2 of permeabilized red muscle fibres (Scaion et al., 2008b) or blood PO2 (Sébert et al., 1997).
By combining the data from all of the pressure-exposed experiments, it was possible to also investigate the influence of acclimation temperature, sex, mass, length and activity on the initial fH and scope for fH in response to compression (Fig. S2). Despite females having a higher resting fH, we report that sex, weight, length and activity did not affect the scope for fH during compression. The lack of an effect of sex on pressure-induced physiology in lumpfish at 10–12°C is consistent with the ṀO2 data for eels at temperatures below 15°C (Scaion et al., 2008a). However, these authors also report that the ṀO2 of female eels was much more sensitive at 22°C as compared to males, and thus the effects of sex on hydrostatic pressure-related changes in the fH of fishes cannot be excluded. Interestingly, the initial fH of lumpfish at 0 bar significantly affected the fH scope in response to the stress of pressure exposure (Fig. S2). Previous research compliments these results, as the capacity for rainbow trout to increase fH in response to exercise was highly dependent on their resting fH (Brijs et al., 2019). It is possible that variation in the intrinsic fH of individuals, or in stress caused by the transfer to the IPOCAMP, resulted in a high allostatic load for some fish and that this reduced their ability to increase fH in response to pressure.
Influence of hydrostatic pressure on the heart rate response to changes in temperature and hypoxia
After lumpfish were exposed to 80 bar of pressure for 1 hour, changing temperature resulted in a linear change in fH. While the relationship was steeper for absolute fH in fish exposed to hydrostatic pressure, the relationship was similar to control fish when the elevated fH in pressure exposed fish at 12°C was taken into account (Fig. 4). These results suggest that while hydrostatic pressure does have an effect on resting fH, it does not influence the sensitivity of fH to changes in temperature. This finding was quite surprising as Scaion et al. (2008a) showed that temperature had a significant effect on the sensitivity of ṀO2 to increases in hydrostatic pressure, and Sébert et al. (1995b) reported that exposing eels to a 5°C temperature increase (from 15°C to 20°C) concomitantly with an increase in pressure to ~101 bar reduced the acute increase in ṀO2 by ~50%. Finally, while tachycardia is seen in pressure-exposed eels at lower temperatures, this response changes to a bradycardia at temperatures near this species’ critical thermal maximum (CTMAX; ~ 31°C) (Belaud et al., 1976; Claësson et al., 2016). The disparity in response to temperature between our study and these studies may be related to species or methodological differences. Most importantly, we exposed the lumpfish to elevated pressure for 1 hour prior to any changes in temperature, whereas the eels were exposed to changes in temperature either before, or in concert with, changes in hydrostatic pressure.
In the lumpfish, decreasing water PO2 at atmospheric pressure from ~100% to ~55% saturation resulted in a slight increase in fH (by ~5 bpm). This minor increase in fH was somewhat surprising as fH generally does not change as water PO2 is lowered to the point of bradycardia. However, such a response has been seen in several other fish species including the Atlantic cod (Gadus morhua) (Gamperl and Driedzic, 2009; Petersen and Gamperl, 2011). Exposure to pressure eliminated the small increase in fH that was observed in the control fish (Fig. 5). This is an interesting observation, and while the mechanism(s) mediating this difference is/are unknown, these data suggest that fish experiencing increased hydrostatic pressure and moderate hypoxia may have a very limited scope for increases in fH. Bradycardia is typically recorded at oxygen levels similar or slightly higher than a species’ critical oxygen tension, Pcrit (e.g. see Marvin and Heath, 1968; Gehrke et al., 1988; Speers-Roesch et al., 2010). Therefore, it is very likely that bradycardia was not recorded in this study because the lumpfish did not reach their Pcrit (~40% air saturation at 12°C; Ern et al., 2016). Future experiments are being planned to examine if hydrostatic pressure affects the oxygen level at which bradycardia is initiated and the magnitude of the decrease in fH.
Ultimately, the most relevant experimental scenario would be one that accurately reflects the environmental and behavioural challenges of a vertical migration, i.e. simultaneous increases in pressure and decreases in temperature and water oxygen levels while the fish is actively swimming (e.g. fish dealing with changing water currents or evading predation).
