Abstract
Study Design.
Retrospective hospital-registry study.
Objective.
To characterize the microbial epidemiology of surgical site infection (SSI) in spinal fusion surgery and the burden of resistance to standard surgical antibiotic prophylaxis.
Summary of Background Data.
SSI persists as a leading complication of spinal fusion surgery despite the growth of enhanced recovery programs and improvements in other measures of surgical quality. Improved understandings of SSI microbiology and common mechanisms of failure for current prevention strategies are required to inform the development of novel approaches to prevention relevant to modern surgical practice.
Methods.
Spinal fusion cases performed at a single referral center between January 2011 and June 2019 were reviewed and SSI cases meeting National Healthcare Safety Network criteria were identified. Using microbiologic and procedural data from each case, we analyzed the anatomic distribution of pathogens, their differential time to presentation, and correlation with methicillin-resistant Staphylococcus aureus screening results. Susceptibility of isolates cultured from each infection were compared with the spectrum of surgical antibiotic prophylaxis administered during the index procedure on a per-case basis. Susceptibility to alternate prophylactic agents was also modeled.
Results.
Among 6727 cases, 351 infections occurred within 90 days. An anatomic gradient in the microbiology of SSI was observed across the length of the back, transitioning from cutaneous (gram-positive) flora in the cervical spine to enteric (gram-negative/anaerobic) flora in the lumbosacral region (correlation coefficient 0.94, P<0.001). The majority (57.5%) of infections were resistant to the prophylaxis administered during the procedure. Cephalosporin-resistant gram-negative infection was common at lumbosacral levels and undetected methicillin-resistance was common at cervical levels.
Conclusion.
Individualized infection prevention strategies tailored to operative level are needed in spine surgery. Endogenous wound contamination with enteric flora may be a common mechanism of infection in lumbosacral fusion. Novel approaches to prophylaxis and prevention should be prioritized in this population.
Keywords: antimicrobial resistance, Epidemiology, gram-negative bacteria, healthcare-associated infection, Microbiology, microbiome, spinal fusion, surgical antibiotic prophylaxis, surgical site infection, wound infection
Surgical site infection (SSI) following procedures with clean (class I)1 skin incisions has traditionally been attributed to gram-positive skin flora such as Staphylococci and Streptococci.2 Accordingly, current infection prevention measures in spine surgery predominantly target this class of bacteria. However, prior reports and clinical experience suggest that the microbiology of SSI in instrumented spine surgery may be more complex, comprising a diverse range of gram-positive, gram-negative, anaerobic, polymicrobial, and culture-negative infections. This complexity is further compounded by a global increase in the proportion of healthcare-associated infection caused by resistant gram-negative organisms.3
To develop more effective infection prevention strategies in spine surgery, a detailed understanding of the microbial epidemiology and burden of antibiotic-resistant infection in modern surgical practice is required. We report the results of a comprehensive survey of all spinal fusion infections at a single, high-volume referral center over an 8-year period and the implications of these findings for future infection prevention strategies in spine surgery.
MATERIALS AND METHODS
Cohort Identification and Case Definition
All SSIs following spinal fusion surgeries performed at Harborview Medical Center (Seattle, WA) between January 1, 2011 and June 1, 2019 were identified by querying the Harborview Infection Prevention and Control Database. This institutional database is a prospectively maintained registry of healthcare-associated infections identified through standardized chart review by trained infection preventionists. For spinal fusion surgery, all SSIs meeting Centers for Disease Control National Healthcare Safety Network (NHSN) criteria4 within the standard 90-day surveillance period are captured. All adult spinal fusion patients meeting these criteria for SSI were eligible for inclusion. Patients with documented surgical infections present at the time of the procedure, those receiving non-standard surgical antimicrobial prophylaxis5,6 (e.g., substitution of prophylactic with therapeutic antibiotics for patients receiving treatment for nonsurgical infections or cases performed during periods of cefazolin shortage) and those for whom culture acquisition was not attempted were excluded.
Data Extraction and Clinical Variable Handling
Because infections following multilevel procedures can rarely be attributed to a specific vertebral level, surgical level was recorded and primarily analyzed as the range of highest vertebral level to lowest vertebral level involved in the procedure, including any non-instrumented procedural elements (e.g., laminectomy) extending the operative field, but not involved in the fusion. In two-stage procedures, levels involved in both stages were counted collectively and collapsed into a single surgical encounter. Spinal levels involved in each operation were identified through manual review of the operative report. All other variables were extracted in an automated fashion from the electronic medical record.
Details of intraoperative surgical antibiotic prophylaxis were obtained from the anesthesia record. Susceptibility or resistance of each SSI-isolate to the antibiotic(s) administered during the index procedure was determined on a percase basis. For cases in which multiple antibiotics were administered (e.g., cefazolin and vancomycin), isolates were designated as resistant if non-susceptible (resistant or intermediate) to all antibiotic agents administered.
