Abstract
Transthyretin amyloidosis involves the deposition of transthyretin amyloid fibrils in the body. We report an unusual case of a young Afro-Caribbean woman harboring a Thr60Ala mutation who presented with clinical signs of heart failure and polyneuropathy confirmed with genetic testing and results of an abdominal fat pad biopsy. (Level of Difficulty: Intermediate.)
Key Words: amyloidosis, cardiomyopathy, early-onset, hereditary, polyneuropathy, transthyretin
Abbreviations and Acronyms: ECG, electrocardiogram; hATTR, hereditary transthyretin amyloidosis; Thr60Ala, alanine for threonine substitution at amino acid 60; TTR, transthyretin
History of Present Illness
The patient is an Afro-Caribbean woman, born in St. Lucia, who presented at 23 years of age with multisystem ailments. She was experiencing gastrointestinal issues suggestive of gastroparesis presenting as daily, epigastric abdominal pain (severity 6 out of 10) that was worse with meals, loss of appetite resulting in unintentional weight loss, nausea, vomiting, and constipation. The patient was also experiencing cardiovascular symptoms such as dizziness, chest pain and palpitations with exertion, fatigue, exhaustion, dyspnea, and edema in her extremities.
Learning Objectives
-
•
To understand that hATTR has a heterogeneous presentation, which often includes autonomic symptoms that can mask cardiac involvement.
-
•
To bring awareness to hATTR, expressing its autosomal dominant mode of inheritance, early onset, and involvement of the heart in more than one-half of cases.
-
•
To show how emerging treatments, including TTR silencers and stabilizers, have shown to be effective for this disorder.
Two years before this visit, the patient had epigastric abdominal pain and was diagnosed with gastritis and treated with a proton pump inhibitor. This gave the patient some relief. However, the following year, her abdominal pain recurred as a stabbing sensation intermittently, along with her previous gastrointestinal symptoms, in addition to bilious vomiting and a weight loss of 30 lbs in a few months. She also presented with new symptoms of numbness and tingling in her hands and feet, dizziness, reduced ability to perform daily chores due to fatigue, dyspnea on exertion and when laying supine, and more swelling in her lower extremities.
Medical History/Prior Hospitalizations
Six years earlier, the patient had been hospitalized in St. Lucia and was treated for gastritis.
Differential Diagnosis
Possible conditions affecting this patient include autonomic polyneuropathy, amyloidosis, autoimmune disorders, hypothyroidism, diabetic polyneuropathy, HIV/AIDS, and vitamin deficiency (B12).
Physical Examination
The patient’s vital signs were as follows: blood pressure, 111/65 mm Hg; heart rate, 99 beats/min; respiratory rate, 16 breaths/min; blood oxygen saturation, 100%; temperature, 36.4°C; weight, 51.4 kg (113.3 lbs); height, 161 cm (5 ft 3 inches); and body mass index, 19.8 kg/m2.
The general appearance of the patient was thin and emaciated. She had a normal cardiac rhythm without any murmurs, rubs, or gallops; however, there was a weak apical impulse, and a displaced left ventricular point of maximal impulse laterally. Her lungs were clear to auscultation, and there was no wheezing, rales, or rhonchi. The abdomen was soft, bowel sounds were present, there was mild tenderness to palpation over the epigastrium, no hepatosplenomegaly, and no masses. The extremities were warm and well perfused, pulses 2+, with edema present in lower extremities. Cranial nerves I to XII were grossly intact. She had full strength in the arms bilaterally and legs, except for 4/5 strength in the extensor hallucis longus; she had preserved deep tendon reflexes, except for absent ankle reflexes. She had distal sensory loss (Table 1).
Table 1.
Nerve Conduction Studies
| June 2017 | June 2018 | July 2019 | |
|---|---|---|---|
| Ulnar nerve, ms | 2.86 | 2.76 | 3.2 |
| Motor amplitude, wrist , mV | 8.8 | 8.1 | 9.4 |
| Motor conduction velocity, m/s | 67 | 58 | 56 |
| Sensory nerve action potential, μV | 12.4 | 8.1 | 11.4 |
| Peroneal nerve | |||
| Distal motor latency, ms | 4.74 | 4.69 | 4.74 |
| Motor amplitude, ankle, mV | 1.7 | 2.1 | 2.2 |
| Motor conduction velocity, m/s | 49 | 48 | 45 m |
| Tibial nerve | |||
| Distal motor latency, m/s | 5.00 | 5.36 | 5.83 |
| Motor amplitude, ankle, mV | 1.0 | 1.1 | 0.9 |
| Motor conduction velocity, m/s | 49 | 42 | 48 |
| Sural nerve | |||
| Sensory nerve action potential, μV | 7.7 | 6.2 | 4.8 |
Her mood and affect are stable. She endorsed prior suicidal ideation but not currently.
