Abstract
Background
Clinical studies have shown that the rapid antidepressant effect of the glutamate N-methyl-D-aspartate receptor antagonist ketamine generally disappears within 1 week but can be maintained by repeated administration. Preclinical studies showed that a single ketamine injection immediately increases the firing and burst activity of norepinephrine (NE) neurons, but not that of serotonin (5-HT) neurons. It also enhances the population activity of dopamine (DA) neurons. In the present study, we investigated whether such alterations of monoamine neuronal firing are still present 1 day after a single injection, and whether they can be maintained by repeated injections.
Methods
Rats received a single ketamine injection or 6 over 2 weeks and the firing activity of dorsal raphe nucleus 5-HT, locus coeruleus NE, and ventral tegmental area DA neurons was assessed.
Results
One day following a single injection of ketamine, there was no change in the firing activity of 5-HT, NE, or DA neurons. One day after repeated ketamine administration, however, there was a robust increase of the firing activity of NE neurons and an enhancement of burst and population activities of DA neurons, but still no change in firing parameters of 5-HT neurons. The increased activity of NE neurons was no longer present 3 days after the last injection, whereas that of DA neurons was still present. DA neurons were firing normally 7 days after repeated injections.
Conclusion
These results imply that the enhanced activity of NE and DA neurons may play a significant role in the maintenance of the antidepressant action of ketamine.
Keywords: ketamine, serotonin, norepinephrine, dopamine, depression, electrophysiology
Significance statement.
Clinical studies have shown that the rapid antidepressant effect of a single infusion of ketamine in patients with treatment-resistant depression disappears within 1 day, and that a repeated thrice-weekly administration prolongs this response. Using this same paradigm in a longitudinal study, we sought to determine whether repeated administration of ketamine maintained the immediate increase of dopamine (DA) and norepinephrine (NE) firing activity previously found with a single administration in rats. Here we showed that although a single-administration regimen did not change the firing activity of serotonin, NE, and DA neurons, repeated ketamine administration resulted in a robust increase of the firing activity of NE neurons that lasted at least 1 day and an enhancement of burst and population activities of DA neurons that was still present after 3 days, while the drug was eliminated. These results indicate that increases in NE and DA neuronal firing activity may play a role in maintaining the antidepressant effect of ketamine.
Introduction
Considerable attention has been dedicated to the glutamate system as a possible contributor to the antidepressant response (Pilc et al., 2013). The most striking breakthrough has been the discovery of the rapid antidepressant effect of a single intravenous infusion of subanesthetic doses of the glutamate N-methyl-D-aspartate (NMDA) receptor noncompetitive antagonist ketamine (Berman et al., 2000; Zarate et al., 2006). This antidepressant response occurs within hours to a day of a single dose in about half of patients with treatment-resistant depression (TRD) (Zarate et al., 2006; Murrough et al., 2013; Sanacora et al., 2017). The drawback of ketamine, however, is that the antidepressant effects of a single dose generally disappear within a week. Several studies have shown, however, that the response can be sustained and even enhanced with repeated infusions (Murrough et al., 2013; Krystal et al., 2019; Phillips et al., 2019). Finally, the response to several infusions can be sustained by decreasing the frequency of administration over time (Phillips et al., 2019; Dale et al., 2020).
Animal studies have shown that in rats subjected to chronic unpredictable mild stress, thrice-weekly administration of ketamine produces a long-lasting amelioration in immobility and sucrose intake, which are respectively modulated by serotonin (5-HT) and dopamine (DA), compared to when given every day for 3 weeks (Zhang et al., 2015a). Another study using the forced swim test has shown that a single ketamine injection induced an increase in the climbing parameter in the forced swim test in rats, which is regulated by norepinephrine (NE; López-Gil et al., 2019). These results indicate that monoamine systems play, at least in part, a role in the antidepressant-like response to ketamine. It is established that monoamine and glutamate systems interact and, consequently, any ketamine-induced alteration in glutamate transmission could directly influence monoamine systems (see Pralong et al., 2002). Behavioral, molecular, and neurochemical studies have shown involvement of monoamines in the effects of an acute injection of ketamine (Belujon and Grace, 2014; El Iskandrani et al., 2015; Witkin et al., 2016; Pham et al., 2017; Fukumoto et al., 2016; López-Gil et al., 2019). Electrophysiologically, a single injection of ketamine acutely increases locus coeruleus (LC) NE neurons firing and burst activity and ventral tegmental area (VTA) DA neuron population activity (Belujon and Grace, 2014; El Iskandrani et al., 2015; Witkin et al., 2016) but not dorsal raphe nucleus (DRN) 5-HT neurons (El Iskandrani et al., 2015). Functionally, studies have found that by antagonizing NMDA receptors ketamine results in incorporation of more α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in synapses and an increased AMPA/NMDA-receptor ratio that leads to excitatory synapses resulting in an enhanced synaptic potentiation (Zanos and Gould, 2018).
In light of these data and clinical results showing that thrice weekly injections of ketamine for 2 weeks maintain its antidepressant effect in most TRD patients, the current study had the following aims: (1) investigating whether a single injection of ketamine induces changes of the firing activity of monoamine neurons that last for at least 1 day, (2) assessing whether any such effects are sustained over time by repeated injections according to the schedule used in clinical studies, and (3) determining the time course for the disappearance of any such potential effects. These experiments may provide evidence for the implication the implication of monoamines in maintaining the antidepressant effects of ketamine.
METHODS
Animals
Male Sprague-Dawley rats, obtained from Charles River (St. Constant, Quebec, Canada) were used. The rats weighed between 250 and 340 g at the time of experiments. Rats were kept in a facility at a constant temperature of 22°C ± 2°C and housed in groups of 2 per cage under standard laboratory conditions (12:12-h light-dark cycles with access to food and water ad libitum). The rats were not used for a week after arrival to allow for habituation. Body temperature was maintained at 37°C during electrophysiological recordings.
