Abstract
Redo tricuspid valve replacement has high surgical operative mortality. Transcatheter valve-in-valve provides a viable option for valve replacement. We discuss the decision-making process involved in performing transcatheter tricuspid valve-in-valve replacement in a 23-week pregnant woman with multiple comorbidities and symptomatic severe bioprosthetic stenosis. (Level of Difficulty: Intermediate.)
Key Words: bioprosthetic valve, tricuspid stenosis, valve-in-valve, valvular heart disease in pregnancy
Abbreviations and Acronyms: CO, cardiac output; LV, left ventricle; RV, right ventricle; TV, tricuspid valve; ViV, transcatheter valve-in-valve
Graphical abstract
A 36-year-old woman G4P0030 with 2 miscarriages and 1 prior termination presented 18 weeks pregnant with symptoms of exertional dyspnea and leg swelling consistent with progressive New York Heart Association class III to IV heart failure, as well as imaging evidence of severe bioprosthetic tricuspid valve (TV) stenosis and moderate tricuspid regurgitation. Physical examination revealed a gravid female with blood pressure 98/60 mm Hg, pulse 85 beats/min, and resting oxygen saturation of 95%. She had jugular venous pulse elevation to 20 cm, pulsatile hepatomegaly, and 1+ lower extremity edema. Cardiac auscultation over her right chest revealed II/VI systolic and II-III/IV diastolic murmurs. She had asymmetric excursion of her chest with inspiration and reduced breath sounds at the base, but no rales.
Learning Objectives
-
•
To appreciate the value of collaborative management strategies of valvular heart disease in pregnancy.
-
•
To demonstrate the feasibility of percutaneous tricuspid valve replacement in failing bioprosthetic valves during pregnancy.
Past Medical History
The patient had a motor vehicle accident in which she sustained multisystem trauma, including a severe crush injury to her chest requiring right pneumonectomy, tracheostomy, and epicardial pacemaker placement in an abdominal pocket 15 years ago. Her postoperative course was complicated by chronic sternal osteomyelitis and TV endocarditis requiring more than 20 surgeries, including chest reconstruction with an Eloesser flap, pacemaker extraction, and bioprosthetic TV replacement (29 mm Carpentier-Edwards 6900 Perimount Plus pericardial valve; Edwards Life Sciences, Irvine, California). In addition, she developed restrictive single lung disease with ambulatory desaturation to 84% on home oxygen therapy and a history of recurrent deep venous thromboses. When she first presented to our clinic in mid-second trimester, she was on enoxaparin 60 mg twice a day and 40 mg furosemide daily, as well as high-dose opioids and benzodiazepines for her chronic pain syndrome.
Differential Diagnoses
Differential diagnoses included bioprosthetic TV degeneration, secondary congestive heart failure, or comorbid condition secondary to high-risk pregnancy.
Investigations
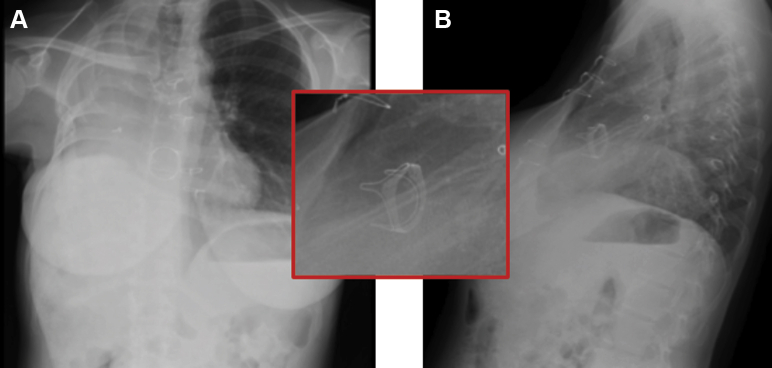
An electrocardiogram revealed right bundle branch block and right ventricle (RV) hypertrophy. Chest radiograph confirmed cardiac dextroposition with right-pneumonectomy changes and clear left lung (Figure 1). Pertinent laboratory values included low hemoglobin of 8.5 g/dl and albumin of 2.3 g/dl. Obstetric ultrasound revealed estimated fetal weight of 537 g (30%) with normal amniotic fluid volume. Prior fetal growth ultrasounds confirmed normal anatomic survey. Echocardiography (Figures 2 and 3, Videos 1, 2, 3, 4A, and 4B) revealed preserved left ventricle (LV) function, low normal RV function, and severe right atrial enlargement with plethoric inferior vena cava (estimated central venous pressure ∼20 cm). The bioprosthetic TV had severe leaflet restriction, mild-to-moderate tricuspid regurgitation, and a 14 mm Hg mean gradient on continuous wave Doppler. Echocardiography-based estimation of the cardiac output (CO) was 3.3 l/min. These findings and symptoms were consistent with severe bioprosthetic TV degeneration with concomitant RV dysfunction.
