Abstract
The systemic immune-inflammatory index (SII) and derived neutrophil-lymphocyte ratio (dNLR) are novel indexes that simultaneously reflect the host inflammatory and immune status and have prognostic value in some cancers. SII was associated with major cardiovascular events in coronary artery disease patients who received percutaneous coronary intervention (PCI). However, dNLR correlations with clinical outcomes in acute coronary syndrome (ACS) patients undergoing PCI remain unclear. This study aimed to elucidate the predictive values of SII and dNLR on the long-term prognosis of patients with ACS undergoing PCI. In total, 1,553 ACS patients undergoing PCI were consecutively enrolled from January 2016 to December 2018. The subjects were divided into high and low SII and dNLR groups for comparison (high vs. low). The SII and dNLR cutoff values for predicting major adverse cardiovascular events (MACE) were calculated using receiver operating characteristic curves, and Kaplan-Meier curves and Cox regression models were used for survival analyses. The endpoint was a MACE, which included all-cause mortality and rehospitalization for severe heart failure during follow-up. The Kaplan-Meier curves showed that a higher SII or dNLR value was associated with a higher risk of MACE (all P < 0.001). Multivariate Cox regression models showed that SII (hazard ratio [HR]: 2.545; 95% confidence interval [CI]: 1.416-4.574; P = 0.002) and dNLR (HR: 2.610, 95% CI: 1.454-4.685, P = 0.001) were independent predictors for MACE. dNLR may be a suitable laboratory marker to identify high-risk ACS patients after PCI.
Keywords: derived neutrophil-lymphocyte ratio, systemic immune-inflammatory index, acute coronary syndrome, percutaneous coronary intervention, prognosis
Introduction
Coronary artery disease (CAD) remains the leading cause of morbidity and mortality worldwide. 1,2 Acute coronary syndrome (ACS) is a severe CAD subtype caused by the rupture of coronary vulnerable atherosclerotic plaques, including acute ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UA). 3 –6 With the increasing burden of ischemic heart disease, percutaneous coronary intervention (PCI) has become a universal ACS treatment strategy, 7 but it is also crucial to explore novel risk factors for assessing and improving the prognosis of ACS patients. 8,9
Overwhelming evidence shows that atherosclerosis is a chronic inflammatory disease. 10 Circulatory inflammatory and immune cells, such as white blood cells (WBC) and WBC subtypes (e.g., neutrophils, monocytes, and lymphocytes), reflect systematic inflammation severity and play an important role in ACS. 11 Currently, various inflammatory ratios relating to the atherosclerotic process, such as the neutrophil-to-lymphocyte ratio, the monocyte-to-lymphocyte ratio, and the platelet-to-lymphocyte ratio, were shown to be potential predictors for poor events in CAD patients. 12,13 The systemic immune-inflammatory index (SII) and derived neutrophil-lymphocyte ratio (dNLR) are novel indexes that simultaneously reflect the host inflammatory and immune status 14,15 and have prognostic value in some cancers. 14 –16 Additionally, SII was associated with adverse outcomes in chronic heart failure and major cardiovascular events in CAD patients who received PCI, and dNLR was associated with short-term mortality in patients with heart failure. 14,17 However, dNLR correlations with clinical outcomes in ACS patients undergoing PCI remain unclear. Therefore, this study aimed to elucidate the predictive values of SII and dNLR on the long-term prognosis of ACS patients undergoing PCI.
Materials and Methods
Study Population
In total, 1,773 patients with ACS undergoing PCI were consecutively enrolled in this prospective cohort study from January 2016 to December 2018 at The Affiliated Hospital of Chengde Medical University. The inclusion criteria were: patients aged ≥40 years, ACS clinical types: UA, NSTEMI, and STEMI, coronary arteriography showing ≥50% stenosis in one or more of the left main, left anterior descending, left circumflex, right coronary, or main branches, and those who underwent PCI (complete revascularization) for the first time. The exclusion criteria were: a coronary artery spasm or other secondary causes of angina or myocardial infarctions, infectious diseases, malignant tumors, blood system diseases (e.g., anemia and leukopenia), severe heart diseases (e.g., aortic dissection and hypertrophic cardiomyopathy), severe systemic disease, systemic inflammatory disorders, glucocorticosteroid therapies within 2 months and chronic kidney disease (stage ≥3). A total of 1,553 patients were included in the final analysis. This study was approved by the Institutional Review Board of The Affiliated Hospital of Chengde Medical University, and all subjects provided written informed consent.
Clinical Data Collection
Demographic and clinical characteristics and information regarding typical ACS clinical risk factors, such as diabetes, hypertension, dyslipidemia, and ischemic stroke, were collected during hospitalization. Hypertension was defined as systolic blood pressure ≥140 mm Hg (1 mm Hg = 0.133 kPa) and diastolic blood pressure ≥90 mm Hg at rest or a previous hypertension diagnosis with antihypertensive therapy. 18 Diabetes mellitus was defined according to the following American Diabetes Association guidelines: a glycated hemoglobin value of ≥6.5%, a fasting plasma glucose value of ≥126 mg/dL (7.0 mmol/L), a 2 h plasma glucose value of ≥200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test using 75 g of glucose, classic hyperglycemia symptoms (e.g., polyuria, polydipsia, and weight loss), or hyperglycemic crisis with a random plasma glucose value of ≥200 mg/dL (11.1 mmol/L). In the absence of unequivocal hyperglycemia, the first 3 criteria were confirmed by repeat testing. 19 Dyslipidemia was defined as a serum total cholesterol value of ≥5.18 mmol/L (200 mg/dL), a high-density lipoprotein cholesterol value of ≤1.04 mmol/L, a low-density lipoprotein cholesterol value of ≥3.37 mmol/L, a triglyceride value of ≥1.7 mmol/L, or a previous dyslipidemia diagnosis with a prescribed medication. 20 Experienced cardiologists performed PCI with the Judkins technique using 6F right and left heart catheters. Procedural success was defined as a reduction in the percent diameter stenosis associated with thrombolysis to <30% in myocardial infarction grades 2 or 3. Angiographic characteristics of all patients were determined and reported by the PCI doctors’ team.