Complete methods for identification and classification of wound culture data including determination of antimicrobial susceptibility are detailed in the Supplemental Methods (see “Handling of microbiology results,” Supplemental Digital Content, http://links.lww.com/BRS/B587). Culture-negative infections were defined as those meeting NHSN criteria1 for SSI and receiving a full course of empiric antimicrobial treatment, but with negative adequate cultures. Although Enterococci and some anaerobic species stain gram-positive, they have intentionally been grouped with gram-negative organisms under the category “enteric” (see Supplemental Table 1, Supplemental Digital Content, http://links.lww.com/BRS/B587) to reflect their primary fecal origin and relative resistance to cephalosporins in contrast to traditional “cutaneous” gram-positive species (see Supplemental Table 2, Supplemental Digital Content, http://links.lww.com/BRS/B587). The term “gram-negative” is used to refer to this group in some instances to reflect common clinical usage, but should be understood to include a small number of gram-positive species.
Statistical Analysis
Normality was evaluated using the Shapiro–Wilk test. Differences in time to presentation were assessed using the Wilcoxon rank sum test and correlation with anatomic level was assessed using the Spearman rank correlation coefficient.
RESULTS
Of 6727 eligible cases performed during the study window, SSI occurred in 351 (5.2%). Cases with pre-existing infection (n=22), receiving non-standard antimicrobial prophylaxis (n=19), or in which culture acquisition was not attempted (n=2) were excluded from analysis, yielding a final study cohort of 308. Patient and procedure characteristics are detailed in Table 1.
TABLE 1.
Characteristics of Study Population
| Age | 58.4 (15.2) |
| Female sex | 44.2% |
| MRSA screen positive | 11.7% |
| BMI | 31.6 (8.6) |
| Diabetes | 16.6% |
| Indication for procedure | |
| Degenerative | 57.1% |
| Fall/trauma | 31.8% |
| Deformity | 5.5% |
| Tumor | 5.2% |
| Vertebral levels | |
| Number involved | 6.6 (3.9) |
| Lowest involved | L4 [T4–S1] |
| Minimally invasive technique | 0.6% |
| Procedure duration, min | 417.6 (159.7) |
| Vancomycin powder | 49.4% |
Values are reported as mean (standard deviation) for continuous variables, as median [interquartile range] for ordinal variables and as percentage for categorical variables.
MRSA indicates methicillin-resistant S. aureus.
Characteristics of Infecting Organisms
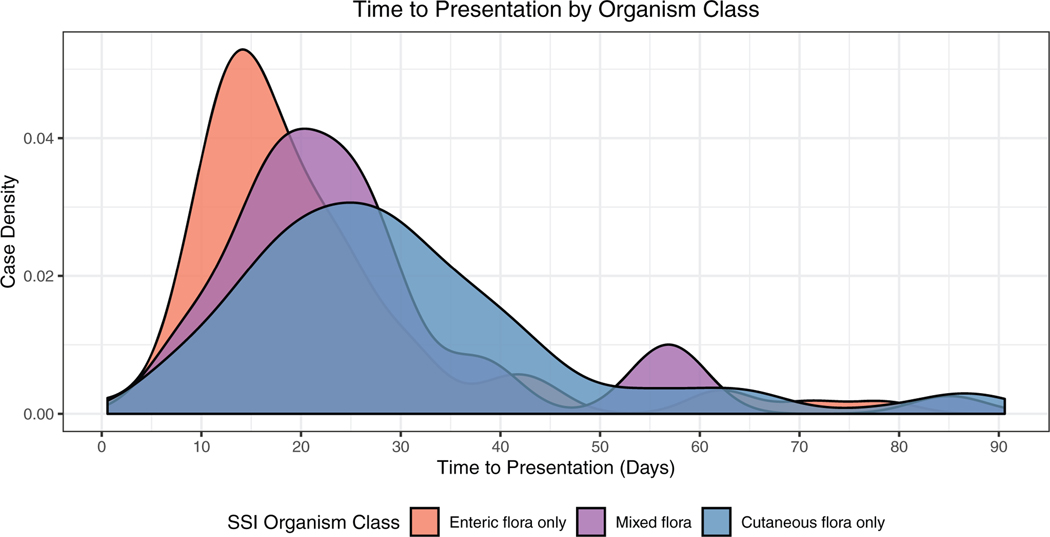
One hundred seventy (55.2%) infections were monomicrobial, 134 (43.5%) were polymicrobial, and four (1.3%) were culture-negative. Among monomicrobial infections, 135 (79.4%) were caused by cutaneous flora (gram-positives other than Enterococcus sp. and fungi) and 35 (20.6%) were caused by enteric species (gram-negatives, Enterococcus sp. and gut-associated anaerobes). Among polymicrobial infections, 30 (22.4%) involved a mixture of cutaneous organisms, 60 (44.8%) involved only enteric organisms, and 44 (32.8%) involved a combination of both cutaneous and enteric species. Gram-negative infections were more likely to be polymicrobial and presented earlier than gram-positive infections (P<0.001) (Figure 1).
Figure 1.
Differential time to presentation between infections caused by cutaneous (gram-positive species other than Enterococcus sp.) versus enteric (gram-negative species and Enterococcus sp.) organisms.