Figure 1.
Chest Radiograph
Posterior-anterior (A) and lateral (B) chest radiograph showing cardiac dextroposition with right-pneumonectomy changes and clear left lung. Bioprosthetic tricuspid valve ring (inset) is visualized.
Figure 2.
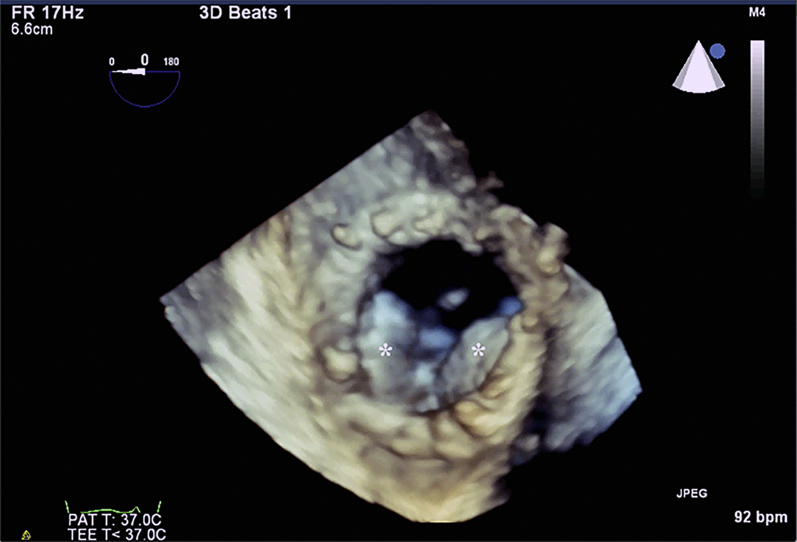
3-Dimensional Echocardiogram of the Tricuspid Valve
Three-dimensional image of the bioprosthetic tricuspid valve in diastole with severe restriction in opening of the leaflets (asterisks).
Figure 3.
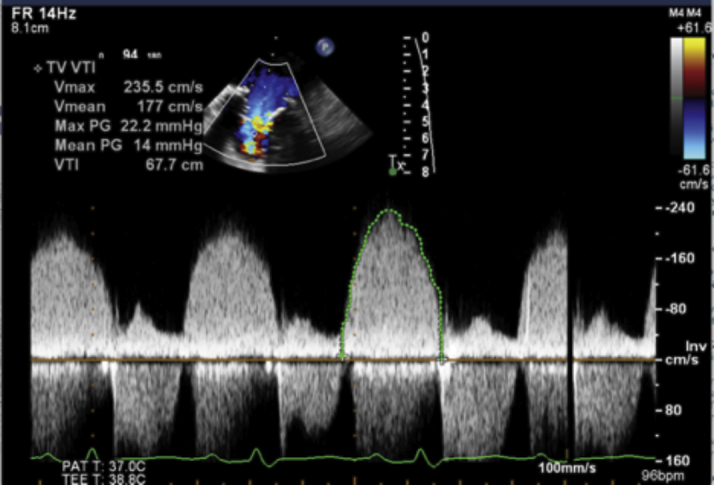
Continuous-Wave Doppler Across the Tricuspid Valve
Mean gradient of 14 mm Hg across the tricuspid valve in diastole.