Laboratory Data
Fasting blood samples were collected within the first 24 h of admission before PCI. WBC, platelet, neutrophil, and lymphocyte counts were assessed using the automatic hematology analyzer (Sysmex XE-2100; Sysmex, Kobe, Japan). dNLR was calculated with the formula: neutrophil count/(WBC count-neutrophil count), 21 and SII was calculated with the formula: platelet count × neutrophil count /lymphocyte count. 14
Follow-Up and Study Endpoints
The study endpoint was a major adverse cardiovascular event (MACE), which included all-cause mortality and rehospitalization for severe heart failure during the follow-up period. All-cause mortality was defined as death from any cause. Severe heart failure was defined as the New York Heart Association (NYHA) Classification Class IV. Follow-up data were collected via clinic visits 1-, 3-, 6-, and 12-months after the procedure and once per year thereafter. The research team was uniformly trained using the same inclusion criteria, measurable criteria, and follow-up protocols.
Statistical Analyses
Statistics analyses were performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA). The Kolmogorov-Smirnov test was used for continuous variables and reported as the mean ± standard deviation for normal distributions and the median and interquartile ranges for skewed distributions. Continuous variables were divided into groups (high and low SII and dNLR), and the differences were compared using the Mann-Whitney U test. Categorical variables were presented as numbers (%) and compared using the chi-squared or Fisher’s exact test. Receiver operating characteristic (ROC) curves were used to calculate the cutoff values for SII and dNLR to predict MACE. Youden’s index (sensitivity + specificity − 1) was used to determine the optimal cutoff point. Survival outcomes differences were investigated using the log-rank test. Kaplan-Meier survival estimates were calculated, and the Cox proportional hazards models were used to identify the independent prognostic factors for MACE with the forward selection method. Two-tailed P-values < 0.05 were considered significant.
Results
Baseline Characteristics
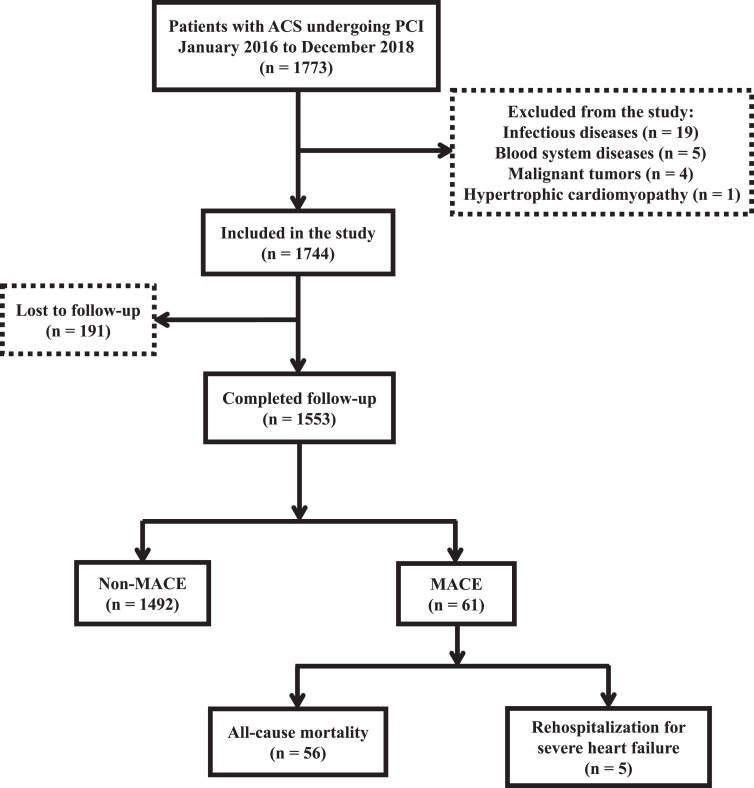
The median follow-up duration was 1,142 days. A total of 1,553 patients were assigned to the following groups: MACE group (n = 61; all-cause mortality [n = 56; death from any cause] and rehospitalization for severe heart failure [n = 5; heart function level IV based on the NYHA classification]) and Non-MACE group (n = 1492; no MACE occurred) (Figure 1). Table 1 presents the patients’ clinical characteristics. The prevalence of UA, STEMI, dyslipidemia, and heart failure (HF) were significantly different between the low SII (n = 812; values < 628.60) and high SII (n = 741; values ≥628.60) groups (all P < 0.05). An elevated SII was associated with increased WBC, platelet, and neutrophil counts (all P < 0.05). There was a greater decrease in lymphocytes in the high SII group than in the low SII group (P < 0.05).
Figure 1.
Patient enrollment and screening flowchart.
Table 1.
Baseline Characteristics of ACS Patients Undergoing PCI.
| Variable | SII < 628.60 (n = 812) | SII ≥ 628.60 (n = 741) | P | dNLR < 2.29 (n = 846) | dNLR ≥ 2.29 (n = 707) |
P |
|---|---|---|---|---|---|---|
| Demographics and clinical data, n (%) | ||||||
| Male | 608 (74.88) | 555 (74.90) | 0.992 | 623 (73.64) | 540 (76.38) | 0.215 |
| Age ≥ 65 years | 186 (22.91) | 183 (24.70) | 0.408 | 190 (22.46) | 179 (25.32) | 0.187 |
| Smoking | 424 (52.22) | 383 (51.82) | 0.876 | 425 (50.24) | 383 (54.17) | 0.122 |
| Dyslipidemia | 153 (18.84) | 186 (25.10) | 0.003 | 167 (19.74) | 172 (24.33) | 0.029 |
| Hypertension | 463 (57.02) | 451 (60.86) | 0.124 | 497 (58.75) | 417 (58.98) | 0.925 |
| Diabetes mellitus | 210 (25.86) | 183 (24.70) | 0.598 | 222 (26.24) | 171 (24.19) | 0.354 |
| History of stroke | 114 (14.04) | 107 (14.44) | 0.821 | 121 (14.30) | 100 (14.14) | 0.929 |
| History of TIA | 2 (0.25) | 3 (0.40) | 0.582 | 4 (0.47) | 1 (0.14) | 0.251 |
| HF | 65 (8.00) | 91 (12.28) | 0.005 | 64 (7.57) | 92 (13.01) | <0.001 |
| CGS | 9 (1.11) | 15 (2.02) | 0.144 | 13 (1.54) | 11 (1.56) | 0.976 |
| Family history of CAD | 130 (16.00) | 89 (12.01) | 0.024 | 127 (15.01) | 92 (13.01) | 0.260 |
| UA | 442 (54.43) | 173 (23.35) | <0.001 | 466 (55.08) | 149 (21.07) | <0.001 |
| STEMI | 252 (31.03) | 440 (59.38) | <0.001 | 260 (30.73) | 432 (61.10) | <0.001 |
