Abstract
Patients with restrictive or hypertrophic cardiomyopathy (HCM) are often ineligible for a left ventricular assist device (LVAD) due to the risk of suction events with a small left ventricular cavity size and left ventricular inflow cannula. We describe an alternative LVAD configuration using a left atrial inflow cannula as a bridge to transplantation in an adult with HCM. (Level of Difficulty: Advanced.)
Key Words: acute heart failure, cardiac assist devices, cardiomyopathy, hemodynamics, left ventricle, pulmonary hypertension
Abbreviations and Acronyms: HCM, hypertrophic cardiomyopathy; LA, left atrium; LV, left ventricle; LVAD, left ventricular assist device; RA, right atrium; RCM, restrictive cardiomyopathy; RVAD, right ventricular assist device
Graphical abstract
Left ventricular assist devices (LVADs) are used as a bridging or destination strategy in select patients with advanced heart failure (1). Patients with restrictive or hypertrophic cardiomyopathy (RCM and HCM, respectively) are often deemed unsuitable for LVAD support due to the risk of suction events with an LV apical inflow cannula and a small LV cavity size. We describe an LA-to-aorta LVAD technique for adults which was previously only reported in children (2,3).
Learning Objectives
-
•
To identify contraindications to advanced heart failure therapies (heart transplantation, LVAD implantation) in a patient with HCM and a small LV cavity.
-
•
To list patient and device criteria that suggest suitability for durable LA-to-aorta LVAD support.
-
•
To understand the principles of management of a patient with a durable LA-to-aorta LVAD, including pump speed and thromboprophylaxis recommendations.
Presentation
A 57-year-old male with nonobstructive HCM presented with progressive dyspnea. Physical examination showed cold extremities; blood pressure of 86/64 mm Hg; a heart rate of 94 beats/min; distended jugular veins; and moderate pedal edema. Serum biochemistry analysis showed creatinine concentration of 2.6 mg/dl; B-type natriuretic peptide level of 1,373 pg/ml; lactate concentration of 2.0 mmol/l; and alanine aminotransferase concentration of 343 U/l. The diagnosis was cardiogenic shock and was treated with intravenous inotropic, vasodilator, and diuretic therapy.
Medical History
The patient had nonobstructive HCM (cardiac myosin-binding protein C gene-positive) and LV systolic dysfunction (left ventricular ejection fraction of 46%; left ventricular end-diastolic dimension, 42 mm); atrial fibrillation, cerebrovascular accident, and a primary prevention implantable cardioverter-defibrillator.
Differential Diagnosis
Cardiogenic shock was diagnosed by clinical, hemodynamic, and laboratory parameters (4). Clinicians must identify the cause of cardiogenic shock (i.e., pump failure, valvular heart disease, pericardial disease) to provide tailored therapy. The most likely cause in this case was an acute decompensation of nonobstructive HCM.
Investigations
We performed Invasive hemodynamic tests while milrinone was administered, 0.375 μg/kg/min intravenously, and showed right atrium (RA) pressure of 3 mm Hg, pulmonary artery pressure of 40/20 mm Hg (29), and wedge pressure of 10 mm Hg. A transpulmonary pressure gradient was measured at 19 mm Hg and a pulmonary artery saturation of 52%, cardiac output (indirect Fick method) of 3.5 l/min, and cardiac index of 2.0 l/min/m2. Combined pre- and post-capillary pulmonary hypertension with a pulmonary vascular resistance of 5.4 WU contraindicated heart transplantation. The patient remained dependent on inotropes for 16 days and required advanced therapies for heart failure treatment. A durable LVAD was considered, but standard LV apical cannulation was not possible due to severe concentric left ventricular hypertrophy and small LV cavity size (Figure 1, Video 1). The patient elected to undergo a nonstandard LVAD implantation instead of an extended hospitalization for inotrope therapy.
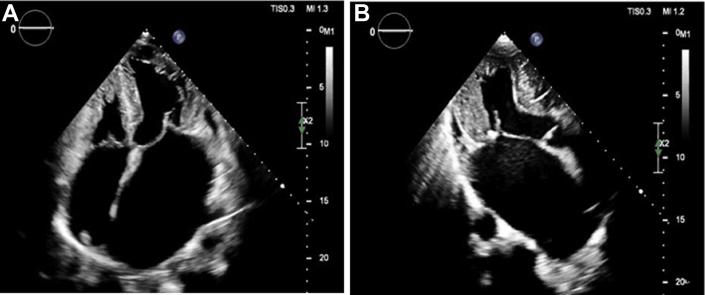
Figure 1.
