Abstract
Deficiencies in the ability to extinguish fear is a hallmark of Trauma- and stressor-related disorders, Anxiety disorders, and certain other neuropsychiatric conditions. Hence, a greater understanding of the brain mechanisms involved in the inhibition of fear is of significant translational relevance. Previous studies in rodents have shown that glutamatergic projections from the infralimbic prefrontal cortex (IL) to basolateral amygdala (BLA) play a crucial instructional role in the formation of extinction memories, and also indicate that variation in the strength of this input correlates with extinction efficacy. To further examine the relationship between the IL→BLA pathway and extinction we expressed three different titers of the excitatory opsin, channelrhodopsin (ChR2), in IL neurons and photostimulated their projections in the BLA during partial extinction training. The behavioral effects of photoexcitation differed across the titer groups: the low titer had no effect, the medium titer selectively facilitated extinction memory formation, and the high titer produced both an acute suppression of fear and a decrease in fear during (light-free) extinction retrieval. We discuss various possible explanations for these titer-specific effects, including the possibility of IL-mediated inhibition of BLA fear-encoding neurons under conditions of sufficiently strong photoexcitation. These findings further support the role of IL→BLA pathway in regulating fear and highlight the importance of methodological factors in optogenetic studies of neural circuits underling behavior.
Keywords: basolateral amygdala, infralimbic cortex, prefrontal cortex, fear, extinction, optogenetics, channelrhodopsin-2
Conditioned fear and extinction have become some of the most widely studied processes in neuroscience. This popularity is due to the simplicity and robustness of many of the typical experimental procedures involved, coupled with their translational relevance to understanding the causes of abnormal fear and extinction in Trauma- and stressor-related disorders and other neuropsychiatric disease states [1–4]. The growing body of data produced by this research has provided major new insights into the neural substrates of fear and extinction [5–8]. At the circuit level, for example, studies using pharmacological inactivation or optogenetic photosilencing approaches have shown that the rodent infralimbic cortex (IL) [9–13] and its reciprocal [14] projections to the basal amygdala (BA) [15–19] instruct cued fear extinction memory [20, 21].
Interestingly, there is also evidence that age- and genotype-related variation in the extent of IL (vmPFC in humans) innervation of the BLA is positively associated with the strength of extinction in mice and humans [22, 23]. These correlative observations suggest that variation in the functional efficacy of the IL→BLA pathway may be a tractable biomarker for predicting individual differences in the strength of fear extinction and associated risk for disorders linked to impaired extinction. To further examine the relationship between extinction and IL→BLA circuit function in the current study, we assessed the effects of optogenetically photostimulating IL→BLA neurons during cued fear extinction learning. By transfecting these neurons with different titers of AAV encoding the excitatory opsin, ChR2, we sought to vary the expression of the opsin and its efficacy for driving neuronal activity, and then test the consequences of this variation for extinction.
Male C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and housed 2 per cage (until surgery) in a temperature- and humidity-controlled vivarium under a 12-hour light/dark cycle (lights on 0630h, testing occurred during light on phase). Following surgery, mice were individually housed to prevent cage-mates damaging the implants. Experimental procedures were approved by the NIAAA Animal Care and Use Committee and followed the NIH guidelines outlined in Using Animals in Intramural Research and the local Animal Care and Use Committees.
To transfect the IL→BLA neurons with ChR2, mice were placed in a stereotaxic alignment system (Kopf Instruments, Tujunga, CA, USA) under isoflurane anesthesia. A viral vector containing ChR2 (rAAV5/CaMKII-hChR2(H134R)-eYFP obtained from the UNC Vector Core (Chapel Hill, NC, USA) or an eYFP control vector (rAAV5/CaMKIIα-eYFP, titer: 4.3 × 1012 molecules/mL) was bilaterally infused into the IL (0.18 μL/hemisphere) over 10 minutes using a Hamilton syringe and 33-gauge needle (with the needle left in place for an additional 5 minutes to ensure diffusion). The coordinates of the IL infusions were anterior/posterior +1.80 mm, medial/lateral ±0.35 mm and dorsal/ventral −2.80 mm, relative to bregma. The ChR2 vector was either unaltered from the original stock received (titer: 1.1 × 1013 molecules/mL = High titer group), diluted 1:2 (titer: 5.5 × 1012 molecules/mL = Medium titer group) or diluted 1:3 (titer: 3.7 × 1012 molecules/mL = Low titer group). The number of mice per group was as follows: eYFP=6, high titer=5, medium=5, low=8.
