Abstract
Objective
Neurodevelopmental impairment (NDI) is a major complication of extreme prematurity. This systematic review was conducted to summarize the worldwide long-term prevalence of NDI associated with extreme prematurity.
Methods
Embase and MEDLINE databases were searched for epidemiologic and observational/real-world studies, published in English between 2011 and 2016, reporting long-term prevalence of NDI (occurring from 1 year) among extremely preterm infants born at gestational age (GA) ≤28 weeks.
Results
Of 2406 articles identified through searches, 69 met the protocol NDI definition (24 North America, 25 Europe, 20 Rest of World). Prevalence of any severity NDI in North America was 8%–59% at 18 months to 2 years, and 11%–37% at 2–5 years; prevalence of moderate NDI in Europe was 10%–13% at 18 months to 2 years, 3% at 2–5 years, and 9%–19% at ≥5 years; prevalence of any NDI in Rest of World was 15%–61% at 18 months to 2 years, and 42% at 2–5 years (no North America/Rest of World studies reported any NDI at ≥5 years). A trend toward higher prevalence of NDI with lower GA at birth was observed.
Conclusions
Extreme prematurity has a significant long-term worldwide impact on neurodevelopmental outcomes.
Keywords: Premature birth, neurodevelopment, developmental disabilities, long-term outcome, prevalence, systematic review
Introduction
Preterm birth is one of the leading causes of infant and childhood death.1,2 Although survival of infants born extremely preterm (birth at gestational age [GA] <28 weeks) has improved in recent decades,3,4 premature infants remain at risk of major complications, including respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage (IVH), and periventricular leukomalacia (PVL). In particular, neurologic injury from IVH and PVL can result in life-long neurodevelopmental impairment (NDI), imposing a significant burden on children, their caregivers, and health systems, with impacts well into adulthood.5–9
Major neurodevelopmental disabilities commonly associated with IVH in extremely preterm infants include cerebral palsy (CP), deafness, blindness, cognitive delay, and behavioral difficulties.10–14 While some of these outcomes can be identified in the first 2–3 years of a child’s life, neurobehavioral and emotional problems emerge later, at school age, and may persist into adulthood.
Because of variations in the way gestational age at birth is reported, variations in the definitions and reporting of NDI, and heterogeneity in the tools used to assess NDI, comparing results across studies is difficult. To the best of our knowledge, at the time this systematic literature review (SLR) was conducted, no SLRs evaluating the global prevalence of NDI in extremely preterm infants had been published.
A greater understanding of the global scope and long-term impact of neurodevelopmental morbidities associated with extreme prematurity may inform parent counseling, early interventions, and resource planning, and identify further research needs for this group of children. However, the systematic identification, collection, and synthesis of data from studies reporting such outcomes is key, to produce unbiased conclusions on the long-term impact of NDI associated with extreme prematurity and allow comparability of findings across studies. For that reason, the current SLR was designed to investigate the following research question: ‘What is the global prevalence of long-term neurodevelopmental impairment in children and adults born at extremely premature gestational ages?’ The aim of this SLR was to evaluate the prevalence of two long-term outcomes that are commonly associated with extreme prematurity: NDI and CP.
Methods
Overall SLR methodology
The SLR was conducted following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, 15 and is registered in the Research Registry (https://www.researchregistry.com; UIN reviewregistry1155). The Embase and MEDLINE databases were searched for articles published in English between January 2011 and June 2016, reflecting a 5-year search, initiated in 2016, that was intended to capture the most current available data. Conference abstracts were excluded, unless they were available in a full-text, peer-reviewed publication format. The search strategy used a combination of free-text and controlled-vocabulary search terms relevant to long-term outcomes of extreme prematurity. Validated search terms were used where available. The full search strategy is available in Supplemental Tables 1–3. The bibliographies of SLRs and meta-analyses published from 2011 to 2016 were reviewed for additional articles. A first level of review identified relevant abstracts and excluded those meeting any of the study exclusion criteria. Publications that were not eliminated passed to the second level of review, which involved the evaluation of the full text. All abstracts were independently screened by one researcher (DM), and 30% were randomly selected for double-screening by two independent researchers (DM and SA). Any disagreement was resolved by a third researcher (GS).
Selection criteria and data extraction
Studies that reported the long-term prevalence of NDI were selected for the present analysis. Inclusion criteria were: (1) humans born at ≤27 weeks 6 days GA–although in prespecified instances, infants born at 28 weeks GA were included in the extremely preterm population to take into account variations in reporting of GA at birth (see Supplemental Table 4 for expanded inclusion criteria); (2) study outcomes that included the prevalence of long-term outcomes; (3) epidemiologic and observational/real-world studies; and (4) English-language publications.
Neurodevelopmental impairment was assessed as at least one type of neurologic (including CP) or sensory disability using the different scales reported by authors in the included studies. Data on NDI severity were extracted where available. ‘Long-term’ was defined as assessment for NDI at ≥12 months after birth. Studies were excluded if they presented results for mixed populations (preterm and term neonates) without a subgroup analysis by GA at birth. Clinical trials, case studies, and protocols were excluded (Supplemental Table 4). Although a study sample size may be a significant factor in determining the generalizability of its findings, no further selection criteria were applied during the present study selection in order to capture all available information on the long-term burden of extreme prematurity. For each included study, data were extracted into a standardized predesigned extraction table by one researcher (DM) and further validated for accuracy and correctness by a second researcher (SA).
Data synthesis
Data were synthesized using qualitative methods to describe the existing body of knowledge on the prevalence of NDI. Studies were grouped according to identified themes, and a narrative synthesis was used to draw on the connections between studies and the objectives of the review. For comparability purposes, data were categorized by geographic location: Europe, North America, and Rest of World. Although CP was considered part of the NDI assessment, the results are presented separately (NDI and CP) where data are available.
For studies reporting long-term outcomes or clinical burden by comparing two groups of extremely preterm infants, with and without specific characteristics (e.g. a specific mode of delivery) or comorbidities (e.g. preterm premature rupture of membranes, IVH, or sepsis), data for the group without the specific characteristics were selected and included in the current SLR. This was done to increase comparability of results across studies and to enhance generalizability of results to the target population (i.e. extremely preterm infants). For studies that reported follow-up data on NDI at several time points, data from the oldest age cohort are presented, unless specified otherwise. Prevalence data for NDI and CP stratified by GA (where available) are presented separately.