| NSTEMI | 118 (14.53) | 128 (17.27) | 0.139 | 120 (14.18) | 126 (17.82) | 0.051 |
| Laboratory data, M(Q1, Q3) | ||||||
| WBC count (109/L) | 6.97 (5.73, 8.70) | 9.39 (7.52, 11.40) | <0.001 | 7.00 (5.79, 8.89) | 9.44 (7.58, 11.46) | <0.001 |
| Platelet count (109/L) | 203.00 (172.00, 240.00) | 223.50 (191.75, 265.25) | <0.001 | 216.00 (180.00, 255.00) | 213.00 (178.00, 247.00) | 0.351 |
| Neutrophil count (109/L) | 3.87 (3.11, 4.84) | 7.53 (5.67, 9.50) | <0.001 | 3.93 (3.11, 4.88) | 7.66 (5.87, 9.59) | <0.001 |
| Lymphocyte count (109/L) | 2.10 (1.60, 2.62) | 1.30 (0.89, 1.70) | <0.001 | 2.10 (1.60, 2.61) | 1.26 (0.88, 1.65) | <0.001 |
| Monocyte count (109/L) | 0.44 (0.34, 0.57) | 0.42 (0.29, 0.60) | 0.107 | 0.45 (0.35, 0.5) | 0.39 (0.28, 0.57) | <0.001 |
| NLR | 1.88 (1.41, 2.44) | 5.34 (3.63, 9.06) | <0.001 | 1.91 (1.43, 2.47) | 5.72 (3.91, 9.38) | <0.001 |
| PLR | 97.76 (74.94, 121.77) | 172.44 (132.81, 237.42) | <0.001 | 104.23 (79.12, 133.62) | 167.18 (121.93, 237.08) | <0.001 |
| MPV (fL) | 10.40 (9.90, 11.00) | 10.30 (9.70, 11.00) | 0.144 | 10.40 (9.80, 10.93) | 10.40 (9.80, 11.00) | 0.268 |
| PDW (%) | 12.10 (11.00, 13.40) | 12.00 (10.90, 13.40) | 0.222 | 12.00 (10.90, 13.30) | 12.10 (11.000, 13.40) | 0.211 |
| ALB (g/L) | 41.60 (38.90, 43.80) | 41.08 (38.48, 43.46) | 0.061 | 41.59 (38.90, 43.80) | 41.00 (38.40, 43.40) | 0.020 |
| CK-MB (U/L) | 13.79 (9.00, 23.00) | 26.00 (12.50, 101.50) | <0.001 | 13.77 (9.00, 22.00) | 27.33 (13.00, 114.00) | <0.001 |
| Cr (μmol/L) | 67.41 (58.47, 78.30) | 67.00 (58.99, 78.31) | 0.441 | 67.71 (58.43, 78.22) | 66.77 (58.95, 78.66) | 0.673 |
| Serum uric acid (μmol/L) | 326.40 (265.70, 384.60) | 328.95 (263.73, 385.6) | 0.767 | 325.38 (261.65, 385.50) | 330.40 (268.46, 384.00) | 0.368 |
| Echocardiography, n (%) | ||||||
| LAa | 167 (23.29) | 190 (27.78) | 0.054 | 182 (24.17) | 175 (27.01) | 0.224 |
| LVEDDa | 164 (22.81) | 188 (27.45) | 0.045 | 173 (22.88) | 179 (27.62) | 0.041 |
| LVEF < 40% | 17 (2.36) | 24 (3.50) | 0.205 | 17 (2.25) | 24 (3.70) | 0.106 |
| Coronary angiogram, n (%) | ||||||
| 1 vessel | 263 (32.39) | 224 (30.23) | 0.360 | 268 (31.68) | 219 (30.98) | 0.766 |
| 2 vessels | 249 (30.67) | 245 (33.06) | 0.311 | 272 (32.15) | 222 (31.40) | 0.752 |
| 3 vessels | 300 (36.95) | 272 (36.71) | 0.922 | 306 (36.17) | 266 (37.62) | 0.554 |
| Drugs, n (%) | ||||||
| Aspirin | 801 (98.65) | 730 (98.52) | 0.829 | 833 (98.46) | 698 (98.73) | 0.661 |
| Clopidogrel | 652 (80.30) | 572 (77.19) | 0.135 | 680 (80.38) | 544 (76.94) | 0.099 |
| Ticagrelor | 147 (18.10) | 158 (21.32) | 0.111 | 152 (17.97) | 153 (21.64) | 0.070 |
| β-blockers | 422 (51.97) | 375 (50.61) | 0.591 | 443 (52.36) | 354 (50.07) | 0.368 |
| ACEI/ARB | 352 (43.35) | 346 (46.69) | 0.186 | 363 (42.91) | 335 (47.38) | 0.077 |
| Statins | 796 (98.03) | 730 (98.52) | 0.464 | 829 (97.99) | 697 (98.59) | 0.372 |
| Diuretic | 48 (5.91) | 62 (8.37) | 0.060 | 51 (6.03) | 59 (8.35) | 0.076 |
Abbreviations: ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; SII, systemic immune-inflammation index; dNLR, derived neutrophil-to-lymphocyte ratio; TIA, transient ischemic attack; HF, heart failure; CGS, cardiogenic shock; CAD, coronary artery disease; UA, unstable angina; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; WBC, white blood cell; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; MPV, mean platelet volume; PDW, platelet distribution width; ALB, albumin; CK-MB, creatine phosphokinase isoenzyme; Cr, creatinine; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin II receptor blocker.
a above the upper limit of normal values.
The prevalence of dyslipidemia, HF, UA, STEMI, and a left ventricular ejection fraction (LVEF) of <40% were significantly different between the low dNLR (n = 846; values < 2.29) and the high dNLR (n = 707; values ≥ 2.29) groups (all P < 0.05). The WBC and neutrophil counts were significantly higher in the high dNLR group (both P < 0.001). A higher dNLR was associated with increased creatine phosphokinase isoenzyme (CK-MB) and an enlarged left ventricular end-diastolic diameter (both P < 0.05). Patients with a dNLR of ≥2.29 were more likely to have a lower lymphocyte count and a decreased level of albumin (ALB) (both P < 0.05), but there was no difference between the high and low dNLR groups regarding the number of diseased vessels and drug use. These results were also observed for the SII groups (both P > 0.05).
