Abstract
A 40-year-old female teacher presented to the rheumatology clinic in 2003 with nonspecific back, knees, and right ankle pain. She was subsequently diagnosed with psoriatic arthritis and was taking methotrexate to control her disease. Over the years, her symptoms were mostly under control. However, in 2018, after adopting a whole food plant-based diet free of added salt, oil, and sugar, she was able to stop taking methotrexate. She was discharged from the rheumatology clinic and has remained symptom-free since. The available literature on managing psoriatic arthritis with diet shows that less than 2% of patients with psoriatic arthritis are able to discontinue medication as a result of disease remission. This case report adds to support and encourages further research in the field of managing inflammatory polyarthropathies with diet and lifestyle.
Keywords: psoriatic arthritis, remission, whole food plant-based, vegan, methotrexate
‘A recent review of PsA [psoriatic arthritis] treatment guidelines discusses pharmacological and nonpharmacological therapies, but at present little can be said about the impact of diet and lifestyle changes on PsA progression.’
Psoriatic arthritis (PsA) is an inflammatory musculoskeletal disorder with 5 domains: peripheral arthritis, axial disease, dactylitis, enthesitis, and skin and nails disease. 1 It affects men and women equally with peak age of diagnosis 40 to 50 years. 2 A recent review of PsA treatment guidelines discusses pharmacological and nonpharmacological therapies, but at present little can be said about the impact of diet and lifestyle changes on PsA progression. Smoking cessation, increased physical activity, and weight loss where necessary is all that can confidently be recommended. 3 In this case report, we describe a patient diagnosed with PsA in 2003 who successfully manages her disease with a whole food plant-based diet (WFPBD), having been tapered off methotrexate in 2018.
Case Presentation
In 2003, a 40-year-old female working as a teacher presented to a rheumatology clinic with a 2-month history of nonspecific back pain, as well as pain in both knees and the right ankle. She also reported malaise and unintentional weight loss. Her past medical history revealed a previous episode of back pain in 1994, suspected to be a prolapsed intervertebral disc. The patient denied any recent infections or skin disease. She had been prescribed Rofecoxib 200 mg twice daily by her general practitioner (GP) prior to the referral. This only partially relieved her symptoms. The patient reported a strong family history of rheumatoid arthritis (RA), affecting her mother, grandmother, and great-grandmother. Furthermore, her mother suffered from psoriasis.
On examination, she had a normal body mass index (BMI) with evidence of muscle wasting. There was severe synovitis of the right ankle with swelling and pedal edema. Small effusions were present in both knees, with a full range of movement, however tight.
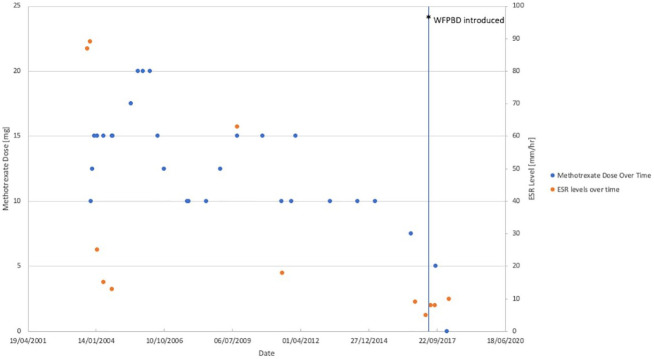
Initial investigations showed raised inflammatory markers with erythrocyte sedimentation rate (ESR) of 87 mm/h and C-reactive protein (CRP) of 128 mg/L. The full blood count, biochemistry results, urate, glucose, and rheumatoid factor were all normal. The patient was not screened for the presence of gluten-related disorders. Serum ESR levels over time are presented in Figure 1.
Figure 1.
Patient’s methotrexate dose and serum ESR (erythrocyte sedimentation rate) level over time. The patient was discharged from Rheumatology clinic in February 2018. Only these results are available from the medical record.
The patient was initially diagnosed with seronegative inflammatory arthropathy but later reclassified as possible psoriatic arthritis in November 2005.