Anatomic Gradient in Microbiology
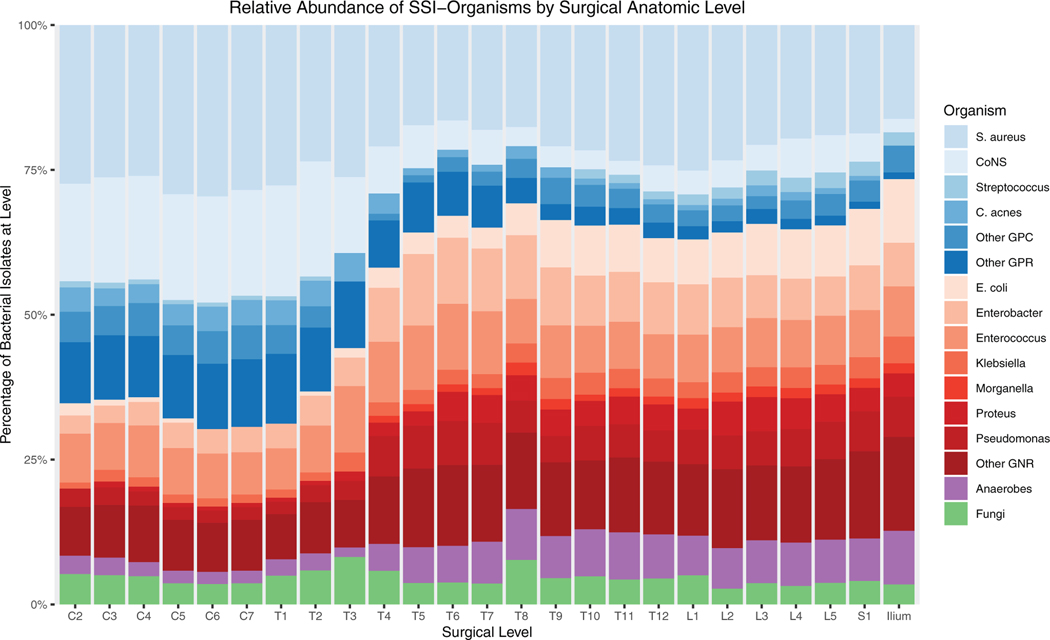
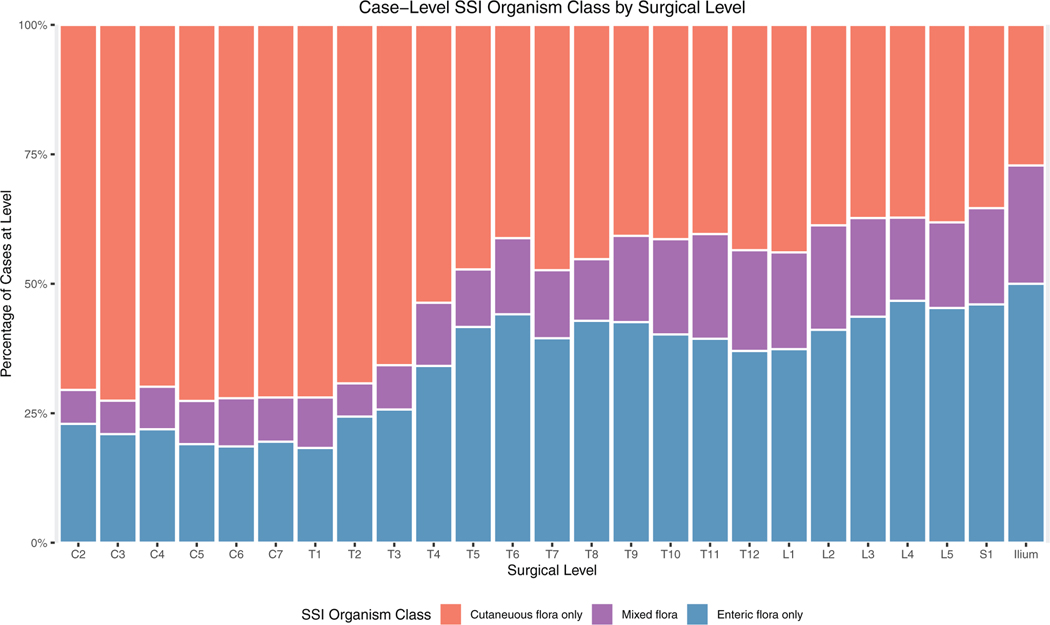
When stratified by operative level, we observed an anatomic gradient in the microbiology of spinal fusion-SSI (Figure 2), transitioning from gram-positive skin commensals in the cervical spine to a predominance of gram-negative enteric organisms in the lumbosacral spine. An inflection point occurred at approximately the T3–4 level. This observation remained stable when aggregated on a per-patient rather than per-isolate basis (Figure 3). The correlation coefficient between vertebral level and infection with one or more enteric flora and was 0.94 (P<0.001). When analyzed according to total species count per level, it is evident that this phenomenon is the result of an absolute, rather than relative, increase in gram-negative infection at lower levels (Supplemental Figure 1, Supplemental Digital Content, http://links.lww.com/BRS/B587).