Ethics Statement
All animals were handled according to the guidelines of the Canadian Council on Animal Care (CCAC) and the local Animal Care Committee (Institute of Mental Health Research, Ottawa, Canada) approved all protocols.
Drug Administration
Ketamine hydrochloride (ERFA Canada Inc.) was diluted in 0.9% saline solution and administered intraperitoneally (ip). Control rats received saline (0.9%, ip). In the single administration paradigm, rats received a single ip injection of ketamine (10 mg/kg), while in the repeated administration regimen, animals received the same dose of ketamine 3 times a week (Monday, Wednesday, and Friday, for 2 weeks, as in clinical studies [Murrough et al., 2013; Phillips et al., 2019]). Electrophysiological experiments were conducted 1, 3, and 7 days after the last administration.
In Vivo Electrophysiological Experiments
Rats were anaesthetized with chloral hydrate (400 mg/kg ip), and placed on a stereotaxic frame (David Kopf, CA, USA) with the skull positioned horizontally. Supplemental doses of anesthetic (100 mg/kg ip) were given to maintain constant anesthesia and prevent any nociceptive response to palpebral reflex or pinching of the hind paw (pedal withdrawal reflex). Body temperature was maintained at 37°C by a thermistor-controlled heating pad. A burr hole was drilled at the stereotaxic coordinates corresponding to the monoaminergic structure of interest. The shape and duration of spikes, as well as the frequency of firing, were used to identify neurons of interest and recorded using the Spike2 program (Cambridge Electronic Design, Cambridge, UK).
Extracellular recordings of 5-HT, NE, and DA neurons were performed using single-barrel glass micropipettes (Stoelting, IL, USA) prepared using a pipette puller (Narishige, Japan) and filled with 2 M NaCl solution at an impedance range of 2–4 MΩ. For every brain structure, several electrode descents were carried out to record a maximum of neurons. Firing rate, number of neurons firing in burst mode, percentage of spikes in burst, and number of neurons per track were determined.
Recording of DRN 5-HT Neurons
A single-barrel glass micropipette was positioned 0.9–1.2 mm anterior to lambda, on the midline, and lowered into the DRN, usually attained at a depth of 4.5–5.5 mm from the brain surface. The presumptive 5-HT neurons were identified according to the following criteria: a slow (0.5–2.5 Hz), regular firing rate, long duration (2–5 milliseconds) bi- or triphasic extracellular waveform (Aghajanian and Vandermaelen, 1982).
Recording of LC NE Neurons
LC NE neurons were recorded with a single-barrel glass micropipette positioned at 1.1–1.2 mm posterior to lambda and 0.9–1.3 mm from the midline suture and at a depth of 4.5–6.0 mm from the surface of the brain. NE neurons were identified by their regular firing rate (0.5–5 Hz), a biphasic action potential of long duration (~2 milliseconds), and a characteristic burst discharge followed by a quiescent period in response to a nociceptive pinch of the contralateral hind paw (Marwaha and Aghajanian, 1982).
Recording of VTA DA Neurons
The number of spontaneously active DA neurons found per track was determined by recording multiple tracks in a grid of 7–9 tracks per rat. Descents were separated by 100 µm and carried out according to the following coordinates from lambda: anterior to posterior: 3.1 to 3.3 mm and 0.7–1.0 mm lateral to the midline and lowered to a depth of 6.5–9 mm from the surface of the brain. The presumed DA neurons were identified by well-established electrophysiological criteria (Ungless and Grace, 2012), including the following: (1) regular or irregular single spiking pattern that may include burst firing with a rate between 2 and 10 Hz; (2) biphasic or triphasic waveforms, with an initial positive deflection (usually notched) followed by a prominent negative phase, with a duration ≥1.1 milliseconds from start to trough of the waveform; (3) long-duration action potentials (2.5–4 milliseconds), and (4) low-pitch sound when monitored by an audio amplifier.
Burst Analysis
Firing activity of monoaminergic neurons was analyzed using spike-sorting software (www.github.com/nno/birstidator/releases). For burst activity, the start of a burst was indicated by the occurrence of 2 spikes with interspike intervals (ISI) <0.08 second for NE and DA neurons and <0.01 second for 5-HT neurons. The termination of a burst was defined as an ISI >0.16 second for NE and DA (Grace and Bunney, 1983) and ISI >0.01 second for 5-HT neurons (Hajos and Sharp, 1996).
Statistical Analysis
Data are presented as mean values ± SEM. Statistical comparisons between control and ketamine groups were carried out using the two-tailed t test when normality passed with the Shapiro-Wilk test. When the normality test failed, the nonparametric Mann-Whitney test was utilized. This test is based on comparing the medians of the groups, and these were added in the tables and figures where it applies. Histograms with individual data were constructed with scripts using matplotlib and seaborn packages in Python 3.7 (Python Software Foundation, Oregon, USA). Statistical comparisons were done using the software SigmaPlot 12.5 (Systat Software Inc, California, USA).
RESULTS
Because monoamine systems are involved in the antidepressant-like effects of ketamine, we tested whether a single ketamine administration can induce a change in the activity of 5-HT, NE, and DA neurons that would persist for at least 1 day, and whether repeated injections would prolong such effects. The following 2 experimental groups were used in this study: (1) a single-administration group whereby ketamine was injected once at a dose of 10 mg/kg (ip) and recordings were carried out 1 day later, and (2) a repeated administration group whereby ketamine was administered 3 times a week (Monday, Wednesday, and Friday) for 2 weeks, and recordings were made 1 day after the last injection and 3 and 7 days later in cases where significant changes occurred. Detailed statistical analyses are included in Tables 1–3.
Table 1.