Maximum exercise and temperature-induced heart rate of lumpfish
Given the low maximum fH recorded in the IPOCAMP at 20°C (63 bpm) and following exercise at 10°C (73–77 bpm), an acute warming protocol was performed under normobaric conditions. The fH of lumpfish increased up to 20.8°C (Q10 = 1.67; Table 1) and began falling as temperatures approached the lumpfish’s CTMAX of 22°C (Fig. 6; Ern et al., 2016). This response is typical of that seen in other fish species, where fH increases (at Q10 values ranging from 1.5 to 2.5) up until ~2°C before the fish’s CTMAX (Gollock et al., 2006; Steinhausen et al., 2008; Gilbert et al., 2019). The highest individual fH recorded in lumpfish was 95 bpm, while the highest average fH at 20°C was 81 bpm. Thus, it appears that lumpfish have a low maximum fH relative to fish species such as the channel catfish Ictalurus punctatus (150 bpm; Burleson and Silva, 2011) and salmonids (105–132 bpm; Clark et al., 2008; Steinhausen et al., 2008; Vornanen et al., 2014; Motyka et al., 2017) and more typical of those recorded in species such as the Atlantic cod (72 bpm; Gollock et al., 2006), winter flounder (Pseudopleuronectes americanus; 73 bpm; Mendonça and Gamperl, 2010) and European perch (Perca fluviatilis; 83 bpm; Jensen et al., 2017).
These results, combined with previous data, suggest that lumpfish are well adapted to a passive, yet still pelagic, lifestyle. Hvas et al. (2018) reported that lumpfish have a low critical swimming speed and aerobic scope due to a limited maximum ṀO2. The results of this study agree with the findings of Hvas et al. (2018), as lumpfish were found to have a low scope for fH and a low maximum fH. Additionally, research shows that lumpfish have relatively low values of exercise-induced cortisol, glucose and lactate, which indicates that lumpfish have a limited capacity to perform exhaustive exercise (Clow et al., 2017; Jørgensen et al., 2017; Hvas et al., 2018). These physiological features are in contrast to most pelagic fish, which are built for strong swimming and aerobic performance, however, not surprising given the lumpfish’s globiform shape, weak tail musculature and uniquely docile nature (Hvas et al., 2018).
Considerations when using data loggers in lumpfish
Temperature had a strong effect on the quality of ECGs recorded by the micro-HRT logger. While most fH values could still be calculated by manually examining the ECG recordings, this is a concern for research being conducted at high temperatures or close to the CTMAX of the species being studied. It has been suggested that low-quality ECGs are related to increased activity at higher temperatures because the potentials from aerobic muscles overlap with the ECG (Altimiras and Larsen, 2000). Interestingly, the percentage of QI0 ECGs also transiently decreased during compression, which was also associated with an increase in activity (Fig. 2). However, we do not believe that this was the main factor affecting ECG quality because the percentage of QI0 recordings in Atlantic salmon during a Ucrit swim test never fell below 50% (Zrini and Gamperl, 2021). Instead, we believe that it was the low amplitude of the signal received by the logger that was the primary issue. In salmon, the R wave amplitude was ~510 mV (see Zrini and Gamperl, 2021), but it was only ~170 mV in the lumpfish (see Fig. S3). This low signal amplitude was not due to the size of the heart as the relative ventricular mass of lumpfish reared at 9°C is 0.94 (Hvas et al., 2018), and within the range of that reported for Atlantic salmon (Deitch et al., 2006; Anttila et al., 2015). Further, it is not that the lumpfish has a particularly large liver (e.g. the hepatosomatic index is only 2.5%; Hvas et al., 2018). However, the heart is relatively deep within the body cavity in lumpfish, and the liver’s position is such that it lies directly between the position of the logger and the heart. This may diminish the strength of the signal received by the data logger. It is possible that modifications may be able to be made to the logger’s design, or to the software/algorithms used to calculate fH, to enhance the logger’s usefulness for this species.
