Abstract
A 50-year-old man presented with an episode of chest pain. Cardiac magnetic resonance revealed the presence of a large ventricular septal aneurysm partially closing a perimembranous ventricular septal defect, prolapsing into the right ventricular outflow tract, and mimicking a mass. We illustrate the diagnostic approach and management of such ventricular septal aneurysms. (Level of Difficulty: Advanced.)
Key Words: cardiac magnetic resonance, congenital heart defect, echocardiography, right ventricular outflow tract mass, ventricular septal aneurysm, ventricular septal defect
Abbreviations and Acronyms: CMR, cardiac magnetic resonance; CT, computed tomography; PA, pulmonary artery; RV, right ventricular; RVOT, right ventricular outflow tract; VSA, ventricular septal aneurysm; VSD, ventricular septal defect
Graphical abstract

History of Presentation
A 50-year-old man presented to the cardiology office with chest pain. He described the pain as sharp, left sided, and so severe in intensity that it nearly caused him to pass out. On physical examination, he had a loud grade 5/6 holosystolic murmur with a thrill heard at the left parasternal area.
Learning Objectives
-
•
Using different imaging modalities for patients VSA to guide decision making.
-
•
For patients with VSA, counseling on treatment options to improve quality of life.
Past Medical History
The patient had a diagnosis of ventricular septal defect (VSD) at the age of 10 years, but did not warrant surgical correction at the time, and he was subsequently lost to follow-up. He has been physically active, playing ice hockey and working as a karate instructor for the past 30 years.
Differential Diagnosis
The differential diagnosis included cardiac tumor, intracardiac thrombus, ventricular septal aneurysm (VSA), pulmonic stenosis, and pulmonic valve endocarditis.
Investigations
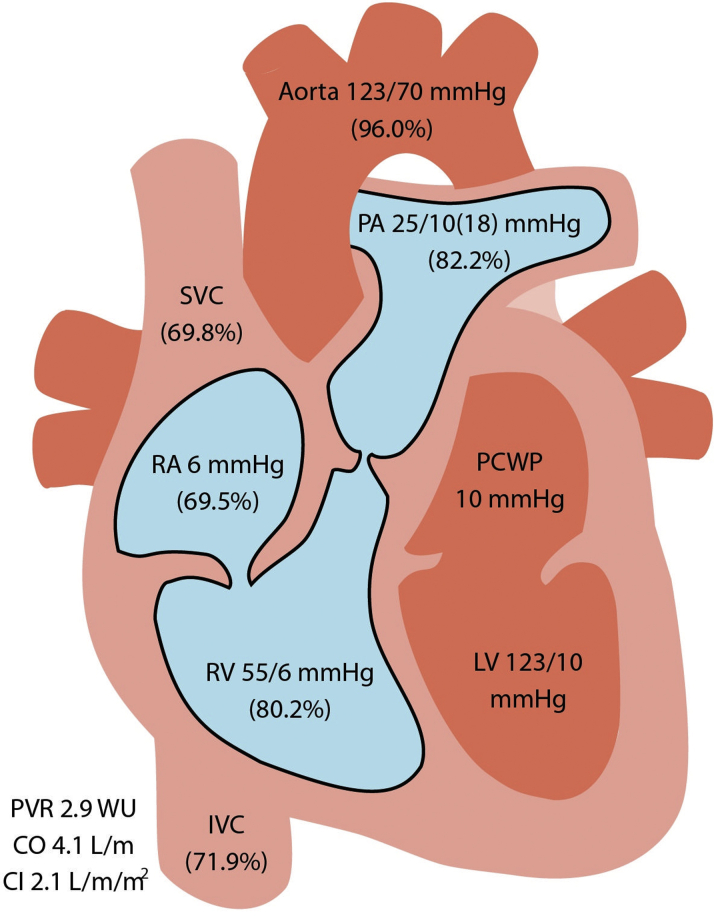
His electrocardiogram and basic laboratory results were unremarkable. An echocardiogram showed normal biventricular size and function with a restrictive perimembranous ventricular septal defect (VSD) (Figure 1A, Video 1). Continuous wave Doppler imaging showed a peak gradient across the VSD of 64 mm Hg (Figures 1B and 1C), as well as an elevated peak velocity in the pulmonic area of 2.5 m/s (Figure 1D). He subsequently underwent combined left- and right-sided heart catheterization that showed normal coronary anatomy, with an oxygen saturation step-up from 69.5% to 80.2% from the right atrium to the right ventricle, a peak-to-peak gradient of 20 mm Hg from the right ventricle to the pulmonary artery (PA), and a pulmonary-to-systemic flow ratio (Qp/Qs ratio) of 1.9 (Figure 2).