Effect of acute (A) and repeated (B) administration of ketamine on discharge parameters of DRN 5-HT neurons 1 day after last injection.
| Firing activity (Hz) |
Burst activity (% neurons firing in burst) |
|||||
|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Rats, n |
Neurons, n | |
| A. Single regimen | ||||||
|
Control (day 1)
Ketamine (day 1) |
1.28 ± 0.07 1.18 ± 0.08 |
1.15 1.06a |
18.57 ± 2.93 19.57 ± 4.05b |
__ __ |
7 7 |
104 101 |
| B. Repeated regimen | ||||||
|
Control (day 1)
Ketamine (day 1) |
1.24 ± 0.08 1.10 ± 0.09 |
1.15 0.91c |
18.4 ± 2.71 17.8 ± 4.31d |
__ __ |
5 6 |
81 87 |
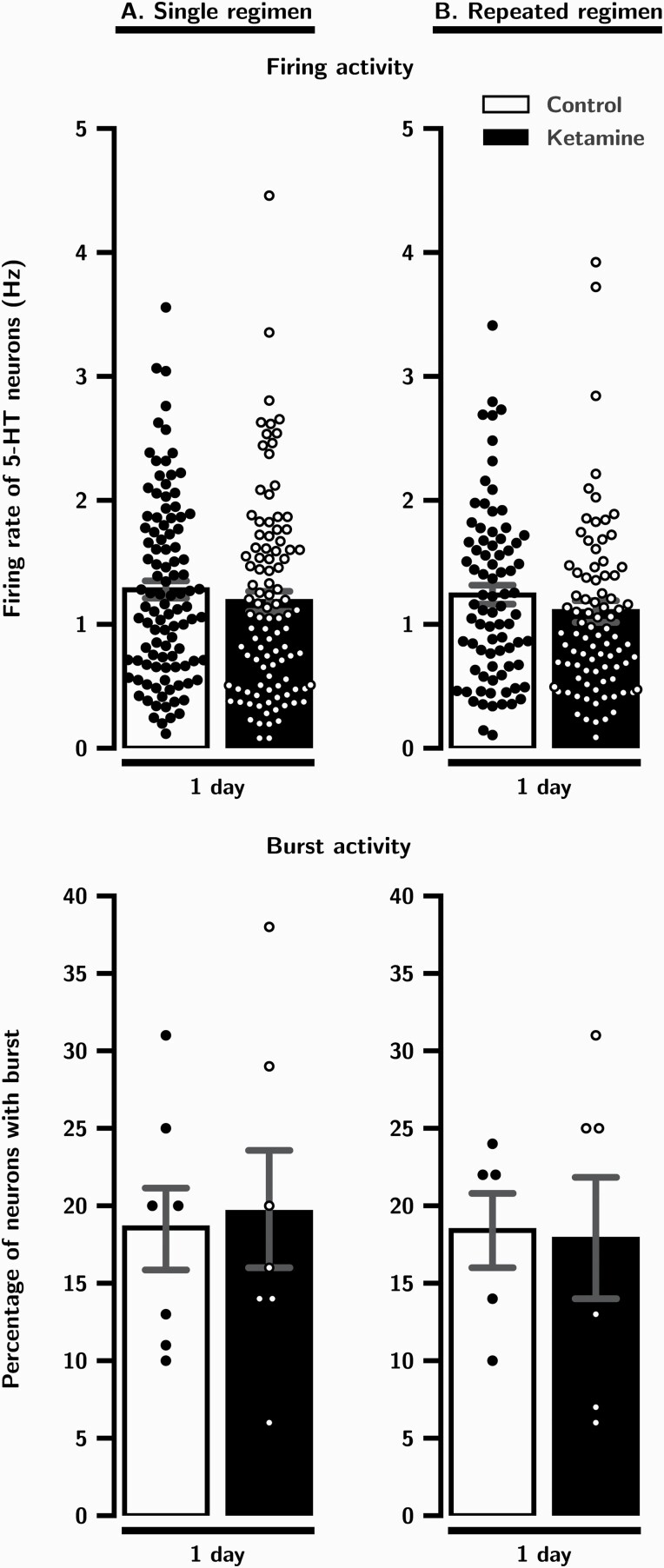
Results are expressed as mean ± SEM. Median values were also shown when Mann-Whitney test was used. (A) Firing activity, aMann-Whitney test, U = 4725, P = .2. Burst activity, bTwo-tailed t test, t(12) = -0.2, P = .8. (B) Firing activity: cMann-Whitney test, U = 2962, P = .08; Burst activity: dTwo-tailed t test, t(9) = 0.1, P = .9.
Table 2.
Effect of acute (A) and repeated (B) administration of ketamine on discharge parameters of LC NE neurons 1 and 3 days after last injection.