Treatment
In October 2003, treatment was initiated with methotrexate 12.5 mg once per week for 4 weeks, further increased to 15 mg once per week, and celecoxib 200 mg once per day. The patient was slowly improving over time. However, she reported right shoulder pain in February 2004, diagnosed as capsulitis with restriction of glenohumeral movement, and required joint injection with 40 mg of Depo-Medrone (prednisolone). Celecoxib dose was reduced down and finally discontinued in May 2004. For the remainder of 2004, she was in remission with ESR value of 15 mm/h or lower, on 15 mg methotrexate weekly.
In 2005, the patient reported a 6-month period of worsening symptoms during which she experienced neck, left shoulder, low back, hands, and wrists discomfort. Furthermore, she suffered from anterior uveitis of the left eye in September. This was managed by increasing the methotrexate dose to 20 mg and administering steroid injections. She developed mouth ulcers, which resolved completely with a folic acid supplement taken 4 times a week.
A year later, the patient reported symptom improvement associated with dietary modification, specifically avoiding dairy products and red meat. Encouraged by the positive change resulting from eliminating certain food products, she further adjusted her diet to eliminate all animal-derived foods, thus essentially adopting a vegan diet. This allowed for lowering the methotrexate dose to 12.5 mg per week. This was particularly important in her case considering a previous decrease in white cell count while on 20 mg methotrexate weekly. Implementing a gluten-free diet prior to that did not improve the patient’s symptoms.
The patient was doing well on a dose of methotrexate ranging from 10 to 15 mg per week. Particularly interesting was the fact that she noticed significant worsening of the symptoms following the festive season over a few years. She reported prolonged morning stiffness and pain, and blamed alcohol and “unhealthy” food consumption during Christmas (this included deep fried foods, rich in salt and sugar).
In 2009, the patient developed iritis of the right eye. At one point that year her ESR increased to 63 mm/h, possibly due to Bartholin’s gland infection. Later, in 2011 and 2013, the patient reported red patches that appeared on her skin, including scalp, periodically, diagnosed as psoriasis. All this time, there were no tender or swollen joints, no enthesitis or dactylitis. In August 2016, the dose of methotrexate was lowered to 7.5 mg per week; however, any further attempts to reduce the dose resulted in the symptoms of pain and stiffness flaring up.
Finally, in the second half of 2017, the patient decided to introduce even further dietary modifications and adopted a WFPBD. It involved no added salt, sugar, or oil; no refined (white flour, polished rice) or processed foods with added preservatives. She ate a variety of fruits, vegetables, beans, wholegrains, nuts, seeds, herbs, and spices (Table 1). Normally, the patient had 3 meals per day with snacks of fruit in between. In September, the dose of methotrexate was lowered to 5 mg per week. Eventually, in February 2018, the patient stopped taking methotrexate and was discharged from the rheumatology clinic soon after as she did not experience any further symptoms associated with her arthritis. The methotrexate dose administered over the years is depicted in Figure 1. In March 2018, her blood test results showed ESR of 10 mm/h and CRP of 2 mg/L. She has not required blood tests since.
Table 1.
Dietary Pattern Before and After Adopting Whole Food Plant-Based Diet (WFPBD).