Results
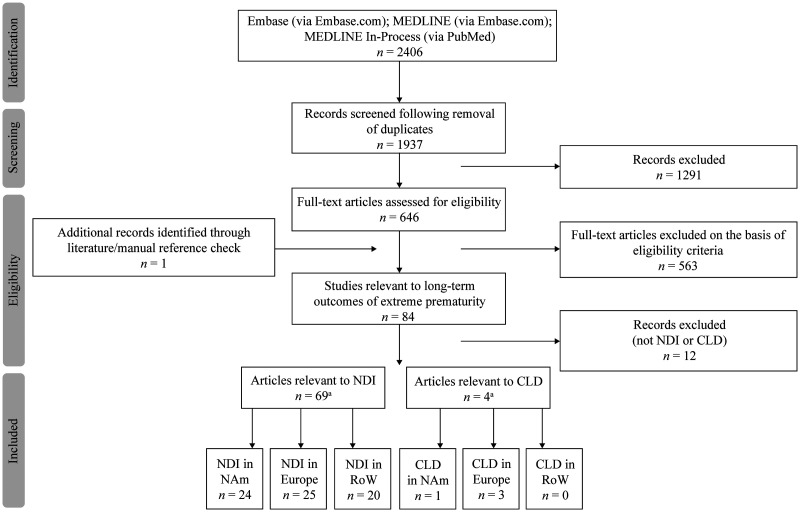
Searches identified 2406 publications. After duplicates were removed, 1937 records were reviewed, and 646 were selected for full-text evaluation. A further 563 publications that did not meet the inclusion criteria were then excluded. The main reasons for exclusion were: a mixed population without separate reporting of data for the population of interest (i.e. term and preterm infants); no outcomes of interest; short-term outcomes; and studies that excluded extremely preterm infants. In total, 69 articles relevant to NDI were included in the present analysis (Figure 1).
Figure 1.
Study selection process for publications reporting on the prevalence of NDI (PRISMA flowchart). CLD, chronic lung disease; NAm, North America; NDI, neurodevelopmental impairment; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RoW, Rest of World. (A total of 4 articles were identified relevant to CLD; these were excluded from the present SLR as not relevant to NDI).
NDI by geographic location
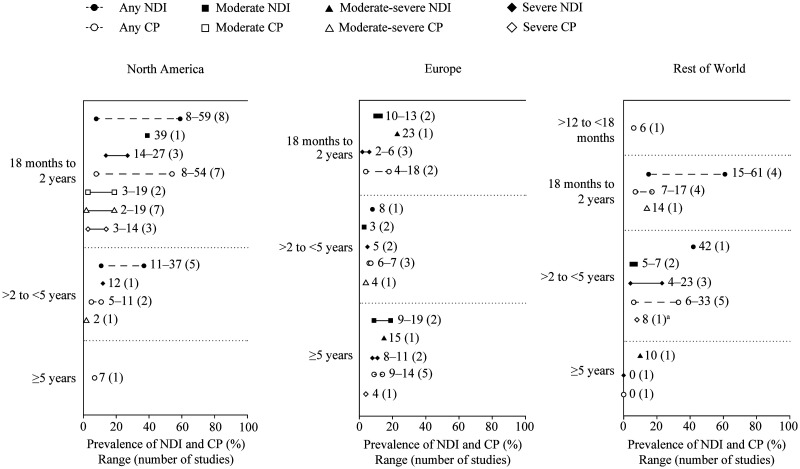
Overall, there were wide variations in prevalence estimates among the included studies, particularly between North America and Europe (Figure 2). Across geographic settings, NDI was commonly assessed using the Bayley Scales of Infant Development (Supplemental Table 5).16,17
Figure 2.
Reported prevalence of NDI in included studies by length of follow-up, in (left to right) North America, Europe, and Rest of World. aIshii 2013 reports ‘profound’ CP. CP, cerebral palsy; NDI, neurodevelopmental impairment.
North America. Twenty-four North American studies reported findings for NDI (United States, 20; Canada, four) (Table 1).18–41 Of those studies, 13 were of prospective design and 11 were retrospective. Sample size varied considerably, ranging from 44 to 3608.20,27 The reported prevalence of any NDI (in studies that reported an aggregate of NDI of any type or severity) among children born extremely preterm ranged from 8% to 59% at 18 months to 2 years follow-up,18,26 and from 11% to 37% between 2 and 5 years follow-up (Figure 2; Table 1).35,39 No studies reported the proportion of children with any NDI at ≥5 years follow-up. The prevalence of severe NDI ranged from 12% to 27% across all follow-up durations (reported in four studies).23,27,28,37 The prevalence of any CP was 8%–54% at 18 months to 2 years follow-up,19,23,31,32 5%–11% between 2 and 5 years follow-up,35,37 and 7% at ≥5 years follow-up. 40
Table 1.
Identified articles reporting on the prevalence of NDI and CP in North America, by length of follow-up period (n = 24).
| Article*; country | Study design; setting | Preterm population definition | Sample size | NDI, % | CP, % |
|---|---|---|---|---|---|
| 18 months to 2 years follow-up (n = 17)a | |||||
| Carlo 2011; 25 USA | Prospective; multicenter | 401–1000 g and 22–25 wGA | 2628g | 49 (any) | 12 (moderate-severe) |
| Hintz 2011; 26 USA | Retrospective; multicenter | 401–1000 g and <25 wGA | 405f | 59 (any) | 15 (moderate-severe) 6 (severe) |
| Natarajan 2012; 33 USA | Prospective; multicenter | 401–1000 g | 556h | NR | 2 (moderate-severe) |
| Stephens 2012; 18 USA | Prospective; multicenter | <27 wGA | 554 | 8 (any) | 3 (moderate) |
| Vohr 2012; 30 USA | Retrospective; multicenter | 401–1000 g and <27 wGA | 922f | NR | 15 (any) 7 (moderate-severe) |
| Goldstein 2013; 22 USA | Retrospective; multicenter | 401–1000 g and 23–28 wGA, with no intracranial hemorrhage or severe intracranial hemorrhage | 3445 | 39 (any) | NR |
| Payne 2013; 19 USA | Prospective; multicenter | <26 weeks 6/7 days estimated GA | 1021b | 10 (NDI <70)c27 (NDI <85)d | 8 (any) 4 (moderate-severe) |
| Salas 2013; 28 USA | Retrospective; single center | 24–29 wGA | 347 | 18 (severe) | NR |
| Tsai 2013; 31 USA | Prospective; NR | Preterm infants with intraparenchymal hemorrhage | 48 | NR | 54 (any) |
| Tsai 2014; 32 USA | Retrospective; single center | <1000 g or <27 wGA, with intraparenchymal hemorrhage | 48 | NR | 54 (any) 19 (moderate) 14 (severe) |
| Miltaha 2015; 27 USA | Prospective; single center | 23 weeks 7 days to 23 weeks 6/7 days GA | 44 | 39 (moderate) 27 (severe) | NR |
| Batton 2016; 21 USA | Prospective; multicenter | 23–26 wGA | 232 | 19 (any) | NR |
| Boghossian 2016; 20 USA | Retrospective; multicenter | 22–28 wGA | 3608e | 16 (any) | 12 (any) 6 (moderate-severe) |
| Lefebvre 2016; 23 Canada | Retrospective; single center | ≤28 wGA | 160 | 47 (any) 14 (severe) | 8 (any) 3 (severe) |
| Leviton 2016; 34 USA | Prospective; multicenter | <28 wGA | 940 | NR | Variousi |
| VanderVeen 2016; 29 USA | Prospective; single center | <28 wGA | 996 | NR | 11 (any) |
| Younge 2016; 24 USA | Retrospective; single center | 22–24 wGA | 187f | 47 (any) | 19 (moderate-severe) |
| >2 years to <5 years follow-up (n = 5)a | |||||
| Lodha 2011; 38 Canada | Retrospective; NR | ≤28 wGA, born to older mothers | 688 early maternal age 28 advanced maternal age | 35 early maternal age 36 advanced maternal age | NR |
| Lodha 2011; 39 Canada | Prospective; single center | ≤28 wGA, cesarean section versus vaginal route | 251 cesarean section 274 vaginal route | 37 cesarean section 36 vaginal route | NR |
| Zayek 2011; 37 USA | Retrospective; single center | <1000 g and 22–26 wGA | 328f | 32 (any) 12 (severe) | 5 (any) 2 (moderate-severe) |
| Wickremasinghe 2012; 35 USA | Prospective; single center | ≤27 weeks 6/7 days GA | 96f | 11 (any) | 11 (any) |
| Alshaikh 2014; 36 Canada | Retrospective; single center | <29 wGA | 105 CoNS 227 no CoNS | 25 CoNS 15 no CoNS | NR |
| ≥5 years follow-up (n = 2)a | |||||
| Sukhov 2012; 41 USA | Prospective; population based | Children with CP | 924 | NR | Variousj |
| Durkin 2015; 40 USA | Prospective; population based | All births surviving to 1 year | 3328 | NR | 7 (any) |
*Articles are listed in each follow-up group chronologically by publication date, then alphabetically by first author.