Patients were also grouped by MACE for analysis. Patients who suffered from a MACE were more likely to be older than 65 years, have a history of stroke, HF, or cardiogenic shock (CGS), a LVEF of <40%, a SII of ≥628.60, and a dNLR of ≥2.29 (all P < 0.05). The prevalence of STEMI and NSTEMI were also higher in the MACE group than in the Non-MACE group, although statistically insignificant. The neutrophil count, CK-MB, and creatinine (Cr) levels (P = 0.001) were higher, and the lymphocyte count and ALB level were lower in the MACE group (all P < 0.05; Table 2).
Table 2.
Baseline Clinical Characteristics Between the MACE and Non-MACE Groups.
| Variable | MACE (n = 61) | Non-MACE (n = 1,492) | χ2/Z | P |
|---|---|---|---|---|
| Demographics and clinical data, n (%) | ||||
| Male | 44 (72.13) | 1,119 (75.00) | 0.256 | 0.613 |
| Age ≥ 65 years | 30 (49.18) | 339 (22.72) | 22.649 | <0.001 |
| Smoking | 29 (47.54) | 779 (52.21) | 0.512 | 0.474 |
| Dyslipidemia | 15 (24.59) | 324 (21.72) | 0.284 | 0.594 |
| Hypertension | 35 (57.38) | 879 (58.91) | 0.057 | 0.811 |
| Diabetes mellitus | 14 (22.95) | 379 (25.40) | 0.186 | 0.666 |
| History of stroke | 16 (26.23) | 205 (13.74) | 7.490 | 0.006 |
| History of TIA | 0 (0.00) | 5 (0.34) | 0.205 | 0.651 |
| HF | 19 (31.15) | 137 (9.18) | 31.291 | <0.001 |
| CGS | 8 (13.11) | 16 (1.07) | 55.857 | <0.001 |
| Family history of CAD | 5 (8.20) | 214 (14.34) | 1.828 | 0.176 |
| UA | 15 (24.59) | 600 (40.21) | 5.981 | 0.014 |
| STEMI | 33 (54.10) | 659 (44.17) | 2.339 | 0.126 |
| NSTEMI | 13 (21.31) | 233 (15.62) | 1.426 | 0.232 |
| Laboratory data, M(Q1, Q3) | ||||
| WBC count (109/L) | 8.48 (7.27, 11.41) | 7.96 (6.37, 10.45) | −1.484 | 0.138 |
| Platelet count (109/L) | 213.50 (178.25, 245.25) | 215.00 (179.00, 252.00) | −0.759 | 0.448 |
| Neutrophil count (109/L) | 6.28 (4.20, 8.96) | 5.18 (3.70, 7.62) | −2.029 | 0.042 |
| Lymphocyte count (109/L) | 1.34 (0.92, 1.83) | 1.67 (1.21, 2.29) | −3.040 | 0.002 |
| Monocyte count (109/L) | 0.50 (0.34, 0.64) | 0.43 (0.32, 0.59) | −1.114 | 0.265 |
| SII ≥ 628.60 | 42 (68.85) | 699 (46.85) | 11.372 | <0.001 |
| dNLR ≥ 2.29 | 41 (67.21) | 666 (44.64) | 12.043 | <0.001 |
| NLR ≥ 2.67 | 46 (75.41) | 779 (52.28) | 12.590 | <0.001 |
| PLR ≥ 237.93 | 16 (26.23) | 181 (12.13) | 10.516 | <0.001 |
| MPV (fL) | 10.50 (9.78, 10.93) | 10.40 (9.80, 11.00) | −0.548 | 0.548 |
| PDW (%) | 12.25 (10.98, 13.43) | 12.10 (10.90, 13.40) | −0.426 | 0.670 |
| ALB(g/L) | 39.70 (37.15, 41.53) | 41.39 (38.80, 43.70) | −3.123 | 0.002 |
| CK-MB (U/L) | 33.34 (11.00, 122.37) | 16.30 (10.00, 48.92) | −2.517 | 0.012 |
| Cr (μmol/L) | 76.56 (63.13, 88.70) | 67.00 (58.52, 78.00) | −3.197 | 0.001 |
| Serum uric acid (μmol/L) | 336.95 (271.40, 395.73) | 327.40 (264.70, 384.60) | −0.681 | 0.496 |
| Echocardiography, n (%) | ||||
| LAa | 20 (35.71) | 337 (25.06) | 3.216 | 0.073 |
| LVEDDa | 18 (14.29) | 334 (24.78) | 1.553 | 0.213 |
| LVEF < 40% | 8 (44.26) | 33 (2.45) | 26.576 | <0.001 |
| Coronary angiogram, n (%) | ||||
| 1 vessel | 16 (26.23) | 471 (31.57) | 0.776 | 0.378 |
| 2 vessels | 21 (34.43) | 473 (31.70) | 0.200 | 0.654 |
| 3 vessels | 24 (39.34) | 548 (36.73) | 0.172 | 0.678 |
Abbreviations: MACE, major adverse cardiovascular events; TIA, transient ischemic attack; HF, heart failure; CGS, cardiogenic shock; CAD, coronary artery disease; UA, unstable angina; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; WBC, white blood cell; SII, systemic immune-inflammation index; dNLR, derived neutrophil to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; MPV, mean platelet volume; PDW, platelet distribution width; ALB, albumin; CK-MB, creatine phosphokinase isoenzyme; Cr, creatinine; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
a Above the upper limit of normal values.
ROC Curve Analyses
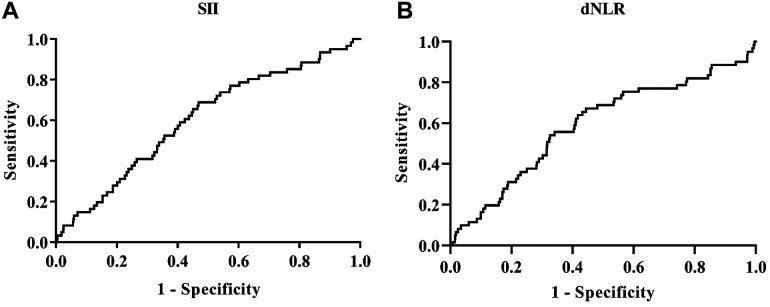
Figure 2 presents the ROC curve analyses to determine the optimal cutoff values for SII and dNLR for MACE evaluation in ACS patients undergoing PCI. The area under the curve (AUC) for SII was 0.604 (P = 0.006, 95% confidence interval [CI]: 0.533-0. 675). The optimal diagnostic cutoff point was 628.60× 109 /L, with a sensitivity of 68.90% and a specificity of 53.20% (Figure 2A). The AUC for dNLR was 0.591 (P = 0.016, 95% CI: 0.513-0.669), and the optimal diagnostic cutoff point was 2.29, with a sensitivity of 67.20% and a specificity of 55.60% (Figure 2B). The AUC for NLR was 0.614 (P = 0.003, 95% CI: 0.541-0.686), and the optimal diagnostic cutoff point was 2.67, with a sensitivity of 75.40% and a specificity of 47.90%. The AUC for PLR was 0.581 (P = 0.032, 95% CI: 0.505-0.657), and the optimal diagnostic cutoff point was 237.93 with a sensitivity of 26.20% and a specificity of 87.90% (Table 3).