| Firing activity (Hz) | Burst activity (% neurons firing in burst) | |||||
|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Rats, n | Neurons, n | |
| A. Single regimen | ||||||
| Control (day 1) | 1.57 ± 0.09 | 1.33 | 38.20 ± 6.41 | __ | 5 | 84 |
| Ketamine (day 1) | 1.60 ± 0.08 | 1.62a | 28.40 ± 6.04b | __ | 5 | 83 |
| B. Repeated regimen | ||||||
| Control (day 1) | 1.58 ± 0.08 | 1.47 | 33.60 ± 6.87 | __ | 5 | 90 |
| Control (day 3) | 1.64 ± 0.08 | 1.60 | 35.17 ± 6.12 | __ | 6 | 67 |
| Ketamine (day 3) | 1.54 ± 0.08 | 1.40d | 37.80 ± 4.96f | __ | 5 | 67 |
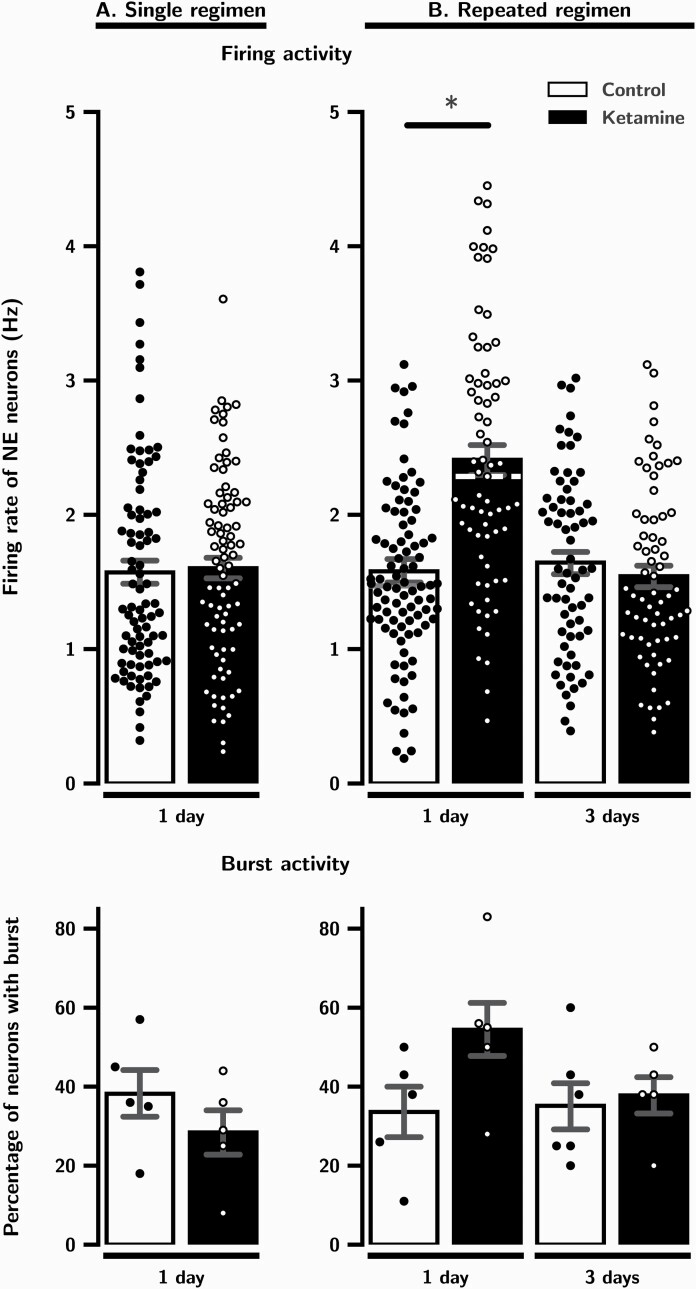
Results are expressed as mean ± S.E.M. Median values were also shown when Mann-Whitney test was used. (A) Firing activity: aMann-Whitney test, U = 3271, P =.5. Burst activity: bTwo-tailed t test, t(8) = 1.1, P =.3. (B) Firing activity: cMann-Whitney test, U = 1471, P <.001; dMann-Whitney test, U = 2036, P =.4. Burst activity: eTwo-tailed t test, t(8) = -1.9, P =.1; fTwo-tailed t test, t(9) = -0.3, P =.8. * indicate significant statistical difference with P <.05.
Table 3.
Effect of acute (A) and repeated (B) administration of ketamine on discharge parameters of VTA DA neurons 1, 3 and 7 days after last injection.
| Firing activity (Hz) | Burst activity (% spikes in burst) | Population activity (number of neurons/track) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Mean | Median | Rats, n | Neuron, n | |
| A. Single regimen | ||||||||
| Control (day 1) | 4.28 ± 0.19 | 4.16 | 33.83 ± 3.39 | 22.38 | 1.98 ± 0.18 | __ | 6 | 87 |
| Ketamine (day 1) | 4.30 ± 0.19 | 4.16a | 34.41 ± 3.56 | 28.41b | 1.47 ± 0.19c | __ | 6 | 68 |
| B. Repeated regimen | ||||||||
| Control (day 1) | 4.45 ± 0.20 | __ | 24.66 ± 3.19 | 14.69 | 1.50 ± 0.14 | __ | 6 | 74 |
| Ketamine (day 1) | 4.82 ± 0.15d | __ | 32.91 ± 2.82 | 26.45g* | 2.19 ± 0.09j* | __ | 5 | 86 |
| Control (day 3) | 3.81 ± 0.18 | 3.57 | 21.39 ± 3.01 | 11.47 | 1.11 ± 0.07 | 1.11 | 7 | 69 |
| Ketamine (day 3) | 4.37 ± 0.20 | 4.37e | 34.71 ± 3.05 | 29.61h* | 2.25 ± 0.35 | 2.14k* | 5 | 96 |
| Control (day 7) | 4.07 ± 0.23 | __ | 30.17 ± 3.27 | 18.67 | 1.36 ± 0.12 | __ | 6 | 75 |
| Ketamine (day 7) | 4.13 ± 0.23f | __ | 31.52 ± 3.56 | 19.54i | 1.44 ± 0.11l | __ | 6 | 66 |
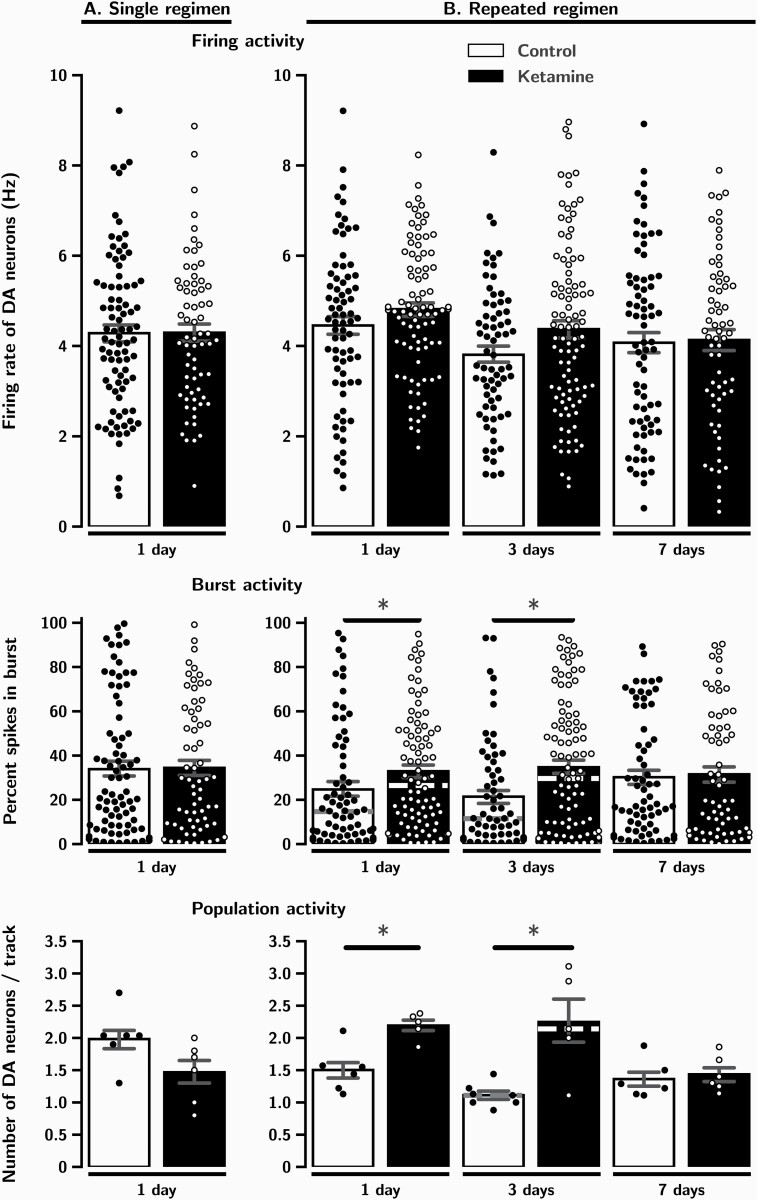
Results are expressed as mean ± SEM. Median values were also shown when Mann-Whitney test was used. (A) Firing activity: aMann-Whitney test, U = 2891, P = .8; Burst activity: bMann-Whitney test, U = 2597, P = .9; Population activity: cTwo-tailed t test, t(10) = 2, P = .08. (B) Firing activity: dTwo-tailed t test, t(158) = -1.4, P = .2; eMann-Whitney test, U = 2793, P = .09; fTwo-tailed t test , t(139) = -0.47, P = .6. Burst activity: gMann-Whitney test, U = 2381, P = .01; hMann-Whitney test, U = 2268, P =.01; iMann-Whitney test, U = 2092, P = .9. Population activity: jTwo-tailed t test , t(9) = -3.9, P = .003; kMann-Whitney test, U = 3.5, P = .02; lTwo-tailed t test , t(10) = -0.5, P =.7. * indicate significant statistical difference with P < .05.
Effects of Single and Repeated Administration of Ketamine on DRN 5-HT Neurons
One day after a single administration of ketamine, there was no significant difference in the mean firing rate of 5-HT neurons in rats that received ketamine versus control animals (P = .2; Table 1A; Figure 1A). Also, there was no change in the percentage of 5-HT neurons firing in bursts (P = .8; Table 1A; Figure 1A).
Figure 1.