aBased on maximum age at follow-up.
bNo peri-intraventricular hemorrhage.
cNDI <70 = composite measure of NDI, defined in the source study as any one of the following: moderate-severe vertebral palsy, severe visual impairment, deafness, or cognitive score <70.
dNDI <85 = composite measure of NDI, defined in the source study as any one of the following: moderate-severe CP, severe visual impairment, deafness, or cognitive score <85.
eNo insulin-dependent diabetes mellitus.
fMost recent period cohort presented in table.
gNo antenatal corticosteroids.
hNo bronchopulmonary dysplasia.
iQuadriparesis, 6%; diparesis, 4%; hemiparesis, 2%.
jSpasticity, 77%; ataxia, 4%; dyskinesis, 2%; hypotonia, 5%; other, 13%. Limb movement: diplegia/paraplegia, 32%; hemiplegia, 12%; monoplegia, 2%; triplegia/quadriplegia, 47%; other CP, 7%.
CP, cerebral palsy; CoNS, positive clinical signs for sepsis and positive cerebrospinal fluid cultures; GA, gestational age; NDI, neurodevelopmental impairment; NR, not reported; wGA, weeks gestational age.
Three studies reported NDI stratified by GA, in which a trend toward a higher prevalence of NDI with lower GA at birth was observed (Supplemental Table 6).22,24,37 Prevalence of any NDI was 48%–65% for infants born at GA 23 weeks versus 26% for those born at GA 27 weeks.22,24,37
Europe. Twenty-five studies in European populations reported findings for NDI (Table 2),8,42–65 over half of which (15/25) were database or registry-based studies. Included studies were conducted in Austria, Denmark, England, Estonia, France, the Netherlands, Norway, Poland, Spain, Sweden, and Switzerland. Sample sizes in included studies ranged considerably from 17 to 1673.48,60 Six studies included secondary analyses of Extremely Preterm Infants in Sweden Study (EXPRESS)–a large, prospective, population-based follow-up study of infants born in Sweden at <27 weeks of GA.
Table 2.
Identified articles reporting the prevalence of NDI and CP in European countries, by length of follow-up period (n = 25).
| Article*; country | Study design; setting | Preterm population definition | Sample size | NDI, % | CP, % |
|---|---|---|---|---|---|
| 18 months to 2 years follow-up (n = 7)a | |||||
| Schlapbach 2011; 46 Switzerland | Prospective; database | 24–28 wGA | 236b | 23 (moderate-severe) | 4 (any) |
| Stoinska 2011; 44 Poland | NR; single center | ELBW/VLBW infants <28 wGA | 227 | 20 (any; 24–26 wGA) 15 (any; 27–28 wGA) | 30 (any; 24–26 wGA) 18 (any; 27–28 wGA) |
| Von Lindern 2011; 47 the Netherlands | Retrospective; multicenter | <28 wGA | 67 | 5 (severe) | NR |
| De Waal 2012; 45 the Netherlands | Prospective; database | 23–27 wGA | 55 (24 wGA) 104 (25 wGA) 130 (26 wGA) | 0 (moderate-severe; 24 wGA) 17 (moderate-severe; 25 wGA) 9 (moderate-severe; 26 wGA) | NR |
| Schlapbach 2012; 43 Switzerland | Prospective; database | 24 weeks 0/7 days to 27 weeks 6/7 days GA | 684 | 13 (moderate) 6 (severe) | NR |
| Toome 2013; 48 Estonia | Prospective; database | Very low GA infants 22–25 wGA | 17 | NR | 18 (any) |
| Morgillo 2014; 42 Switzerland | Prospective; single center | 23–28 wGA | 147 | 10 (moderate) 2 (severe) | NR |
| >2 years to <5 years follow-up (n = 8)a | |||||
| Grahn 2012; 53 Sweden | Prospective; database | <27 wGA | 107 | NR | 6 (any) |
| Kerstjens 2012; 55 the Netherlands | Prospective; multicenter | ≤28 wGA | 150 | NR | NR |
| Moore 2012; 8 England | Prospective; multicenter | 22–26 wGA | 576 | 3 (moderate) 5 (severe) | NR |
| Skiöld 2012; 51 Sweden | Prospective; database | <27 wGA | 91 | 9 (24 wGA) 3 (25 wGA) | 7 (any) |
| Boulet 2014; 49 France | Prospective; single center | 22–28 wGA | 67 | 3 (moderate) 5 (severe) | NR |
| Holmström 2014; 54 Sweden | Prospective; database | <27 wGA | 411 | NR | 7 (any)4 (moderate-severe) |
| Källén 2015; 50 Sweden | Prospective; database | <27 wGA | 456 | 8 (any) | NR |
| Serenius 2015; 52 Sweden | Prospective; database | <27 wGA | 456 | 27–30 (22–26 wGA) | NR |
| ≥5 years follow-up (n = 10)a | |||||
| Leversen 2011; 56 Norway | Prospective; multicenter | 22–27 wGA or BW 500–999 g | 306 | 9 (moderate) 8 (severe) | 4 (severe) |
| Mathiasen 2011; 65 Denmark | Retrospective; database | Infants stratified by GA | 1078 | NR | NR |
| Camba 2012; 59 Spain | Retrospective; database | <26 wGA | 9 (23 wGA)32 (24 wGA)65 (25 wGA) | 13 (moderate; 24 wGA) 6 (moderate; 25 wGA) 25 (severe; 24 wGA)11 (severe; 25 wGA) | NR |
| Elgen 2012; 57 Norway | Prospective; database | <28 wGA or BW <1000 g | 255 | 15 (moderate-severe) | NR |
| Klebermass-Schrehof 2012; 62 Austria | Prospective; single center | <28 wGA | 320e | NR | 14 (any) |
| Marret 2013; 64 France | Prospective; multicenter | 22–26 wGA | 610 | NR | 13 (moderate; 24–26 wGA) 9 (moderate; 27–28 wGA) 7 (severe; 24–26 wGA) 5 (severe; 27–28 wGA) |
| Mitha 2013; 63 France | Prospective; multicenter | 22–28 wGA | 436 | NR | 14 (any) |
| Serenius 2014; 58 Sweden | Prospective; database | <27 wGA | 445 | 19 (moderate) 11 (severe) | 9 (any) |
| Trønnes 2014; 60 Norway | Retrospective; registry | 23–27 wGA | 1673 | NR | 9 (any) |
| Skromme 2015; 61 Norway | Prospective; database | <28 wGA or BW <1000 g | 281c | NR | 9d |
*Articles are listed in each follow-up group chronologically by publication date, then alphabetically by first author.