Figure 2.
Cutaneous flora (blue shades) predominate in cervical and upper thoracic regions while enteric flora (red shades) are more common in cases extending below the midthoracic region. Surgical level (horizontal axis) for each case includes all operative levels involved in the procedure.
Figure 3.
Per-patient analysis of SSI microbiology by level, accounting for polymicrobial infection. SSI indicates surgical site infection.
Given the increase in gram-negative infection with inferior (caudal) extent of the surgical field, we performed a parallel, post-hoc analysis stratified by the lowest vertebral level involved in the procedure. Using lowest vertebral level as an indicator, the inflection point between gram-positive and gram-negative infection occurred lower, near the thoracolumbar junction (Supplemental Figure 2, Supplemental Digital Content, http://links.lww.com/BRS/B587).
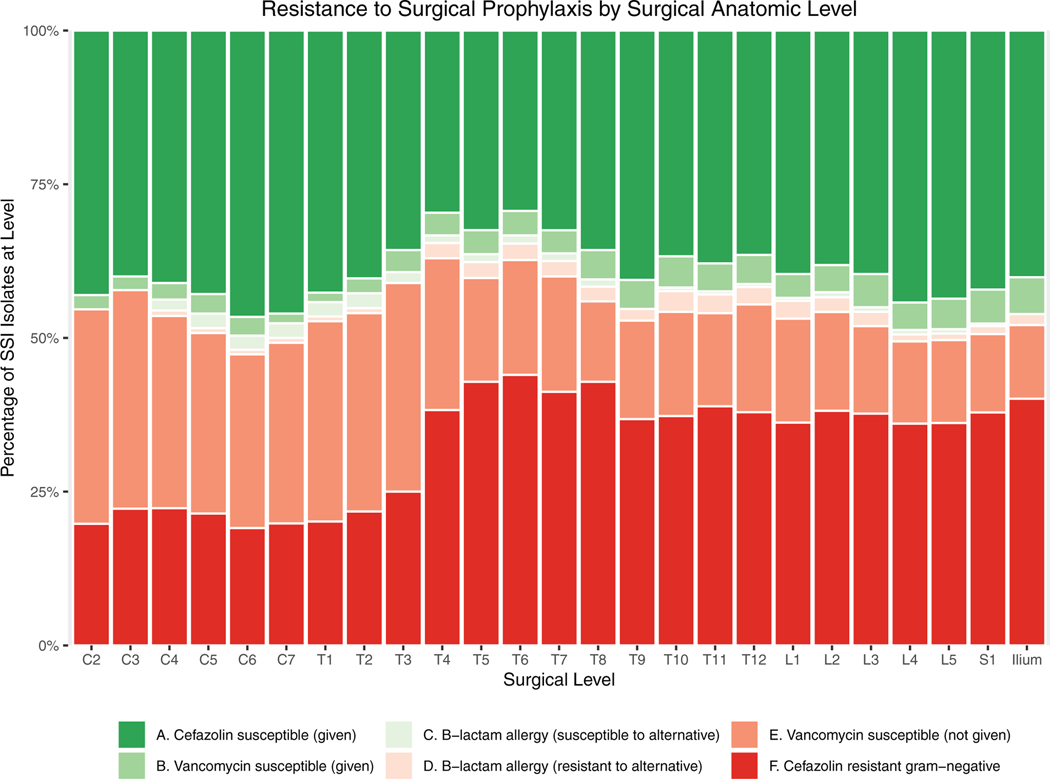
Resistance to Surgical Prophylaxis
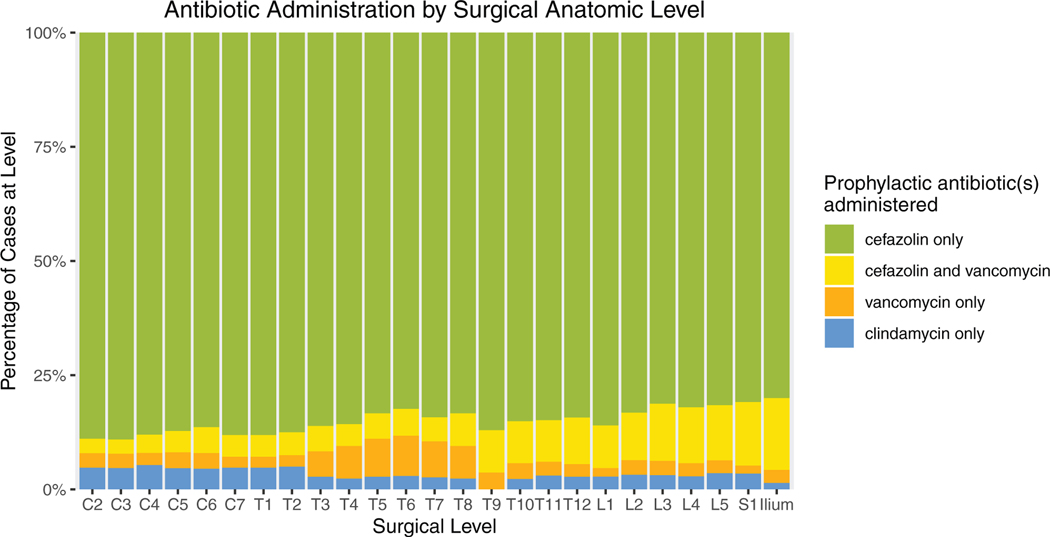
All patients received surgical antibiotic prophylaxis, which consisted of cefazolin (84.7%), cefazolin plus vancomycin (8.8%), vancomycin only (3.2%), or clindamycin only (3.2%). Susceptibility to the prophylactic agent(s) administered during the index procedure could reliably be determined for 97.4% of isolates. One hundred seventy seven SSI cases (57.5%) grew at least one organism resistant to the prophylaxis administered during the index procedure. Categories of concordance and discordance between the spectrum of activity of the prophylactic agent administered and susceptibility of organisms subsequently isolated from SSIs are reported in Table 2. Because current guidelines for antibiotic prophylaxis in spine surgery are the same for procedures performed at all surgical levels, actual antibiotic administration was uniform across spinal regions (Figure 4). As a result, resistance of SSI isolates to prophylaxis also varied across the length of the back (Figure 5).