Figure 1.
Ventricular Septal Aneurysm
(A) Apical 4-chamber view showing a perimembranous ventricular septal defect (green line) with ventricular septal aneurysm (red arrow). (B) Color Doppler image showing flow across the ventricular septal defect. (C) Parasternal short-axis view showing a ventricular septal aneurysm (red arrow) causing right ventricular outflow tract obstruction (green line). (D) Spectral continuous wave Doppler image showing a peak velocity of 4 m/s (red arrow) equating to a gradient of 64 mm Hg across the ventricular septal defect; ECG tracing in green. (E) Spectral continuous wave Doppler image showing peak velocity of 2.5 m/s (red arrow) across the right ventricular outflow tract; ECG tracing in green.
Figure 2.
Right-Sided Heart Catheterization With Shunt Run
This study shows step-up from the right atrium (RA) (69.5%) to the right ventricle (RV) (80.2%). CI = cardiac index; CO = cardiac output; IVC = inferior vena cava; LV = left ventricle; PA = pulmonary artery; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; SVC = superior vena cava.
Given these findings, cardiac magnetic resonance (CMR) imaging was performed, and it showed the presence of a perimembranous VSD with a Qp/Qs ratio of 1.6 and a large right ventricular (RV) outflow tract (RVOT) mass measuring 2.8 × 2.7 cm with systolic and diastolic movement (Videos 2, 3, and 4). The mass demonstrated increased perfusion with late gadolinium enhancement, central clearing, and rim enhancement (Figure 3). This was thought to be a vascular mass of unclear origin.
Figure 3.

Cardiac Magnetic Resonance Sagittal View With Late Gadolinium Enhancement
Cardiac magnetic resonance shows rim enhancement with central clearing (red arrow).
Management
Given the location, size, and appearance of the mass on CMR, the patient was referred for open heart surgery to a center with experience managing coexisting adult congenital heart defects. Further review of echocardiographic and CMR images demonstrated the possibility of a VSA causing nearly complete closure of the VSD and mimicking the appearance of an intracardiac mass. This VSA likely prolapsed into the RVOT in response to higher left ventricular pressure creating partial outflow obstruction and the RV-PA pressure gradient. Given the lack of significant flow across the VSD, the left and right ventricles were of normal anatomic size and function. The CMR appearance of a vascular mass with central clearing most likely represented a susceptibility artifact from turbulent flow across the restrictive VSD. “Rim enhancement” was attributed to static flow in areas adjacent to the VSA that created a high signal on late gadolinium enhancement.
Given the appearance of the VSA on CMR, the patient was scheduled for cardiac computed tomography (CT), which confirmed the absence of any intracardiac mass or thrombus. Because our patient did not have recurrent symptoms, and the VSA was thought to be chronic, with no immediate risk of rupture, the decision was made to pursue conservative management with continued surveillance.
Discussion
Membranous VSA is a rare congenital anomaly, occurring in 0.3% of patients with congenital heart disease (1). It is usually found in association with an uncorrected congenital VSD, by partially or completely closing the VSD and preventing the development of hemodynamically significant intracardiac shunts (2). Such VSAs are usually a few millimeters in size, but they can rarely expand to obstruct the RVOT completely or extend into the cavity of the right atrium (3). Conversely, aneurysms in the muscular portion of the ventricular septum are often the result of myocardial infarction.
Little is understood about the natural history of VSAs and whether they progress to any extent over time. A leading theory on the mechanism of development of large VSAs is related to the delayed natural closure of VSDs, causing the membranous tissue of the interventricular septum to expand under a higher left ventricular systolic pressure (4). The VSA usually incorporates redundant tissue from the septal leaflet of the tricuspid valve but the valve itself remains structurally and functionally intact. Such aneurysms are not considered dangerous or at risk of rupture, but are in fact helpful in partially closing a VSD.
Transthoracic echocardiography, transesophageal echocardiography, and 3-dimensional echocardiography are the mainstays of diagnosis, showing a thin membrane within the RV cavity. They also help assess biventricular function and the extent of shunting across the VSD. However, echocardiography has several limitations and artifacts. Multidetector cardiac CT and CMR provide better spatial resolution, free of imaging planes or acoustic shadowing. Multidetector CT provides high-resolution 3D anatomic detail of VSAs, requiring less time and sedation than CMR, as well as less post-processing time (5). It is not uncommon for cardiac catheterization to demonstrate a mild step-up (<15%) in oxygen saturation on the right side, along with a small gradient across the RVOT from the right ventricle to the PA (2). In most cases these findings are hemodynamically insignificant.