Transthoracic Echocardiography
Transthoracic echocardiography shows apical 4-chamber (A) and apical 3-chamber (B) views in a patient with advanced heart failure due to hypertrophic cardiomyopathy. These views demonstrate severe left ventricular hypertrophy, small left ventricular cavity, and severe biatrial hypertrophy.
Management
To provide LVAD support as a bridge to candidacy, we implanted a HeartWare Ventricular Assist Device (Medtronic, Minneapolis, Minnesota) with an LA inflow cannula, using a transseptal RA approach. The HVAD device was selected for its smaller size and for experience with this technique in pediatric patients (2,3). This technique was feasible due to markedly dilated atria that could accommodate device components and suction (Figure 1, Video 1). A right atriotomy was performed through a median sternotomy approach with bicaval cannulation, and the heart arrested. A 20-mm atrial septal defect was created by excising the fossa ovalis and surrounding atrial septal tissue. A 20-mm ring-reinforced Gore-Tex graft (WL Gore, Newark, Delaware) was sewn to the atrial septal defect and cut to the pre-measured distance from the RA free wall to the septum (5 cm) (Video 2). The inflow cannula was placed into the graft and fixed with zip ties (Leco Plastics, Saddlebrook, New Jersey). The right atriotomy was closed around the base of the inflow cannula. The outflow graft was trimmed to reach the ascending aorta and anastomosed in the routine fashion. The patient was weaned from the cardiopulmonary bypass. The HVAD was set at a speed of 2,800 rpm; however, the aortic valve remained closed and spontaneous echocardiography contrast rapidly developed in the LV (Video 3).
An LA-to-aorta LVAD configuration results in reduced blood flow and increased afterload to the LV. This may result in the absence of LV ejection through the aortic valve and the rapid development of LV thrombus due to blood stasis. Therefore, the pump speed was lowered to 2,600 rpm. Echocardiography showed partial opening of the aortic valve with every cardiac cycle, mild mitral regurgitation, and laminar flow in the inflow and outflow cannulae (Figure 3, Video 4), with peak velocities of 0.4 and 1.0 m/s, respectively. The patient’s post-operative course was uncomplicated. Therapy with acetylsalicylic acid (ASA, aspirin), 325 mg daily, and warfarin, international normalized ratio (INR) target of 2.5 to 3.0 was started. Chest radiographs showed appropriate LVAD positioning (Figure 2). The patient was discharged home on post-operative day 15 and was followed with monthly echocardiograms to assess LV unloading and exclude device-related complications, such as intracardiac thrombus. In follow-up examinations, there were no device alarms, and the HVAD waveform remained flat. There were no hemocompatibility issues over the 4 months of LVAD support.
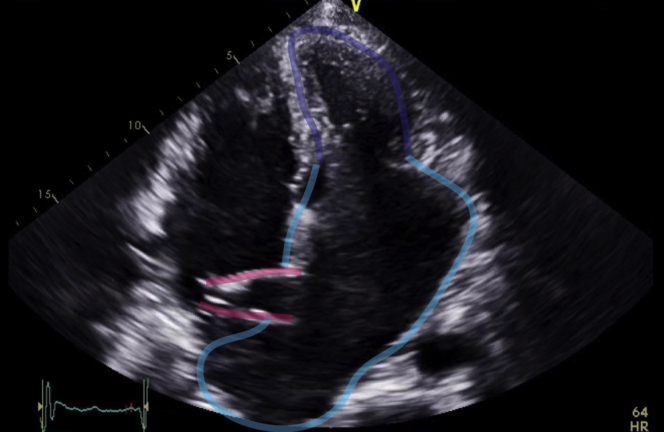
Figure 3.
Transthoracic Echocardiography, Apical 4-Chamber View
Apical 4-chamber transthoracic echocardiography shows a small and hypertrophic left ventricle (purple) and a severely dilated left atrium (blue), and the transseptal left atrial inflow cannula (pink) of a left atrium-to-aorta HeartWare Ventricular Assist Device.