Conclusions
The effects of hydrostatic pressure on the cardiovascular system of fish are poorly understood, and this is often attributed to the difficulty of obtaining physiological data while fish are at pressure (Guerrero et al., 2000; Shillito et al., 2014). With the miniaturization and growing popularity of biologgers for use in fish (Wilson et al., 2015), we are learning about the vertical movement patterns of marine species, but this also leads to further questions such as the following: how physiological perturbations associated with pressure influence their capacity to deal with other environmental challenges or how simultaneous changes in conditions such as temperature and oxygen levels affect the heart’s response to pressure. Star-Oddi micro-HRT loggers and IPOCAMP chambers were successfully used in this research to show that acute exposure to hydrostatic pressure produced a tachycardia in lumpfish, but that this had no effect on the slope of the temperature-fH relationship when this pressure-induced increase was taken into account. In contrast, the minor increase in the fH of control fish to decreasing water PO2 was eliminated by exposure to hydrostatic pressure. Lastly, lumpfish were found to have a low maximum fH in response to exercise or a temperature increase close to their CTMAX relative to other fishes. Our research suggests that pressure can influence the fH response to environmental challenges and provides the first evidence that lumpfish have a limited capacity to increase fH.
Funding
This work was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant [#2016-0448 to A.K.G.] and partially supported by a Memorial University School of Graduate Studies Fellowship [to Z.A.Z.].
Supplementary Material
Acknowledgements
We thank Danny Boyce, Jessica Fry, Jennifer Monk and Ellen Peroni, for assistance with fish care; and Drs. Bill Driedzic, Iain McGaw, Erika Eliason and Travis Van Leeuwan, for comments on earlier versions of this manuscript (Z.A.Z. M.Sc. thesis). A special thanks to Asgeir Bjarnason from Star-Oddi for technical assistance with the micro-HRT loggers.
Supplementary material
Supplementary material is available at Conservation Physiology online.
References
- Altimiras J, Larsen E (2000) Non-invasive recording of heart rate and ventilation rate in rainbow trout during rest and swimming. Fish go wireless. J Fish Biol 57: 197–209. [Google Scholar]
- Andrzejaczek S, Gleiss AC, Pattiaratchu CB, Meekan MG (2019) Patterns and drivers of vertical movements of the large fishes of the epipelagic. Rev Fish Biol Fisheries 29: 335–354. [Google Scholar]
- Anttila K, Lewis M, Prokkola JM, Kanerva M, Seppӓnen E, Kolari I, Nikinmaa M (2015) Warm acclimation and oxygen depletion induce species-specific responses in salmonids. J Exp Biol 218: 1471–1477. [DOI] [PubMed] [Google Scholar]
- Armstrong JD (1986) Heart rate as an indicator of activity, metabolic rate, food intake and digestion in pike, Esox lucius. J Fish Biol 29: 207–221. [Google Scholar]
- Belaud A, Barthélémy L, Le Saint J, Oeyraud C (1976) Trying to explain an effect per se hydrostatic pressure on heart rate in fish. Aviat Space Environ Med 47: 252–257. [PubMed] [Google Scholar]
- Blacker RW (1983) Pelagic records of the lumpsucker, Cyclopterus lumpus L. J Fish Biol 23: 405–417. [Google Scholar]
- Brauer RW, Beaver RW, Hogue CD, Ford B, Goldman SM, Venters RT (1974) Intra- and interspecies variability of vertebrate high pressure neurological syndrome. J Appl Physiol 37: 844–851. [DOI] [PubMed] [Google Scholar]
- Brill RW, Lowe TE, Cousins KL (1998) How water temperature really limits the vertical movements of tunas and billfishes – it’s the heart stupid. In K Gamperl, A Farrell, D Mackinlay, eds, International Conference on the Biology of Fish. American Fisheries Society, Baltimore, pp. 57–62. [Google Scholar]
- Brijs J, Sandblom E, Rosengren M, Sundell K, Berg C, Axelsson M, Grӓns A (2019) Prospects and pitfalls of using heart rate bio-loggers to assess the welfare of rainbow trout (Oncorhynchus mykiss) in aquaculture. Aquaculure 509: 188–197. [Google Scholar]
- Burleson ML, Silva PE (2011) Cross tolerance to environmental stressors: effects of hypoxic acclimation on cardiovascular responses of channel catfish (Ictalurus punctatus) to a thermal challenge. J Therm Biol 36: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claësson D, Wang T, Malte H (2016) Maximal oxygen consumption increases with temperature in the European eel (Anguilla anguilla) through increased heart rate and arteriovenous extraction. Conserv Physiol 4: cow027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TD, Sandblom E, Cox GK, Hinch SG, Farrell AP (2008) Circulatory limits to oxygen supply during an acute temperature increase in Chinook salmon (Oncorhynchus tsawytscha). Am J Physiol Integr Comp Physiol 295: 1631–1639. [DOI] [PubMed] [Google Scholar]
- Clow KA, Short CE, Driedzic WR (2017) Low levels of extracellular glucose limit cardiac anaerobic metabolism in some species of fish. J Exp Biol 220: 2970–2979. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSEWIC (2017) COSEWIC assessment and status report on the lumpfish, Cyclopterus lumpus in Canada.