Investigations
Results of the patient’s laboratory tests showed the following: urinalysis and urine culture were both normal; white blood cell count, 7,100/mm3; hemoglobin, 12.4 g/dl; mean cell volume, 87.9 μm; hematocrit, 37%; platelet, 223,000/mm3; total protein, 7.1 g/dl; albumin, 3.9 g/dl; total bilirubin, 0.9 mg/dl; direct bilirubin, 0.5 mg/dl; alanine transaminase, 13 U/l; alkaline phosphatase, 27 U/l; amylase, 9 U/l; and lipase, 14 U/l.
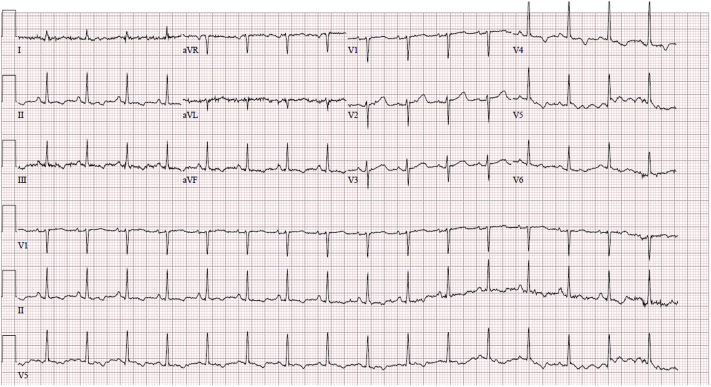
An electrocardiogram revealed the following: sinus tachycardia, heart rate of 108 beats/min, normal axis, no low voltage, no interval changes, T-wave flattening, normal R-wave progression, and no ST-segment changes (Figure 1).
Figure 1.
Electrocardiogram Demonstrating Sinus Tachycardia With T-Wave Flattening, Normal R-Wave Progression, and No ST-Segment Changes
A computed tomography scan of the abdomen and pelvis revealed no evidence of bowel obstruction. The appendix was unremarkable. There was focal irregular narrowing within the transverse colon, possibly transient peristalsis or a structure (although primary neoplasm was not excluded despite the patient’s age). There was no colonic dilation proximal to this point.
In terms of nerve conductions, the distal motor latency, evoked response amplitude, and motor conduction velocity were normal in the left ulnar nerve. The distal motor latency was normal, with reduced evoked response amplitude and normal conduction velocity in the left tibial and fibular nerves. The evoked response amplitude and sensory conduction velocity were normal in the left ulnar nerve. The evoked response amplitude was reduced in the left sural nerve, with normal sensory conduction velocity. The electro-diagnostic findings are compatible with an axonal sensorimotor polyneuropathy.