| Usual diet before WFPBD | |
|---|---|
| Grains | Pasta, white flour, wholemeal flour, white rice |
| Pulses | Red lentils |
| Vegetables | 2 portions daily; potatoes, carrots, peas, sweetcorn, tomatoes, cucumbers |
| Fruit | 2 portions daily; apples, bananas, oranges |
| Nuts and seeds | None |
| Processed foods | Oil, textured vegetable protein, vegan cheese, vegan sausages, soya cream, vegan mayonnaise, soya margarine, pastries, cakes, biscuits, ice cream, hummus, sugar |
| WFPBD adopted by the patient | |
| Grains | Wholemeal bread, wholemeal pasta, amaranth, millet, oat groats, wholegrain rice, barley, rye, buckwheat |
| Pulses | 2+ servings daily; chickpeas, black beans, kidney beans, peas; occasionally, tofu and tempeh |
| Vegetables | 5+ portions daily; mushrooms, onions, garlic, tomatoes, chives, potatoes, sweet potatoes, carrots, parsnips, artichokes, yakon, radish, pumpkin, cauliflower, broccoli, green leafy vegetables, seaweed |
| Fruit | 5+ portions daily; berries, apples, pears, peaches, bananas, oranges, grapes, mango |
| Nuts and seeds | 1+ portion daily; flaxseed, walnuts, almonds, cashews, peanuts, chia seeds, pumpkin seeds, sesame seeds, sunflower seeds |
| Processed foods | 1 portion daily of soya milk; sauerkraut; occasionally soya sauce, miso |
Outcome and Follow-up
The patient has not been taking methotrexate or any other medications since February 2018, and her PsA has remained dormant. The patient, however, says that she “knows the disease is still there.” She reports an episode when she was making plant-based cupcakes. On tasting a tablespoon of chocolate non-butter cream topping, she immediately felt her fingers stiffen and “lock” around the electric whisk. The patient had to use her other hand to peel back her fingers. This further encouraged her to maintain a WFPBD free of added oil, salt, and sugar to stay symptom-free and pain-free.
Currently, the patient does ceramics and does not have any problems applying pressure on the clay with her fingers. She also runs 10 km regularly and is never limited by her disease. Her BMI has stayed within healthy range with no significant weight loss.
Discussion
The aim of conventional therapy for PsA is to control symptoms and ideally induce remission. A recent meta-analysis of 258 publications, including 114 651 patients, reported a remission rate of 13.1% to 42.1% depending on the method used to measure disease activity. 4 Cure and elimination of medication is not considered possible for the vast majority of patients, with studies reporting a medication discontinuation rate due to sustained remission of only 1.7%. 5 Lifestyle modifications are recommended and concentrate on reducing weight if required, smoking cessation, and regular physical activity. 3 Yet the case presented suggests that dietary modifications may play a role in sustaining remission and aiding the discontinuation of medication.
Our patient followed a WFPBD. It is one that is centered on fruits, vegetables, whole grains, legumes, nuts, and seeds, while avoiding (or minimizing) animal-derived foods and processed foods and includes a vegetarian and vegan diet pattern. This diet pattern is recommended by the American College of Lifestyle Medicine as being optimal for disease prevention and management.6-8 The health benefits of a WFPBD are increasingly recognized and can play a role in the prevention and treatment of cardiovascular disease (CVD),9-11 type 2 diabetes, 12 and certain cancers. 13 Those following a vegetarian or vegan diet also have a lower BMI compared to meat consumers.14,15 Chronic conditions such as CVD or type 2 diabetes occur with increased frequency in patients with PsA and therefore there is the potential to improve long-term health as well. 16
In the field of rheumatology, the association between diet, lifestyle, and disease development and management has been investigated. The most widely studied diet pattern is the Mediterranean diet (MD), which is based on the consumption of whole grains, legumes, fruit, vegetables, nuts, and seeds and also includes regular intake of olive oil, as well as moderate intake of fish and dairy. Its health benefits are well established and are predominately related to the consumption of whole plant foods 17 and in part related to its ability to reduce inflammation. 18 There are limited data on the role of a MD for patients with PsA, but studies do suggest adherence to this diet pattern is beneficial.19,20 The MD has been more extensively studied in RA. It has been shown to potentially lower the incidence of RA but predominantly in men and only for seropositive RA.21,22 Based on the favorable results of interventional studies, it is the most commonly recommended healthy diet pattern for patients. 23 Omega-3 fatty acids are thought to be an important component of the MD and are mostly derived from fish. However, our patient has excluded fish consumption and achieved PsA remission with omega-3 fats obtained exclusively from plant sources. For osteoarthritis (OA), studies have shown a reduction in development, progression, and pain perception in patients adopting a MD.24,25
An exclusively plant-based diet is an attractive option for patients with various forms of arthritis given its anti-inflammatory properties, a consequence of the vast quantities of antioxidant compounds and phytonutrients and their ability to lower markers of inflammation such as CRP.26,27 A few intervention studies have been performed and show promise. A randomized study of a WFPBD in patients with OA was able to show improvement in self-reported pain and function in the intervention group. 28 Fasting followed by the introduction of a low-fat vegan diet has also been shown to be beneficial in PsA, improving symptoms and reducing medication usage. 29 A small study of a low-fat WFPBD in patients with RA resulted in a significant reduction in CRP and rheumatoid factor activity, while also improving symptoms. 30 The other mechanisms that may explain the benefits seen are reduced exposure to dietary antigens, improvement in the health of gut microbiome, and elimination of inflammatory foods in the diet such as red and processed meats.27,31-33
A recent case report has highlighted the impact of fasting followed by the introduction of a WFPBD for a patient with PsA. 29 Our case adds further evidence for the potential of a WFPBD to support patients with PsA and the possibility to eliminate the need for medication. It also provides evidence to support the design of intervention studies to test the efficacy of a WFPBD on a larger scale for this disease.