aBased on maximum age at follow-up.
bUninfected (no sepsis).
cPediatrician assessment.
dCP, blindness, or complete deafness.
eNo intraventricular hemorrhage.
BW, birth weight; CP, cerebral palsy; ELBW, extremely low birth weight; GA, gestational age; NDI, neurodevelopmental impairment; NR, not reported; VLBW, very low birth weight; wGA, weeks gestational age.
The prevalence of NDI as a complication of extreme prematurity was most frequently reported as moderate or severe NDI. The prevalence of moderate NDI was 10%–13% at 18 months to 2 years follow-up,42,43 3% between 2 and 5 years follow-up,8,49 and 9%–19% at ≥5 years follow-up (Figure 2; Table 2).56,58 The reported prevalence of severe NDI ranged from 2% to 11% across all follow-up durations (reported in seven studies).8,42,43,47,49,56,58 In the included studies, CP was classified using different diagnostic criteria: the child’s ability to walk without aids, by International Statistical Classification of Diseases and Related Health Problems codes, and using the Gross Motor Function Classification System (Supplemental Table 5). 66 Most studies (15/25 [including either prospective or retrospective study design]) reported follow-up at <5 years. The prevalence of any CP was 4%–18% at 18 months to 2 years follow-up,46,48 6%–7% between 2 and 5 years follow-up,51,53,54 and 9%–14% at ≥5 years follow-up (Figure 2; Table 2).58,60,62,63
Four European studies reported NDI stratified by GA. The overall trend in findings was for a higher prevalence of NDI (any, moderate, severe) with lower GA at birth (Supplemental Table 6).44,45,51,59 The trend for increasing NDI prevalence of any severity with lower GA was less clearly defined in European studies than in North American studies, with one study reporting 0% prevalence in infants born at 24 weeks, but 17% in those born at 25 weeks and 9% in those born at 26 weeks. 45 A decline in prevalence of NDI (any severity) with higher GA at birth was reported in two other studies, each comparing two different GA groups.44,45,51 The prevalence of moderate or severe NDI was 13% and 25%, respectively, for infants with GA of 24 weeks versus 6% and 11%, respectively, for infants born at 25 weeks GA. 59
Rest of World. Twenty studies from outside North America and Europe reported findings for NDI (Table 3).12,67–85 Sample size ranged from eight (infants with focal intestinal perforation) to 2883.73,83 Most (17/20) studies were retrospective. Just over half of the studies (11/20) were conducted in Australia; the others were conducted in China, Japan, Singapore, and South Africa. NDI was not consistently defined across studies.
Table 3.
Identified articles reporting on the prevalence of NDI and CP from Rest of World, by length of follow-up period (n = 20).
| Article*; country | Study design; setting | Preterm population definition | Sample size | NDI, % | CP, % |
|---|---|---|---|---|---|
| 12 months to <18 months follow-up (n = 1)a | |||||
| Heald 2012; 67 Australia | Retrospective; single center | <29 wGA | 97 (64b) | NR | 6 (any)b,c |
| 18 months to 2 y follow-up (n = 8)a | |||||
| Ballot 2012; 75 South Africa | Prospective; single center | VLBW | 26 | NR | NR |
| Sugiura 2012; 73 Japan | Retrospective; multicenter | <33 wGA | 2883 | NR | 13 (<24 wGA) 5 (24 wGA) 9 (25 wGA) 5 (26 wGA) 6 (27 wGA) 4 (28 wGA) |
| Cheong 2013; 68 Australia | Retrospective; NR | <28 wGA or BW <1000 g | 256d | 15 (any) | 7 (any) |
| Chang 2015; 70 China | Retrospective; single center | ELBWe | 45 (control) | 28 (any) | 14 (moderate-severe) |
| Hayakawa 2015; 71 Japan | Retrospective; multicenter | BW ≤1500 g; underwent laparotomy | 44 (NEC)47 (FIP) | 61 (NEC) 47 (FIP) | NR |
| Orton 2015; 69 Australia | Retrospective; single center | <28 wGA and/orBW <1000 g | 109 | 16 (abnormal neurologic examination) | 7 (any) |
| Sun 2015; 74 China | Retrospective; single center | ≤32 wGA, BW <1500 g | 70 | NR | 17 (any) |
| Ohhashi 2016; 72 Japan | Retrospective; multicenter | <1500 g | 633 | NR | 14 (any) |
| >2 y to <5 y follow-up (n = 9)a | |||||
| Kent 2012; 82 Australia | Retrospective; multicenter (tertiary) | <29 wGA | 1473 | NR | NR |
| Ishii 2013; 78 Japan | NR; multicenter (tertiary) | 22–25 wGA | 562 | 42 (any NDI) 23 (profound NDI) | 14 (any CP) 8 (profound CP) |
| Abdel-Latif 2014; 80 Australia | Retrospective; multicenter (tertiary) | <29 wGA | 1473 | NR | 9 (any)c |
| Bolisetty 2014; 12 Australia | Retrospective; multicenter (tertiary) | 23–28 wGA | 1472 | NR | 7 (any)g |
| Keir 2014; 81 Australia | Retrospective; single center | ≥22 wGA and BW ≤500 g | 14 | NR | 33 (any) |
| Wong 2014; 79 Australia | Retrospective; multicenter (tertiary) | <29 wGA | 1472 | NR | 6 (any)c,f |
| Janz-Robinson 2015; 76 Australia | Retrospective; multicenter | <29 wGA | 826 non-PDA 569 PDA medical78 PDA surgery | 6 (moderate [–1 SD to –2 SD]) 4 (severe [<–2 SD]) | NR |
| Rodrigues 2015; 77 Australia | Retrospective; multicenter | <29 wGA | 1205 urban 268 rural | 7 (moderate; urban [–1 SD to –2 SD]) 5 (moderate; rural [–1 SD to –2 SD]) 5 (severe; urban [<–2 SD]) 5 (severe; rural [<–2 SD]) | NR |
| Tanaka 2015; 83 Japan | Retrospective; single center | BW <1500 g; infants who underwent laparotomy for FIP or NEC | 8 FIP 24 VLBW | NR | NR |
| ≥5 y follow-up (n = 2)a | |||||
| Roberts 2011; 85 Australia | Prospective; multicenter | 22–27 wGA or BW 500–999 g | 132 | NR | NR |
| Poon 2013; 84 Singapore | Retrospective; single center | ≤26 wGA | 10 (<23 wGA;n = 0 at 8 y) 23 (24 wGA; n = 6 at 8 y) 27 (25 wGA; n = 8 at 8 y) 41 (26 wGA; n = 11 at 8 y) | 10 (moderate-severe; 23–25 wGA) 0 (severe; 23–26 wGA) | 0 (24–25 wGA) |
*Articles are listed in each follow-up group chronologically by publication date, then alphabetically by first author.
aBased on maximum age at follow-up.
bNo insulin.
cRequiring walking aids.
d2005 cohort presented.
eReceived prophylactic indomethacin.
fNo antenatal steroids (9/143).
gNo intraventricular hemorrhage (68/1043).