Effects of a single (A) and repeated (B) administration of ketamine (10 mg/kg/day) on DRN 5-HT neurons firing and burst activity 1 day after the last injection. Each dot indicates a value for an individual data point. Histograms show data as mean values ± SEM. Statistical significance is indicated where it applies.
One day after repeated administration of ketamine, there was still no significant difference compared with controls in the mean firing rate of 5-HT neurons (P = .08; Table 1B, Figure 1B) or the number firing in burst mode (P = .9; Table 1B, Figure 1B).
Effects of Single and Repeated Administration of Ketamine on LC NE Neurons
As illustrated in Figure 2A and shown in Table 2A, the mean firing rate of NE neurons and the percentage of those firing in burst mode was not significantly altered 1 day after a single administration of ketamine (P = .5 and .3, respectively).
Figure 2.
The effects of a single (A) and repeated (B) administration of ketamine (10 mg/kg/day) on LC neurons firing and burst activity 1 and 3 days after the last injection. Experiments are carried out until significant effects on firing and burst activity disappear. Each dot indicates a value for an individual data point. Histograms show data as mean values ± SEM. Medians are indicated in dashed lines when Mann-Whitney test was used. Statistical significance is indicated where it applies. *P < .05 when compared to control.
The mean firing rate of NE neurons was significantly increased by 53% 1 day after repeated administration of ketamine (P < .001; Table 2B; Figure 2B). This enhancement was no longer present 3 days after last injection (P = .4; Table 2B; Figure 2B). Despite an increase of 62% in the ketamine group compared to controls, the percentage of NE neurons firing in burst mode was not statistically significant after 1 day and remained at control level 3 days after multiple injections (P = .1 and P = .8, respectively; Table 2B; Figure 2B).
Effects of Single and Repeated Administration of Ketamine on VTA DA Neurons
One day after a single administration of ketamine, neither the firing rate (P = .8; Table 3A; Figure 3A) nor the percentage of spikes occurring in bursts (P = .9; Table 3A; Figure 3A) were significantly different in rats that received ketamine compared to controls. The number of spontaneously firing DA neurons encountered per track (population activity) was also unaltered 1 day after a single administration of ketamine (P = .08; Table 3A; Figure 3A).
Figure 3.
The effects of a single (A) and repeated (B) administration of ketamine (10 mg/kg/day) on VTA DA neurons firing, burst and population activity, 1, 3 and 7 days after the last injection. Experiments are carried out until significant effects on firing and burst activity disappear. Each dot indicates a value for an individual data point. Histograms show data as mean values ± SEM. Medians are indicated in dashed lines when the Mann-Whitney test was used. Statistical significance is indicated where it applies. * P < .05 when compared to control.
As illustrated in Figure 3B and shown in Table 3B, following 6 repeated administrations of ketamine, there was no alteration of the firing activity of DA neurons 1 day after the last injection (P = .2). However, this same regimen induced a significant 33% increase in the percentage of spikes occurring in bursts (P = .01). This burst activity was further enhanced to 62% after 3 days (P = .01), but was no longer present after 7 days (P = .7). The mean number of spontaneously firing DA neurons recorded per track was increased by 46% after 1 day and by 103% after 3 days (P = .003 and P = .02, respectively). These marked enhancements were no longer present 7 days after the last injection of ketamine (P = .7).
