Abstract
All-trans retinoic acid (ATRA) is the mainstay of treatment in patients with acute promyelocytic leukemia. Despite being effective, it can lead to cardiac complications either as a component of ATRA syndrome or an isolated form denominated as ATRA-induced isolated perimyocarditis. We present a case of this complication and review the literature. (Level of Difficulty: Intermediate.)
Key Words: drug toxicity, imaging, myocarditis
Abbreviations and Acronyms: APL, acute promyelocytic leukemia; ATRA, all-trans retinoic acid; CRP, C-reactive protein; CT, computed tomography; LVEF, left ventricle ejection fraction; MRI, magnetic resonance imaging; TTE, transthoracic echocardiography
Graphical abstract
A 23-year-old woman diagnosed with acute promyelocytic leukemia (APL) was admitted to the hematology unit. Induction therapy consisting of idarubicin 20 mg/day (on the second, fourth, sixth, and eighth days) and all-trans retinoic acid (ATRA) at a dose of 45 mg/m2/day was initiated. The patient had been well until 18th day from the initiation of therapy. However, she experienced a typical chest pain in the left-retrosternal region without radiating; thus, she was referred to the cardiology clinic for evaluation of chest pain on the 18th day of induction therapy. Her temperature was 36.8°C, blood pressure was 120/70 mm Hg, the heart rate was 86 beats/min, respiratory rate was 12 breaths/min, and the oxygen saturation was 98%. The patient looked anxious.