To determine the levels of eYFP fluorescence associated with each virus titer as an indicator of transfection, mice were deeply anaesthetized with sodium pentobarbital (50–60 mg/kg) at the completion of behavioral testing (see procedures below) and transcardially perfused with PBS followed by 4% PFA. After suspension in 4% PFA overnight followed by 0.1M PB at 4°C for 1–2 days, 50 μm thick coronal sections were cut with a Leica VT1200 S vibratome (Leica Biosystems, Buffalo Grove, IL, USA) and coverslipped with Vectashield HardSet mounting medium with DAPI (Vector Laboratories, Inc, Burlingame, CA, USA).
Images were acquired from separate brain sections containing the BLA or the IL using Olympus BX41 fluorescent microscope and a DP73 camera (Olympus Corporation, Center Valley, PA, USA). The same exposure duration settings were applied to acquire images across all mice in the green (eYFP) and blue (DAPI) channels. Representative images containing the IL were inspected for eYFP expression in the IL (Figure S1). A total of 10 images (5 per hemisphere) from consecutive 50 μm thick coronal sections containing the BLA were acquired from each mouse. Two mice were excluded from the quantification due to an insufficient number of sections for imaging. Fluorescence intensity within the defined area of the BLA measured from TIFF images by using the Fiji (‘Fiji Is Just ImageJ’) open source image processing package [23]. The BLA and neighboring CeA were manually defined using DAPI labeling and reference to a mouse atlas.
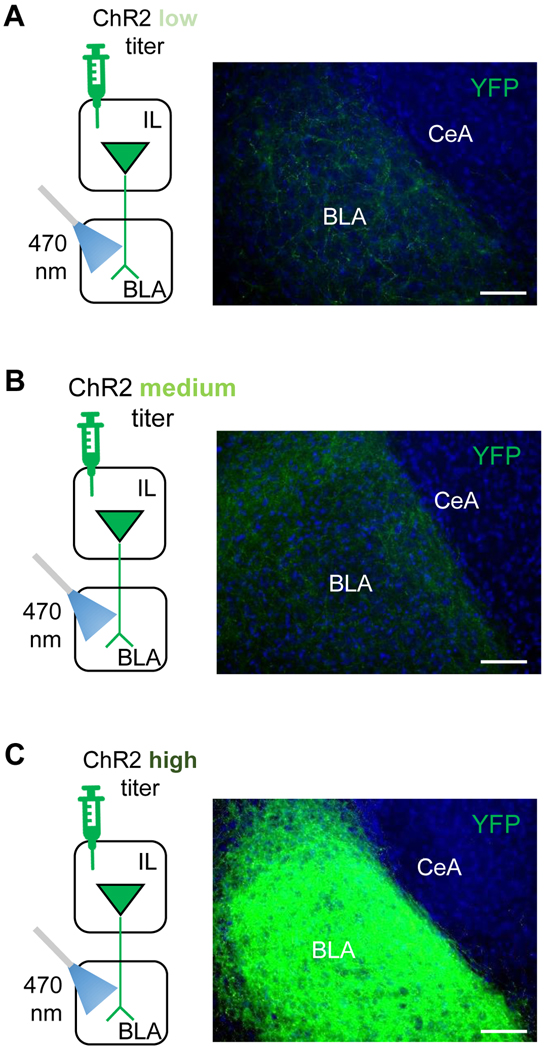
For each hemisphere, the level of fluorescence in the BLA was normalized to the largely-fluorescence devoid CeA and averaged across the 10 coronal sections. Consistent with prior studies that have transfected neurons with ChR2 in the IL or mPFC more broadly [11, 15, 24–26], there was eYFP labeling in the high titer group throughout most of the anterior extent of the BLA and particularly the more dorsomedial portion (average fluorescence intensity=103.0±9.3 arbitrary units (a.u.). Comparatively, fluorescence was 10-fold lesser in mice transfected with the medium (8.2 ± 2.3 a.u.) and low (10.9 ± 4.2 a.u.) titer ChR2 groups (Figure 1).
Figure 1: BLA expression of ChR2-YFP fluorescence from IL projections.