BW, birth weight; CP, cerebral palsy; ELBW, extremely low birth weight; FIP, focal intestinal perforation; NDI, neurodevelopmental impairment; NEC, necrotizing enterocolitis; NR, not reported; PDA, patent ductus arteriosus; SD, standard deviation; VLBW, very low birth weight; wGA, weeks gestational age; y, years.
Among included studies, the prevalence of any NDI ranged from 15% to 61% at 18 months to 2 years follow-up,68,71 and was 42% between 2 and 5 years follow-up, 78 summarized in Figure 2 and Table 3. No studies reported prevalence of any (aggregate of any severity) NDI at ≥5 years follow-up; prevalence of moderate-severe NDI was 10%. 84 The prevalence of any CP was 6% between 12 and 18 months follow-up, 67 7%–17% at 18 months to 2 years follow-up,68,69,74 6%–33% between 2 and 5 years follow-up,79,81 and 0% at ≥5 years follow-up. 84
Two studies reported NDI stratified by GA. There was some evidence of a trend toward a higher prevalence of NDI at lower GAs, but there was variability across studies (Supplemental Table 6).78,84
Discussion
This SLR provides global-based evidence that children born extremely preterm are at high risk for developing long-term neurodevelopmental adverse outcomes. Overall, the search identified 69 articles that fulfilled the study criteria for NDI. The higher prevalence of NDI in North America versus Europe was notable, particularly among infants with a shorter duration of follow-up. This is likely attributable to the following factors: (1) differences in the methodology of data collection and assessment tools; (2) resuscitation practices; and (3) differences between study populations, such as GA, and neonatal and maternal comorbid conditions. An additional consideration is that studies in North America tended to report the prevalence of any NDI, whereas European studies were more likely to report NDI prevalence by severity. Across follow-up ages, the prevalence of any NDI in North America was 8%–59%. The prevalence of moderate NDI was 39% in the single North American study that reported moderate NDI, 27 versus 3%–19% in included European studies. A meta-analysis that included birth cohorts in a median year of 2000 or later (median age at assessment, 28 months) estimated that 52% of surviving infants born extremely preterm worldwide develop NDI (defined as cognitive, motor, hearing, and visual impairment) to some degree; 86 this is in line with the NDI prevalence in North America found in the present SLR of more recent data.
A trend towards increased risk of NDI with decreasing GA at birth was observed in the present SLR among infants born at 23 weeks versus 27 weeks of GA (NDI prevalence, 48%–65% versus 26%, respectively). These findings are broadly consistent with data published later than the date cut-off used to identify the literature included in this study, including a 2018 meta-analysis that reported the prevalence of moderate-to-severe NDI at 18 months to 3 years (assessed using the Bayley Scales of Infant Development II or III) to be 50% among infants born at 23 weeks of GA and 17% among infants born at 27 weeks after 1994 in high-income countries. 87 Similar results were reported in a 2019 meta-analysis of studies with follow-up of extremely preterm infants (born in 1995 or later and aged 4–10 years at assessment), conducted in Australia, Europe, Japan, and the USA. 88 The risk of moderate-to-severe NDI (based on measurement of cognitive ability) significantly declined with each 1-week increase in GA, with NDI rates of 41% at 23 weeks’ GA and 23% at 25 weeks’ GA. However, this SLR had restricted population criteria (22–25 weeks) and looked at a later age range at follow-up, leaving a critical window of follow-up at 1–2 years after birth. Early follow-up is important, as most clinical interventions are initiated during this early life period in an effort to improve later-life outcomes.
Compared with the present SLR, these meta-analyses were more limited regarding the overall populations studied. Furthermore, each analysis employed a different definition of NDI, all of which were more specific than the broader scope applied in the present review. The heterogeneity of our findings (>50% of included studies) precluded any follow-up statistical analysis (i.e. meta-analysis).
Retrospective and observational studies conducted in North America, South Korea, and Poland have also provided evidence that NDI prevalence increases with lower GA.13,89,90 In these studies, NDI as well as individual cognitive and motor components, CP, and hearing impairment were reported more frequently with lower GA in extremely preterm infants. Comparison of infants born at 22–24 weeks with those born at 25–26 weeks revealed significant differences in prevalence rates for NDI (28% versus 14%), moderate-to-severe cognitive delay (35% versus 24%), and moderate-to-severe motor impairment (16% versus 6%). 13 In a study in France, moderate/severe NDI was reported in 28% and 12%, and mild NDI was reported in 38.5% and 34% of infants born at 24–26 weeks and 32–34 weeks, respectively. 91 These data indicate that the burden of NDI among extremely preterm infants is highest for infants born at the lowest GA.
The prevalence of any CP for different follow-up durations was broadly similar across geographical regions in the present SLR, with 18 months to 2 years’ follow-up showing the greatest variation (4%–54%). In agreement with earlier studies,7,30 findings from a recent US cohort study suggested that CP in extremely preterm infants may be declining in severity over time. 13 Over the four-year study period of this US study, the percentage of infants with CP decreased from 16% to 9%, reflecting a 43% decrease in prevalence of severe CP and a 13% increase in prevalence of mild CP.
Strengths of the current study include the global focus of the review, the strict criteria used to capture the target population of extremely preterm infants and the systematic approach used to gather the data. Our findings have implications for further research, including the need for additional longitudinal studies to capture the long-term complications in children born extremely preterm, to identify risk factors associated with these outcomes and evaluate new interventions to alter the life-long trajectory of these complications. The scope of previous SLRs reporting long-term outcomes of NDI was different from the scope of the present SLR, as they have either considered a wider population focus (prematurity in general) in specific settings (low and middle-income countries only), 92 have considered a very narrow definition of extreme prematurity (e.g. those born between 22 and 25 weeks' gestation), 93 or have restricted the follow-up time frame (18–24 months). 94 The present results may be limited by variations in the tools used to assess NDI and in the definitions of NDI, which may have limited direct comparison of results across studies. A further consideration, which is inherent to observational research, is the potential bias due to patients lost to follow-up in included studies. Methodological variation and incomplete reporting are common issues encountered in studies evaluating NDI in extremely preterm children. 95 For example, in a previous evaluation of 14 cohort studies, information about several aspects of outcome assessment was lacking and most studies failed to report complete details of data analysis, including masking, subgroup analyses, and handling of missing data. 95 Such shortfalls in reporting affect the interpretation of study results and, thus, affect the conclusions made in systematic reviews, impeding evidence-based clinical decisions.