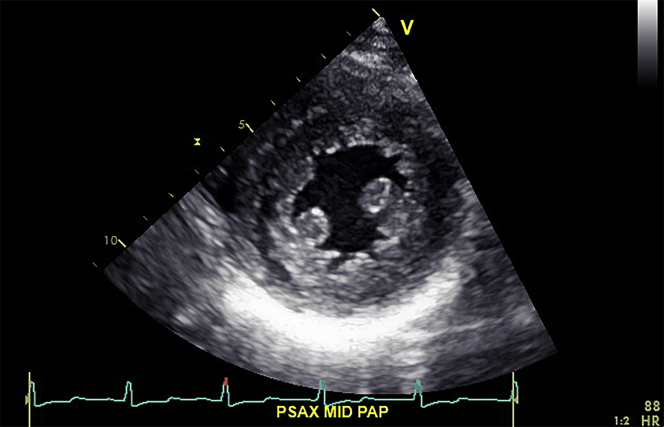
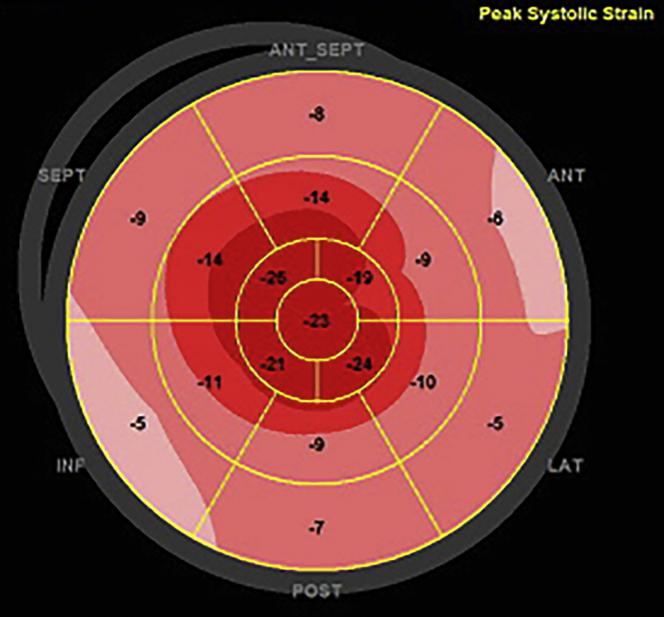
On autonomic testing, the average heart rate difference, with deep breathing, was 2.9 (5th percentile). An echocardiogram revealed the following: interventricular septum of 15 mm, a nondilated left ventricle (25 mm), preserved ejection fraction (52%), myocardial contraction fraction (38.5%), and low global strain (–11%) (Video 1, Figures 2 and 3).
Figure 2.
Short-Axis at the Papillary Muscle Level
Figure 3.
Left Ventricular Strain Displayed by Bull’s Eye Showing a Low Global Strain of –11%
Results of an abdominal fat pad biopsy revealed amyloidosis, and transthyretin (TTR) was confirmed by immunohistochemistry.
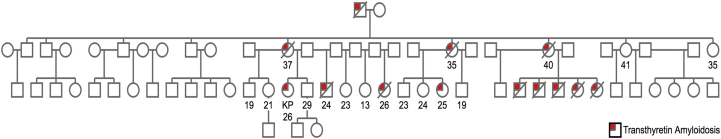
In the pedigree analysis, 13 family members were determined to also be affected with similar symptoms (Figure 4); several of these family members had died at any early age.
Figure 4.
Pedigree Spanning 3 Generations Showing the Penetrance of Transthyretin Amyloidosis in the Patient’s Family
KP = patient of interest.
Management
The patient was diagnosed with TTR amyloidosis manifesting with both a polyneuropathy and cardiomyopathy. She was found to be a carrier of the Thr60Ala mutation. She was enrolled in the APOLLO (The Study of an Investigational Drug, Patisiran, for the Treatment of Transthyretin TTR Amyloidosis) trial (1) and started on patisiran 0.3 mg/kg every 3 weeks.
Discussion
TTR amyloidosis has a broad spectrum of genotypes and heterogeneous phenotypes (2). TTR mutations usually occur within presumed ethnic groups and have an age-dependent penetrance. Val122Ile is most prevalent in people of West African descent and typically presents as a cardiomyopathy in the sixth or seventh decade. Thr60Ala mutation appears most commonly in people of Scottish or Irish descent with onset also in the sixth or seventh decade, typically presenting as cardiomyopathy and polyneuropathy (3). We report an unusual case of a 26-year old Afro-Caribbean woman harboring the Thr60Ala mutation who presented with clinical signs of TTR amyloidosis polyneuropathy and clinical evidence of cardiomyopathy.
This condition has an autosomal dominant inheritance pattern. The patient has a pedigree spanning 3 generations, providing evidence regarding the penetrance of TTR amyloidosis in her family. Pedigree analysis showed that of the 13 individuals in her family affected by the disease, 11 are now deceased, all before the age of 41 years (Figure 4). Although hereditary transthyretin amyloidosis (hATTR) has an age-dependent penetrance, the onset of disease in this family was earlier than has been previously reported, which may have an as yet undefined genetic or environmental cause. Thus, although certain mutations are associated with specific ethnic groups and ages of onset, this case highlights the heterogeneity of hATTR and variability in its presentation, emphasizing that consideration of this disorder in one’s differential is key to establishing a diagnosis.