Representative images of YFP expression in the BLA following injection of low (A), medium (B) or high (C) titers of a YFP-fused ChR2-containing AAV construct into the IL. All 3 titer groups had optical fibers chronically implanted in BLA to shine blue light onto the transfected IL projections in BA. Scale bars = 50 μm.
Comparison of expression across different studies must be made with caution due to variations in the methods used. Nevertheless, fluorescence levels in the BLA in the high and medium titer groups are generally similar to those previously reported in ex vivo studies where IL or PL-originating ChR2-transfected neurons were optically-stimulated to generate robust ex vivo optically-evoked excitatory and inhibitory responses in the BLA [15, 19, 24, 25, 27]. As such, though we did not obtain direct measures of the functional efficacy of the virus (e.g., electrophysiological or immediate-early gene induction), we can tentatively conclude the expression levels in the high and medium titer groups was sufficient to drive increased IL→BLA transmission in response to blue light; a conclusion borne out by the behavioral effects evident in these groups (see below).
To assess behavioral differences between titer groups, ceramic ferrules were chronically implanted to bilaterally direct optic fibers at the BLA to direct light at virally transfected IL projections in the BLA. Ferrule affixation was performed during the same surgery as virus was infused in the IL (i.e., as described above and previously [11, 28]). The coordinates for the BLA implants were anterior/posterior −1.40 mm, medial/lateral ±3.22 mm and dorsal/ventral −4.70 mm relative to bregma. Optic fibers (5 mm length, cut from prefabricated fibers, 200 μm core, 0.39 NA, Thorlabs, Newton, NJ, USA) were dipped in the red dye solution (DiI, cat#468495, Sigma Aldrich, St Louis, MO, USA) to mark the location during histology (as described above) and secured to the skull using cyanoacetate and acrylic cement. The estimated location of the fiber tips is depicted in Figure S2. To minimize variability between groups, all surgeries were completed within 10 days and testing was subsequently conducted within a 10-day period. Six weeks after surgery, mice were handled for 2 minutes a day for 3 days and then habituated to being connected to the optical fibers for 30 minutes in the home-cage each day for 2 days.
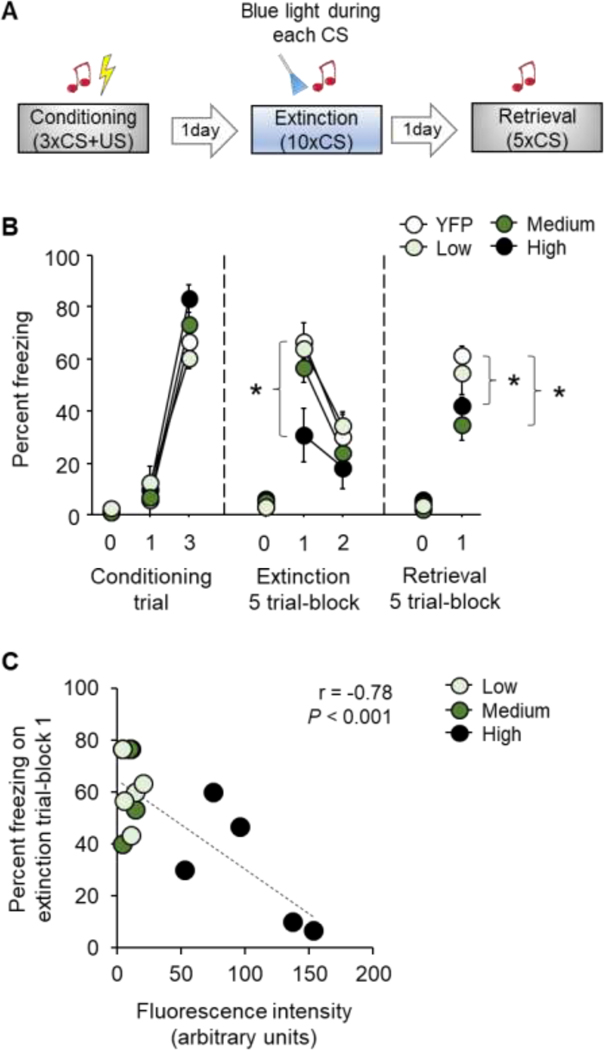
Cued fear conditioning, extinction training and extinction retrieval was conducted across 3 consecutive days as previously described [29–31] (Figure 2A). Each session began with a 3-minute habituation period. For conditioning, there were 3 pairings (variable 60–180 second interpairing interval) of a 30-second, 75 dB, white noise conditioned stimulus (CS) that co-terminated with a 2-second, 0.6 mA scrambled footshock unconditioned stimulus (US). For extinction training, a partial (10-trial) design was employed to produce suboptimal extinction and thereby enable sufficient range (avoid a floor effect) to detect of photostimulation-induced memory facilitation [10, 11]. There were 10 x CS-only presentations (5-second inter CS interval).
