Abstract
Papillary muscle rupture (PMR) is a catastrophic complication of acute myocardial infarction (AMI). We report on 3 consecutive patients with AMI cardiogenic shock due to PMR, treated with combined venoarterial extracorporeal membrane oxygenation and Impella-CP axial flow circulatory support as a bridge to definitive surgery. (Level of Difficulty: Intermediate.)
Key Words: acute myocardial infarction, cardiogenic shock, combined VA-ECMO and Impella-CP, mechanical circulatory support, papillary muscle rupture
Abbreviations and Acronyms: AMI, acute myocardial infarction; CI, cardiac index; ECG, electrocardiography; LV, left ventricular; MR, mitral regurgitation; PMR, papillary muscle rupture; VA-ECMO, venoarterial extracorporeal membrane oxygenation
Graphical abstract
Case 1
A 70-year-old female with a history of diabetes presented after 3 days of chest pain and shortness of breath. Physical examination revealed a grade 3/6 holosystolic murmur in the left lower sternal border with bilateral inspiratory crackles. Notable laboratory results included a troponin concentration of 6 ng/ml and a brain natriuretic peptide concentration of 837 pg/ml. Electrocardiography (ECG) showed diffuse ST-segment depressions. Coronary angiography demonstrated an acutely thrombotic distal circumflex artery lesion. Left ventricular (LV) angiography showed she had hyperdynamic LV function with 4+ mitral regurgitation (MR). Right heart catheterization revealed a severely depressed cardiac index (CI) of 1.8 l/min/m2 with reduced cardiac power output (CPO) of 0.45 W and elevated pulmonary capillary wedge pressure (PCWP) of 40 mm Hg. Echocardiography demonstrated severe MR with a flail posterior mitral valve leaflet and an ejection fraction (EF) of 65%.
Learning Objectives
-
•
To understand the role of a strategy combining mechanical circulatory support using venoarterial extracorporeal membrane oxygenation and Impella-CP in cases of cardiogenic shock due to papillary muscle rupture.
-
•
To recognize the benefit of hemodynamic stabilization in cases of acute papillary muscle rupture to facilitate definitive surgical repair.
An intra-aortic balloon pump (IABP) was initially placed for afterload reduction. However, the patient’s clinical condition deteriorated, as she developed lactic acidosis, acute kidney injury, and shock liver. Given her high operative risk (calculated by Society of Thoracic Surgery [STS] risk of mortality of 16% and morbidity/mortality rate of 83%), a multidisciplinary heart team decision was made to provide cardiopulmonary support with venoarterial extracorporeal membrane oxygenation (VA-ECMO) and to vent the left ventricle by using an Impella-CP (Abiomed, Danvers, Massachusetts). Figures 1, 2 and 3 show echocardiographic images following escalation of mechanical circulatory support (MCS). Her hemodynamic values and markers of end-organ perfusion improved (Table 1). On day 7 of hospitalization, she underwent surgical mitral valve replacement (MVR) with coronary artery bypass graft, and VA-ECMO was decannulated following surgery. She was discharged to a rehabilitation center 20 days later.
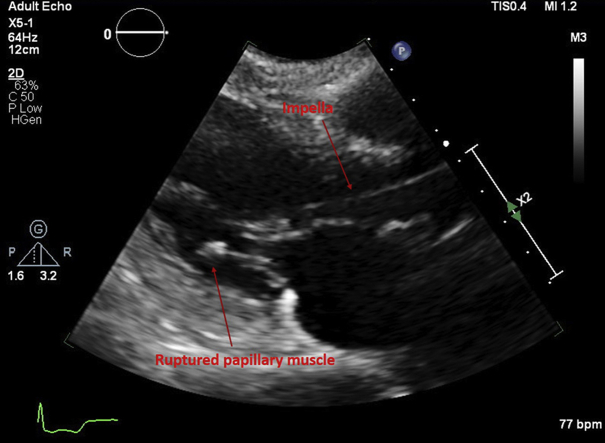
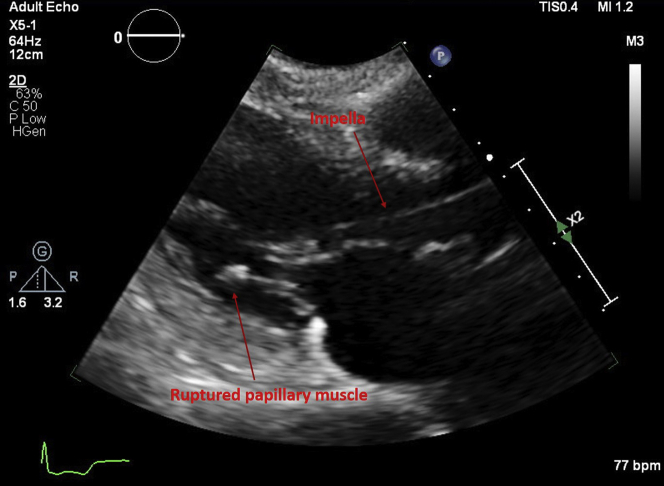
Figure 1.