Discussion
There is ample evidence supporting the notion that an enhancement of 5-HT transmission can exert a significant role in antidepressant-like responses of various pharmacological and brain stimulation strategies. One parameter that can contribute to an enhancement of 5-HT transmission is an increase of the firing activity of 5-HT neurons above their baseline, as was documented with agomelatine, bupropion, mirtazapine, and vagus nerve stimulation (Blier and El Mansari, 2013). The results of the present experiments indicate that ketamine did not significantly alter the firing rate of 5-HT neurons in the rat DRN 24 hours after a single injection, which is consistent with an unaltered extracellular level of 5-HT in the mouse DRN, but unexpectedly not with a concomitant suppressed firing of 5-HT neurons (Pham et al., 2017). The current observations taken together with the unmodified firing activity of 5-HT neurons within 2 hours after single administration indicate that ketamine, despite its moderate affinity for the 5-HT transporter (Martin et al., 1990; El Iskandrani et al., 2015) does not inhibit 5-HT reuptake to a significant extent in the DRN sufficiently to inhibit firing, as SSRIs do. Repeated administration of ketamine for 2 weeks also did not modify this parameter. These results stand in contrast with the enhanced c-fos expression in DRN 5-HT neurons following systemic administration of ketamine and its microinjection in mouse medial prefrontal cortex (mPFC; Fukumoto et al., 2016).
The seemingly unaltered firing activity of 5-HT neurons does not, however, eliminate the possibility that the 5-HT system is a contributor to the antidepressant response of ketamine, because in the mPFC, a single subcutaneous injection of ketamine enhances the extracellular 5-HT level after 1 day (Pham et al., 2017). In this same brain region, it was also found that a single injection of ketamine increased 5-HT–induced excitatory potentials 1 day after its administration (Li et al., 2010). Furthermore, it was reported that 5-HT synthesis inhibition using para-chlorophenylanine prevented the decrease in immobility in the forced swim test produced 1 day after a single administration of ketamine (Gigliucci et al., 2013; Fukumoto et al., 2016; Pham et al., 2017). Although the present results showed that 5-HT neuronal activity was unaltered, it is interesting that the latter did not decrease after either single or repeated administration of ketamine, whereas medications such as 5-HT reuptake inhibitors and monoamine oxidase inhibitors that indirectly activate 5-HT1A autoreceptors initially inhibit this activity. Clearly, the present results showed that an increase in the firing activity of DRN 5-HT neurons did not play a role in maintaining the antidepressant effect of ketamine. Therefore, net 5-HT transmission will have to be assessed in postsynaptic brain structures, as all antidepressant treatments studied so far showed an enhancement of tonic activation of the 5-HT1A receptors of hippocampus CA3 pyramidal neurons (Blier and El Mansari, 2013).
Various strategies used to treat MDD can enhance noradrenergic transmission, as is the case for the 5-HT system. For instance, long-term administration of mirtazapine, as well as prolonged vagus nerve stimulation, enhance the firing rate of NE neurons above their baseline (Haddjeri et al., 1997; Manta et al., 2013). Prior results showed that the mean firing rate of NE neurons was increased and the percentage of neurons firing in bursts was doubled 30 minutes to 2 hours after a single ketamine injection. As well, the firing rate of NE neurons was still elevated immediately after 3 daily injections, but the percentage of neurons firing in bursts was no longer enhanced (El Iskandrani et al., 2015). In the current experiments, 24 hours after such a single dose of ketamine, the NE neuronal firing and burst activity was no longer enhanced. In contrast, 24 hours after a thrice weekly regimen of ketamine over 2 weeks, the firing rate of NE neurons was significantly increased, but dissipated three days after the last injection. These results indicate that repeated administration of ketamine can sustain an enhancement of NE neuronal firing by ketamine after its washout.
The enhancement of the firing activity of NE neurons by ketamine is unlikely due to a direct blockade of NMDA receptors in the LC because local application of the NMDA antagonist AP-5 does not alter the baseline firing activity of LC NE neurons (Jodo and Aston-Jones, 1997). Rather, it is likely that the increase in firing of NE neurons by ketamine results from accrued activity at AMPA receptors, because this rapid enhancement of firing is prevented by systemic administration of the AMPA antagonist NBQX (El Iskandrani et al., 2015). These receptors may be located within the LC, because local application of AMPA itself and AMPA receptor agonists in the LC enhance the firing, but not the burst activity, of NE neurons (Olpe et al., 1989; Rasmussen et al., 1996). The increased firing activity of NE neurons could also be mediated through excitatory glutamatergic afferents to the LC (Szabadi, 2013). Specifically, it was reported that ketamine, by blocking NMDA receptors on GABA neurons, increases the firing activity of mPFC pyramidal neurons (Jackson et al., 2004; Homayoun and Moghaddam, 2007), and presumably results in enhanced activity of NE neurons (Jodo et al., 1998). This possibility is supported by the observation that this enhancement of activity is absent in rats anesthetized with ketamine, where the frontal cortex rather exerts an inhibitory influence on NE neuronal firing (Sara and Hervé-Minvielle, 1995). Whether this increase in activity is due to enhancement in AMPA or a prolonged blockade of NMDA in the mPFC remains to be elucidated. Altogether, the present results showed that repeated administration of ketamine can sustain an increase in the firing rate of NE neurons and that it may play a role in maintaining the antidepressant response to ketamine.