Figure 2: Effects of photoexcitation of the IL→BLA pathway on fear and extinction.
(A) Schematic of experimental design showing photoexcitation of the IL→BLA pathway during CS presentation during partial (10-trial) extinction training. (B) Relative to YFP controls photoexcitation of IL→BLA pathway decreased freezing on the first trial-block of extinction training and on subsequent (light-free) retrieval in mice expressing a high-titer viral construct, decreased freezing on retrieval only in medium titer expressing mice, and was without effect at any stage of testing in low titer expressing mice. (C) Freezing during first trial-block of extinction training inversely correlated with fluorescence intensity in the BA. Data are means ± SEM. eYFP n=6, high titer n=5, medium n=5, low n=8. *P<0.05 versus YFP.
During each CS of extinction training, blue light shone in the BLA at 20 Hz through a 200 μm patch cable connected to intensity division fiberoptic rotary joint (Doric Lenses Inc, Quebec, Canada) coupled to a 100 mW, 473 nm, laser system (Opto Engine, Midvale, UT, USA) interfaced with the Med Associates VideoFreeze system (Med Associates Inc, Fairfax, VT, USA) to deliver TTL pulses to a laser driver in synchrony with the CS. Laser power at the tip of the fiber was measured before each test using a power meter (PM20, Thorlabs, Newton, NJ, USA) and adjusted to achieve 10 mW (irradiance 0.5 mm from the fiber tip estimated at 4.73 mW/mm2). For extinction retrieval, there were 5 x CS-only presentations (5-second inter CS interval), with the optic fibers connected (but no light delivered) to ensure this part of the procedure was consistent with extinction training.
Regardless of titer group, freezing increased significantly from the first to the final US-paired CS during conditioning (ANOVA effect of trial: F1,20=370.80, P<0.01; effect of group: F3,20=0.98, P=0.42; CS x group interaction: F3,20=3.15, P=0.05) (Figure 2B). During extinction training, freezing decreased across CS trial-blocks in the eYFP, low and medium titer groups as compared to eYFP controls, whereas the high titer group did not show a decrease across trial-blocks due to lesser freezing on the first trial-block (ANOVA effect of trial-block: F1,20=127.30, P<0.01; effect of group: F3,20=3.17, P=0.05; trial-block x group interaction: F3,20=4.13, P=0.02, followed by Dunnett’s post hoc tests) (Figure 2B).
On the light-free extinction retrieval test, the high and medium but not low, titer groups froze less than eYFP controls (ANOVA effect of group: F3,20=3.18, P=0.05, followed by post hoc post hoc tests). Only the medium titer group froze less on retrieval as compared to the first trial-block of extinction training, indicative of a facilitation of extinction memory (paired t-test: t(4)=3.90, P=0.02) (Figure 2B). Finally, correlations between freezing and YFP fluorescence across individual mice in the 3 titer groups found a significant inverse correlation between fluorescence and freezing during first (Pearson’s r = −0.78, P<0.001) (Figure 2C) and last (Pearson’s r = −0.53, P<0.05) trial-blocks of extinction training, but not extinction retrieval (Pearson’s r = −0.06, P>0.05).
The absence of behavioral effects in the low titer group is parsimoniously explained by a level of ChR2 expression that is inadequate to excite IL input above a threshold sufficient to alter performance. The pattern of results in the medium titer group, however, is consistent with the facilitation of extinction memory formation and replicates the findings of our earlier study in which a similar IL→BLA optogenetic photoexcitation approach was used [11]. As such, these results confirm prior studies demonstrating a key contribution of IL inputs to BLA in the instantiation of extinction memory [20, 21], presumably through the promotion of plastic changes in the BLA that remain to be fully elucidated.