Figure 2.
SII and dNLR ROC curve analyses for predicting MACE. A, The SII AUC. B, The dNLR AUC.
Table 3.
ROC Curve Analyses of the MACE and Non-MACE Groups.
| Variable | AUC | SE | P | 95% CI | Se (%) | Sp (%) | Cut off |
|---|---|---|---|---|---|---|---|
| SII(109/L) | 0.604 | 0.036 | 0.006 | 0.533-0.675 | 68.90 | 53.20 | 628.60 |
| dNLR | 0.591 | 0.040 | 0.016 | 0.513-0.669 | 67.20 | 55.60 | 2.29 |
| NLR | 0.614 | 0.037 | 0.003 | 0.541-0.686 | 75.40 | 47.90 | 2.67 |
| PLR | 0.581 | 0.039 | 0.032 | 0.505-0.657 | 26.20 | 87.90 | 237.93 |
Abbreviations: ROC, receiver operating characteristic; MACE, major adverse cardiovascular events; AUC, area under the curve; SE, standard error; CI, confidence interval; Se, sensitivity; Sp, specificity; SII, systemic immune-inflammation index; dNLR, derived neutrophil to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
Correlation Analysis
In the Spearman correlation analysis, SII was significantly positively correlated with dNLR, NLR and PLR (all P < 0.001). dNLR was also positively correlated with SII, NLR and PLR (all P < 0.05; Table 4).
Table 4.
Correlations of SII and dNLR With Other Markers.
| Variable | SII | dNLR | ||
|---|---|---|---|---|
| r | P | r | P | |
| SII | - | - | 0.834 | <0.001 |
| dNLR | 0.834 | <0.001 | - | - |
| NLR | 0.897 | <0.001 | 0.927 | <0.001 |
| PLR | 0.761 | <0.001 | 0.550 | <0.001 |
Abbreviations: SII, systemic immune-inflammation index; dNLR, derived neutrophil-to-lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
Survival Analyses
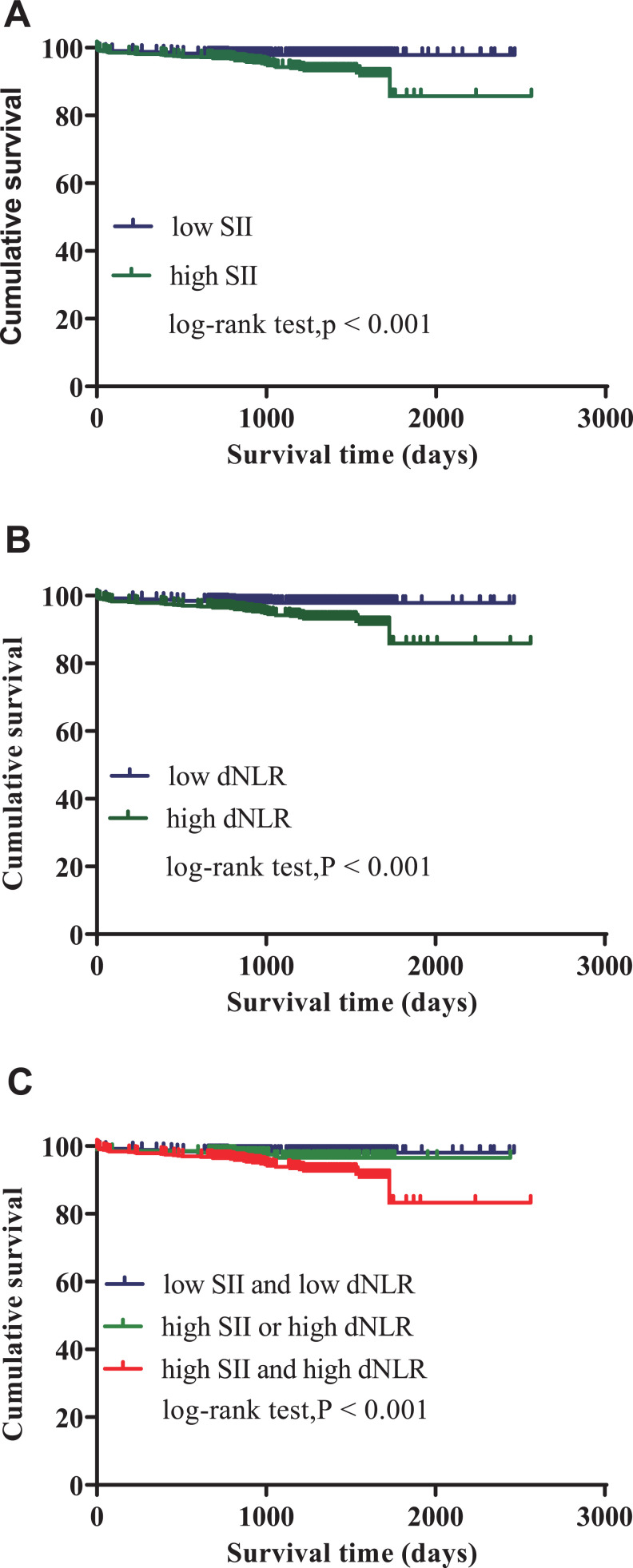
The Kaplan-Meier curve was plotted with the follow-up survival data. The cumulative survival rates of the high SII (values ≥ 628.60) and dNLR (values ≥ 2.29) groups were significantly lower than the low groups (log-rank tests: all P < 0.001; Figure 3A, B). Patients in both the high SII and dNLR groups had significantly higher all-cause mortality and rehospitalization for severe heart failure than patients in only one of the high groups (SII or dNLR) or both low groups (log-rank tests: P < 0.001; Figure 3C).
Figure 3.