- Davenport J (1985) Synopsis of biological data on the lumpsucker (Cyclopterus lumpus Linnaeus, 1758). FAO Fish Synop 147: 31. [Google Scholar]
- Deitch EJ, Fletcher GL, Petersen LH, Costa IA, Shears MA, Driedzic WR, Gamperl AK (2006) Cardiorespiratory modifications, and limitations, in post-smolt growth hormone transgenic Atlantic salmon Salmo salar. J Exp Biol 209: 1310–1325. [DOI] [PubMed] [Google Scholar]
- Eliason EJ, Clark TD, Hinch SG, Farrell AP (2013) Cardiorespiratory collapse at high temperature in swimming adult sockeye salmon. Conserv Physiol 1: cot008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ern R, Norin T, Gamperl AK, Esbaugh AJ (2016) Oxygen dependence of upper thermal limits in fishes. J Exp Biol 219: 3376–3383. [DOI] [PubMed] [Google Scholar]
- Farrell AP, Smith F (2017) Cardiac form, function and physiology. In AK Gamperl, TE Gillis, AP Farrell, eds, The Cardiovascular System: Morphology, Control and Function. Elsevier Inc., Cambridge, MA, pp. 155–264. [Google Scholar]
- Gamperl AK, Driedzic WR (2009) Cardiovascular function and cardiac metabolism. In J Richards, AP Farrell, CJ Brauner, eds, Fish Physiology: Hypoxia, Ed 1 Vol. 27. Academic Press, Massachusetts, pp. 301–360. [Google Scholar]
- Gehrke PC, Fielder DR (1988) Effects of temperature and dissolved oxygen on heart rate, ventilation rate and oxygen consumption of spangled perch, Leiopotherapon unicolor (Günther 1859), (Percoidei, Teraponidae). J Comp Physiol B 157: 771–782. [Google Scholar]
- Gilbert MJ, Rani V, McKenzie SM, Farrell AP (2019) Autonomic cardiac regulation facilitates acute heat tolerance in rainbow trout: in situ and in vivo support. J Exp Biol 222: jeb194365. [DOI] [PubMed] [Google Scholar]
- Gollock MJ, Currie S, Petersen LH, Gamperl AK (2006) Cardiovascular and haematological responses of Atlantic cod (Gadus morhua) to acute temperature increase. J Exp Biol 209: 2961–2970. [DOI] [PubMed] [Google Scholar]
- Grӓns A, Sandblom E, Kiesslin A, Axelsson M (2014) Post-surgical analgesia in rainbow trout: is reduced cardioventilatory activity a sign of improved animal welfare or the adverse effects of an opioid drug? PLoS One 9: e95283. 10.1371/journal.pone.0095283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M, Jaenicke R (1994) Proteins under pressure: the influence of high hydrostatic pressure on structure, function and assembly of proteins and protein complexes. Eur J Biochem 221: 617–630. [DOI] [PubMed] [Google Scholar]
- Guerrero F, Theron M, Sébert P (2000) In vitro reactivity of central aorta to acetylcholine and noradrenaline in yellow freshwater eel (Anguilla anguilla L.) acclimatized to 10.1 MPa hydrostatic pressure. Can J Physiol Parmacol 78: 897–903. [PubMed] [Google Scholar]
- Hill J, Forster ME (2004) Cardiovascular responses of Chinook salmon (Oncorhynchua tshawytscha) during rapid anaesthetic induction and recovery. Comp Biochem Physiol C 137: 167–177. [DOI] [PubMed] [Google Scholar]
- Hvas M, Oppedal F (2019) Physiological responses of farmed Atlantic salmon and two cohabitant species of cleaner fish to progressive hypoxia. Aquaculture 512: 734353. [Google Scholar]
- Hvas M, Folkedal O, Imsland A, Oppedal F (2018) Metabolic rates, swimming capabilities, thermal niche and stress response of the lumpfish, Cyclopterus lumpus. Biol Open 7: bio036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvas M, Folkedal O, Oppedal F (2020) Heart rate bio-loggers as welfare indicators in Atlantic salmon (Salmo salar) aquaculture. Aquaculture 529: 735630. [Google Scholar]