The most striking and novel behavioral effects were seen in the high titer group. Here, photoexcitation of IL→BLA projections produced lesser freezing on light-free retrieval, similar to that observed in the medium titer group. However, there was also a marked decrease in freezing during the first 5-trial block of extinction training. This decrease represents a decrease in the retrieval/expression of the original fear memory, rather than a rapid promotion of extinction learning within the first 5 training trials, because further inspection of the data showed freezing did not decrease across these trials. The finding that higher BLA fluorescence intensity associated with lesser freezing on this initial trial-block indicates a relationship between greater excitation of IL input in BLA and a reduction in fear. While these findings are apparently at odds with the view that the IL and the IL→BLA pathway is involved in regulating fear extinction but not fear expression per se [7, 12, 15, 24], some [10], but not other [12], recent optogenetic studies have shown that locally (within the IL) photoexciting IL neurons can, produce an acute suppression of fear prior to extinction, similar to our data in the high titer IL→BLA group.
How could strong drive of IL input to the BLA produce an acute suppression of CS-related fear? First, it should be noted that we did not find evidence that this reduction in freezing reflected increased levels of active forms of defensive behavior, such as ambulation and grooming (Figure S3). The YFP and high titer groups also did not differ in the magnitude of the flinch response to the shock during conditioning (YFP = 1.5 ± 0.1, high = 1.7 ± 0.2, on a manually scored 3-point flinch scale), suggesting that lesser freezing on extinction in the high titer group was not the result of lesser sensitive to the unconditioned stimulus.
Other technical explanations include the possibility that the high titer virus compromised IL neuronal function, due to toxicity, or that the greater efficiency of the higher titer meant it spread to other BLA-projecting prefrontal regions around the injection site which, when photoexcited, accounted for the acute reduction in fear. This former explanation seems unlikely given pre-conditioning lesioning or inactivation of the IL does not reduce fear expression [32, 33]. The latter explanation is also improbable, however, given that, although some spread into the prelimbic cortex (PL) was indeed apparent in this group (see Figure S1), the PL has a role in promoting not reducing fear [5–8].
An alternative explanation is that transfection with the high titer virus may have encompassed IL projections beyond the boundaries of BLA, such as the intercalated cell masses (ITCs), and that the photoexcitation of these targets (alone or in combination with the BLA produced reduced fear expression. This possibility cannot currently be ruled out, though the degree to which the ITCs may be involved in fear expression is unclear and the question of whether IL directly innervates these cells is itself controversial [11, 15, 19]. In an another scenario, relatively strong IL→BLA photoexcitation in the high titer group could have caused antidromic propagation of action potentials back to the IL and a resultant activation of additional downstream targets of the IL that have the effect of acutely reducing fear [30].
Finally, when sufficiently excited, IL projections could have suppressed the activity of fear-encoding BLA neurons to reduce fear expression, for example by generating interneuron-mediated feed-forward inhibition. In support of this interpretation, in vivo recordings have found CS and freezing-related activity in a subpopulation of BLA neurons [30, 34], while ex vivo physiological studies demonstrate that ChR2-mediated excitation of IL projections recruits inhibitory responses in the BLA [15, 19, 24, 25, 27]. Nonetheless, this scheme remains hypothetical in the absence of more direct evidence such as that obtained from combining IL→BLA photoexcitation with parallel in vivo BA neuronal recordings. It is also important to bear in mind that if such a mechanism could be demonstrated, it would still remain unclear whether an equivalently high level of excitation as that produced optogenetically can occur under physiological conditions to account for differences in fear behavior.
In summary, the current data show that IL→BLA photoexcitation during extinction training can cause a selective facilitation of extinction memory or an acute decrease in fear expression (or null effects) depending the titer of ChR2 containing virus used. It is likely that similar differences could be produced by varying other methodological factors in this type of study, such as the power, duration and frequency of light stimulation [10]. More broadly, these findings provide further evidence for an important contribution of the IL→BLA pathway in regulating fear, with implications for understanding the neural circuit dysfunctions underlying fear-related disorders.
Supplementary Material
Figure 1: IL expression of ChR2-YFP fluorescence. Representative examples and population heat map estimates of fluorescence in the IL following injection of low (A), medium (B) or high (C) titer YFP-fused ChR2-containing AAV. High titer n=5, medium n=5, low n=8. Scale bars = 500 μm.