Impella-CP in Left Ventricle
Transthoracic echocardiogram parasternal long-axis view shows Impella in the left ventricle and ruptured papillary muscle attached to the posterior leaflet of the mitral valve.
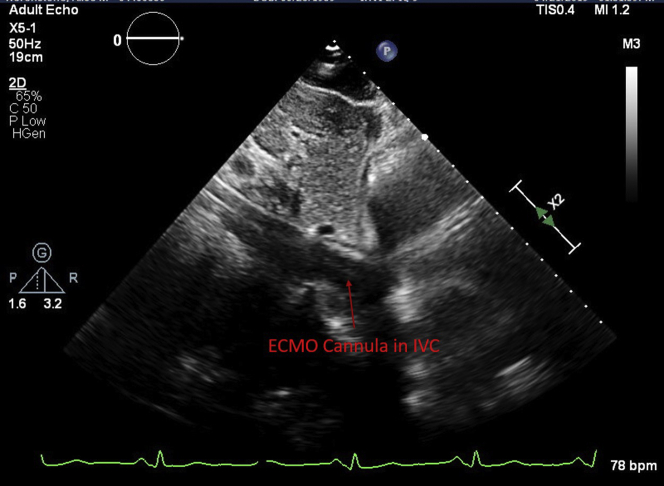
Figure 2.
ECMO Cannula in Inferior Vena Cava
Transthoracic echocardiogram subcostal view shows extracorporeal membrane oxygenation (ECMO cannula in the inferior vena cava (IVC).
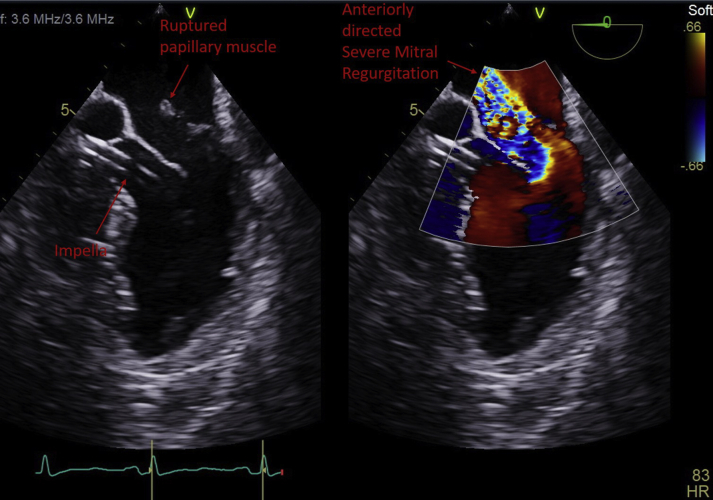
Figure 3.
Transesophageal Echocardiogram Upper Esophageal View Shows an Impella-CP Device Traversing the Left Ventricular Outflow Tract Into the Left Ventricle
The ruptured papillary muscle attached to the posterior mitral valve leaflet. Color flow imaging shows anteriorly directed severe mitral regurgitation.
Table 1.