The SII and dNLR Kaplan-Meier survival curves for ACS patients undergoing PCI for MACE. A, SII curves for low (<628.60) and high (≥628.60) groups (log-rank test: P < 0.001). B, dNLR curves for low (<2.29) and high (≥2.29) groups (log-rank test: P < 0.001). C, SII and dNLR combined curves.
Cox Regression Analyses
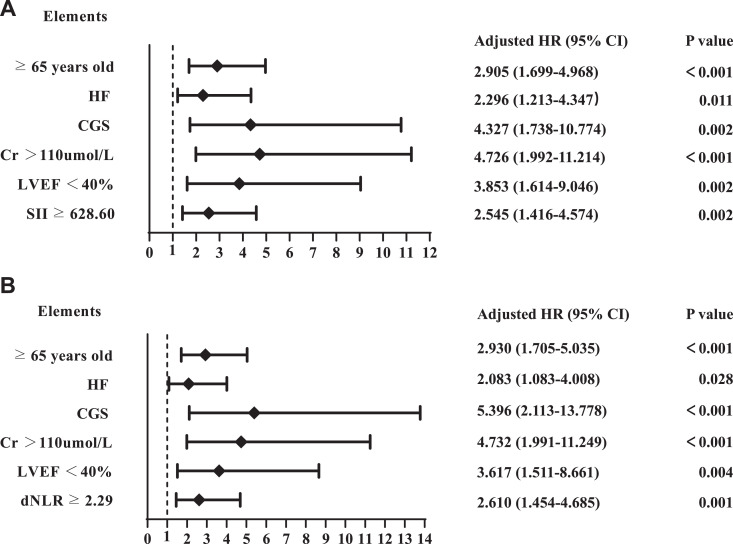
Figure 4 and Table 5 present the results of univariate and multivariate analysis using a Cox regression model to determine independent predictors for MACE. In Model 1, the results demonstrated that age ≥65 years old (hazard ratio [HR]: 2.905, 95% CI: 1.699-4.968, P < 0.001), HF (HR: 2.296, 95% CI: 1.213-4.347, P = 0.011), CGS (HR: 4.327, 95% CI: 1.738-10.774, P = 0.002), Cr > 110 µmol/L (HR: 4.726, 95% CI: 1.992-11.214, P < 0.001), LVEF < 40% (HR: 3.853, 95% CI: 1.614-9.046, P = 0.002), and an SII of ≥628.60 (HR: 2.545, 95% CI: 1.416-4.574, P = 0.002) were independent predictors of MACE. Model 2 showed that age ≥65 years old (HR: 2.930, 95% CI: 1.705 −5.035, P < 0.001), HF (HR: 2.083, 95% CI: 1.083-4.008, P = 0.028), CGS (HR: 5.396, 95% CI: 2.113-13.778, P < 0.001), Cr > 110 µmol/L (HR: 4.732, 95% CI: 1.991-11.249, P < 0.001), LVEF < 40% (HR: 3.617, 95% CI: 1.511-8.661, P = 0.004), and a dNLR of ≥2.29 (HR: 2.610, 95% CI: 1.454-4.685, P = 0.001) were independent prognostic factors for MACE. Model 3 showed that age ≥65 years old (HR: 2.881, 95% CI: 1.682-4.934, P < 0.001), HF (HR: 2.263, 95% CI: 1.190-4.303, P = 0.013), CGS (HR: 5.319, 95% CI: 2.119-13.349, P < 0.001), Cr > 110 µmol/L (HR: 4.332, 95% CI: 1.820-10.315, P < 0.001), LVEF < 40% (HR: 3.337, 95% CI: 1.418-7.851, P = 0.006), and a NLR ≥2.67 (HR: 2.927, 95% CI: 1.530-5.602, P = 0.001) were independent prognostic factors for MACE. Model 4 showed that age ≥65 years old (HR: 2.788, 95% CI: 1.638-4.744, P < 0.001), HF (HR: 2.306, 95% CI: 1.200-4.429, P = 0.012), CGS (HR: 4.682, 95% CI: 1.830-11.979, P = 0.001), LVEF < 40% (HR: 3.324, 95% CI: 1.370-8.063, P = 0.008), and a PLR ≥237.93 (HR: 2.118, 95% CI: 1.147-3.911, P = 0.017) were independent prognostic factors for MACE.
Figure 4.
Forest graphs based on Cox proportional hazards regression models for MACE risk factors. A, Model 1 (SII). B, Model 2 (dNLR).
Table 5.
Cox Regression Model for Predicting MACE in ACS Patients Undergoing PCI.
| Variables | Univariate | Multivariate model 1 | Multivariate model 2 | Multivariate model 3 | Multivariate model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| ≥65 years old | 3.314 (2.005-5.478) | <0.001 | 2.905 (1.699-4.968) | <0.001 | 2.930 (1.705-5.035) | <0.001 | 2.881 (1.682-4.934) | <0.001 | 2.788 (1.638-4.744) | <0.001 |
| HF | 4.588 (2.666-7.896) | <0.001 | 2.296 (1.213-4.347) | 0.011 | 2.083 (1.083-4.008) | 0.028 | 2.263 (1.190-4.303) | 0.013 | 2.306 (1.200-4.429) | 0.012 |
| CGS | 11.432 (5.415-24.136) | <0.001 | 4.327 (1.738-10.774) | 0.002 | 5.396 (2.113-13.778) | <0.001 | 5.319 (2.119-13.349) | <0.001 | 4.682 (1.830-11.979) | 0.001 |
| Cr > 110 μmol/L | 4.157 (1.788-9.664) | <0.001 | 4.726 (1.992-11.214) | <0.001 | 4.732 (1.991-11.249) | <0.001 | 4.332 (1.820-10.315) | <0.001 | - | - |
| LVEF <40% | 6.897 (3.252-14.629) | <0.001 | 3.853 (1.614-9.046) | 0.002 | 3.617 (1.511-8.661) | 0.004 | 3.337 (1.418-7.851) | 0.006 | 3.324(1.370-8.063) | 0.008 |
| SII ≥ 628.60 | 2.558 (1.487-4.401) | <0.001 | 2.545 (1.416-4.574) | 0.002 | - | - | - | - | - | - |
| dNLR ≥ 2.29 | 2.572 (1.507-4.392) | <0.001 | - | - | 2.610 (1.454-4.685) | 0.001 | - | - | - | - |
| NLR ≥ 2.67 | 2.857 (1.594-5.118) | <0.001 | - | - | - | - | 2.927 (1.530-5.602) | 0.001 | - | - |
| PLR ≥ 237.93 | 2.704 (1.526-4.790) | <0.001 | - | - | - | - | - | - | 2.118 (1.147-3.911) | 0.017 |
Abbreviations: MACE, major adverse cardiovascular events; ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; CI, confidence interval; HF, heart failure; CGS, cardiogenic shock; Cr, creatinine; LVEF, left ventricular ejection fraction; SII, systemic immune-inflammation index; dNLR, derived neutrophil-to-lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio.