Management
Despite our recommendation to consider termination, the patient was clear in her resolve to continue with her pregnancy despite heightened risks to herself and the fetus. Multidisciplinary discussions between maternal-fetal-medicine and the Heart Valve Team (cardiology, cardiothoracic surgery, and cardiac anesthesiology) concluded that she was failing optimal medical therapy and needed TV intervention. She had worsening RV failure in light of failure to increase CO appropriately in a parturient. There was concern that she would not be able to tolerate further expansion in blood volume and CO with pregnancy progression. This would limit left-sided function, decrease uteroplacental perfusion, and place the fetus at risk for intrauterine growth restriction.
Her Society of Thoracic Surgeons Score for open TV reoperation mortality was approximately 10%. This was likely an underestimate in view of her hostile chest and restrictive lung disease. In the setting of progressive symptoms along with collaborative decision making with the patient, she agreed to undergo transcatheter tricuspid valve-in-valve replacement (ViV).
At 23-weeks’ gestation, the patient was placed under general anesthesia due to high anesthetic risk, restrictive lung disease, and high opioid and benzodiazepine tolerance. Normal fetal heart rate was confirmed by ultrasound before the procedure. She had continuous arterial and central venous monitoring. Inotropes were initiated for right heart support. She was placed in a leftward tilt during the procedure for ensuring adequate uterine blood flow. Percutaneous access was obtained and a 5-F pacing catheter was advanced into the LV. Right atrial pressure was measured at 17 mm Hg with a and v waves measured at 22 mm Hg with a mean gradient across the TV of 15 mm Hg. The TV was crossed with the aid of a steerable catheter and an extra-small Safari wire was positioned in the RV apex using a marker pigtail. A total of 11,000 U of heparin was administered to achieve a peak activated clotting time of 240 s.
Using fluoroscopic and transesophageal echocardiography guidance, a 29-mm Edwards Sapien 3 (S3) balloon-expandable valve was advanced with image detector angulation at 60o of right anterior oblique across the existing bioprosthesis and deployed during rapid LV pacing at 170 beats/min (Figures 4 and 5). Following valve deployment, transesophageal echocardiography demonstrated optimal valve position. The tricuspid gradient was reduced to 2 mm Hg without tricuspid regurgitation (Figures 6 and 7, Videos 5A and 5B) and the CO increased from 3.3 l/min to 5.3 l/min. She was extubated a few hours later with stable fetal readings and an immediate improvement in heart failure symptoms.
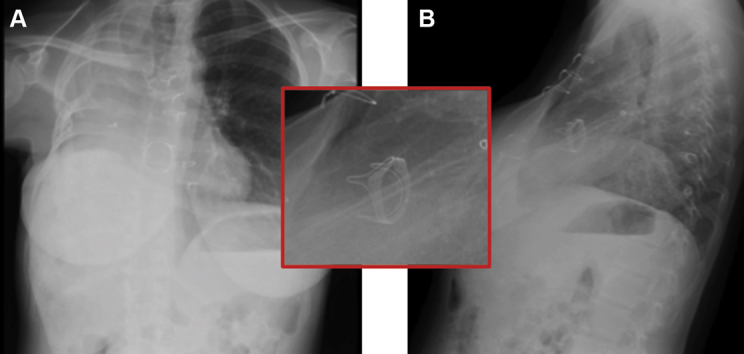
Figure 4.