TABLE 2.
Concordance Between Surgical Antimicrobial Prophylaxis and SSI Isolates
| Group | Prophylactic Regimen | Organism Susceptibility | Number of Isolates (%) | |
|---|---|---|---|---|
| Susceptible (concordant) | A | Cefazolin only (standard prophylactic regimen) | Cefazolin susceptible (e.g., methicillin-susceptible S. aureus) | 228 (43.8%) |
| B | Cefazolin + vancomycin (preoperative MRSA swab positive) | Cefazolin-resistant, vancomycin-susceptible (e.g., methicillin-resistant S. aureus) | 23 (4.4%) | |
| C | Clindamycin or vancomycin monotherapy (β lactam allergy) | Susceptible to alternative administered | 7 (1.3%) | |
| Resistant (discordant) | D | Cefazolin only (standard prophylactic regimen) | Cefazolin-resistant, vancomycin-resistant (e.g., E. coli, Proteus sp.) | 153 (29.4%) |
| E | Cefazolin only (standard prophylactic regimen) | Cefazolin-resistant, vancomycin-susceptible (e.g., methicillin-resistant S. aureus, Enterococcus) | 102 (19.6%) | |
| F | Clindamycin or vancomycin monotherapy (β - lactam allergy) | Resistant to alternative administered | 7 (1.3%) |
SSI indicates surgical site infection.
Figure 4.
Actual antibiotic administration stratified by range of operative levels. Because current guidelines for surgical antibiotic prophylaxis are the same for spine surgeries performed at all operative levels, observed patterns of antibiotic administration were uniform across anatomic regions and did not mirror the gradient observed in SSI microbiology. SSI indicates surgical site infection.
Figure 5.
Methicillin-resistant, vancomycin-susceptible infection is rare among patients receiving prophylactic regimens including vancomycin (B), but is common in cervical fusions in which vancomycin is not administered (E). Most cefazolin-resistant gram-negative infections (F) occurred below the midthoracic level.
Among resistant organisms, cefazolin-resistant enteric species (Table 2-D) were the most common cause of resistance to surgical prophylaxis, accounting for 58.4% of discordant infections. Bacteria in this class were predominantly cultured from lower thoracic and lumbosacral regions (Figure 5F). The most commonly isolated species were Enterobacter sp. (21.2%), Enterococcus sp. (15.3%), Pseudomonas aeruginosa (14.7%), Proteus sp. (8.8%), and Escherichia coli (7.6%). Overall, 68.8% of gram-negative SSI isolates were resistant to cefazolin, 53.4% were resistant to ceftriaxone, and 15.9% were resistant to gentamicin.
Methicillin-resistant gram-positive infections among patients not receiving vancomycin (Table 2-E) constituted the second-largest class of resistance to prophylaxis (38.9%). These occurred predominantly in the cervical and upper thoracic regions (Figure 5E) and most frequently involved methicillin-resistant S. aureus (MRSA) (42.2%) or methicillin-resistant coagulase-negative Staphylococci (34.3%).The majority (61.4%) of MRSA infections occurred in patients screening negative for MRSA preoperatively.
Infections caused by organisms resistant to a non-β-lactam antibiotic administered in cases of allergy were comparatively infrequent. Among SSI cases, 13.3% had a reported penicillin or cephalosporin allergy at the time of surgery, approximately half of whom received an alternative (non-β-lactam) antibiotic for surgical prophylaxis. Infections caused by organisms resistant to this alternative (e.g., clindamycin or vancomycin monotherapy) were predominantly gram-negative and accounted for only 2.7% of resistant isolates (Figure 5D, Table 2-F).