Learning Objectives
-
•
To recognize ATRA-related cardiac complications.
-
•
To understand the clinical course of ATRA-induced perimyocarditis.
-
•
To take action against this complication.
Medical History
The patient had no medical history or family history of cardiovascular diseases or risk factors. She did not report history of smoking, medication, or drug abuse. Findings on a screening electrocardiogram were normal (Figure 1A). Transthoracic echocardiography (TTE) before the induction therapy revealed no wall motion abnormality and a normal left ventricle ejection fraction (LVEF).
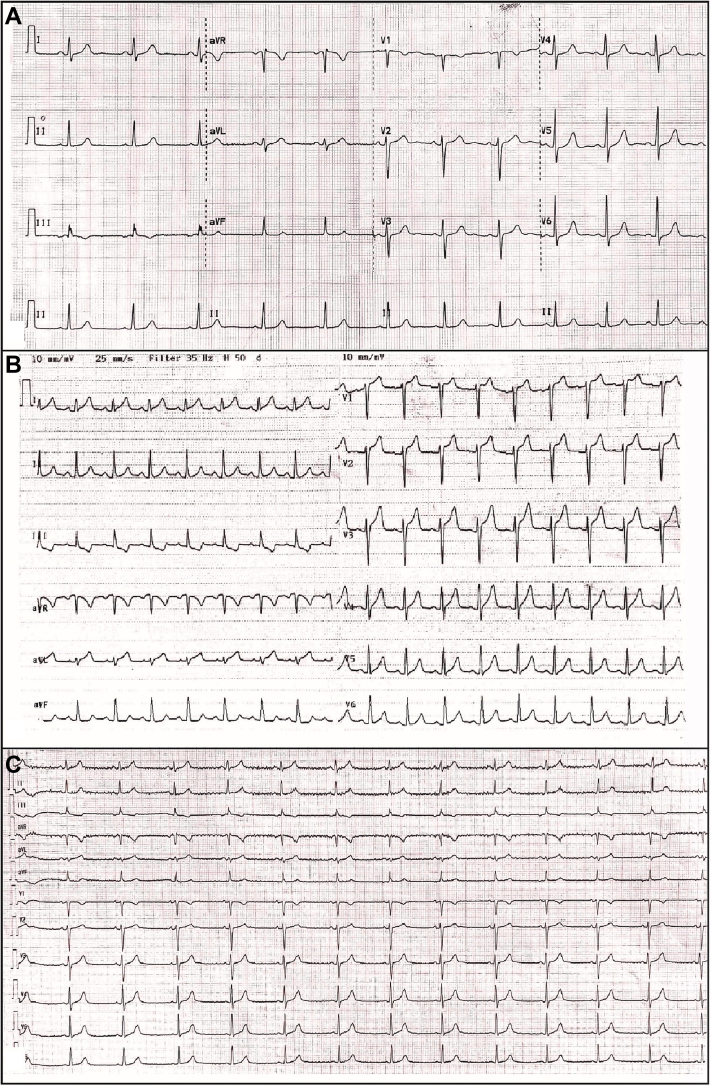
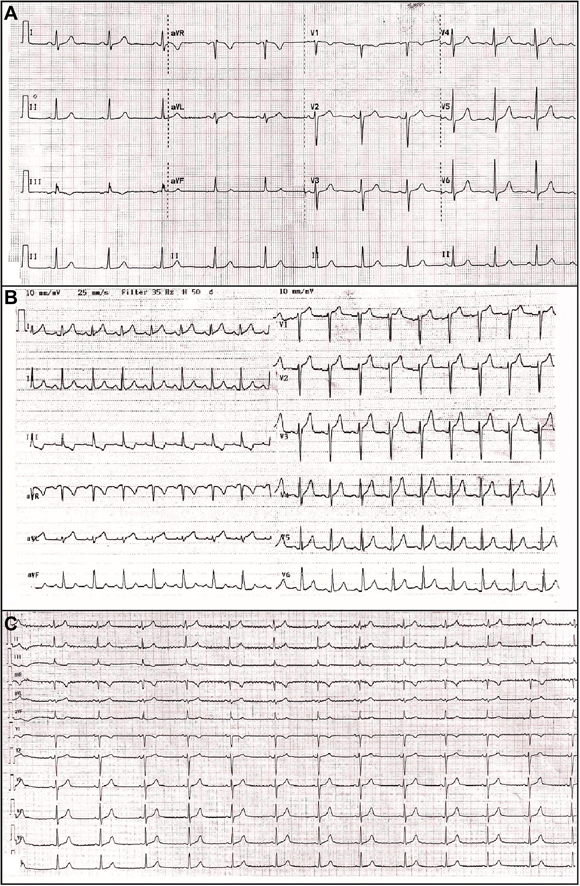
Figure 1.
The Electrocardiographic Records of the Patient
(A) Baseline electrocardiography before all-trans retinoic acid treatment. (B) Electrocardiography shows ST-segment elevation, mainly in the anterior and lateral derivations during chest pain. (C) Electrocardiography shows resolution of ST-segment elevation after the discontinuation of all-trans retinoic acid.
Differential Diagnosis
The differential diagnosis for chest pain in this patient included acute coronary syndrome, viral myocarditis and/or pericarditis, stress cardiomyopathy, and endocarditis.