Discussion
This study found that a high SII and dNLR were independent risk factors for predicting all-cause mortality and rehospitalization for severe heart failure. Patients with a high SII and dNLR had a greater risk of long-term clinical cardiovascular outcomes than patients with one increased risk factor or no risk factors. SII and dNLR had the similar diagnostic value for MACE in patients with ACS after PCI. To our knowledge, this is the first study to report associations between dNLR and the clinical outcomes of ACS patients undergoing PCI.
Increasing evidence shows that ACS is an inflammatory disease that includes vascular inflammation, the perturbation of lipid metabolism, and the rupture of vulnerable plaques with complex interactions of innate and adaptive immune systems. 22 –24 Former studies highlighted the importance of inflammation in atherosclerosis. The CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) demonstrated that anti-inflammatory treatment with IL (interleukin)-1β reduced the risk of recurrent cardiovascular events, supporting the atherosclerotic inflammation hypothesis. 25 Hence, it is important to identify at-risk patients using convenient inflammation markers. Complete blood cell counts are widely used to reflect the host’s inflammatory status. Previous reports indicated SII was associated with major cardiovascular events in CAD patients who received PCI, which are consistent with our results. Therefore, SII may be used as a marker in cancer patients as well as CAD patients. 14,15
dNLR is a novel index, with much less supporting evidence as a predictive factor in CAD than SII. Proctor et al initially defined dNLR as the neutrophil count divided by the (WBC count-neutrophil count). 26 Numerous studies regarded dNLR as an inflammation marker that reflected the severity of systematic inflammation in cancers, such as non-small cell lung cancer, 21 gastrointestinal cancer, 27 and breast cancer, 28 but the roles of dNLR in ACS have not yet been established. Interestingly, a possible association between an increased dNLR and high short-term mortality in heart failure patients with a reduced ejection fraction was reported. 17 dNLR may also reflect the subclinical inflammation in young, healthy adults with obesity (a CAD risk factor). 29,30 Our study found a higher prevalence of dyslipidemia and HF and increased CK-MB levels in patients with a higher dNLR.
The neutrophil count divided by the (WBC count-neutrophil count) is an alternative way to calculate dNLR using only WBC and neutrophil counts. WBCs are mainly composed of neutrophils and lymphocytes. Therefore, the WBC count minus the neutrophil count is primarily lymphocytes. 27 Neutrophils have a major role in the inflammatory process, and the lymphocyte count is a regulatory pathways marker. Classical and generally accepted proinflammatory cytokines, such as IL-6, IL-8, tumor necrosis factor-α (TNF-α), may trigger neutrophil extracellular traps (NET) formation. 31 Stimulated neutrophils activate their NADPH oxidase to generate large amounts of superoxide anion and ensuing reactive oxygen species (ROS). 32 Excessive ROS leads to oxidative stress, which has been demonstrated in the vascular oxidative stress in atherosclerosis by previous studies. 33 NET from activated or suicidal neutrophils comprise a net-like structure of DNA strands, 34 resulting in a pro-inflammatory immune response and playing a causative role in atherosclerotic plaque formation and thrombosis. 35 Oxidatively modified low-density lipoprotein also plays an important role in atherosclerosis through foam cell formation, enhancing NET-mediated inflammatory responses in vascular endothelial cells through neutrophil activation. 36 Previous reports indicated that neutrophils are strongly associated with in-hospital mortality and major adverse cardiovascular and cerebrovascular events. 37 A lower lymphocyte count was associated with atherosclerosis progression and adverse clinical outcomes in patients with myocardial infarction. 38 –40 This study found that patients with an elevated dNLR had higher WBC and neutrophil counts but a lower lymphocyte count. Consequently, an elevated dNLR was linked to aggravated inflammation and poor ACS outcomes.
NLR and PLR have emerged as universal laboratory markers for predicting proinflammatory and prothrombotic diseases. 41 Our study found that SII, dNLR NLR, and PLR had similar diagnostic value for MACE and the correlations of these markers were very good. Multivariate Cox regression models showed that these markers were all independent predictors for MACE, with similar hazard ratios. Thus, dNLR may be a suitable clinical laboratory marker to identify high-risk ACS patients after PCI.
Limitation
Our study has several limitations. First, our data were from a single-center, and there might be selection bias. Second, post-discharge dNLR changes were not recorded, and the effects of these changes on all-cause mortality and rehospitalization for severe heart failure remain uncertain. Third, long duration of patients recruitment might lead to possible seasonal fluctuations of blood cell ratios. Further studies are needed to fill in this knowledge gap.
Conclusion
We found that a higher SII and dNLR was independently associated with a higher risk of developing all-cause mortality and rehospitalization for severe heart failure in patients with ACS undergoing PCI. SII and dNLR had the similar diagnostic value for MACE in patients with ACS after PCI. These results indicate that dNLR may be a suitable laboratory marker for identifying high-risk ACS patients after PCI.
Footnotes
Authors’ Note: Ethical approval to report this case was obtained from the Institutional Review Board of The Affiliated Hospital of Chengde Medical University (Number: LL087). Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Hebei Province Government Science and Technology Agency (Grant no. 17277769D) awarded to Dr. Lixian Sun, the Technology Innovation Guidance Project-Science and Technology Work Conference from Hebei Provincial Department of Science and Technology (202011) awarded to Dr. Lixian Sun, and the Hebei Provincial Department of Education Graduate Innovation Funding Project (Grant no. CXZZSS2021138) awarded to Wenjun Fan.