Figure 2: Optical fiber placements in BA. Cartoon depicting optical fiber tip locations in the BLA of the low (A), medium (B) or high (C) titer groups. High titer n=5, medium n=5, low n=8.
Figure 3: Additional behavioral measures. No differences in grooming, ambulation or rearing between the YFP and high titer groups during extinction training (A) or retrieval (B). High titer n=5, YFP n=6.
Acknowledgements
Research supported by the NIAAA Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Milton AL, Holmes A, Editorial: the psychopharmacology of extinction-from theory to therapy, Psychopharmacology (Berl) 236(1) (2019) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fanselow MS, Poulos AM, The neuroscience of mammalian associative learning, Annu Rev Psychol 56 (2005) 207–34. [DOI] [PubMed] [Google Scholar]
- [3].Hartley CA, McKenna MC, Salman R, Holmes A, Casey BJ, Phelps EA, Glatt CE, Serotonin transporter polyadenylation polymorphism modulates the retention of fear extinction memory, Proc Natl Acad Sci U S A 109(14) (2012) 5493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Singewald N, Holmes A, Rodent models of impaired fear extinction, Psychopharmacology (Berl) 236(1) (2019) 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tovote P, Fadok JP, Luthi A, Neuronal circuits for fear and anxiety, Nat Rev Neurosci 16(6) (2015) 317–31. [DOI] [PubMed] [Google Scholar]
- [6].Bukalo O, Pinard CR, Holmes A, Mechanisms to medicines: elucidating neural and molecular substrates of fear extinction to identify novel treatments for anxiety disorders, Br J Pharmacol 171(20) (2014) 4690–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Milad MR, Quirk GJ, Fear extinction as a model for translational neuroscience: ten years of progress, Annu Rev Psychol 63 (2012) 129–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Likhtik E, Johansen JP, Neuromodulation in circuits of aversive emotional learning, Nat Neurosci 22(10) (2019) 1586–1597. [DOI] [PubMed] [Google Scholar]
- [9].Laurent V, Westbrook RF, Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction, Learn Mem 16(9) (2009) 520–9. [DOI] [PubMed] [Google Scholar]
- [10].Do-Monte FH, Manzano-Nieves G, Quinones-Laracuente K, Ramos-Medina L, Quirk GJ, Revisiting the role of infralimbic cortex in fear extinction with optogenetics, J Neurosci 35(8) (2015) 3607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bukalo O, Pinard CR, Silverstein S, Brehm C, Hartley ND, Whittle N, Colacicco G, Busch E, Patel S, Singewald N, Holmes A, Prefrontal inputs to the amygdala instruct fear extinction memory formation, Sci Adv 1(6) (2015) e1500251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim HS, Cho HY, Augustine GJ, Han JH, Selective Control of Fear Expression by Optogenetic Manipulation of Infralimbic Cortex after Extinction, Neuropsychopharmacology 41(5) (2016) 1261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bloodgood DW, Sugam JA, Holmes A, Kash TL, Fear extinction requires infralimbic cortex projections to the basolateral amygdala, Transl Psychiatry 8(1) (2018) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Little JP, Carter AG, Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala, J Neurosci 33(39) (2013) 15333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cho JH, Deisseroth K, Bolshakov VY, Synaptic encoding of fear extinction in mPFC-amygdala circuits, Neuron 80(6) (2013) 1491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ, Ferenczi E, Gunaydin LA, Mirzabekov JJ, Ye L, Kim SY, Lei A, Deisseroth K, Basomedial amygdala mediates top-down control of anxiety and fear, Nature 527(7577) (2015) 179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, Fadok JP, Muller C, Letzkus JJ, Luthi A, Long-range connectivity defines behavioral specificity of amygdala neurons, Neuron 81(2) (2014) 428–37. [DOI] [PubMed] [Google Scholar]
- [18].Klavir O, Prigge M, Sarel A, Paz R, Yizhar O, Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex, Nat Neurosci 20(6) (2017) 836–844. [DOI] [PubMed] [Google Scholar]
- [19].Strobel C, Marek R, Gooch HM, Sullivan RKP, Sah P, Prefrontal and Auditory Input to Intercalated Neurons of the Amygdala, Cell Rep 10(9) (2015) 1435–1442. [DOI] [PubMed] [Google Scholar]