Culture-Negative Infection and Molecular Diagnostics
Among the four cases meeting clinical criteria for SSI which remained culture negative, all underwent molecular testing for the presence of bacterial and fungal DNA. Although 16S/ITS polymerase chain reaction (PCR) amplicon sequencing provided diagnostic confirmation in many cases of delayed culture positivity, we observed no instances of culture-negative infection resolved by this method.
DISCUSSION
In the largest review of spinal fusion SSIs to date, including 351 consecutive infections from a single referral center over an 8-year period, we observed a strong anatomic gradient in the microbiology of SSI. Gram-positive infection occurred at all anatomic levels, but predominated in the cervical and upper thoracic regions. A significant excess of gram-negative infection occurred in lumbosacral procedures. Most infections were caused by strains resistant to the surgical antibiotic prophylaxis administered during the index case. Cefazolin-resistant gram-negative organisms were the most common form of resistant infection, followed by methicillin-resistant Staphylococci. These findings support the potential importance of endogenous gram-negative infection in the pathogenesis of spine SSI and suggest important future directions for infection prevention in spine surgery.
Importance of Gram-Negative Infection in Lumbosacral Fusion
The continuous increase in gram-negative infection with inferior extension of the surgical field is a striking feature of the epidemiology in this cohort. The majority of infections in cases extending to T4 or below (encompassing 75.6% of patients in this study) involved one or more enteric pathogens. The greatest proportion of these occurred in the sacroiliac region. This observation is consistent with the results of another case series including 239 spine SSIs between 2005 and 2010 in which an excess of gram-negative infections was observed in lower lumbar and sacral regions.7 Taken together, these data demonstrate that the microbiology of SSI in spine surgery varies in a predictable anatomic fashion and suggests that distinct approaches to prevention are required, depending on operative level.
Because current infection prevention practices such as cefazolin-based surgical prophylaxis, nasal decolonization, and screening for MRSA predominantly target gram-positive organisms, the development of parallel interventions targeting gram-negative SSI represents a critical opportunity for quality improvement in spine surgery. An essential next step in the development of such measures is identifying the mechanisms by which gram-negative wound contamination occurs in lumbosacral spine surgery. Both intraoperative and postoperative factors may contribute to this phenomenon. Distinguishing between these two potential routes is essential as they are amenable to distinct forms of intervention.
Intraoperative Routes of Gram-Negative Wound Contamination
Intraoperative contamination of clean (class I) wounds is commonly caused by bacteria colonizing patient skin at the site of incision.2 Human skin flora have traditionally been considered to comprise gram-positive species such as Staphylococci and Streptococci based on their avid growth in culture. However, culture-free technologies such as 16S PCR have revealed much greater bacterial diversity within the human skin microbiome.8 A high degree of variation in the composition of “normal” skin flora is now known to exist between individuals and across anatomic sites. This includes the presence of several clinically relevant gram-negative species such as P. aeruginosa and E. coli in particular skin regions.9 It is therefore possible that the observed anatomic gradient in spine SSI simply reflects a normal gradient in the composition of skin flora along the length of the back. Unfortunately, the back is one of the most poorly characterized regions of the human microbiome10 and this hypothesis remains to be tested.
Established measures for the prevention of intraoperative wound contamination with patient skin flora include the application of topical skin antisepsis and administration of surgical antimicrobial prophylaxis prior to incision. Given the large number of gram-negative infections in lumbosacral procedures and their high degree of resistance to cefazolin (68.8% in this study and 61.6% in another case-series7), the suitability of cefazolin as a sole prophylactic agent in this subgroup must be critically examined. The utility of gram-negative prophylaxis in spine surgery as a whole was previously explored in a meta-analysis of studies on the introduction of surgical antimicrobial prophylaxis to spine surgery in the 1980s.11 This meta-analysis found no difference in the efficacy of regimens containing gram-positive coverage alone versus those containing both gram-positive and gram-negative coverage and is referenced in current guidelines for antibiotic prophylaxis in spine surgery.6 However, the prophylactic regimens and patient characteristics of the included studies were heterogeneous and rates of cephalosporin-resistance have increased substantially over the intervening decades.
Our data suggest that an individualized approach, matching the spectrum of prophylactic activity to the microbiology of the surgical site may be more effective. Patients undergoing lumbosacral spine surgery would be a high priority population in which to study the impact of targeted gram-negative prophylaxis in modern surgical practice. Based on patterns of susceptibility observed in this study, the addition of gentamicin to cefazolin may be a more rational selection than substitution of cefazolin with a higher-generation cephalosporin such as ceftriaxone.
Postoperative Routes of Gram-Negative Wound Contamination
Postoperative factors may also play a role in the pathogenesis of gram-negative SSI. Saturation of the surgical dressing and contamination with fecal material may occur in the setting of impaired mobility during the early recovery period. A primary fecal origin for endogenous gram-negative infection in spine surgery would be highly consistent with the excess of enteric infections observed in lumbosacral procedures. The plausibility of this mechanism is further supported by numerous prior reports of an association between gram-negative spine SSI and postoperative incontinence.12–14 The earlier presentation of gram-negative versus gram-positive SSI in this study may seem inconsistent with a postoperative origin for gram-negative infection. However, this difference may be more reflective of bacterial growth characteristics than the timing of wound inoculation.