It is now well documented that shortly after an acute injection of ketamine there is an increase in population activity of VTA DA neurons, without any alteration of their mean firing rate or burst activity in naïve rats (El Iskandrani et al., 2015; Witkin et al., 2016) and a restoration of DA neuron population activity in a behavioral model of depression (Belujon and Grace, 2014). This escalation of activity in the VTA is mediated by AMPA receptors since prior systemic injection of NBQX prevents the enhancement (El Iskandrani et al., 2015; Witkin et al., 2016). In the current experiments, such an increase was no longer present 24 hours after a single injection, thus suggesting that the prompt rise in population activity was not sustained over time at least in naïve rats. This enhancement was, however, sustained after 24 hours postinjection in helpless Wistar-Kyoto rats (Belujon and Grace, 2014). This discrepancy may have stemmed from the fact that helpless rats used in the latter study presented dampened DA neuron population activity at baseline. Similarly, 3 consecutive injections of ketamine in helpless rats increased population activity of DA neurons after 1 day (Belujon et al., 2014), although it did not do so in naïve rats (El Iskandrani et al., 2015). As shown herein in these rats, however, 6 injections repeated over 2 weeks increased DA neuron population activity for 3 days after the last administration. These results indicate that the increase in burst and population activity of DA neurons required longer term administration to reliably manifest itself, at least in naïve rats. In addition, once this upregulation is consolidated, it was sustained for at least 3 days after the last injection following a schedule of injections used in the clinic (Phillips et al., 2019).
The increases in DA neurons population and burst activity are controlled by different mechanisms. On the one hand, an enhancement in population activity is regulated by the ventral subiculum (vSub) since its activation by infusion of NMDA in that structure induces an increase in such population activity, but not bursting activity. On the other hand, activation of the pedunculopontine tegmental nucleus (PPTg) by local application of NMDA in that structure results in a significant increase in DA neuronal burst firing, but not population activity. Furthermore, both parameters are increased when vSub and PPTg are coactivated (Lodge and Grace, 2006). Accordingly, since ketamine is a noncompetitive NMDA receptor antagonist, its blockade of NMDA receptors resulting from systemic administration should have produced a decrease rather than the observed increase in population and burst activities. Instead, at least the increase in burst activity observed herein after 2 weeks with 6 repeated injections of ketamine may have stemmed from a direct effect on DA neurons. Indeed, direct activation of AMPA and NMDA receptors plays a role in increasing burst activity of VTA DA neurons (Chergui et al., 1993; Zhang et al., 1997; Zakharov et al., 2016). Since ketamine blocks NMDA receptors yet increases AMPA receptors throughput, the latter action was hypothesized to underlie its antidepressant effect (Zarate and Machado-Vieira, 2016); it is possible that the ketamine-induced increase in burst activity is due to an increase in AMPA receptor density. It is also conceivable that the maintenance of the increase in burst activity for 3 days after ketamine has long been eliminated (Sato et al., 2004; Mion and Villevieille, 2013) may be due to a sustained enhancement of AMPA GluA1 subunits within the VTA itself. Whether there is an increase of AMPA signaling in the VTA by ketamine remains to be investigated. However, the increase in AMPA GluA1 subunits in the medial prefrontal cortex and dentate gyrus was shown to be present 8 days following a single systemic injection in mice subjected to the social defeat paradigm (Zhang et al., 2015b). It is thus possible that such a phenomenon may have maintained the increase in burst and population activity of DA neurons seen in the present experiments after ketamine was no longer present.
Given that cocaine and ketamine both impact the DA system, it is important to consider similarities and differences between both agents. Although a single cocaine exposure increases burst activity of VTA DA neurons for up to 5 days, this effect was abolished by the NMDA receptor antagonist MK-801 (Creed et al., 2016), suggesting that NMDA receptor activation is necessary to induce changes in activity. However, in the case of ketamine herein, when NMDA receptors had been blocked by ketamine, there was still an increase in burst activity but that only lasted 3 days, presumably through an action on AMPA receptors (see discussion above). In addition, a single cocaine administration increases the rate of firing activity of DA neurons, but ketamine did not, as shown here. Finally, while repeated ketamine exposure increased the population activity of DA neurons, previous studies had shown that repeated cocaine decreased population activity 14 days after the last injection (Ackerman and White, 1992; Shen et al., 2007). Altogether, these discrepancies do not suggest a common mechanism of action between cocaine and ketamine. Whether ketamine has addictive effects still needs to be properly assessed in a paradigm of self administration. On the clinical side, so far there has been no study showing addictive properties of ketamine administered at low doses in a monitored environment for the treatment of depression.
In summary, the current study documented an increase in firing of NE neurons by repeated ketamine administration that persisted at least for 1 day, and a sustained enhancement of population and burst activity of DA neurons that lasted, respectively, 1 and 3 days. It is intriguing that these brain adaptions induced by ketamine would persist for days while the drug is rapidly eliminated. Consequently, these monoaminergic alterations produced by ketamine appear more consistent with the maintenance than the immediate manifestation of its antidepressant response.
Acknowledgments
This research was conducted as part of the Canadian Biomarker Integration Network for Depression (CAN-BIND) program through the Ontario Brain Institute, an independent nonprofit corporation, funded partially by the Government of Ontario. The opinions, results, and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. A part of this research was also funded by the Canadian Institutes of Health Research (grant PJT-169078 to P.B.).
Statement of Interest
C.M.O., R.H., and M.E.M. declare no conflict of interest. P.B. received grant funding and/or honoraria for lectures, expert testimony, and/or participation in advisory boards for Allergan, Bristol Myers Squibb, Eli Lilly, Janssen, Lundbeck, Otsuka, Pfizer, Pierre Fabre Médicaments, Valeant, and Takeda.
Author Contribution
M.E.M. and P.B. designed the study and wrote the protocol. C.M.O., R.H., and M.E.M. carried out the experiments and undertook the statistical analysis. M.E.M. wrote the first draft of the manuscript. All authors contributed to writing the final manuscript.