One important aspect of imaging is to exclude a sinus of Valsalva aneurysm, which can cause saccular dilatation of the aortic sinus and follows a more aggressive course, often requiring surgical intervention. Patients with perimembranous VSAs seldom develop symptoms or warrant surgical intervention (2). Current guidelines recommend a 3- to 5-year medical surveillance period with echocardiography to screen for possible complications in the absence of symptoms (6). Complications may include thrombus formation within the VSA, endocarditis, systemic emboli, cardiac arrhythmias, and intracardiac shunting secondary to aneurysmal rupture. Given the anatomic location of the VSA, surgical resection can cause tricuspid regurgitation, or it can create atrioventricular blocks if conduction tissue (especially the atrioventricular node) lies adjacent to resected aneurysmal tissue. In a single study, the diameter and indexed cross-sectional area of the VSA were found to be predictive of prognosis and successful surgical closure (7). VSA prolapse into the RVOT that causes a subpulmonic gradient is an unusual and infrequent presentation rarely reported. Given our patient’s moderate gradient in the RVOT, his normal biventricular size and function, and his lack of symptoms, no further intervention was planned. This patient’s data were de-identified, and this report was exempted from Institutional Review Board approval.
Follow-Up
On outpatient follow-up, no new clinical symptoms have developed, and the patient continues to do well overall.
Conclusions
We present an unusual case of perimembranous VSD with a large VSA prolapsing into the RVOT and mimicking the appearance of an RVOT mass on CMR. This condition produced a subpulmonic gradient seen on echocardiography and an oxygen saturation step up seen on cardiac catheterization that, given its hemodynamic insignificance and minimal risk of rupture, did not warrant surgical intervention.
Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Apical 4-Chamber View on Echocardiography Showing Flow Across the Perimembranous Ventricular Septal Defect and Ventricular Septal Aneurysm
Cardiac Magnetic Resonance Sagittal View Showing the Ventricular Septal Aneurysm. The ventricular septal aneurysm (red arrow) is mimicking a large right ventricular outflow tract mass with systolic and diastolic motion.
Cardiac Magnetic Resonance 4-Chamber Stack Showing the Ventricular Septal Defect. Cardiac magnetic resonance shows left-to-right flow (red arrow).
Cardiac Magnetic Resonance Cross-Sectional Stack. Cardiac magnetic resonance shows the ventricular septal defect and ventricular septal aneurysm (red arrow).
References
- 1.Carcano C., Kanne J.P., Kirsch J. Interventricular membranous septal aneurysm: CT and MR manifestations. Insights Imaging. 2016;7:111–117. doi: 10.1007/s13244-015-0456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain A.C., Rosenthal R. Aneurysm of the membranous ventricular septum. Br Heart J. 1967;29:60–63. doi: 10.1136/hrt.29.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould L.A., Betzu R., Lin C.S., Judge D., Taddeo M., Lee J. Aneurysm of the membranous ventricular septum. N Y State J Med. 1988;88:157. [PubMed] [Google Scholar]
- 4.Edelstein J Charms B.L. Ventricular septal aneurysms. A report of two cases. Circulation. 1965;32:981–984. doi: 10.1161/01.cir.32.6.981. [DOI] [PubMed] [Google Scholar]
- 5.Goo H.W., Park I., Ko J. CT of congenital heart disease: normal anatomy and typical pathologic conditions. Radiographics. 2003;23:S147–S165. doi: 10.1148/rg.23si035501. [DOI] [PubMed] [Google Scholar]
- 6.Warnes C.A., Williams R.G., Bashore T.M. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;118:2395–2451. doi: 10.1161/CIRCULATIONAHA.108.190811. [DOI] [PubMed] [Google Scholar]
- 7.Miyake T., Shinohara T., Nakamura Y. Aneurysm of the ventricular membranous septum: serial echocardiographic studies. Pediatr Cardiol. 2004;25:385–389. doi: 10.1007/s00246-003-0572-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apical 4-Chamber View on Echocardiography Showing Flow Across the Perimembranous Ventricular Septal Defect and Ventricular Septal Aneurysm
Cardiac Magnetic Resonance Sagittal View Showing the Ventricular Septal Aneurysm. The ventricular septal aneurysm (red arrow) is mimicking a large right ventricular outflow tract mass with systolic and diastolic motion.
Cardiac Magnetic Resonance 4-Chamber Stack Showing the Ventricular Septal Defect. Cardiac magnetic resonance shows left-to-right flow (red arrow).
Cardiac Magnetic Resonance Cross-Sectional Stack. Cardiac magnetic resonance shows the ventricular septal defect and ventricular septal aneurysm (red arrow).