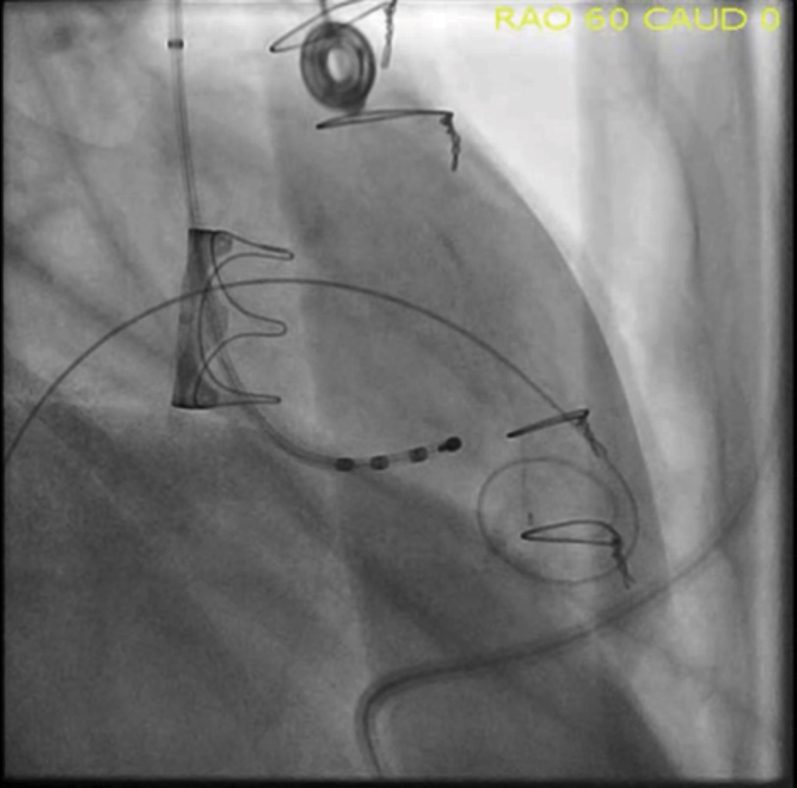
Pre-Procedure Fluoroscopy
Surgical Edwards Model 6900P valve on fluoroscopy in steep right anterior oblique angle 60o projection.
Figure 5.
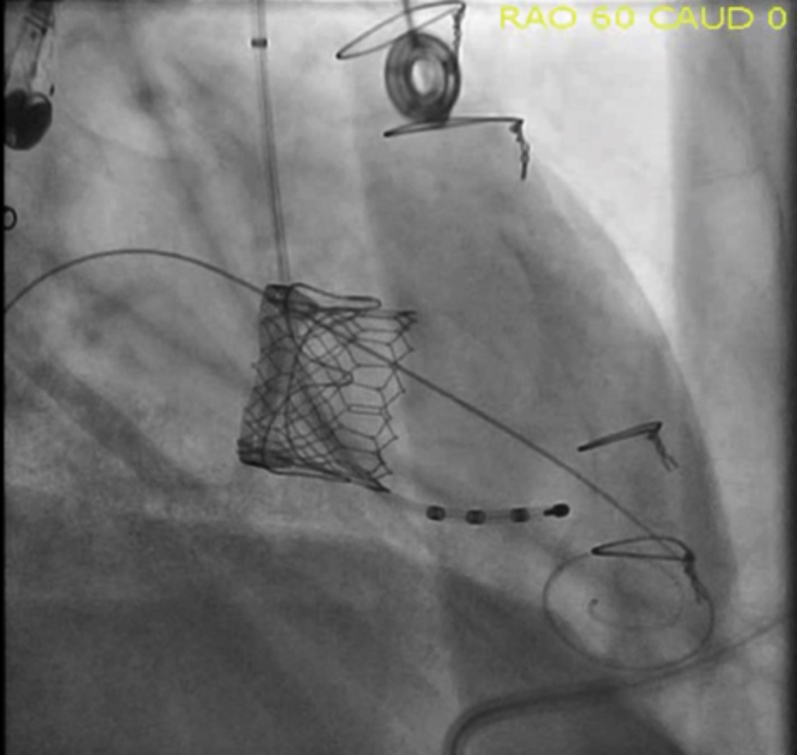
Post-Procedure Fluoroscopy
Tricuspid surgical bioprosthetic valve after deployment of valve-in-valve in steep right anterior oblique angle 60o projection.
Figure 6.