Cardiac Hemodynamic Indices and Markers of Perfusion Pre- and Post-Impella-CP Support
| Patient 1 |
Patient 2 |
Patient 3 |
||||
|---|---|---|---|---|---|---|
| Pre-MCS | Post-MCS | Pre-MCS | Post-MCS | Pre-MCS | Post-MCS | |
| RA, mm Hg | 15 | 7 | 4 | 7 | 20 | 8 |
| RV, mm Hg | 56/18 | 50/8 | ||||
| PA, mm Hg | 65/35 (45) | 36/19 (25) | 44/20 (28) | 33/15 (21) | 50/29 (36) | 35/10 (18) |
| PAPi | 2 | 2.4 | 6 | 2.5 | 1.1 | 3.1 |
| PCWP, mm Hg | 40 | 20 | 32 | |||
| CO (Fick), l/min | 3.6 | 5.3 | 3.1 | 5.5 | 3.6 | 5.5 |
| CI | 1.8 | 2.6 | 1.76 | 3.2 | 2 | 3 |
| CPO, W | 0.45 | 0.83 | 0.42 | 0.97 | 0.5 | 0.94 |
| Lactate, mmol/l | 4.1 | 1.3 | 1.6 | 5.6 | 1.2 | |
| Cr, mg/dl | 1.6 | 0.8 | 1.1 | 0.9 | 0.9 | 0.8 |
| AST/ALT, U/l | 145/117 | 73/54 | 26/15 | 36/25 | 191/164 | 53/77 |
Values in parentheses are the mean pulmonary artery pressure.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; CI = cardiac index; CO = cardiac output; CPO = cardiac power output; Cr = creatinine; MCS = mechanical circulatory support; PA = pulmonary artery pressure; PAPi = pulmonary artery pulsatility index; PCWP = pulmonary capillary wedge pressure; RA = right atrium; RV = right ventricle.
Case 2
A 79-year-old female without a cardiac history presented to an outside hospital with chest pain and hypotension. Initial troponin concentration was 3 ng/ml, and ECG traces were consistent with posterior ST-segment elevation myocardial infarction (STEMI). Coronary angiography demonstrated an acutely thrombotic mid-right coronary artery lesion which was treated with 2 drug-eluting stents. She subsequently stabilized clinically. Her initial echocardiogram demonstrated an EF of 65% with no significant valvulopathy. On day 4, she developed cardiogenic shock (CS), as shown by elevated PCWP of 20 mm Hg, a reduced CI of 1.76, and a CPO of 0.42 W. A repeated echocardiogram showed severe MR with flail posterior mitral leaflet, and she was transferred to the authors’ institution for emergent surgery. Given her refractory CS with hypoxic respiratory failure and metabolic derangements (calculated STS risk of mortality of 18% and morbidity/mortality of 78%), a multidisciplinary heart team decision was made to stabilize her hemodynamic symptoms using VA-ECMO and provide left ventricular venting using the Impella-CP. Her respiratory and hemometabolic status improved, and she underwent surgical MVR on day 5. The patient was subsequently discharged to a rehabilitation center 32 days later.
Case 3
A 46-year-old male with no cardiac history presented to the hospital with chest pain. Initial ECG demonstrated inferior STEMI. Emergent coronary angiography revealed thrombotic occlusion of the distal right coronary artery, which was successfully treated with 1 drug-eluting stent. LV angiography demonstrated an EF of 35% with inferior akinesia and 4+ MR. Right heart catheterization demonstrated biventricular CS with right atrial (RA) pressure of 20 mm Hg, a PCWP of 32 mm Hg, a CPO of 0.5 W, and a pulmonary artery pulsatility index of 1.1. An IABP was emergently placed for afterload reduction. Echocardiography confirmed severe MR with ruptured chord and flail anterior leaflet. In the cardiac intensive care unit, the patient remained in refractory CS with biventricular failure and escalating vasopressor requirements, and his lactate concentration rose to 3 mmol/l. Given his multiorgan system dysfunction, the patient was deemed to be a prohibitive surgical risk (calculated STS risk of mortality of 7% and morbidity/mortality of 88%), and the multidisciplinary heart team decided to provide cardiopulmonary support with VA-ECMO and venting of the left ventricle using an Impella-CP. His hemodynamic values stabilized over the next 2 days. His hospital course was complicated, however, by large right middle cerebral artery stroke and pneumonia. The patient did not stabilize medically, so the family elected for comfort measures only, and the patient died.
Discussion
Despite a decline in incidence over the past 2 decades, papillary muscle rupture complicating acute myocardial infarction is still associated with a high rate of mortality due to refractory cardiogenic shock and multiorgan system failure (1). Although MVR remains the treatment of choice, high operative risks have compromised the overall effectiveness of this definitive surgical therapy (2).
The hemodynamic support equation has been proposed to understand the treatment objective in the management of CS: coronary reperfusion, circulatory support to maintain a viable arterial pressure, and ventricular unloading to mitigate the effects of elevated afterload (3). VA-ECMO relies on retrograde aortic flow to maintain end-organ perfusion. A recognized limitation of this strategy is the resultant increase in LV afterload, which increases myocardial work. This is particularly deleterious in papillary muscle rupture, as it can worsen valvular regurgitation and pulmonary edema. Strategies to unload the left ventricle in this situation include inodilators, IABP, Impella-CP, atrial septostomy, and direct pulmonary arterial puncture. Contemporary observational studies suggest that LV venting in CS patients supported with VA-ECMO may be associated with hemodynamic improvement, successful weaning from cardiopulmonary support, and improved survival in VA-ECMO patients treated with LV unloading (4, 5, 6).