Investigations
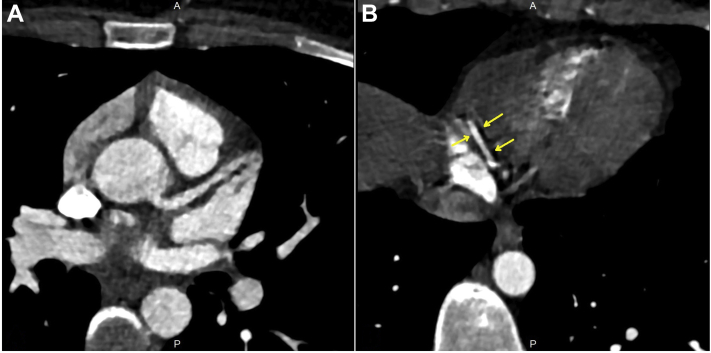
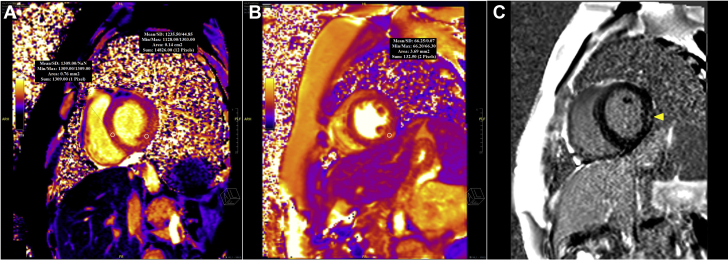
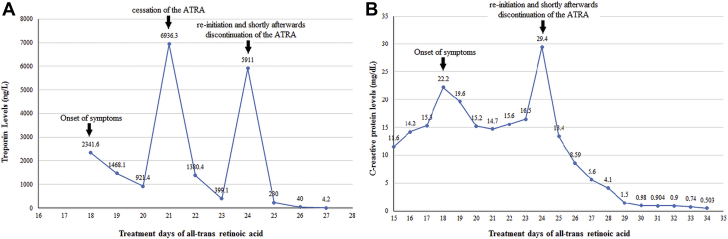
Electrocardiography revealed ST-segment elevation, mainly in the anterior and lateral derivations (Figure 1B). TTE revealed globally reduced LV systolic function with an EF of 43% (Videos 1, 2, and 3). Speckle tracking echocardiography in the apical 3-chamber view also showed reduced global longitudinal myocardial deformation of –14.0% (Video 4). Laboratory test findings indicated a white blood cell count of 3.9 × 103/μl, troponin I of 2,341.6 ng/l (range: 14 to 42.9 ng/l), C-reactive protein (CRP) of 22.2 mg/dl (range: 0 to 0.8 mg/dl), and procalcitonin of 0.21 ng/ml (range: 0 to 0.14 ng/ml). There were no signs or symptoms suggestive of infection. Coronary computed tomography (CT) was performed promptly and revealed that there was no obstructive lesion in a given coronary artery (Figure 2). In the view of the possibility of ATRA-related toxicity, we ceased to give the ATRA as the troponin level peaked (6,936.3 ng/l) on the 21st day of treatment. Three days later, we reinitiated the drug as troponin values started to decrease below 400 ng/l. Nonetheless, because chest pain was exacerbated and troponin (5,911 ng/l) and CRP (29.4 mg/dl) levels peaked again, the drug was discontinued, and cardiac magnetic resonance imaging (MRI) was performed on the same day. T1 (Figure 3A) and T2 (Figure 3B) mapping showed prolongation in relaxation time in the basal inferolateral region and all segments in the septum, consistent with interstitial edema. In addition, the post-gadolinium T1 mapping showed late enhancement in the inferolateral wall with an extracellular volume fraction of 59% (Figure 3C). LVEF determined by cardiac MRI was 45% and similar to that measured by TTE.
Figure 2.
The Cardiac Computed Tomography of the Patient
Cardiac computed tomography reveals no coronary obstructive lesion in the (A) left and (B) right system. Arrows indicate the crux segment of the right coronary artery.
Figure 3.
Cardiac Magnetic Resonance Imaging Findings of the Patient
Cardiac magnetic resonance imaging reveals prolonged native myocardial relaxation times in T1-weighted mapping.
A region of interest placed in the septum and the inferolateral wall shows an increase in T1 relaxation times. (A) T1 signal is 1,309 ms for the septum and 1,235 ms for the inferolateral wall, consistent with interstitial edema. (For normal myocardium, the mean/2SD value is 950/42 ms). (B) In the T2 mapping, the area of interstitial edema in the inferior wall has an increased relaxation time of 66 ms (for normal myocardium, mean/2 standard deviations is 50/4 ms). (C) The post-gadolinium T1 mapping shows late enhancement in the inferolateral wall (yellow arrowhead) with an extracellular volume fraction of 59%. max = maximum; min = minimum; SD = standard deviation.