Learning Points
Few PsA patients stop taking DMARDs (disease-modifying antirheumatic drugs) because of sustained disease remission.
A WFPBD free from refined and processed food, added salt, oil, and sugar led to PsA remission in a 55-year-old lady previously dependent on methotrexate for 15 years.
Adopting a WFPBD may contribute to PsA symptom improvement directly and indirectly (through weight loss) as well as reduce the risk of CVD, which is increased in PsA patients.
Footnotes
Author Contributions: ML was responsible for writing the first draft of the full article. KD reviewed the case description. SK reviewed the discussion.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
ORCID iD: Marta Lewandowska  https://orcid.org/0000-0002-4172-9717
https://orcid.org/0000-0002-4172-9717
Contributor Information
Marta Lewandowska, Royal Surrey County Hospital, Guildford, UK.
Kate Dunbar, Plant-Based Health Professionals UK, Bordon, UK.
Shireen Kassam, King’s College Hospital, London, UK; Winchester University, Hampshire, UK.
References
- 1.Moll JMH, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55-78. [DOI] [PubMed] [Google Scholar]
- 2.Kerschbaumer A, Fenzl KH, Erlacher L, Aletaha D. An overview of psoriatic arthritis—epidemiology, clinical features, pathophysiology and novel treatment targets. Wien Klin Wochenschr. 2016;128:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogdie A, Coates LC, Gladman DD. Treatment guidelines in psoriatic arthritis. Rheumatology (Oxford). 2020;59(suppl 1):i37-i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagège B, Tan E, Gayraud M, Fautrel B, Gossec L, Mitrovic S. Remission and low disease activity in psoriatic arthritis publications: a systematic literature review with meta-analysis. Rheumatology (Oxford). 2020;59:1818-1825. [DOI] [PubMed] [Google Scholar]
- 5.Navarini L, Costa L, Tasso M, et al. Retention rates and identification of factors associated with anti-TNFα, anti-IL17, and anti-IL12/23R agents discontinuation in psoriatic arthritis patients: results from a real-world clinical setting. Clin Rheumatol. 2020;39:2663-2670. [DOI] [PubMed] [Google Scholar]
- 6.Norman K, Klaus S. Veganism, aging and longevity: new insight into old concepts. Curr Opin Clin Nutr Metab Care. 2020;23:145-150. [DOI] [PubMed] [Google Scholar]
- 7.Hever J, Cronise RJ. Plant-based nutrition for healthcare professionals: implementing diet as a primary modality in the prevention and treatment of chronic disease. J Geriatr Cardiol. 2017;14:355-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hever J. Plant-based diets: a physician’s guide. Perm J. 2016;20:15-082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001-2007. [DOI] [PubMed] [Google Scholar]
- 10.Esselstyn CB, Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD. J Fam Pract. 2014;63:356-364b. [PubMed] [Google Scholar]
- 11.Satija A, Bhupathiraju SN, Spiegelman D, et al. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in US adults. J Am Coll Cardiol. 2017;70:411-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57:3640-3649. [DOI] [PubMed] [Google Scholar]
- 14.Spencer EA, Appleby PN, Davey GK, Key TJ. Diet and body mass index in 38 000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int J Obes Relat Metab Disord. 2003;27:728-734. [DOI] [PubMed] [Google Scholar]