- [20].Arruda-Carvalho M, Clem RL, Prefrontal-amygdala fear networks come into focus, Front Syst Neurosci 9 (2015) 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marek R, Sun Y, Sah P, Neural circuits for a top-down control of fear and extinction, Psychopharmacology (Berl) 236(1) (2019) 313–320. [DOI] [PubMed] [Google Scholar]
- [22].Gee DG, Fetcho RN, Jing D, Li A, Glatt CE, Drysdale AT, Cohen AO, Dellarco DV, Yang RR, Dale AM, Jernigan TL, Lee FS, Casey BJ, Consortium P, Individual differences in frontolimbic circuitry and anxiety emerge with adolescent changes in endocannabinoid signaling across species, Proc Natl Acad Sci U S A 113(16) (2016) 4500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, Huang C, Cravatt BF, Glatt CE, Hill MN, Casey BJ, Lee FS, FAAH genetic variation enhances fronto-amygdala function in mouse and human, Nat Commun 6 (2015) 6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Arruda-Carvalho M, Clem RL, Pathway-selective adjustment of prefrontal-amygdala transmission during fear encoding, J Neurosci 34(47) (2014) 15601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arruda-Carvalho M, Wu WC, Cummings KA, Clem RL, Optogenetic Examination of Prefrontal-Amygdala Synaptic Development, J Neurosci 37(11) (2017) 2976–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hubner C, Bosch D, Gall A, Luthi A, Ehrlich I, Ex vivo dissection of optogenetically activated mPFC and hippocampal inputs to neurons in the basolateral amygdala: implications for fear and emotional memory, Front Behav Neurosci 8 (2014) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cheriyan J, Kaushik MK, Ferreira AN, Sheets PL, Specific Targeting of the Basolateral Amygdala to Projectionally Defined Pyramidal Neurons in Prelimbic and Infralimbic Cortex, eNeuro 3(2) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Halladay LR, Kocharian A, Piantadosi PT, Authement ME, Lieberman AG, Spitz NA, Coden K, Glover LR, Costa VD, Alvarez VA, Holmes A, Prefrontal Regulation of Punished Ethanol Self-administration, Biol Psychiatry (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sengupta A, Holmes A, A Discrete Dorsal Raphe to Basal Amygdala 5-HT Circuit Calibrates Aversive Memory, Neuron 103(3) (2019) 489–505 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gunduz-Cinar O, Brockway E, Lederle L, Wilcox T, Halladay LR, Ding Y, Oh H, Busch EF, Kaugars K, Flynn S, Limoges A, Bukalo O, MacPherson KP, Masneuf S, Pinard C, Sibille E, Chesler EJ, Holmes A, Identification of a novel gene regulating amygdala-mediated fear extinction, Mol Psychiatry 24(4) (2019) 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rovira-Esteban L, Gunduz-Cinar O, Bukalo O, Limoges A, Brockway E, Muller K, Fenno L, Kim YS, Ramakrishnan C, Andrasi T, Deisseroth K, Holmes A, Hajos N, Excitation of Diverse Classes of Cholecystokinin Interneurons in the Basal Amygdala Facilitates Fear Extinction, eNeuro 6(6) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sierra-Mercado D Jr., Corcoran KA, Lebron-Milad K, Quirk GJ, Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction, Eur J Neurosci 24(6) (2006) 1751–8. [DOI] [PubMed] [Google Scholar]
- [33].Sierra-Mercado D, Padilla-Coreano N, Quirk GJ, Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear, Neuropsychopharmacology 36(2) (2011) 529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A, Switching on and off fear by distinct neuronal circuits, Nature 454(7204) (2008) 600–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1: IL expression of ChR2-YFP fluorescence. Representative examples and population heat map estimates of fluorescence in the IL following injection of low (A), medium (B) or high (C) titer YFP-fused ChR2-containing AAV. High titer n=5, medium n=5, low n=8. Scale bars = 500 μm.
Figure 2: Optical fiber placements in BA. Cartoon depicting optical fiber tip locations in the BLA of the low (A), medium (B) or high (C) titer groups. High titer n=5, medium n=5, low n=8.
Figure 3: Additional behavioral measures. No differences in grooming, ambulation or rearing between the YFP and high titer groups during extinction training (A) or retrieval (B). High titer n=5, YFP n=6.