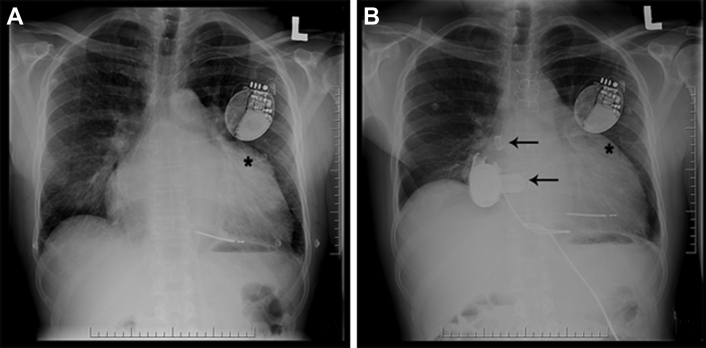
Figure 2.
Chest Radiography
Chest radiograph (A) shows an enlarged cardiopericardial silhouette, left atrial enlargement, and left atrial appendage enlargement (∗) in a patient with hypertrophic cardiomyopathy. (B) Chest radiography shows appropriate inflow and outflow cannulae positions (black arrows) and a decrease in size of the left atrial appendage following implantation of a left ventricular assist device in the same patient by a transseptal left atrial approach.
Discussion
Most LVAD patients have a dilated LV cavity that can accommodate standard LV apical cannulation. Patients with RCM and HCM are often deemed unsuitable for LVAD support due to the higher risk of complications including suction events, RV failure, and death (5); indeed, only 2% of patients with LVADs have RCM or HCM (6).
An LA-to-aorta LVAD configuration using a transseptal LA approach is an alternative to standard LV apical cannulation for select patients and has shown acceptable outcomes in pediatric patients (2,3). This technique can provide durable LVAD support as a bridge to transplantation for select adults, particularly those with pre-capillary pulmonary hypertension. The LA cannula will markedly unload the LA and likely result in suction across the pulmonary circulation, resulting in a wedge pressure of 0 mm Hg. With this technique, it is unclear whether the wedge pressure can estimate LA pressure and/or transpulmonary gradient to determine reversibility of pre-capillary pulmonary hypertension. This technique is limited by a less favorable hemodynamic profile (with higher LV peak systolic pressures and diastolic volumes) (7), risk of LV thrombus formation, and difficulty with RVAD placement if required. Therefore, these authors reserve this technique for patients who are otherwise ineligible for standard LV apical cannulation and who have severe LA dilation to accommodate an LA inflow cannula. To prevent LV thrombosis, pump speeds are reduced to allow for LV washing, and thromboprophylaxis is applied using ASA therapy, 325 mg daily and vitamin K antagonists with an INR target range of 2.5 to 3.0. The advantages of this technique include expanding LVAD candidacy to those who are otherwise ineligible.
Follow-Up
Pre-capillary pulmonary hypertension resolved after 4 months of LVAD support (Table 1). Heart transplantation was subsequently performed (Figure 4). The explanted heart and LVAD showed patches with a small amount of thrombus along the outer surface of the inflow cannula in the RA but no thrombus at the junction of the cannula orifice and LA. The postoperative course was complicated by vasoplegia and biventricular primary graft dysfunction, which required 4 days of venoarterial extracorporeal membrane oxygenation. The patient was discharged home on post-operative day 15.
Table 1.
Invasive Hemodynamic Measurements Performed Before and After Implantation of a HeartWare HVAD (Medtronic) in a Left Atrium-to-Aorta Configuration
| Pre-LVAD | Post-LVAD | |
|---|---|---|
| Right atrium, mm Hg | 3 | 6 |
| Pulmonary artery, mm Hg | 40/20 (29) | 25/6 (14) |
| Wedge, mm Hg | 10 | 0 |
| Cardiac output, l/min | 3.5 | 5.25 |
| Cardiac index, l/min/m2 | 2.0 | 2.73 |
| Right ventricular stroke work index | 8 | 5.72 |
| Mixed venous oxygen saturation, % | 52 | 59 |
| Transpulmonary gradient, mm Hg | 19 | 14 |
| Diastolic pulmonary gradient, mm Hg | 10 | 6 |
| Pulmonary vascular resistance, WU | 5.4 | 2.7 |
| Milrinone infusion, μg/kg/min | 0.375 | – |
| Pump speed, rpm | – | 2,600 |
| Power, W | – | 3.3 |
| Flow, l/min | – | 2.7 |
| Alarms | – | None |
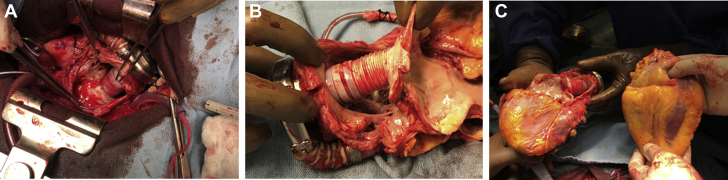
Figure 4.