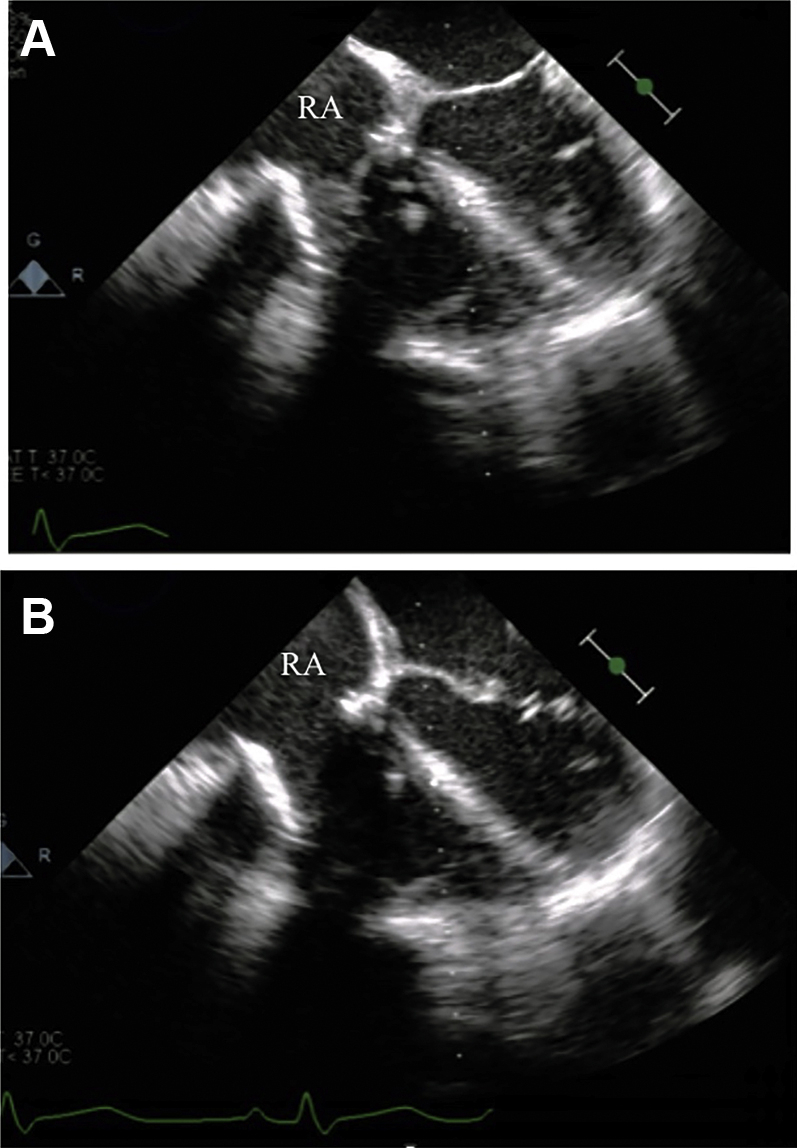
Post-Procedure Echocardiogram of the Tricuspid Valve
Normal functioning tricuspid bioprosthesis post deployment in (A) systole and (B) diastole. RA = right atrium.
Figure 7.
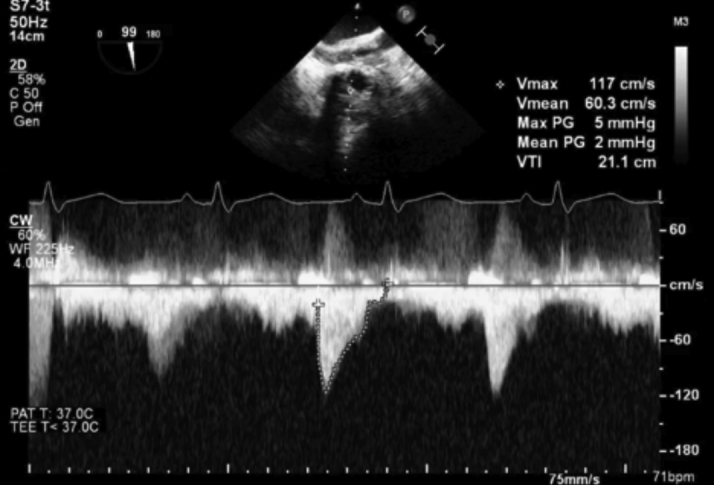
Post-Procedure Continuous-Wave Doppler Across the Tricuspid Valve
Mean gradient of 2 mm Hg post deployment across the tricuspid bioprosthesis in diastole.