In conclusion, children born extremely preterm experience negative long-term neurodevelopmental outcomes. Wide variations among prevalence estimates of NDI and a trend toward a higher prevalence of long-term NDI among infants born at the lowest GA were noted. Further work is needed to identify and minimize the risk of NDI in this vulnerable patient population.
Supplemental Material
Supplemental material, sj-pdf-2-imr-10.1177_03000605211028026 for Global prevalence of long-term neurodevelopmental impairment following extremely preterm birth: a systematic literature review by Sujata P. Sarda, Grammati Sarri and Csaba Siffel in Journal of International Medical Research
Acknowledgements
Seye Abogunrin and Dušan Milenković, formerly employees of Evidera, performed research (screening and data extraction) for this study. Under direction of the authors, Rosalind Bonomally, of Excel Medical Affairs, provided writing assistance for this publication. Editorial assistance in formatting, proof reading, and copy editing was also provided by Excel Medical Affairs. Takeda provided funding to Excel Medical Affairs for support in writing and editing this manuscript.
Footnotes
Declaration of conflicting interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SPS is currently employed by Apellis Pharmaceuticals. GS is currently employed by Visible Analytics, Ltd and was a paid consultant to Shire, a Takeda company, in relation to this study. CS is an employee of and holds stock/stock options in Takeda.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Shire, a member of the Takeda group of companies.
ORCID iD: Csaba Siffel https://orcid.org/0000-0002-6491-6953
Supplemental material: Supplemental material for this article is available online.
References
- 1.Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013; 10: S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379: 2151–2161. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Preterm birth, http://www.who.int/mediacentre/factsheets/fs363/en/ (2018, accessed 16 July 2019).
- 4.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015; 314: 1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008; 371: 261–269. [DOI] [PubMed] [Google Scholar]
- 6.March of Dimes, The Partnership for Maternal Newborn & Child Health, Save the Children, et al. Born too soon: the global action report on preterm birth, https://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf (2012, accessed 16 July 2019).
- 7.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ 2012; 345: e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007; 357: 1946–1955. [DOI] [PubMed] [Google Scholar]
- 10.O'Shea TM, Allred EN, Kuban KC, et al. Intraventricular hemorrhage and developmental outcomes at 24 months of age in extremely preterm infants. J Child Neurol 2012; 27: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis AS, Hintz SR, Goldstein RF, et al. Outcomes of extremely preterm infants following severe intracranial hemorrhage. J Perinatol 2014; 34: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolisetty S, Dhawan A, Abdel-Latif M, et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 2014; 133: 55–62. [DOI] [PubMed] [Google Scholar]
- 13.Adams-Chapman I, Heyne RJ, DeMauro SB, et al. Neurodevelopmental impairment among extremely preterm infants in the Neonatal Research Network. Pediatrics 2018; 141: e20173091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers EE, Hintz SR. Early neurodevelopmental outcomes of extremely preterm infants. Semin Perinatol 2016; 40: 497–509. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayley N. Bayley scales of infant and toddler development. 2nd ed. San Antonio, TX: The Psychological Corporation, 1993. [Google Scholar]
- 17.Bayley N. Bayley scales of infant and toddler development. 3rd ed. San Antonio, TX: The Psychological Corporation, 2006. [Google Scholar]
- 18.Stephens BE, Bann CM, Watson VE, et al. Screening for autism spectrum disorders in extremely preterm infants. J Dev Behav Pediatr 2012; 33: 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne AH, Hintz SR, Hibbs AM, et al. Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA Pediatr 2013; 167: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boghossian NS, Hansen NI, Bell EF, et al. Outcomes of extremely preterm infants born to insulin-dependent diabetic mothers. Pediatrics 2016; 137: e20153424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batton B, Li L, Newman NS, et al. Early blood pressure, antihypotensive therapy and outcomes at 18–22 months' corrected age in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2016; 101: F201–F206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein RF, Cotten CM, Shankaran S, et al. Influence of gestational age on death and neurodevelopmental outcome in premature infants with severe intracranial hemorrhage. J Perinatol 2013; 33: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefebvre F, Gagnon MM, Luu TM, et al. In extremely preterm infants, do the Movement Assessment of Infants and the Alberta Infant Motor Scale predict 18-month outcomes using the Bayley-III? Early Hum Dev 2016; 94: 13–17. [DOI] [PubMed] [Google Scholar]
- 24.Younge N, Smith PB, Gustafson KE, et al. Improved survival and neurodevelopmental outcomes among extremely premature infants born near the limit of viability. Early Hum Dev 2016; 95: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlo WA, McDonald SA, Fanaroff AA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks' gestation. JAMA 2011; 306: 2348–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hintz SR, Kendrick DE, Wilson-Costello DE, et al. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks' gestational age. Pediatrics 2011; 127: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miltaha HR, Fahey LM, Sajous CH, et al. Influence of perinatal factors in short- and long-term outcomes of infants born at 23 weeks of gestation. Am J Perinatol 2015; 32: 627–632. [DOI] [PubMed] [Google Scholar]