Anatomy of the Heart Explant
(A) In situ view of the Gor-Tex graft (housing the inflow cannula) sewn to the atrial septum within the right atrium. (B) Close-up view of the Gor-Tex graft as it traversed the right atrium to join the left atrium at the atrial septum. (C) Explanted heart with ventricular assist device attached (left), and donor heart (right).
Conclusions
Patients with RCM and HCM who are ineligible for the standard LV-to-aorta LVAD configuration may be candidates for an LA-to-aorta LVAD configuration using a transseptal LA inflow cannula. To the authors’ knowledge, this is the first report of a durable LVAD implantation in an adult with severe HCM using this technique.
Author Disclosures
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Marat Fudim, MD, served as Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Apical 4-chamber transthoracic echocardiographic view shows severe LV hypertrophy, small left ventricular cavity size, and severe biatrial enlargement in a patient with advanced heart failure due to hypertrophic cardiomyopathy.
Transesophageal 3D echocardiographic left atrial view of the interatrial septum shows the transseptal LA inflow cannula orifice.
Transesophageal echocardiogram mid-esophageal long-axis aortic valve view shows the presence of spontaneous echocardiographic contrast in the LV outflow tract and absence of aortic valve opening with an HVAD pump speed of 2,800 rpm.
Transthoracic echocardiogram apical 4-chamber view shows the inflow cannula of an LA-to-aorta HVAD implanted by using a transseptal LA approach. There is laminar flow and a peak velocity of 0.4 m/s in the inflow cannula at a pump speed of 2,600 rpm.
References
- 1.Stewart G.C., Givertz M.M. Mechanical circulatory support for advanced heart failure: patients and technology in evolution. Circulation. 2012;125:1304–1315. doi: 10.1161/CIRCULATIONAHA.111.060830. [DOI] [PubMed] [Google Scholar]
- 2.Ma M., Yarlagadda V.V., Rosenthal D.N., Maeda K. A novel inflow cannulation strategy for pediatric mechanical circulatory support in small left ventricles. J Thorac Cardiovasc Surg. 2017;154:e47–e48. doi: 10.1016/j.jtcvs.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K., Nasirov T., Yarlagadda V. Novel trans-septal left atrial VAD cannulation technique for hypertrophic/restrictive cardiomyopathy. J Heart Lung Transplant. 2019;38:S479. [Google Scholar]
- 4.Diepen S van, Katz J.N., Albert N.M. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 5.Sreenivasan J., Kaul R., Khan M.S. Left ventricular assist device implantation in hypertrophic and restrictive cardiomyopathy. ASAIO J. 2020 July 28 doi: 10.1097/MAT.0000000000001238. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Patel S.R., Saeed O., Naftel D. Outcomes of restrictive and hypertrophic cardiomyopathies after LVAD: an INTERMACS analysis. J Card Fail. 2017;23:859–867. doi: 10.1016/j.cardfail.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Korakianitis T., Shi Y. Numerical comparison of hemodynamics with atrium to aorta and ventricular apex to aorta VAD support. ASAIO J. 2007;53:537–548. doi: 10.1097/MAT.0b013e318142bfce. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apical 4-chamber transthoracic echocardiographic view shows severe LV hypertrophy, small left ventricular cavity size, and severe biatrial enlargement in a patient with advanced heart failure due to hypertrophic cardiomyopathy.
Transesophageal 3D echocardiographic left atrial view of the interatrial septum shows the transseptal LA inflow cannula orifice.
Transesophageal echocardiogram mid-esophageal long-axis aortic valve view shows the presence of spontaneous echocardiographic contrast in the LV outflow tract and absence of aortic valve opening with an HVAD pump speed of 2,800 rpm.
Transthoracic echocardiogram apical 4-chamber view shows the inflow cannula of an LA-to-aorta HVAD implanted by using a transseptal LA approach. There is laminar flow and a peak velocity of 0.4 m/s in the inflow cannula at a pump speed of 2,600 rpm.