Management
A beta-blocker and angiotensin-converting enzyme inhibitor were initiated as soon as systolic dysfunction was detected. Because of low platelet count (20,000 × 103/μl), neither an antiplatelet nor an anticoagulant agent could be given. No additional medical treatment was given.
Discussion
ATRA syndrome is characterized by fever, hypotension, pulmonary infiltration, and pericardial effusion; it is seen in one-fourth of the patients receiving ATRA. Cardiac involvement can be seen both as a component of ATRA syndrome and as an isolated perimyocarditis. The clinical picture may resemble acute coronary syndrome and viral myocarditis. It can be difficult to ascertain whether the perimyocarditis is a component of the ATRA syndrome or is a purely drug-related toxic phenomenon. However, it occurs, the pathological mechanism is thought to be caused by ATRA-induced promyelocyte maturation, cytokine release causing increased capillary permeability and endothelial damage, and subsequent extravasation and tissue infiltration of APL cells (1). In our case, the patient did not express other findings suggestive of ATRA syndrome, such as fever, hypotension, respiratory distress, and weight gain due to generalized edema and pleural effusion. Although increase in troponin level, typical chest pain, and changes in electrocardiography and echocardiography findings suggested acute coronary syndrome, the patient’s characteristics and the absence of cardiovascular risk factors raised the suspicion of a drug-related complication. Hence, we performed coronary CT to exclude coronary artery occlusion. Mitigating symptoms with the cessation of the drug and peaking troponin levels after re-initiation of the drug indicated that this clinical picture was ATRA-induced perimyocarditis (Figure 4A). Concomitant increase in CRP (Figure 4B) and procalcitonin levels also suggested the inflammatory nature of the underlying event. Eventually, the diagnosis was confirmed with cardiac MRI findings.
Figure 4.
The laboratory Findings of the Patient
Graph of (A) troponin I and (B) C-reactive protein levels according to treatment days of the ATRA. ATRA = all-trans retinoic acid.
Thus far, there are limited numbers of studies reporting the cases of ATRA-induced isolated perimyocarditis in the literature (Table 1). In these cases, almost all patients were young (9 to 58 years) and experienced the disease within 3 weeks following the initiation of the induction therapy. Our patient’s age (23 years) and the emergence of the disease (18th day) are consistent with the previous cases. Different electrocardiographic changes and different myocardial segment involvements on echocardiography reported in the literature indicate that ATRA does not exhibit a specific involvement pattern. We did not consider anti-inflammatory therapy. In many of the previous case reports, nevertheless, anti-inflammatory therapy such as dexamethasone and prednisone, along with conventional heart failure therapy, was initiated to alleviate myocardial and pericardial inflammation.
Table 1.
All-trans Retinoic Acid–Induced Isolated Perimyocarditis Cases in the Literature
| First Author (Ref. #) | Year | Age (yrs), Sex | Day of Occurrence∗ | Ejection Fraction, % | Wall Motion Abnormalities | Treatment | Changes on Electrocardiography |
|---|---|---|---|---|---|---|---|
| Choi et al. (2) | 2011 | 39, female | 18 | 38 | Basal and midanterior | Dexamethasone and diuretic | No change |
| Ben El Makki et al. (3) | 2019 | 27, male | 10 | 33 | Diffuse | Vasopressors, diuretic, ACE inhibitor, beta blocker |
Diffuse ST-segment elevation |
| Klein et al. (4) | 2007 | 34, female | 19 | No data | No data | No specific treatment | Diffuse ST-segment elevation |
| Klein et al. (4) | 2007 | 46, male | 23 | No data | Diffuse | No specific treatment | Diffuse ST-segment elevation |
| Fabbiano et al. (5) | 2005 | 45, male | 23 | No data | Posterolateral | No specific treatment | Lateral ST-segment elevation V1 to V2 ST-segment depression |
| Van Rijssel et al. (6) | 2010 | 58, male | 21 | No data | No data | The patient died 2 days later | No data |
| Carcelero et al. (7) | 2018 | 35, male | 16 | 35 | Diffuse | Anti-inflammatory agents, diuretics, ACE inhibitor, and dobutamine | Diffuse ST-segment elevation |
| Isik et al. (8) | 2010 | 9, female | Within first week | 40 | Diffuse | Prednisolone, inotropics, and diuretics | Diffuse voltage depression |
ACE = angiotensin-converting enzyme.