- Imsland AK, Reynolds P, Eliassen G, Hangstad TA, Foss A, Vikinsta E, Elvegard TA (2014) The use of lumpfish (Cyclopterus lumpus L.) to control sea lice (Lepeophtheirus salmonid Krøyer) infestations in intensively farmed Atlantic salmon (Salmo salar L.). Aquaculture 424–425: 18–23. [Google Scholar]
- Imsland AK, Reynolds P, Eliassen G, Hangstad TA, Nytro AV, Foss A, Vikingstad E, Elvegard TA (2015) Assessment of suitable substrates for lumpfish in sea pens. Aquaculture 23: 639–645. [Google Scholar]
- Jensen DL, Overgaard J, Wang T, Gesser H, Malte H (2017) Temperature effects on aerobic scope and cardiac performance of European perch (Perca fluviatilis). J Therm Biol 68: 162–169. [DOI] [PubMed] [Google Scholar]
- Jørgensen EH, Haatuft A, Puvanendran V, Mortensen A (2017) Effects of reduced water exchange rate and oxygen saturation on growth and stress indicators of juvenile lumpfish (Cyclopterus lumpus L.) in aquaculture. Aquaculture 474: 26–33. [Google Scholar]
- Kennedy J, Jónsson S, Ólafsson HG, Kasper JM (2016) Observations of vertical movements and depth distribution of migrating female lumpfish (Cyclopterus lumpus) in Iceland from data storage tags and trawl surveys. ICES J Mar Sci 73: 1160–1169. [Google Scholar]
- Leclercq E, Zerafa B, Brooker AJ, Davie A, Migaud H (2018) Application of passive-acoustic telemetry to explore the behaviour of ballan wrasse (Labrus bergylta) and lumpfish (Cyclopterus lumpus) in commercial Scottish salmon sea-pens. Aquaculture 495: 1–12. [Google Scholar]
- Lucas MC (1994) Heart rate as an indicator of metabolic rate and activity in adult Atlantic salmon, Salmo salar. J Fish Biol 44: 889–903. [Google Scholar]
- Macdonald AG, Gilchrist I, Wardle CS (1987) Effects of hydrostatic pressure on the motor activity of fish from shallow water and 900 m depths; some results of Challenger Cruise 6B/85. Comp Biochem Physiol 88: 543–547. [DOI] [PubMed] [Google Scholar]
- Marvin DE, Heath AG (1968) Cardiac and respiratory responses to gradual hypoxia in three ecologically distinct species of fresh-water fish. Comp Biochem Physiol A 27: 349–355. [Google Scholar]
- Mendonça PC, Gamperl AK (2010) The effects of acute changes in temperature and oxygen availability on cardiac performance in winter flounder (Pseudopleuronectes americanus). Comp Biochem Physiol A 155: 245–252. [DOI] [PubMed] [Google Scholar]
- Morris JP, Thatje S, Cottin D, Oliphant A, Brown A, Shillito B, Ravaux J, Hauton C (2015) The potential for climate-driven bathymetric range shifts: sustained temperature and pressure exposures on a marine ectotherm, Palaemonetes varians. R Soc Open Sci 2: 150472. 10.1098/rsos.150472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyka R, Norin T, Petersen LH, Huggett DB, Gamperl AK (2017) Long-term hypoxia exposure alters the cardiorespiratory physiology of steelhead trout (Oncorhynchus mykiss), but does not affect their upper thermal tolerance. J Therm Biol 68: 149–161. [DOI] [PubMed] [Google Scholar]
- Petersen LH, Gamperl AK (2011) Cod (Gadus morhua) cardiorespiratory physiology and hypoxia tolerance following acclimation to low-oxygen conditions. Physiol Biochem Zool 84: 18–31. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S (2020) nlme: linear and nonlinear mixed effects models. R package version 3.1–148. https://CRAN.R-project.org/package=nlme.