References
- Ackerman JM, White FJ (1992) Decreased activity of rat A10 dopamine neurons following withdrawal from repeated cocaine. Eur J Pharmacol 218:171–173. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Vandermaelen CP (1982) Intracellular recordings from serotonergic dorsal raphe neurons: pacemaker potentials and the effect of LSD. Brain Res 238:463–469. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA (2014) Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Blier P, El Mansari M (2013) Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc Lond B Biol Sci 368:20120536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Charléty PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G (1993) Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci 5:137–144. [DOI] [PubMed] [Google Scholar]
- Creed M, Kaufling J, Fois GR, Jalabert M, Yuan T, Lüscher C, Georges F, Bellone C (2016) Cocaine exposure enhances the activity of ventral tegmental area dopamine neurons via calcium-impermeable NMDARs. J Neurosci 36:10759–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RM, Bryant KA, Thompson NR (2020) Metabolic syndrome rather than body mass index is associated with treatment response to ketamine infusions. J Clin Psychopharmacol 40:75–79. [DOI] [PubMed] [Google Scholar]
- El Iskandrani KS, Oosterhof CA, El Mansari M, Blier P (2015) Impact of subanesthetic doses of ketamine on AMPA-mediated responses in rats: an in vivo electrophysiological study on monoaminergic and glutamatergic neurons. J Psychopharmacol 29:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Chaki S (2016) The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 41:1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A (2013) Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology 228:157–166. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS (1983) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–1. Identification and characterization. Neuroscience 10:301–315. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C (1997) Effects of long-term treatment with the alpha 2-adrenoceptor antagonist mirtazapine on 5-HT neurotransmission. Naunyn Schmiedebergs Arch Pharmacol 355:20–29. [DOI] [PubMed] [Google Scholar]
- Hajos M, Sharp T (1996) Burst-firing activity of presumed 5-HT neurones of the rat dorsal raphe nucleus: electrophysiological analysis by antidromic stimulation. Brain Res 740:162–168. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B (2004) NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A 101:8467–8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Aston-Jones G (1997) Activation of locus coeruleus by prefrontal cortex is mediated by excitatory amino acid inputs. Brain Res 768:327–332. [DOI] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G (1998) Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience 83:63–79. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS (2019) Ketamine: a paradigm shift for depression research and treatment. Neuron 101:774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA (2006) The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology 31:1356–1361. [DOI] [PubMed] [Google Scholar]
- López-Gil X, Jiménez-Sánchez L, Campa L, Castro E, Frago C, Adell A (2019) Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci 10:3318–3326. [DOI] [PubMed] [Google Scholar]
- Manta S, El Mansari M, Debonnel G, Blier P (2013) Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int J Neuropsychopharmacol 16:459–470. [DOI] [PubMed] [Google Scholar]
- Martin DC, Introna RP, Aronstam RS (1990) Inhibition of neuronal 5-HT uptake by ketamine, but not halothane, involves disruption of substrate recognition by the transporter. Neurosci Lett 112:99–103. [DOI] [PubMed] [Google Scholar]
- Marwaha J, Aghajanian GK (1982) Relative potencies of alpha-1 and alpha-2 antagonists in the locus ceruleus, dorsal raphe and dorsal lateral geniculate nuclei: an electrophysiological study. J Pharmacol Exp Ther 222:287–293. [PubMed] [Google Scholar]
- Mion G, Villevieille T (2013) Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 19:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpe HR, Steinmann MW, Brugger F, Pozza MF (1989) Excitatory amino acid receptors in rat locus coeruleus. An extracellular in vitro study. Naunyn Schmiedebergs Arch Pharmacol 339:312–314. [DOI] [PubMed] [Google Scholar]
- Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ, Gardier AM (2017) Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology 112:198–209. [DOI] [PubMed] [Google Scholar]
- Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, Owoeye O, Batten LA, Blier P (2019) Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry 176:401–409. [DOI] [PubMed] [Google Scholar]
- Pilc A, Wierońska JM, Skolnick P (2013) Glutamate-based antidepressants: preclinical psychopharmacology. Biol Psychiatry 73:1125–1132. [DOI] [PubMed] [Google Scholar]
- Pralong E, Magistretti P, Stoop R (2002) Cellular perspectives on the glutamate-monoamine interactions in limbic lobe structures and their relevance for some psychiatric disorders. Prog Neurobiol 67:173–202. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Kendrick WT, Kogan JH, Aghajanian GK (1996) A selective AMPA antagonist, LY293558, suppresses morphine withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropsychopharmacology 15:497–505. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments (2017) A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 74:399–405. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Hervé-Minvielle A (1995) Inhibitory influence of frontal cortex on locus coeruleus neurons. Proc Natl Acad Sci U S A 92:6032–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Kobayashi E, Hakamata Y, Kobahashi M, Wainai T, Murayama T, Mishina M, Seo N (2004) Chronopharmacological studies of ketamine in normal and NMDA epsilon1 receptor knockout mice. Br J Anaesth 92:859–864. [DOI] [PubMed] [Google Scholar]
- Shen RY, Choong KC, Thompson AC (2007) Long-term reduction in ventral tegmental area dopamine neuron population activity following repeated stimulant or ethanol treatment. Biol Psychiatry 61:93–100. [DOI] [PubMed] [Google Scholar]
- Szabadi E (2013) Functional neuroanatomy of the central noradrenergic system. J Psychopharmacol 27:659–693. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Grace AA (2012) Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM (2016) The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 358:71–82. [DOI] [PubMed] [Google Scholar]
- Zakharov D, Lapish C, Gutkin B, Kuznetsov A (2016) Synergy of AMPA and NMDA receptor currents in dopaminergic neurons: a modeling study. Front Comput Neurosci 10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Machado-Vieira R (2016) GSK-3: a key regulatory target for ketamine’s rapid antidepressant effects mediated by enhanced AMPA to NMDA throughput. Bipolar Disord 18:702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME (1997) Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther 281:699–706. [PubMed] [Google Scholar]
- Zhang GF, Liu WX, Qiu LL, Guo J, Wang XM, Sun HL, Yang JJ, Zhou ZQ (2015a) Repeated ketamine administration redeems the time lag for citalopram’s antidepressant-like effects. Eur Psychiatry 30:504–510. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Han M, Hashimoto K (2015b) Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology 232:4325–4335. [DOI] [PubMed] [Google Scholar]