A recent analysis of the Nationwide Inpatient Sample from 2002 to 2014, evaluating the use of MCS in patients with chordae tendinae and papillary muscle rupture complicating STEMI, demonstrated a mortality rate of 46% despite a high rate of the use of MCS (7). The use of MCS in this cohort remained almost exclusively limited to IABP (91%) with ECMO and percutaneous ventricular assist devices used 5% and 4.1% of the time, respectively. Although a mortality benefit has not been demonstrated in randomized clinical trials, axial and centrifugal flow devices provide superior hemodynamic support compared to IABP (8). Still, it remains unclear how other devices such as Tandem Heart (LivaNova, London, United Kingdom) or interatrial septostomy compare to Impella-CP as LV venting methods when combined with VA-ECMO (9).
In the present series of cases, the patients achieved hemometabolic stabilization following initiation of circulatory support with VA-ECMO and Impella-CP as shown by lactate clearance, rise in CPO, and decrease in mean pulmonary artery pressure (Table 1). Two of the patients eventually underwent surgical MVR on hospital days 5 to 7 and were successfully discharged.
Conclusions
A strategy of combined VA-ECMO and Impella circulatory support may serve to hemodynamically stabilize and bridge appropriately selected patients with acute myocardial infarction-cardiogenic shock complicated by papillary muscle rupture to surgery. The multidisciplinary heart team is pivotal in determining which patients are not at prohibitive risk for intervention and evaluating patient- specific factors to ascertain suitability for MCS. At the authors’ institution, hemodynamic support was provided for 5 to 7 days which enabled unloading of the left ventricle; optimization of end-organ perfusion; and decreased myocardial inflammation from recent infarct before proceeding with definitive surgical intervention.
Given the presence of concomitant cardiogenic shock and respiratory failure in this cohort, VA-ECMO is pivotal for cardiorespiratory support. Further research with multicenter registries and prospective randomized trials is warranted to study the safety and efficacy of different LV venting methods such as Impella-CP, Tandem Heart (LivaNova, London, United Kingdom) or interatrial septostomy.
Author Disclosures
Dr. Tehrani has served as a consultant and speaker for Medtronic. Dr. Batchelor has served as a consultant for Abbott, Boston Scientific, and vWave; and has served as a speaker for Abbott. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.O'Gara P.T., Kushner F.G., Ascheim D.D. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 2.French J.K., Hellkamp A.S., Armstrong P.W. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEX-AMI) Am J Cardiol. 2010;105:59–63. doi: 10.1016/j.amjcard.2009.08.653. [DOI] [PubMed] [Google Scholar]
- 3.Esposito M.L., Kapur N.K. Acute mechanical circulatory support for cardiogenic shock: the “door to support” time. F1000Res. 2017;6:737. doi: 10.12688/f1000research.11150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel S.M., Lipinski J., Al-Kindi S.G. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with Impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J. 2019;65:21–28. doi: 10.1097/MAT.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 5.Pappalardo F., Schulte C., Pieri M. Concomitant implantation of Impella on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017;19:404–412. doi: 10.1002/ejhf.668. [DOI] [PubMed] [Google Scholar]
- 6.Russo J.J., Aleksova N., Pitcher I. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73:654–662. doi: 10.1016/j.jacc.2018.10.085. [DOI] [PubMed] [Google Scholar]
- 7.Pahuja M., Singh M., Patel A. Utilization of mechanical circulatory support devices in chordae tendinae and papillary muscle rupture complicating ST-elevation myocardial infarction: insights from Nationwide Inpatient Sample. J Am Coll Cardiol. 2018;71:A219. [Google Scholar]
- 8.Thiele H., Jobs A., Ouweneel D.M. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017;38:3523–3531. doi: 10.1093/eurheartj/ehx363. [DOI] [PubMed] [Google Scholar]
- 9.DiVita M., Visveswaran G.K., Makam K. Emergent TandemHeart-ECMO for acute severe mitral regurgitation with cardiogenic shock and hypoxaemia: a case series. Eur Heart J Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytz234. [DOI] [PMC free article] [PubMed] [Google Scholar]