ORCID iDs: Wenjun Fan  https://orcid.org/0000-0002-9760-4331
https://orcid.org/0000-0002-9760-4331
Ying Zhang  https://orcid.org/0000-0002-4822-4295
https://orcid.org/0000-0002-4822-4295
Xiuxin Gao  https://orcid.org/0000-0003-0602-4305
https://orcid.org/0000-0003-0602-4305
Yixiang Liu  https://orcid.org/0000-0001-7185-0369
https://orcid.org/0000-0001-7185-0369
Fei Shi  https://orcid.org/0000-0002-8779-3263
https://orcid.org/0000-0002-8779-3263
Lixian Sun  https://orcid.org/0000-0001-9814-0965
https://orcid.org/0000-0001-9814-0965
References
- 1. Arora S, Stouffer GA, Kucharska-Newton AM, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139(8):1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blumenthal D, Hsiao W, Blumenthal DM. Caring for coronary artery disease in China: managing modernization. JAMA Intern Med. 2016;176(4):521–523. [DOI] [PubMed] [Google Scholar]
- 3. Roffi M, Patrono C, Collet JP; et al. ESC Scientific Document Group. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315. [DOI] [PubMed] [Google Scholar]
- 4. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368(21):2004–2013. [DOI] [PubMed] [Google Scholar]
- 5. Eisen A, Giugliano RP, Braunwald E. Updates on acute coronary syndrome: a review. JAMA Cardiol. 2016;1(6):718–730. [DOI] [PubMed] [Google Scholar]
- 6. Amsterdam EA, Wenger NK, Brindis RG; et al. ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130(25):e344–e426. [DOI] [PubMed] [Google Scholar]
- 7. Zheng X, Curtis JP, Hu S; et al. China PEACE Collaborative Group. Coronary catheterization and percutaneous coronary intervention in China: 10-year results from the China PEACE-retrospective CathPCI study. JAMA Intern Med. 2016;176(4):512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290(7):891–897. [DOI] [PubMed] [Google Scholar]
- 9. Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290(7):898–904. [DOI] [PubMed] [Google Scholar]
- 10. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horne BD, Anderson JL, John JM; et al. Intermountain Heart Collaborative Study Group. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–1643. [DOI] [PubMed] [Google Scholar]
- 12. Fan Z, Li Y, Ji H, Jian X. Prognostic utility of the combination of monocyte-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with NSTEMI after primary percutaneous coronary intervention: a retrospective cohort study. BMJ Open. 2018;8(10):e023459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vakili H, Shirazi M, Charkhkar M, Khaheshi I, Memaryan M, Naderian M. Correlation of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio with thrombolysis in myocardial infarction frame count in ST-segment elevation myocardial infarction. Eur J Clin Invest. 2017;47(4):322–327. [DOI] [PubMed] [Google Scholar]
- 14. Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5):e13230. [DOI] [PubMed] [Google Scholar]
- 15. Yucel S, Bilgin B. The prognostic values of systemic immune-inflammation index and derived neutrophil-lymphocyte ratio in EGFR-mutant advanced non-small cell lung cancer. J Oncol Pharm Pract. 2021;27(1):71–77. [DOI] [PubMed] [Google Scholar]
- 16. Liu C, Li L, Lu WS, et al. A novel combined systemic inflammation-based score can predict survival of intermediate-to-advanced hepatocellular carcinoma patients undergoing transarterial chemoembolization. BMC Cancer. 2018;18(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sadeghi MT, Esgandarian I, Nouri-Vaskeh M, Golmohammadi A, Rahvar N, Teimourizad A. Role of circulatory leukocyte based indices in short-term mortality of patients with heart failure with reduced ejection fraction. Med Pharm Rep. 2020;93(4):351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334–1357. [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. da Silva PM, Duarte JS, von Hafe P, Gil V, de Oliveira JN, de Sousa G. Standardization of laboratory and lipid profile evaluation: a call for action with a special focus in 2016 ESC/EAS dyslipidaemia guidelines—Full report. Atheroscler Suppl. 2018;31:e1–e12. [DOI] [PubMed] [Google Scholar]
- 21. Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muhammad K, Ayoub MA, Iratni R. Vascular inflammation in cardiovascular disease: is immune system protective or bystander. Curr Pharm Des. 2021. doi: 10.2174/1381612827666210118121952 [DOI] [PubMed] [Google Scholar]
- 23. Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74(12):1608–1617. [DOI] [PubMed] [Google Scholar]
- 24. Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13(6):368–380. [DOI] [PubMed] [Google Scholar]
- 25. Ridker PM, Everett BM, Thuren T; et al. CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 26. Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107(4):695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu F, Luo H, Zhu Z, Zhu P, Huang J. Prognostic significance of peripheral blood-derived neutrophil/lymphocyte ratio in patients with digestive cancer. J Cell Physiol. 2019;234(12):22775–22786. [DOI] [PubMed] [Google Scholar]
- 28. Ren K, Yin Y, He F, Shao Y, Wang S. Prognostic role of derived neutrophil-to-lymphocyte ratio in surgical triple-negative breast cancer. Cancer Manag Res. 2018;10:4891–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234(10):16812–16823. [DOI] [PubMed] [Google Scholar]
- 30. Osadnik T, Bujak K, Osadnik K, et al. Novel inflammatory biomarkers may reflect subclinical inflammation in young healthy adults with obesity. Endokrynol Pol. 2019;70(2):135–142. [DOI] [PubMed] [Google Scholar]
- 31. Klopf J, Brostjan C, Eilenberg W, Neumayer C. Neutrophil extracellular traps and their implications in cardiovascular and inflammatory disease. Int J Mol Sci. 2021;22(2):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annu Rev Biochem. 2016;85:765–792. [DOI] [PubMed] [Google Scholar]
- 33. Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120(4):713–735. [DOI] [PubMed] [Google Scholar]
- 34. Döring Y, Libby P, Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ Res. 2020;126(9):1228–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–743. [DOI] [PubMed] [Google Scholar]
- 36. Obama T, Itabe H. Neutrophils as a novel target of modified low-density lipoproteins and an accelerator of cardiovascular diseases. Int J Mol Sci. 2020;21(21):8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang J, Zhang Q, Wang R, et al. Systemic immune-inflammatory index predicts clinical outcomes for elderly patients with acute myocardial infarction receiving percutaneous coronary intervention. Med Sci Monit. 2019;25:9690–9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marçula M, de Souza Buto MF, Madaloso BA, et al. Lymphocyte count and prognosis in patients with heart failure. Int J Cardiol. 2015;188:60–62. [DOI] [PubMed] [Google Scholar]
- 39. Vaduganathan M, Ambrosy AP, Greene SJ, et al. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. 2012;5(6):750–758. [DOI] [PubMed] [Google Scholar]
- 40. Li J, Ley K. Lymphocyte migration into atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2015;35(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39(4):345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]