Changes to antimicrobial prophylaxis are unlikely to prevent infections originating after the time of surgery. To the extent that postoperative mechanisms contribute to SSI in spine surgery, measures specifically targeting these routes of infection may be required. Examples include implementation of improved protocols for mobility, toileting, and pain control, standardized drain management, and use of incisional negative-pressure wound therapy (NPWT). The use of incisional NPWT as a method of reducing SSI is supported by high quality evidence in some procedural groups,15–17 but has demonstrated lack of benefit in others.18–20 Its efficacy in spine surgery is unknown. Routine application of incisional NPWT in long-segment thoracolumbar spine fusions at a single center was associated with reductions in SSI and wound dehiscence in one retrospective study21 and two randomized trials of incisional NPTW in major spine surgery are ongoing (NCT03632005 and NCT03820219).
Need for Improved Detection of MRSA
The majority of MRSA infections occurred in patients with a negative preoperative MRSA screen. This unexpected finding suggests either the acquisition of healthcare-associated strains or lack of sensitivity of routine screening protocols. Preoperative screening for nasal carriage of MRSA at our institution is performed using chromogenic agar. Sampling of multiple sites (e.g., oropharyngeal, rectal, skin)22–24 and use of PCR-based assays25–27 have been shown to improve screening sensitivity. These strategies may not be cost-effective if applied to surgical patients broadly,28 but may warrant consideration in high risk groups such as those undergoing cervical and upper-thoracic spinal fusion surgery.
LIMITATIONS
The single-center nature of this study limits its generalizability to other practice settings. For example, variations in practice such as use of minimally invasive surgical techniques and povidone-iodine irrigation are uncommon at our center, but may impact SSI rates and microbiology. Despite this, the reported findings are consistent with those of the next-largest case series of spine SSI.7 The use of an institutional antibiogram to infer antimicrobial susceptibility of isolates not subjected to direct testing further limits the generalizability of findings related to antibiotic-resistant infection. Our institutional antibiogram and rates of MRSA colonization are similar to national norms, but vary from those in many regions outside of North America. A significant correlation between anatomic level and microbiology was observed, but causality cannot be inferred from this unadjusted analysis and other factors such as procedural indication may underlie this association. Finally, only spinal fusion procedures were analyzed, and the findings may not apply to other classes of spine surgery.
CONCLUSION
The microbiology of spinal fusion SSI transitions from gram-positive to gram-negative infection across the length of the back. The pathogenesis of these two classes of infection may differ and future studies should consider these as distinct, potentially competing outcomes with unique risk profiles. Before changes to practice recommendations can be made, further work is required to determine the relevant routes of gram-negative wound contamination in spine surgery and identify whether intraoperative interventions such as targeted use of gram-negative surgical prophylaxis or postoperative changes in wound care are more likely to be effective. Individualized strategies targeting the patient microbiome may afford opportunities to reduce rates of SSI in spine surgery while minimizing the risk of antimicrobial resistance.
Supplementary Material
Key Points.
An anatomic bacterial gradient exists in the microbiology of SSI in spinal fusion surgery.
Cefazolin-resistant gram-negative bacteria are a common cause of SSI in lumbosacral fusions.
Methicillin-resistant gram-positive organisms are common in cervical and upper thoracic fusions.
The majority of bacteria cultured in cases of SSI are resistant to the standard surgical antimicrobial prophylaxis administered during the procedure.
The majority of MRSA infections occur in patients screening negative for MRSA by culture of preoperative nasal swabs.
Acknowledgment
The authors would like to thank the Center for Perioperative & Pain initiatives in Quality Safety Outcome (PPiQSO) at the University of Washington, Seattle for providing elements of the data extract analyzed in the present work.
The manuscript submitted does not contain information about medical device(s)/drug(s).
NIH T32 training grant (GM086270) and Society for Healthcare Epidemiology of America 2019 Epi Competition Award funds were received in support of this work.
Relevant financial activities outside the submitted work: grants.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appearing in the printed text are provided in the HTML and PDF version of this article on the journal’s Web site (www.spinejournal.com).