- Powell A, Pooley C, Scolamacchia M, Garcia de Leaniz C (2017) Review of lumpfish biology. In JW Treasurer, ed, Cleaner Fish Biology and Aquaculture Applications. 5M Publishing Ltd, Sheffield, pp. 98–121. [Google Scholar]
- Powell A, Treasurer JW, Pooley CL, Keay AJ, Lloyd R, Imsland AK, Garcia de Leaniz C (2018) Use of lumpfish for sea-lice control in salmon farming: challenges and opportunities. Rev Aquacult 10: 683–702. [Google Scholar]
- Pennec J-P, Wardle CS, Harper AA, Macdonald AG (1988) Effects of high hydrostatic pressure on the isolated hearts of shallow water and deep sea fish; results of Challenger cruise 6B/85. Comp Biochem Physiol A 89: 215–218. [Google Scholar]
- Penney CM, Nash GW, Gamperl AK (2014) Cardiorespiratory responses of seawater-acclimated adult Arctic char (Salvelinus alpinus) and Atlantic salmon (Salmo salar) to an acute temperature increase. Can J Fish Aquat Sci 71: 1096–1105. [Google Scholar]
- Rosen S, Holst JC (2013) DeepVision in-trawling imaging: sampling the water column in four dimensions. Fish Res 148: 64–73. [Google Scholar]
- Rosen S, Jörgensen T, Hammersland-White D, Holst JC (2013) DeepVision: a stereo camera system provides highly accurate counts and lengths of fish passing inside a trawl. Can J Fish Aquat Sci 70: 1456–1467. [Google Scholar]
- R Core Team (2015) R: a language and environment for statistical computing. In R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/. [Google Scholar]
- Rothwell SE, Black SE, Jerret AR, Forster ME (2005) Cardiovascular changes and catecholamine release following anaesthesia in Chinook salmon (Oncorhynchus tshawytscha) and snapper (Pagrus auratus). Comp Biochem Physiol A 140: 289–298. [DOI] [PubMed] [Google Scholar]
- Scaion D, Belhomme M, Sébert P (2008a) Pressure and temperature interactions on aerobic metabolism of migrating European silver eel. Resp Physiol Neurobiol 164: 319–322. [DOI] [PubMed] [Google Scholar]
- Scaion D, Vettier A, Sébert P (2008b) Pressure and temperature interactions on aerobic metabolism in migrating silver eels: results in vitro. Undersea Hyperb Med 35: 27–33. [PubMed] [Google Scholar]
- Sébert P, Barthélémy L (1985a) Effects of high hydrostatic pressure per se, 101 atm on eel metabolism. Resp Physiol 62: 349–357. [DOI] [PubMed] [Google Scholar]
- Sébert P, Barthélémy L (1985b) Hydrostatic pressure and adrenergic drugs (agonists and antagonists): effects and interactions in fish. Comp Biochem Physiol C 82: 207–212. [DOI] [PubMed] [Google Scholar]
- Sébert P, Macdonald AG (1993) Fish. In AG Macdonald, ed, Effects of High Pressure on Biological Systems. Springer-Verlag, Berlin, pp. 147–196. [Google Scholar]
- Sébert P, Barthélémy L, Caroff J, Hourmant A (1987) Effects of hydrostatic pressure per se (101 ATA) on energetic processes in fish. Comp Biochem Physiol A 86: 491–495. [DOI] [PubMed] [Google Scholar]
- Sébert P, Simon B, Barthélémy L (1993) Hydrostatic pressure induces a state resembling histotoxic hypoxia in Anguilla anguilla. Comp Biochem Physiol 105: 255–258. [Google Scholar]
- Sébert P, Pequeux A, Simon B, Barthélémy L (1995a) Effects of hydrostatic pressure and temperature on the energy metabolism of the Chinese crab (Eriocheir sinensis) and the yellow eel (Anguilla anguilla). Comp Biochem Physiol 112: 131–136. [Google Scholar]
- Sébert P, Simon B, Barthélémy L (1995b) Effects of a temperature increase on oxygen consumption of yellow freshwater eels exposed to high hydrostatic pressure. Exp Physiol 80: 1039–1046. [DOI] [PubMed] [Google Scholar]