- 28.Salas AA, Faye-Petersen OM, Sims B, et al. Histological characteristics of the fetal inflammatory response associated with neurodevelopmental impairment and death in extremely preterm infants. J Pediatr 2013; 163: 652–657.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderVeen DK, Allred EN, Wallace DK, et al. Strabismus at age 2 years in children born before 28 weeks' gestation: antecedents and correlates. J Child Neurol 2016; 31: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vohr BR, Stephens BE, Higgins RD, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr 2012; 161: 222–228.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai AJ, Evans PW, Kennedy KA. Factors affecting neurodevelopmental outcomes in preterm infants with intraparenchymal hemorrhage. J Investig Med 2013; 61: 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai AJ, Lasky RE, John SD, et al. Predictors of neurodevelopmental outcomes in preterm infants with intraparenchymal hemorrhage. J Perinatol 2014; 34: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natarajan G, Pappas A, Shankaran S, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev 2012; 88: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leviton A, Allred EN, Kuban KC, et al. Brain disorders associated with corticotropin-releasing hormone expression in the placenta among children born before the 28th week of gestation. Acta Paediatr 2016; 105: e7–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wickremasinghe AC, Rogers EE, Piecuch RE, et al. Neurodevelopmental outcomes following two different treatment approaches (early ligation and selective ligation) for patent ductus arteriosus. J Pediatr 2012; 161: 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alshaikh B, Yee W, Lodha A, et al. Coagulase-negative staphylococcus sepsis in preterm infants and long-term neurodevelopmental outcome. J Perinatol 2014; 34: 125–129. [DOI] [PubMed] [Google Scholar]
- 37.Zayek MM, Trimm RF, Hamm CR, et al. The limit of viability: a single regional unit's experience. Arch Pediatr Adolesc Med 2011; 165: 126–133. [DOI] [PubMed] [Google Scholar]
- 38.Lodha A, Sauve R, Tand S, et al. Does advanced maternal age affect long term neurodevelopmental outcomes in preterm infants at 3 years of age? Paediatr Child Health 2011; 16: 33A. [Google Scholar]
- 39.Lodha A, Wood S, Creighton DE, et al. Mode of delivery does not affect neurodevelopmental outcomes at 3 years of age in preterm infants born ≤28 weeks' gestational age. Paediatr Child Health 2011; 16: 33A. [Google Scholar]
- 40.Durkin MS, Maenner MJ, Benedict RE, et al. The role of socio-economic status and perinatal factors in racial disparities in the risk of cerebral palsy. Dev Med Child Neurol 2015; 57: 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sukhov A, Wu Y, Xing G, et al. Risk factors associated with cerebral palsy in preterm infants. J Matern Fetal Neonatal Med 2012; 25: 53–57. [DOI] [PubMed] [Google Scholar]
- 42.Morgillo D, Morgillo-Mitchell J, Fontanta M, et al . Outcome of extremely low gestational age newborns (ELGANs) following a pro-active treatment approach: a Swiss single centre experience over 10 years. Swiss Med Wkly 2014; 144: w14014. [DOI] [PubMed] [Google Scholar]
- 43.Schlapbach LJ, Adams M, Proietti E, et al. Outcome at two years of age in a Swiss national cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatr 2012; 12: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoinska B, Gadzinowski J. Neurological and developmental disabilities in ELBW and VLBW: follow-up at 2 years of age. J Perinatol 2011; 31: 137–142. [DOI] [PubMed] [Google Scholar]
- 45.De Waal CG, Weisglas-Kuperus N, van Goudoever JB, et al. Mortality, neonatal morbidity and two year follow-up of extremely preterm infants born in the Netherlands in 2007. PLoS One 2012; 7: e41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlapbach LJ, Aebischer M, Adams M, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011; 128: e348–e357. [DOI] [PubMed] [Google Scholar]
- 47.Von Lindern JS, Khodabux CM, Hack KE, et al. Long-term outcome in relationship to neonatal transfusion volume in extremely premature infants: a comparative cohort study. BMC Pediatr 2011; 11: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toome L, Varendi H, Männamaa M, et al. Follow-up study of 2-year-olds born at very low gestational age in Estonia. Acta Paediatr 2013; 102: 300–307. [DOI] [PubMed] [Google Scholar]
- 49.Boulet C, Debillon T, Cottereau S, et al. Outcome at 4-year-old children born before 29 weeks of gestation. J Matern Fetal Neonatal Med 2014; 27(S1): 367. [Google Scholar]
- 50.Källén K, Serenius F, Westgren M, et al. Impact of obstetric factors on outcome of extremely preterm births in Sweden: prospective population-based observational study (EXPRESS). Acta Obstet Gynecol Scand 2015; 94: 1203–1214. [DOI] [PubMed] [Google Scholar]
- 51.Skiöld B, Vollmer B, Böhm B, et al. Neonatal magnetic resonance imaging and outcome at age 30 months in extremely preterm infants. J Pediatr 2012; 160: 559–566.e1. [DOI] [PubMed] [Google Scholar]
- 52.Serenius F, Blennow M, Maršál K, et al. Intensity of perinatal care for extremely preterm infants: outcomes at 2.5 years. Pediatrics 2015; 135: e1163–e1172. [DOI] [PubMed] [Google Scholar]
- 53.Grahn A, Horsch S, Skiöld B, et al. Prevalence of hearing impairments at age four years in extremely preterm infants. Arch Dis Child 2012; 97: A360–A361. [Google Scholar]
- 54.Holmström GE, Källen K, Hellström A, et al. Ophthalmologic outcome at 30 months' corrected age of a prospective Swedish cohort of children born before 27 weeks of gestation: the Extremely Preterm Infants in Sweden Study. JAMA Ophthalmol 2014; 132: 182–189. [DOI] [PubMed] [Google Scholar]
- 55.Kerstjens JM, De Winter AF, Bocca-Tjeertes IF, et al. Risk of developmental delay increases exponentially as gestational age of preterm infants decreases: a cohort study at age 4 years. Dev Med Child Neurol 2012; 54: 1096–1101. [DOI] [PubMed] [Google Scholar]
- 56.Leversen KT, Sommerfelt K, Rønnestad A, et al. Prediction of neurodevelopmental and sensory outcome at 5 years in Norwegian children born extremely preterm. Pediatrics 2011; 127: e630–e638. [DOI] [PubMed] [Google Scholar]
- 57.Elgen SK, Leversen KT, Grundt JH, et al. Mental health at 5 years among children born extremely preterm: a national population-based study. Eur Child Adolesc Psychiatry 2012; 21: 583–589. [DOI] [PubMed] [Google Scholar]
- 58.Serenius F, Ewald U, Fellman V, et al. Neurodevelopmental outcome of extremely preterm infants at 6.5 years of age; Extremely Preterm Infants Study in Sweden (EXPRESS). Arch Dis Child 2014; 99: A131. [Google Scholar]
- 59.Camba F, Cespedes M, Felipe A, et al. Results of the follow-up program of extremely preterm infants born at 23 to 25 weeks' gestation. J Matern Fetal Neonatal Med 2012; 25: 92. [Google Scholar]
- 60.Trønnes H, Wilcox AJ, Lie RT, et al. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: a national cohort study. Dev Med Child Neurol 2014; 56: 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skromme K, Leversen KT, Eide GE, et al. Respiratory illness contributed significantly to morbidity in children born extremely premature or with extremely low birthweights in 1999–2000. Acta Paediatr 2015; 104: 1189–1198. [DOI] [PubMed] [Google Scholar]
- 62.Klebermass-Schrehof K, Czaba C, Olischar M, et al. Impact of low-grade intraventricular hemorrhage on long-term neurodevelopmental outcome in preterm infants. Childs Nerv Syst 2012; 28: 2085–2092. [DOI] [PubMed] [Google Scholar]
- 63.Mitha A, Foix-L'Hélias L, Arnaud C, et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics 2013; 132: e372–e380. [DOI] [PubMed] [Google Scholar]