References
- 1.Van Wicklin SA, Brubaker SA. Surgical wound classification. AORN J 2015;102:299–308. [Google Scholar]
- 2.Charles F, Brunicardi DA, Billiar TR, Dunn DL, Hunter JG, Raphael E. Schwartz’s Manual of Surgery. New York, NY: Mcgraw-Hill Medical; 2009; 159. [Google Scholar]
- 3.Diekema DJ, Hsueh P-R, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 2019;63:e00355–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Procedure-associated Module: Surgical Site Infection (SSI) Event. Centers for Disease Control; 2020. Available at:https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf. Accessed January 29, 2020. [Google Scholar]
- 5.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013;70:195–283. [DOI] [PubMed] [Google Scholar]
- 6.Shaffer WO, Baisden J, Fernand R, et al. Antibiotic Prophylaxis in Spine Surgery. North American Spine Society; 2013. [DOI] [PubMed] [Google Scholar]
- 7.Abdul-Jabbar A, Berven SH, Hu SS, et al. Surgical site infections in spine surgery: identification of microbiologic and surgical characteristics in 239 cases. Spine (Phila Pa 1976) 2013;38:E1425–31. [DOI] [PubMed] [Google Scholar]
- 8.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011;9:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouslimani A, Porto C, Rath CM, et al. Molecular cartography of thehumanskinsurfacein3D.ProcNatlAcadSciUSA 2015;112:E2120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIH Human Microbiome Project. Human Microbiome Project Data Portal; 2017. Available at: https://portal.hmpdacc.org. Accessed October 5, 2019.
- 11.Barker FG. Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis. Neurosurgery 2002;51:391–400; discussion 400–401. [PubMed] [Google Scholar]
- 12.Sponseller PD, LaPorte DM, Hungerford MW, et al. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine (Phila Pa 1976) 2000;25:2461–6. [DOI] [PubMed] [Google Scholar]
- 13.Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg 2003;98:149–55. [PubMed] [Google Scholar]
- 14.Perry JW, Montgomerie JZ, Swank S, et al. Wound infections following spinal fusion with posterior segmental spinal instrumentation. Clin Infect Dis 1997;24:558–61. [DOI] [PubMed] [Google Scholar]
- 15.Hasselmann J, Björk J, Svensson-Björk R, et al. Inguinal vascular surgical wound protection by incisional negative pressure wound therapy: a randomized controlled trial—INVIPS trial. Ann Surg 2019;271:48–53. [DOI] [PubMed] [Google Scholar]
- 16.Javed AA, Teinor J, Wright M, et al. Negative pressure wound therapy for surgical-site infections: a randomized trial. Ann Surg 2019;269:1034–40. [DOI] [PubMed] [Google Scholar]
- 17.Newman JM, Siqueira MBP, Klika AK, et al. Use of closed incisional negative pressure wound therapy after revision total hip and knee arthroplasty in patients at high risk for infection: a prospective, randomized clinical trial. J Arthroplasty 2019;34:554.e1–9.e1. [DOI] [PubMed] [Google Scholar]
- 18.Hussamy DJ, Wortman AC, McIntire DD, et al. Closed incision negative pressure therapy in morbidly obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol 2019;134:781–9. [DOI] [PubMed] [Google Scholar]
- 19.Masden D, Goldstein J, Endara M, et al. Negative pressure wound therapy for at-risk surgical closures in patients with multiple comorbidities: a prospective randomized controlled study. Ann Surg 2012;255:1043–7. [DOI] [PubMed] [Google Scholar]
- 20.Murphy PB, Knowles S, Chadi SA, et al. Negative pressure wound therapy use to decrease surgical nosocomial events in colorectal resections (neptune): a randomized controlled trial. Ann Surg 2019;270:38–42. [DOI] [PubMed] [Google Scholar]
- 21.Adogwa O, Fatemi P, Perez E, et al. Negative pressure wound therapy reduces incidence of postoperative wound infection and dehiscence after long-segment thoracolumbar spinal fusion: a single institutional experience. Spine J 2014;14:2911–7. [DOI] [PubMed] [Google Scholar]
- 22.Batra R, Eziefula AC, Wyncoll D, et al. Throat and rectal swabs may have an important role in MRSA screening of critically ill patients. Intensive Care Med 2008;34:1703–6. [DOI] [PubMed] [Google Scholar]
- 23.Currie A, Davis L, Odrobina E, et al. Sensitivities of nasal and rectal swabs for detection of methicillin-resistant Staphylococcus aureus colonization in an active surveillance program. J Clin Microbiol 2008;46:3101–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popovich KJ, Aroutcheva A, Hota B, et al. Anatomic sites of colonization with community-associated methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2014; 35:1192–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang STJ, McHugh MP, Guerendiain D, et al. Underestimation of Staphylococcus aureus (MRSA and MSSA) carriage associated with standard culturing techniques: one third of carriers missed. Bone Joint Res 2018;7:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolk DM, Marx JL, Dominguez L, et al. Comparison of MRSA-Select Agar, CHROMagar Methicillin-Resistant Staphylococcus aureus (MRSA) medium, and Xpert MRSA PCR for detection of MRSA in Nares: diagnostic accuracy for surveillance samples with various bacterial densities. J Clin Microbiol 2009;47:3933–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paule SM, Mehta M, Hacek DM, et al. Chromogenic media vs real-time PCR for nasal surveillance of methicillin-resistant Staphylococcus aureus: impact on detection of MRSA-positive Persons. Am J Clin Pathol 2009;131:532–9. [DOI] [PubMed] [Google Scholar]
- 28.Murthy A, De Angelis G, Pittet D, et al. Cost-effectiveness of universal MRSA screening on admission to surgery. Clin Microbiol Infect 2010;16:1747–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.