Day of occurrence indicates how many days the event occurred after induction therapy.
Follow-Up
The patient was completely asymptomatic and the troponin level (4.2 ng/l) was within normal limits 3 days after the discontinuation. CRP (0.503 mg/dl) and procalcitonin (0.016 ng/ml) also returned to their normal levels 10 days after the discontinuation. Electrocardiogram showed resolution of ST-segment elevation (Figure 1C). TTE revealed normal LV systolic function with an EF of 56% (Videos 5, 6, and 7). Speckle tracking echocardiography in the apical 3-chamber view indicated normal global longitudinal myocardial deformation of –27.7% (Video 8). Arsenic trioxide has been initiated for leukemia treatment.
Conclusions
ATRA-induced isolated perimyocarditis should be kept in mind in patients with APL. Although rare, it warrants high suspicion and early detection because it can cause devastating complications. In the present case, we demonstrated the ATRA-induced isolated perimyocarditis by using a variety of imaging modalities including 2- and 3-dimensional echocardiography, coronary CT, and cardiac MRI. In our case, the complete recovery of myocardial function was achieved by discontinuation of ATRA and conventional heart failure therapy.
Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
References
- 1.Fenaux P., De Botton S. Retinoic acid syndrome. Recognition, prevention and management. Drug Safety. 1998;18:273–279. doi: 10.2165/00002018-199818040-00003. [DOI] [PubMed] [Google Scholar]
- 2.Choi S., Kim H.S., Jung C.S. Reversible symptomatic myocarditis induced by all-trans retinoic acid administration during induction treatment of acute promyelocytic leukemia: rare cardiac manifestation as a retinoic acid syndrome. J Cardiovasc Ultrasound. 2011;19:95–98. doi: 10.4250/jcu.2011.19.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben El Makki A., Mahtat E.M., Kheyi J., Bouzelmat H., Chaib A. A rare case of perimyocarditis induced by all-trans retinoic acid administration during induction treatment of acute promyelocytic leukemia. Med Pharm Rep. 2019;92:418–420. doi: 10.15386/mpr-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein S.K., Biemond B.J., van Oers M.H. Two cases of isolated symptomatic myocarditis induced by all-trans retinoic acid (ATRA) Ann Hematol. 2007;86:917–918. doi: 10.1007/s00277-007-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabbiano F., Magrin S., Cangialosi C., Felice R., Mirto S., Pitrolo F. All-trans retinoic acid induced cardiac and skeletal myositis in induction therapy of acute promyelocytic leukaemia. Br J Haematol. 2005;129:444–445. doi: 10.1111/j.1365-2141.2005.05465.x. [DOI] [PubMed] [Google Scholar]
- 6.van Rijssel R.H., Wegman J., Oud M.E., Pals S.T., van Oers M.H. A case of ATRA-induced isolated myocarditis in the absence of circulating malignant cells: demonstration of the t(15;17) translocation in the inflammatory infiltrate by in situ hybridisation. Leuk Res. 2010;34:e142–e144. doi: 10.1016/j.leukres.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Carcelero San Martin E., Riu Viladoms G., Creus Baro N. Severe myopericarditis following induction therapy with idarubicin and transretinoic acid in a patient with acute promyelocytic leukemia. Med Clin (Barc) 2018;150:492–493. doi: 10.1016/j.medcli.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Isik P., Cetin I., Tavil B. All-transretinoic acid (ATRA) treatment-related pancarditis and severe pulmonary edema in a child with acute promyelocytic leukemia. J Pediatr Hematol Oncol. 2010;32:e346–e348. doi: 10.1097/MPH.0b013e3181e75731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.