- 15.Turner-McGrievy G, Mandes T, Crimarco A. A plant-based diet for overweight and obesity prevention and treatment. J Geriatr Cardiol. 2017;14:369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah K, Paris M, Mellars L, Changolkar A, Mease PJ. Real-world burden of comorbidities in US patients with psoriatic arthritis. RMD Open. 2017;3:e000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lăcătușu CM, Grigorescu ED, Floria M, Onofriescu A, Mihai BM. The Mediterranean diet: from an environment-driven food culture to an emerging medical prescription. Int J Environ Res Public Health. 2019;16:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsigalou C, Konstantinidis T, Paraschaki A, Stavropoulou E, Voidarou C, Bezirtzoglou E. Mediterranean diet as a tool to combat inflammation and chronic diseases. An overview. Biomedicines. 2020;8:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caso F, Navarini L, Carubbi F, et al. Mediterranean diet and psoriatic arthritis activity: a multicenter cross-sectional study. Rheumatol Int. 2020;40:951-958. [DOI] [PubMed] [Google Scholar]
- 21.Johansson K, Askling J, Alfredsson L, Di Giuseppe D; EIRA Study Group. Mediterranean diet and risk of rheumatoid arthritis: a population-based case-control study. Arthritis Res Ther. 2018;20:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y, Costenbader KH, Gao X, Hu FB, Karlson EW, Lu B. Mediterranean diet and incidence of rheumatoid arthritis in women. Arthritis Care Res (Hoboken). 2015;67:597-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gioia C, Lucchino B, Tarsitano MG, Iannuccelli C, Di Franco M. Dietary habits and nutrition in rheumatoid arthritis: can diet influence disease development and clinical manifestations? Nutrients. 2020;12:1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales-Ivorra I, Romera-Baures M, Roman-Viñas B, Serra-Majem L. Osteoarthritis and the Mediterranean diet: a systematic review. Nutrients. 2018;10:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veronese N, Koyanagi A, Stubbs B, et al. Mediterranean diet and knee osteoarthritis outcomes: a longitudinal cohort study. Clin Nutr. 2019;38:2735-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haghighatdoost F, Bellissimo N, Totosy De, Zepetnek JO, Rouhani MH. Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2017;20:2713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alwarith J, Kahleova H, Rembert E, et al. Nutrition interventions in rheumatoid arthritis: the potential use of plant-based diets. A review. Front Nutr. 2019;6:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinton CM, O’Brien S, Law J, Renier CM, Wendt MR. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis. 2015;2015:708152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frey AR, Frey J. Case study: water-only fasting and an exclusively whole plant foods diet in the management of psoriatic arthritis. Int J Dis Reversal Prev. 2020;2:7. [Google Scholar]
- 30.McDougall J, Bruce B, Spiller G, Westerdahl J, McDougall M. Effects of a very low-fat, vegan diet in subjects with rheumatoid arthritis. J Altern Complement Med. 2002;8:71-75. [DOI] [PubMed] [Google Scholar]
- 31.Khanna S, Jaiswal KS, Gupta B. Managing rheumatoid arthritis with dietary interventions. Front Nutr. 2017;4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomova A, Bukovsky I, Rembert E, et al. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr. 2019;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horta-Baas G, Romero-Figueroa MDS, Montiel-Jarquín AJ, Pizano-Zárate ML, García-Mena J, Ramírez-Durán N. Intestinal dysbiosis and rheumatoid arthritis: a link between gut microbiota and the pathogenesis of rheumatoid arthritis. J Immunol Res. 2017;2017:4835189. [DOI] [PMC free article] [PubMed] [Google Scholar]