- Sébert P, Simon B, Péqueux A (1997) Effects of hydrostatic pressure on energy metabolism and osmoregulation in crab and fish. Comp Biochem Physiol 116: 281–290. [Google Scholar]
- Shillito B, Gaill F, Ravaux J (2014) The IPOCAMP pressure incubator for deep-sea fauna. J Mar Sci Tech 22: 97–102. [Google Scholar]
- Simon B, Sébert P, Barthélémy L (1989) Effects of long-term exposure to hydrostatic pressure per se (101 ATA) on eel metabolism. Can J Physiol Pharmacol 67: 1247–1251. [DOI] [PubMed] [Google Scholar]
- Simpson MR, Gauthier J, Benoît HP, Macdonald D, Hedges K, Collins R, Mello L, Miri C (2016) A pre-COSEWIC assessment of the common lumpfish (Cyclopterus lumpus, Linnaeus 1758) in Canadian Atlantic and Arctic waters. DFO Canadian Science Advisory Secretariat Research Document 2016/068. v + 135 p. Ottawa, ON, Canada.
- Singmann H, Hervé M, Love J, Buerkner P (2019) emmeans: estimating marginal means, aka least-square means. R package version 1.3.4.
- Skar MW, Haughland GT, Powell MD, Wergeland HI, Samuelsen OB (2017) Development of anaesthetic protocols for lumpfish (Cyclopterus lumpus L.): effect of anaesthetic concentrations, sea water temperature and body weight. PLoS One 12: e0179344. 10.1371/journal.pone.0179344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adapatation will determine ‘winners’ and ‘losers. J Exp Biol 213: 912–920. [DOI] [PubMed] [Google Scholar]
- Speers-Roesch B, Lingwood D, Stevens ED (2004) Effects of temperature and hydrostatic pressure on routine oxygen uptake of the bloater (Coregonus hoyi). J Great Lakes Res 30: 70–81. [Google Scholar]
- Speers-Roesch B, Sandblom E, Lau GY, Farrell AP, Richards JG (2010) Effects of environmental hypoxia on cardiac energy metabolism and performance in tilapia. Am J Physiol Regul Integr Comp Physiol 298: 104–119. [DOI] [PubMed] [Google Scholar]
- Steinhausen MF, Sandblom E, Eliason EJ, Verhille C, Farrell AP (2008) The effect of acute temperature increases on the cardiorespiratory performance of resting and swimming sockeye salmon (Oncorhynchus nerka). J Exp Biol 211: 3915–3926. [DOI] [PubMed] [Google Scholar]
- Vettier A, Székely C, Sébert P (2003) Are yellow eels from Lake Balaton able to cope with high pressure encountered during migration to the Sargossa sea? The case of energy metabolism. Anim Biol 53: 329–338. [Google Scholar]
- Vettier A, Amérand A, Cann-Moisan C, Sébert P (2005) Is the silvering process similar to the effects of pressure acclimatization on yellow eels? Resp Physiol Neurobiol 145: 243–250. [DOI] [PubMed] [Google Scholar]
- Vettier A, Labbe C, Amérand A, Da Costa G, Le Reumeur E, Moisan C, Sébert P (2006) Hydrostatic pressure effects on eel mitochondrial functioning and membrane fluidity. Undersea Hyperb Med 33: 149–156. [PubMed] [Google Scholar]
- Vornanen M, Haverinen J, Egginton S (2014) Acute heat tolerance of cardiac excitation in the brown trout (Salmo trutta fario). J Exp Biol 217: 299–309. [DOI] [PubMed] [Google Scholar]
- Wilson ADM, Wikelski M, Wilson RP, Cooke SJ (2015) Utility of biological sensor tags in animal conservation. Conserv Biol 29: 1065–1075. [DOI] [PubMed] [Google Scholar]
- Zrini ZA, Gamperl AK (2021) Validating heart rate and acceleration data storage tags for use in Atlantic salmon (Salmo salar). Anim Biotelem 9: 12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