Discussion
To our knowledge, this is the first publication of tricuspid ViV replacement being performed during pregnancy. There were critical concerns with this pregnancy related to both the mother and fetus. In addition to her severe bioprosthetic TV stenosis with New York Heart Association functional class III to IV heart failure, she had 1 lung with anatomic distortion of her thorax and exertional desaturation. She was also on high doses of narcotics and antianxiolytics. Stenotic valvular disease even in the right heart portends enhanced risk during pregnancy. A pregnant patient has increased blood volume and low systemic vascular resistance, and should be able to increase her CO by approximately 45% at 24 weeks, which is equivalent to 6 to 7 l/min (1). In comparison, our patient’s estimated pre-procedure CO was approximately 3.3 l in her second trimester with potential untoward effects to mother and fetus.
In caring for our patient, individual autonomy and informed consent were paramount, especially in relation to pregnancy (2). Although medically indicated procedures should not be delayed in pregnancy, the second trimester is generally considered the safest time for surgery in pregnancy, with lower miscarriage risk and a decrease in teratogenic risk for any medication and radiation exposure compared with the first trimester. In addition, intervention before fetal viability (24 weeks in New York State) was preferred in this case to avoid the potential of resuscitation of an extremely preterm infant in case delivery became indicated due to deterioration of either maternal or fetal status. To minimize the risk of radiation, the procedure was conducted with low-dose fluoroscopy without the use of cinefluorography after consultation with a radiation physicist.
Dysfunctional TV bioprostheses can be challenging to manage because patients often have comorbidities and have undergone prior sternotomies (3). TV surgery may be considered for isolated TV disease in patients with severe symptomatic disease, although mortality rates with open surgery are reported as high as 37% in patients undergoing TV replacement after previous repair (4). Tricuspid ViV was first reported in humans by Van Garsse et al (5). Limited international registry data suggest that the procedure can be performed with low incidence of adverse events and good procedural success (6). In our patient with severe symptomatic bioprosthetic TV dysfunction and multiple comorbidities, redo sternotomy and cardiopulmonary bypass were considered prohibitive. Tricuspid ViV was considered the best option for treating her bioprosthetic dysfunction and advanced heart failure symptoms.
Our case adds to the current evidence (6) that tricuspid ViV can be safely performed even in complex clinical scenarios by an experienced Heart Valve Team with favorable outcomes. Important factors to consider when planning a high-risk procedure are patient comorbidities, choice of access (transfemoral, transjugular, or transatrial), and a knowledge of the size and design of the failed bioprosthesis, including the relationship between the strut and the sewing ring for correctly positioning the valve.
In treating pregnant women with cardiac disease, the focus remains primarily on the mother with the imperfect mantra that “healthy mom equals healthy baby.” However, therapeutic decisions must consider fetal risk. Multidisciplinary decision making was imperative to determine procedural feasibility and optimal timing of the intervention.
Follow-Up
The patient was discharged home on postprocedural day 4. She eventually had an uncomplicated vaginal labor with telemetry monitoring at 37 weeks. She delivered a small for gestational age infant with Apgar scores of 8 and 9 and birthweight of 2.0 kg.
Conclusion
Tricuspid ViV is feasible and effective in pregnant and severely ill patients for whom surgery is prohibitive. Decision making in these cases demands a robust multidisciplinary team and careful preprocedural planning.
Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transthoracic echocardiography showing normal left ventricular function, mildly reduced right ventricular function, severely enlarged right atrium with bowing of the interatrial septum into the left atrium.
Dilated inferior vena cava with elevated estimated right atrial pressure of 20 mm Hg.
Three-dimensional superior view of the tricuspid bioprosthetic valve with severely limited opening in diastole.