- 64.Marret S, Marchand-Martin L, Picaud JC, et al. Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS One 2013; 8: e62683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathiasen R, Hansen BM, Forman JL, et al. The risk of psychiatric disorders in individuals born prematurely in Denmark from 1974 to 1996. Acta Paediatr 2011; 100: 691–699. [DOI] [PubMed] [Google Scholar]
- 66.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997; 39: 214–223. [DOI] [PubMed] [Google Scholar]
- 67.Heald A, Abdel-Latif ME, Kent AL. Insulin infusion for hyperglycaemia in very preterm infants appears safe with no effect on morbidity, mortality and long-term neurodevelopmental outcome. J Matern Fetal Neonatal Med 2012; 25: 2415–2418. [DOI] [PubMed] [Google Scholar]
- 68.Cheong JL, Anderson P, Roberts G, et al. Postnatal corticosteroids and neurodevelopmental outcomes in extremely low birthweight or extremely preterm infants: 15-year experience in Victoria, Australia. Arch Dis Child Fetal Neonatal Ed 2013; 98: F32–F36. [DOI] [PubMed] [Google Scholar]
- 69.Orton LJ, McGinley JL, Fox LM, et al. Challenges of neurodevelopmental follow-up for extremely preterm infants at two years. Early Hum Dev 2015; 91: 689–694. [DOI] [PubMed] [Google Scholar]
- 70.Chang HY, Lung HL, Li ST, et al . Outcomes of prophylactic indomethacin for extremely low birth weight infants. Hong Kong J Paediatr 2015; 20: 62–70. [Google Scholar]
- 71.Hayakawa M, Taguchi T, Urushihara N, et al. Outcome in VLBW infants with surgical intestinal disorder at 18 months of corrected age. Pediatr Int 2015; 57: 633–638. [DOI] [PubMed] [Google Scholar]
- 72.Ohhashi M, Yoshitomi T, Sumiyoshi K, et al. Magnesium sulphate and perinatal mortality and morbidity in very-low-birthweight infants born between 24 and 32 weeks of gestation in Japan. Eur J Obstet Gynecol Reprod Biol 2016; 201: 140–145. [DOI] [PubMed] [Google Scholar]
- 73.Sugiura T, Goto T, Ueda H, et al. Periventricular leukomalacia is decreasing in Japan. Pediatr Neurol 2012; 47: 35–39. [DOI] [PubMed] [Google Scholar]
- 74.Sun H, Zhou Y, Xiong H, et al. Prognosis of very preterm infants with severe respiratory distress syndrome receiving mechanical ventilation. Lung 2015; 193: 249–254. [DOI] [PubMed] [Google Scholar]
- 75.Ballot DE, Potterton J, Chirwa T, et al. Developmental outcome of very low birth weight infants in a developing country. BMC Pediatr 2012; 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janz-Robinson EM, Badawi N, Walker K, et al. Neurodevelopmental outcomes of premature infants treated for patent ductus arteriosus: a population-based cohort study. J Pediatr 2015; 167: 1025–1032.e3. [DOI] [PubMed] [Google Scholar]
- 77.Rodrigues AN, Bajuk B, Oei J, et al. Neurodevelopmental outcome of extremely preterm infants born to rural and urban residents' mothers in Australia. Early Hum Dev 2015; 91: 437–443. [DOI] [PubMed] [Google Scholar]
- 78.Ishii N, Kono Y, Yonemoto N, et al. Outcomes of infants born at 22 and 23 weeks' gestation. Pediatrics 2013; 132: 62–71. [DOI] [PubMed] [Google Scholar]
- 79.Wong D, Abdel-Latif ME, Kent AL, et al. Antenatal steroid exposure and outcomes of very premature infants: a regional cohort study. Arch Dis Child Fetal Neonatal Ed 2014; 99: F12–F20. [DOI] [PubMed] [Google Scholar]
- 80.Abdel-Latif ME, Bajuk B, Oei J, et al. Population study of neurodevelopmental outcomes of extremely premature infants admitted after office hours. J Paediatr Child Health 2014; 50: E45–E54. [DOI] [PubMed] [Google Scholar]
- 81.Keir A, McPhee A, Wilkinson D. Beyond the borderline: outcomes for inborn infants born at ≤500 grams. J Paediatr Child Health 2014; 50: 146–152. [DOI] [PubMed] [Google Scholar]
- 82.Kent AL, Wright IMR, Abdel-Latif ME. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics 2012; 129: 124–131. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka Y, Uchida H, Kawashima H, et al. Influence of surgical intervention on neurodevelopmental outcome in infants with focal intestinal perforation. Pediatr Int 2015; 57: 687–689. [DOI] [PubMed] [Google Scholar]
- 84.Poon WB, Ho SKY, Yeo CL. Short- and long-term outcomes at 2, 5 and 8 years old for neonates at borderline viability–an 11-year experience. Ann Acad Med Singap 2013; 42: 7–17. [PubMed] [Google Scholar]
- 85.Roberts G, Anderson PJ, Davis N, et al. Developmental coordination disorder in geographic cohorts of 8-year-old children born extremely preterm or extremely low birthweight in the 1990s. Dev Med Child Neurol 2011; 53: 55–60. [DOI] [PubMed] [Google Scholar]
- 86.Blencowe H, Lee AC, Cousens S, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res 2013; 74: 17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Myrhaug HT, Brurberg KG, Hov L, et al. Survival and impairment of extremely premature infants: a meta-analysis. Pediatrics 2019; 143: e20180933. [DOI] [PubMed] [Google Scholar]
- 88.Ding S, Lemyre B, Daboval T, et al. A meta-analysis of neurodevelopmental outcomes at 4-10 years in children born at 22-25 weeks gestation. Acta Paediatr 2019; 108: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 89.Wroblewska-Seniuk K, Dabrowski P, Greczka G, et al. Sensorineural and conductive hearing loss in infants diagnosed in the program of universal newborn hearing screening. Int J Pediatr Otorhinolaryngol 2018; 105: 181–186. [DOI] [PubMed] [Google Scholar]
- 90.Youn Y, Lee SM, Hwang JH, et al. National Registry data from Korean Neonatal Network: two-year outcomes of Korean very low birth weight infants born in 2013–2014. J Korean Med Sci 2018; 33: e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pierrat V, Marchand-Martin L, Marret S, et al. Neurodevelopmental outcomes at age 5 among children born preterm: EPIPAGE-2 cohort study. BMJ 2021; 373: n741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milner KM, Neal EF, Roberts G, et al. Long-term neurodevelopmental outcome in high-risk newborns in resource-limited settings: a systematic review of the literature. Paediatr Int Child Health 2015; 35: 227–242. [DOI] [PubMed] [Google Scholar]
- 93.Jarjour IT. Neurodevelopmental outcome after extreme prematurity: a review of the literature. Pediatr Neurol 2015; 52: 143–152. [DOI] [PubMed] [Google Scholar]
- 94.Guillen U, DeMauro S, Ma L, et al. Relationship between attrition and neurodevelopmental impairment rates in extremely preterm infants at 18 to 24 months: a systematic review. Arch Pediatr Adolesc Med 2012; 166: 178–184. [DOI] [PubMed] [Google Scholar]
- 95.Ding S, Mew EJ, Chee-A-Tow A, et al. Neurodevelopmental outcome descriptions in cohorts of extremely preterm children. Arch Dis Child Fetal Neonatal Ed 2020; 105: 510–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-2-imr-10.1177_03000605211028026 for Global prevalence of long-term neurodevelopmental impairment following extremely preterm birth: a systematic literature review by Sujata P. Sarda, Grammati Sarri and Csaba Siffel in Journal of International Medical Research