TTR is a protein made up of ∼127 amino acids, structurally composed of beta sheets synthesized primarily in the liver. When soluble, it takes on a tetrameric complex form consisting of 4 single-chain monomers of TTR. ATTRm is the nomenclature designated for hereditary or mutant amyloidosis caused by a TTR gene mutation. When mutations in ATTRm lead the tetrameric TTR to dissociate into its monomeric subunits, they misfold and aggregate into insoluble fibrillar structures that deposit in organs and tissues (2).
Kinetic stability of TTR is quantifiable by subunit exchange assay. It occurs when tagged TTR homotetramers are added to untagged homotetramers at equal concentrations to measure the rate at which the subunits exchange. The kinetic stability of TTR was analyzed by using a subunit exchange assay. The subunit exchange assay showed the lower kinetic stability of TTR compared with that of wild-type controls (k-ex [-h] 0.0167 ± 0.0012 vs 0.0084 ± 0.0001) due to the presence of the mutant subunits in the TTR heterotetramer (4). The native TTR concentration was reduced 10-fold compared with that of healthy controls (28.7 ± 0.2 μg/ml vs. 222.7 ± 5.6 μg/ml). The remaining plasma TTR is 2-fold more unstable than healthy wild-type controls. In an untreated patient with this condition, the prognosis would be poor, leading to more advanced heart failure and poor neurological outcome.
Follow-Up
While taking patisiran for several years, the patient’s cardiac biomarkers (Table 2) revealed undetectable troponin I (<0.10 ng/ml), and N-terminal pro–B-type natriuretic peptide level was originally 130 pg/ml and is currently 161 pg/ml. Her gastrointestinal symptoms have improved. After treatment for 4 years with patisiran, neurological examination showed no signs of progression, with weakness remaining confined to the toe extensors and distal sensory loss. She continues to experience orthostatic hypotension. Her cardiovascular symptom of shortness of breath on exertion has diminished. She has more functional capacity then when first diagnosed with the condition.
Table 2.
Cardiac Biomarkers
| 2015 (Baseline) | 2018 | 2019 | Reference Range | |
|---|---|---|---|---|
| Troponin I, ng/ml | <0.10 | <0.10 | <0.10 | <0.10 |
| N-terminal pro–B-type natriuretic peptide, pg/ml | 130 | 126 | 161 | ≤124 |
Conclusions
This case shows the heterogeneous nature of TTR amyloidosis, presenting as a mixed phenotype with both cardiac and neurological involvement, with a mutation not commonly seen in the patient’s country of origin and occurring at an unusually early onset. Further study of correlations between biochemical measures of TTR stability and clinical phenotype may have the potential to elucidate the biological underpinnings of this devastating disease and be useful in identifying the mechanism of early disease.
Author Disclosures
Dr. Maurer has received grant support from the National Institutes of Health (R01HL139671-01, R21AG058348, and K24AG036778); consulting income from Pfizer, EIdos, Prothena, Akcea, and Alnylam; and clinical trial funding from Pfizer, Prothena, Eidos, and Alnylam to his institution. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The completion of this paper would have not been possible without the assistance of all of the authors involved. Special consideration for the patient involved in this piece who provided us with the permission to report her case.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Apical 4-Chamber View Echocardiogram. Interventricular septum of 15 mm, nondilated left ventricle (25 mm), preserved ejection fraction (52%), myocardial contraction fraction (38.5%), and low global strain (–11%).
References
- 1.Adams D., Gonzalez-Duarte A., O’Riordan W. Patisiran, an RNAi therapeutic for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 2.Kristen A.V., Maurer M.S., Rapezzi C., Mundayat R., Suhr O., Damy T. Impact of genotype and phenotype on cardiac biomarkers in patients with transthyretin amyloidosis—report from the Transthyretin Amyloidosis Outcome Survey (THAOS) Plos One. 2017;12 doi: 10.1371/journal.pone.0173086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunjes D.L., Castano A., Clemons A., Rubin J., Maurer M. Transthyretin cardiac amyloidosis in older Americans. J Card Fail. 2016;22:996–1003. doi: 10.1016/j.cardfail.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rappley I., Monteiro C., Novais M. Quantification of transthyretin kinetic stability in human plasma using subunit exchange. Biochemistry. 2014;53:1993–2006. doi: 10.1021/bi500171j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apical 4-Chamber View Echocardiogram. Interventricular septum of 15 mm, nondilated left ventricle (25 mm), preserved ejection fraction (52%), myocardial contraction fraction (38.5%), and low global strain (–11%).