Online Video 4A(A) Transesophageal echocardiography through the tricuspid bioprosthesis showing right atrial enlargement. (B) Transesophageal echocardiography with color Doppler through the tricuspid bioprosthesis showing right atrial enlargement and mild-to-moderate tricuspid regurgitation.
Online Video 4B(A) Transesophageal echocardiography through the tricuspid bioprosthesis showing right atrial enlargement. (B) Transesophageal echocardiography with color Doppler through the tricuspid bioprosthesis showing right atrial enlargement and mild-to-moderate tricuspid regurgitation.
(A) Postprocedure transesophageal echocardiography showing good position of the new transcatheter valve-in-valve. (B) Postprocedure transesophageal echocardiography with color Doppler showing good position of the new transcatheter valve-in-valve as well as reduction in tricuspid regurgitation.
(A) Postprocedure transesophageal echocardiography showing good position of the new transcatheter valve-in-valve. (B) Postprocedure transesophageal echocardiography with color Doppler showing good position of the new transcatheter valve-in-valve as well as reduction in tricuspid regurgitation.
References
- 1.Hunter S., Robson S.C. Adaptation of the maternal heart in pregnancy. Br Heart J. 1992;68:540–543. doi: 10.1136/hrt.68.12.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali N., Coonrod D.V., McCormick T.R. Ethical issues in maternal-fetal care emergencies. Crit Care Clin. 2016;32:137–143. doi: 10.1016/j.ccc.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Guenther T., Noebauer C., Mazzitelli D., Busch R., Tassani-Prell P., Lange R. Tricuspid valve surgery: a thirty-year assessment of early and late outcome. Eur J Cardiothorac Surg. 2008;34:402–409. doi: 10.1016/j.ejcts.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Jeganathan R., Armstrong S., Al-Alao B., David T. The risk and outcomes of reoperative tricuspid valve surgery. Ann Thorac Surg. 2013;95:119–124. doi: 10.1016/j.athoracsur.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 5.Van Garsse L.A.F.M., Ter Bekke R.M.A., van Ommen V.G.V.A. Percutaneous transcatheter valve-in-valve implantation in stenosed tricuspid valve bioprosthesis. Circulation. 2011;123:e219–e221. doi: 10.1161/CIRCULATIONAHA.110.972836. [DOI] [PubMed] [Google Scholar]
- 6.McElhinney D.B., Cabalka A.K., Aboulhosn J.A. Transcatheter tricuspid valve-in-valve implantation for the treatment of dysfunctional surgical bioprosthetic valves: an international, multicenter registry study. Circulation. 2016;133:1582–1593. doi: 10.1161/CIRCULATIONAHA.115.019353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiography showing normal left ventricular function, mildly reduced right ventricular function, severely enlarged right atrium with bowing of the interatrial septum into the left atrium.
Dilated inferior vena cava with elevated estimated right atrial pressure of 20 mm Hg.
Three-dimensional superior view of the tricuspid bioprosthetic valve with severely limited opening in diastole.
Online Video 4A(A) Transesophageal echocardiography through the tricuspid bioprosthesis showing right atrial enlargement. (B) Transesophageal echocardiography with color Doppler through the tricuspid bioprosthesis showing right atrial enlargement and mild-to-moderate tricuspid regurgitation.
Online Video 4B(A) Transesophageal echocardiography through the tricuspid bioprosthesis showing right atrial enlargement. (B) Transesophageal echocardiography with color Doppler through the tricuspid bioprosthesis showing right atrial enlargement and mild-to-moderate tricuspid regurgitation.
(A) Postprocedure transesophageal echocardiography showing good position of the new transcatheter valve-in-valve. (B) Postprocedure transesophageal echocardiography with color Doppler showing good position of the new transcatheter valve-in-valve as well as reduction in tricuspid regurgitation.
(A) Postprocedure transesophageal echocardiography showing good position of the new transcatheter valve-in-valve. (B) Postprocedure transesophageal echocardiography with color Doppler showing good position of the new transcatheter valve-in-valve as well as reduction in tricuspid regurgitation.